Introduction
Gastric cancer (GC) is a highly heterogeneous
malignant disease characterized by a unique pattern of genome
driver aberrations. Some of these aberrations are used to predict
development of the disease or guide therapy (1–3). For
example, overexpression of human epidermal growth factor receptor 2
(HER2) is detected in GC and can be considered a novel
therapeutic agent (4–6). However, the genome aberration profile
can change throughout the course of therapy, and many patients with
GC develop acquired drug resistance along with tumor evolution
(7,8). Detecting variations prior to and during
therapy is therefore crucial to improve patient outcome. However,
repeated invasive tissue biopsies of GC are not feasible due to the
clinical risk of tumor spread. Cell-free circulating tumor DNA
(ctDNA) has attracted increasing attention and may be considered a
potential tumor marker. In addition, its detection is convenient
and non invasive (9). Analysis of
ctDNA presents therefore a potentially clinical prospect in the
treatment and auxiliary diagnosis of GC.
Numerous approaches, including the BEAMing (beads,
emulsion, amplification, and magnetics) method, the Scorpion ARMS
method that detects epidermal growth factor receptor aberration and
the droplet digital polymerase chain reaction method that detect
HER2 amplification, have been successfully used to identify
ctDNA aberrations in patients with various types of cancer
(10–15). Furthermore, a previous study using
next generation sequencing (NGS) to detect ctDNA in the bloodstream
of patients with GC has identified concordant variations between
ctDNA and tumor DNA (tDNA); however, this study only primarily
focused on a small cohort of genes, including tumor protein p53
(TP53) (16). However, due to
the high heterogeneity of GC, numerous genes may be involved and
available for analysis. To explore the association between ctDNA
and the clinical characteristics of patients with GC, the present
study used a targeted capture sequencing method to detect
variations at known hot-spot loci of 545 cancer-associated genes in
tumor and plasmatic ctDNA from nine patients with GC.
Materials and methods
Patients and samples
The present study was approved by the Ethics
Committees of the First Affiliated Hospital of Soochow University.
All patients provided written informed consent for the use of their
blood and tumor samples. Nine patients diagnosed with advanced GC
and received surgery or palliative surgery were involved in this
study. Tumor staging was performed according to the 7th American
Joint Committee on Cancer (AJCC) TNM system (17). All samples and medical data used in
this study have been irreversibly anonymized. Gastric tumor and
plasma samples from nine patients with GC were analyzed (Table I). All 9 patients, including six men
and three women, were diagnosed with adenocarcinoma. Smoking
history was not assessed. Tumor tissues obtained from biopsies
taken at diagnosis or during surgery were fixed in formalin at room
temperature for 6–48 h, then embedded in paraffin as previously
described (18). HER2
Immunohistochemistry (IHC) was carried out on formalin-fixed, 5-µm
thick, paraffin-embedded (FFPE) tissue sections (Ventana; Roche)
using a pre-diluted antibody (ready to use) of monoclonal rabbit
PATHWAY anti-HER2 (4B5; Bench Mark GX; Roche Diagnostics K.K.).
Briefly, the FFPE sections were deparaffinized. After cell
conditioning, it was incubated with primary monoclonal rabbit
PATHWAY anti-HER2 at 37°C for 30 min. Counterstaining was performed
by incubation with hematoxylin at room temperature for 8 min,
followed by incubation with building reagent for 12 min. Staining
was scored as follows: 0, no membrane staining or no reactivity;
+1, cancer cell cluster with a barely/faint perceptible membranous
reactivity; +2, tumor cell cluster with a weak to moderate
complete, basolateral, or lateral membranous reactivity; +3, tumor
cell cluster with a strong complete, basolateral, or lateral
membranous reactivity. Tissues with a score of +3, or +2 in
addition to fluorescence in situ hybridization (FISH) positivity,
were considered as HER2 positive. Peripheral blood samples were
collected from patients one week prior to surgery.
 | Table I.Clinicopathological characteristics
of the nine patients with gastric cancer. |
Table I.
Clinicopathological characteristics
of the nine patients with gastric cancer.
|
Characteristics | Number (%) |
|---|
| Age (years) |
|
| Mean
(SD) | 62.89 (9.27) |
| Median
(range) | 64 (46–77) |
| Sex |
|
|
Male | 3 (33.33%) |
|
Female | 6 (66.67%) |
| Pathological
diagnosis |
|
| Gastric
adenocarcinoma | 9 (100%) |
| Tumor stage |
|
| II | 3 (33.33%) |
|
III | 5 (55.56%) |
| IV | 1 (11.11%) |
Sample processing and DNA
extraction
Two types of samples were collected from each
patient, tumor tissue (fresh and FFPE) and 20 ml peripheral blood
(PB) prior to surgery. DNA was extracted from fresh tissue using
E.Z.N.A. Tissue DNA kit (Omega Bio-Tek), and from FFPE tissue using
QIAamp DNA FFPE Tissue kit (Qiagen) according to the manufacturer's
instructions. EDTA tubes containing blood samples were centrifuged
for 10 min at 1,000 × g at 4°C. Cell layer containing peripheral
blood lymphocytes (PBLs) was collected and transferred into 1-ml
Eppendorf tubes and stored at −20°C until further use. Supernatants
were further centrifuged at 10,000 × g at 4°C for 10 min and plasma
was collected and stored at −80°C until further use. DNA from PBLs
was extracted using the E.Z.N.A. Blood DNA kit (Omega Bio-Tek),
whereas ctDNA was extracted from at least 1 ml plasma using QIAamp
Circulating Nucleic Acid kit (Qiagen) following the manufacturer's
instructions. DNA was quantified with the Qubit 2.0 Fluorometer and
the Qubit dsDNA HS Assay kit (Life Technologies; Thermo Fisher
Scientific, Inc.) according to the recommended protocols.
Sequencing library construction and
target enrichment
DNA (1 µg) from tissue and PBLs was cropped into
300-bp fragments with a Covaris S2 ultrasonicator as previously
described (19). Libraries of DNA
from tissue, PBLs germline and circulating DNA were prepared with
the KAPA Library Preparation kit (Kapa Biosystems) according to the
manufacturer's protocol. A custom SeqCap EZ Library (Roche
NimbleGen, Inc.) was designed for targeted capture. To explore the
comprehensive genetic properties of GC, the capture probe was
designed according to genomic regions (total approximately 1.7 Mb
in size, data not shown) of the 545 genes most frequently mutated
in gastric tumor and other common solid tumors. Capture
hybridization was carried out according to the manufacturer's
protocol.
NGS sequencing
Sequencing was carried out using Illumina 2×100 bp
paired-end reads on an Illumina HiSeq 3000 instrument according to
the manufacturer's recommendations and using TruSeq PE Cluster
Generation Kit v3 and the TruSeq SBS Kit v3 (Illumina, Inc.).
Analysis of sequencing data
After removal of terminal adaptor sequences and
low-quality data, reads were mapped to the reference human genome
and aligned as previously described (19). The Genome Analysis Toolkit
(https://www.broadinstitute.org/gatk/)
and MuTect (20) were used to call
somatic small insertions and deletions and single nucleotide
variants by filtering PBL germline mutations. The following somatic
mutations obtained were further filtered as follows: i) All
mutations from tissues and plasma should present ≥5 and ≥2 mutated
reads, respectively; ii) the frequency of mutations in tissue
should be ≥3%; and iii) mutated reads of each mutations should be
observed on both strands. Copy number variations (CNV) were
generated using Copy Number Targeted Resequencing Analysis
(http://contra-cnv.sourceforge.net;
version 2.0.3) (21). BreakDancer
algorithm was used to detect tumor-associated structure variations
(22). The final candidate variants
were manually verified with the Integrative Genomics Viewer (IGV)
browser (https://software.broadinstitute.org/software/igv)
(23). COSMIC database (https://cancer.sanger.ac.uk/cosmic) was used to
determine the occurrence of variants.
Statistical analysis
Pearson's correlation and one-way ANOVA followed by
Bonferroni correction post-hoc test were performed using SPSS
software (version 16.0; SPSS, Inc.) to analyze the correlation
between ctDNA fraction and clinical characteristics, including
metastasis lymph node number and lactate dehydrogenase (LDH)
content, as previously described (24). P<0.05 was considered to indicate a
statistically significant difference.
Results
Sequencing coverage of the target
region
Of all nine paired samples, capture sequencing data
demonstrated a mean coverage of 904× in tissues (ranging from 275×
to 1,255×; data not shown) and of 1,375× in plasmas (ranging from
965× to 2,203×; data not shown). Furthermore, approximately 99% of
the target region was covered at >20×. For each sample type, the
gene coverage was uniformly distributed, with >167× in 92.73% of
tissue genes and >500× in 87.71% of plasma genes (data not
shown). In this case, mutations of most genes in both sample types
could possess at least 5 support reads at a frequency of
approximately 1% in plasma or approximately 3% in tissue.
Somatic mutations in tissue and plasma
samples
Somatic mutations were detected in all tissues and
matched plasma samples (100%). The number of non-synonymous somatic
mutations detected in tissues ranged from 2 to 46, with a mean
value of 16. The mean variant allele fraction (VAF) was 18.85%. In
plasma, a total of 80 non-synonymous somatic mutations were
detected, with a mean VAF of 1.90%. Among all mutations, 32
mutations in tissues and 17 mutations in plasma were confirmed in
COSMIC database.
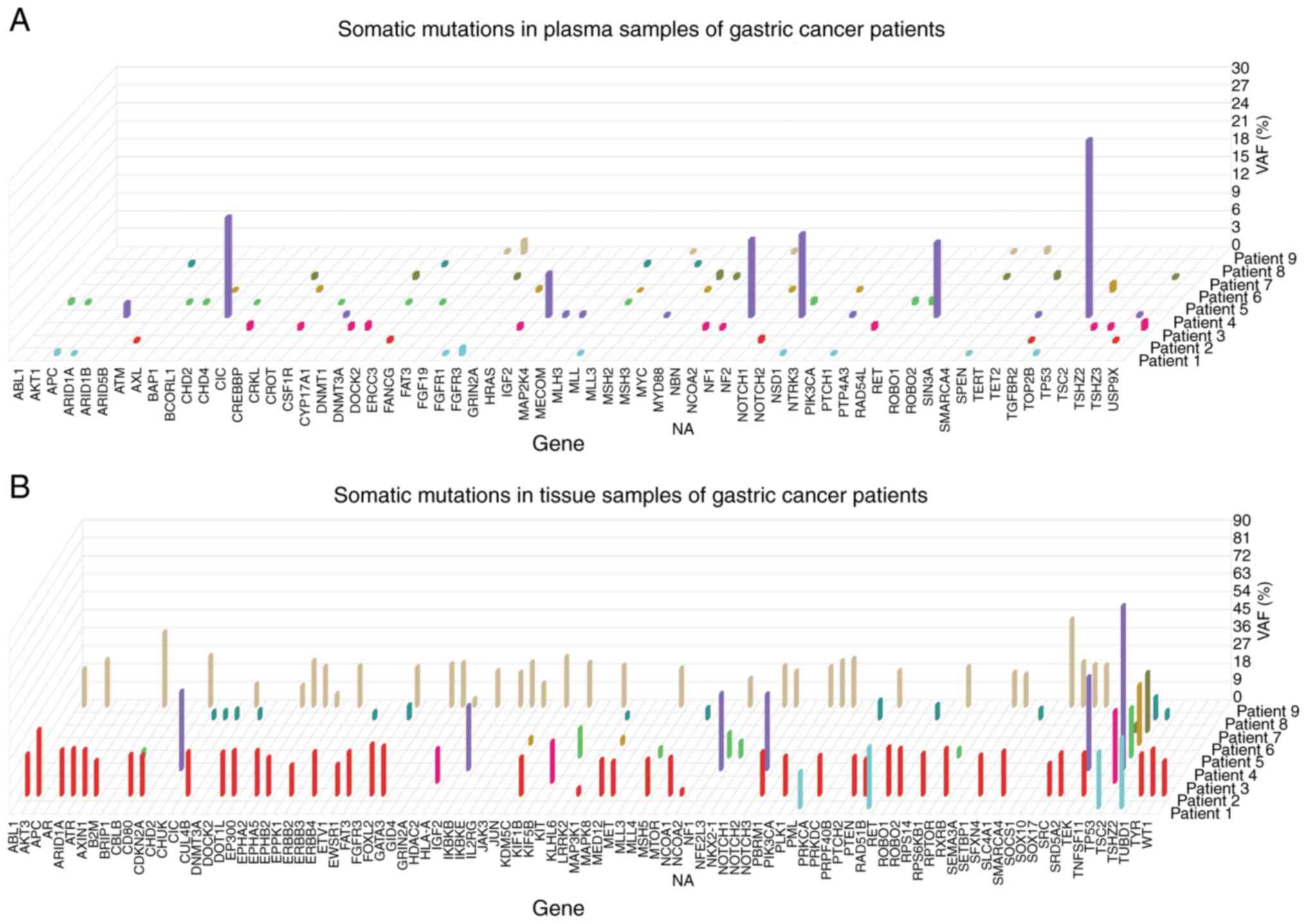
Mutation spectrums of the 9 GC tissues revealed
great inter-individual tumor genetic heterogeneity (Fig. 1). Notably, seven patients (78%)
presented TP53 gene mutations, which occurred at six
different amino acid positions (p.T211Nfs*5, p.C176F, p.P190L,
p.R213*, p.E271V and p.G245S). However, the structure variations
were not detected in tissues and plasma samples.
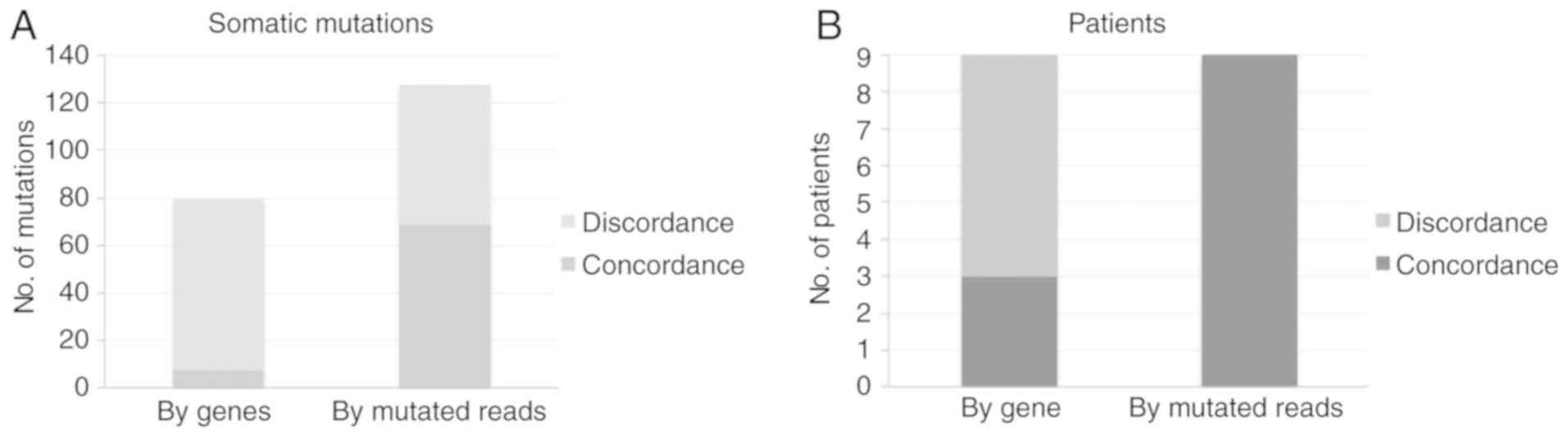
Mutation concordance between tissue
and plasma
In all detected non-synonymous somatic mutations,
capture sequencing identified a total of eight concordant mutations
in both tissue and plasma samples in four of the nine patients with
GC (44%). Notably, in patient 4, who was the only patient diagnosed
with distant metastasis, five out of six tumor-derived mutations
were found in plasma ctDNA. In addition, the results from further
analysis of plasma samples sequencing data demonstrated that 45% of
mutation in tissue presented concordant mutation in the plasma
ctDNA of all patients (Fig. 2).
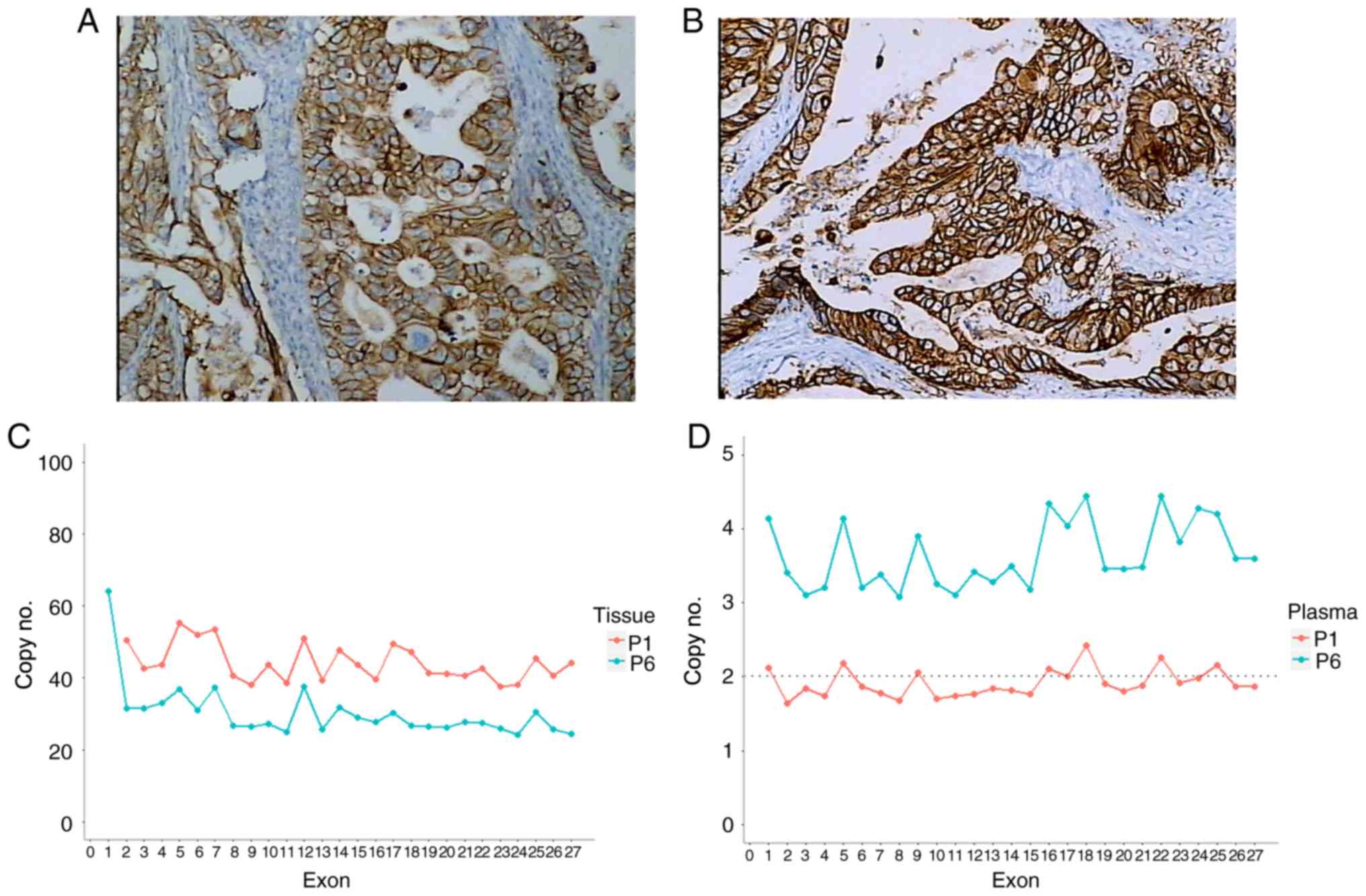
CNV amplification of HER2 in FFPE and
plasma samples
Prior to sequencing, immunohistochemistry (IHC) was
performed on GC FFPE to assess HER2 expression. The results
demonstrated that only two patients (22.22%) expressed HER2
(Fig. 3). Based on capture
sequencing, CNV of HER2 was analyzed in tissue and matched
plasma by comparing reads depth with PBL. Significant copy number
gains of HER2 in tissue samples was detected in these two
patients (22.22%) [patient no. 1 (P1), copy no.=46.2, P<0.01;
patient no. 6 (P6), copy no.=30.3, P<0.01]. Other CNV negative
results were in accordance with IHC assess (25). Furthermore, only P6 presented a
significant HER2 gene amplification in plasma ctDNA
(P<0.01), and the fold-change of copy no. was only 3.6. In
addition, analysis of plasma ctDNA from P1 demonstrated relative
depth of all HER2 exons that fluctuated around 2.
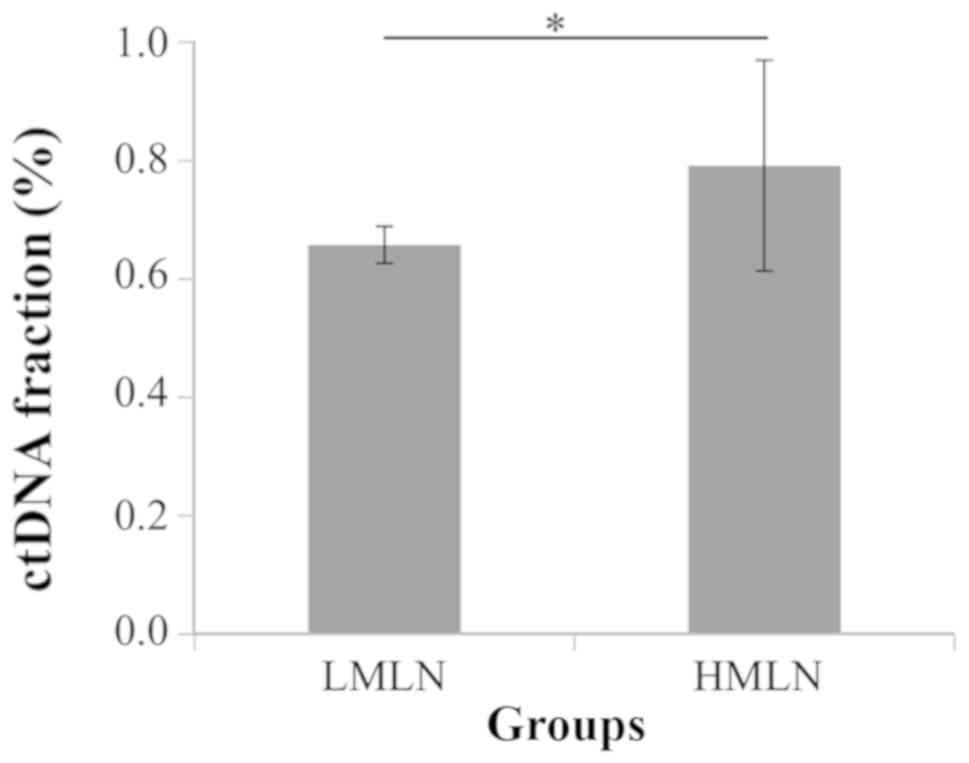
Correlation between ctDNA fraction and
clinical characteristics of patients with GC
The correlation between ctDNA fraction and clinical
characteristics of patients with GC was analyzed. Based on the
number of metastasis lymph nodes, patients were divided into two
groups, a low metastasis lymph node (LMLN) group including N1 and
N2 patients and a high metastasis lymph node (HMLN) group including
N3 patients. The mean of ctDNA fraction in HMLN group was
significantly higher than in LMLN group (P=0.03; Fig. 4). In addition, the ctDNA fraction and
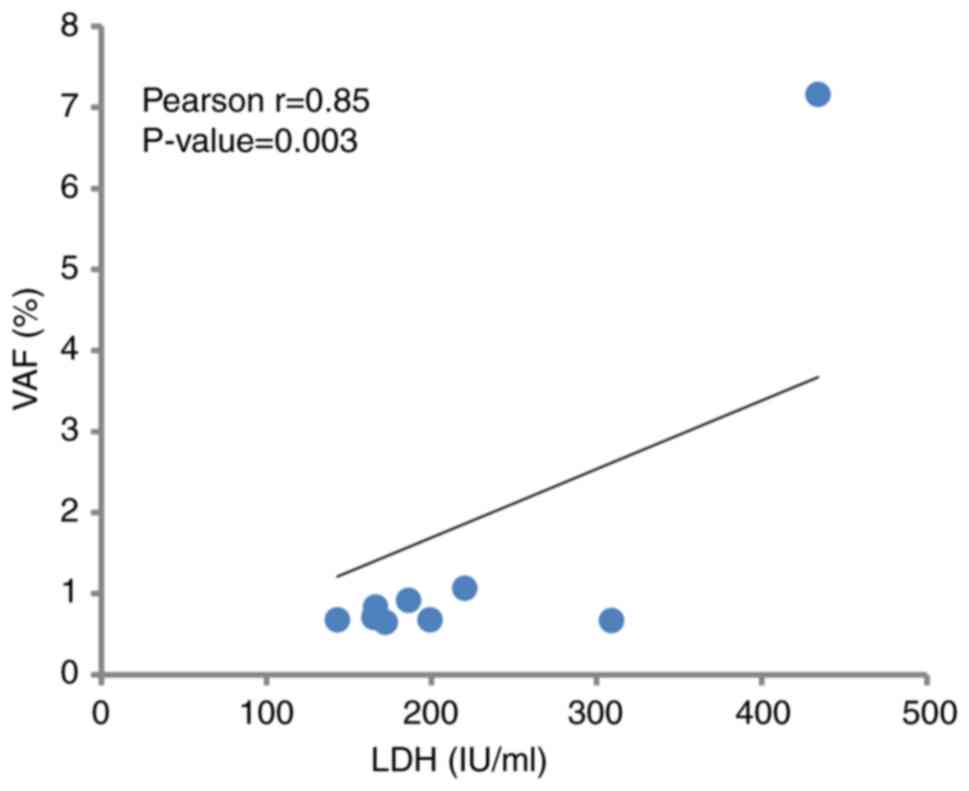
the LDH level were positively correlated in all groups (r=0.85;
P=0.003; Fig. 5).
Discussion
Targeted capture sequencing is an economical and
effective method used to explore the genomic characteristic
(26–28). By using capture sequencing of 545
genes at a mean depth of 904× in tissues and 1375× in plasma, the
present study reported numerous inter-individual molecular
differences among patients with GC. Mutations frequently occurred
on TP53 gene and occurred at six different amino acid
positions, which suggested that this PCR-based method could only be
applied in a limited number of patients with hot-spot mutations.
However, capture sequencing, whole exome sequencing or whole genome
sequencing may be more suitable to identify cancer mutations, and
would decrease the cost of sequencing (29).
The present study detected the mutation in plasma
samples of patients with GC in a non-invasive way. The results
demonstrate that 45% mutations in paired GC tissues presented
concordant mutated reads in plasma samples from all 9 patients.
Furthermore, additional de novo mutations in the DNA in the
plasma can be induced by spatial heterogeneity of the lesion
(30). A previous study reported
that, in a case of metastatic breast cancer, multiregional tumor
biopsies vary from each other, and that ctDNA present the mutations
of both primary tumor and metastases (31). Similarly, a study revealed that ctDNA
contains variations from heterogeneous regions in the primary lung
cancer lesion (32). Notably, in the
only stage IV patient (P6) with distant metastasis from the present
study, the consistency of mutations in plasma and tissue was of
83%, which may be due to the high ctDNA level of patients with
distant metastasis (33). This
result indicated that the non-invasive ctDNA detection may offer
more benefit in late-stage patients.
One crucial purpose of molecular diagnosis in
patients with cancer is to determine sensitive drug targets
(34), including HER2, which
could be specifically bound by herceptin, which is a monoclonal
antibody used in anticancer therapy (35). The present study identified two
true-positive HER2 CNV in patients. Regarding plasma
samples, despite the high dilution of cfDNA, the true positive
HER2 gain was detected in one case, which suggested that
non-invasive ctDNA analysis in CNV is a viable method to determine
target drugs for patients with GC. However, it is crucial to
improve the sensitivity of ctDNA CNV detection.
The correlation between ctDNA fraction and clinical
characteristics from patients with GC was determined. The results
demonstrated that ctDNA fraction was abundant in patients with more
metastasis lymph nodes. This result suggested that metastasis
ability of tumor may be associated with ctDNA fraction in plasma.
In addition, this result further explained the high ctDNA level
observed in one case of stage IV GC (P6), which caused the high
consistency of mutations between ctDNA and tDNA. Future studies
should involve the detection of more clinical serum biomarker,
including carcinoembryonic antigen, CA19-9 and HER2
expression level (36,37). Detection of ctDNA as a biomarker has
been considered a sensitive and specific method in the prognosis
and monitoring of breast and colorectal cancers (38,39).
However, ctDNA were not monitored for disease progression or
remission, following surgery, and were not investigated following
chemotherapy. This was an inevitable limitation of the present
study. Since such investigation has not been made in GC, future
study will involve ctDNA monitoring following treatment in GC. The
results from the present study need to be further confirmed in a
larger patient population. This could provide important findings on
the use of ctDNA in GC. As a promising tool, the noninvasive
detection of ctDNA may represent a promising tool in the individual
treatment and monitoring of patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 81702048) and the Suzhou
Science and Technology Bureau project (grant no. SYS201609).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ZY and HQ designed the study. JL and ZY collected
samples and clinical data. JL, YL, YG and LC peformed the analysis
and interpretation of the data. JL, YL, YG and LC wrote the
manuscript. All authors contributed to the drafting and revision of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Soochow University.
All patients provided written informed consent prior to the study
start.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roviello G, Ravelli A, Polom K, Petrioli
R, Marano L, Marrelli D, Roviello F and Generali D: Apatinib: A
novel receptor tyrosine kinase inhibitor for the treatment of
gastric cancer. Cancer Lett. 372:187–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Espelin CW, Leonard SC, Geretti E, Wickham
TJ and Hendriks BS: Dual HER2 targeting with trastuzumab and
liposomal-encapsulated doxorubicin (MM-302) demonstrates
synergistic antitumor activity in breast and gastric cancer. Cancer
Res. 76:1517–1527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen K, Yang D, Li X, Sun B, Song F, Cao
W, Brat DJ, Gao Z, Li H, Liang H, et al: Mutational landscape of
gastric adenocarcinoma in Chinese: Implications for prognosis and
therapy. Proc Natl Acad Sci USA. 112:1107–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park H, Cho SY, Kim H, Na D, Han JY, Chae
J, Park C, Park OK, Min S, Kang J, et al: Genomic alterations in
BCL2L1 and DLC1 contribute to drug sensitivity in gastric cancer.
Proc Natl Acad Sci USA. 112:12492–12497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hutchinson L: Biomarkers:
ctDNA-identifying cancer before it is clinically detectable. Nat
Rev Clin Oncol. 12:3722015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taniguchi K, Uchida J, Nishino K, Kumagai
T, Okuyama T, Okami J, Higashiyama M, Kodama K, Imamura F and Kato
K: Quantitative detection of EGFR mutations in circulating tumor
DNA derived from lung adenocarcinomas. Clin Cancer Res.
17:7808–7815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinugasa H, Nouso K, Tanaka T, Miyahara K,
Morimoto Y, Dohi C, Matsubara T, Okada H and Yamamoto K: Droplet
digital PCR measurement of HER2 in patients with gastric cancer. Br
J Cancer. 112:1652–1655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HR, Lee SY, Hyun DS, Lee MK, Lee HK,
Choi CM, Yang SH, Kim YC, Lee YC, Kim SY, et al: Detection of EGFR
mutations in circulating free DNA by PNA-mediated PCR clamping. J
Exp Clin Cancer Res. 32:502013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douillard JY, Ostoros G, Cobo M, Ciuleanu
T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T
and McCormack R: Gefitinib treatment in EGFR mutated caucasian
NSCLC: Circulating-free tumor DNA as a surrogate for determination
of EGFR status. J Thorac Oncol. 9:1345–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamakawa T, Kukita Y, Kurokawa Y, Miyazaki
Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Taniguchi K,
Takiguchi S, et al: Monitoring gastric cancer progression with
circulating tumour DNA. Br J Cancer. 112:352–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shoda K, Ichikawa D, Fujita Y, Masuda K,
Hiramoto H, Hamada J, Arita T, Konishi H, Komatsu S, Shiozaki A, et
al: Monitoring the HER2 copy number status in circulating tumor DNA
by droplet digital PCR in patients with gastric cancer. Gastric
Cancer. 20:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kukita Y, Matoba R, Uchida J, Hamakawa T,
Doki Y, Imamura F and Kato K: High-fidelity target sequencing of
individual molecules identified using barcode sequences: De novo
detection and absolute quantitation of mutations in plasma
cell-free DNA from cancer patients. DNA Res. 22:269–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Washington K: 7th Edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagahashi M, Shimada Y, Ichikawa H,
Nakagawa S, Sato N, Kaneko K, Homma K, Kawasaki T, Kodama K, Lyle
S, et al: Formalin-fixed paraffin-embedded sample conditions for
deep next generation sequencing. J Surg Res. 220:125–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X, Chu Y, Zhang R, Han Y, Zhang L, Fu
Y, Li D, Peng R, Li D, Ding J, et al: Technical validation of a
next-generation sequencing assay for detecting clinically relevant
levels of breast cancer-related single-nucleotide variants and copy
number variants using simulated cell-free DNA. J Mol Diagn.
19:525–536. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES,
Getz G, et al: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–219. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Lupat R, Amarasinghe KC, Thompson
ER, Doyle MA, Ryland GL, Tothill RW, Halgamuge SK, Campbell IG and
Gorringe KL: CONTRA: Copy number analysis for targeted
resequencing. Bioinformatics. 28:1307–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen K, Wallis JW, McLellan MD, Larson DE,
Kalicki JM, Pohl CS, McGrath SD, Wendl MC, Zhang Q, Locke DP, et
al: BreakDancer: An algorithm for high-resolution mapping of
genomic structural variation. Nat Methods. 6:677–681. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson JT, Thorvaldsdottir H, Winckler
W, Guttman M, Lander ES, Getz G and Mesirov JP: Integrative
genomics viewer. Nat Biotechnol. 29:24–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan ZY, Gao SG, Mu JW, Xue Q, Mao YS,
Wang DL, Zhao J, Gao YS, Huang JF and He J: Prognostic value of
preoperative serum lactate dehydrogenase in thymic carcinoma. J
Thoracic Dis. 8:2464–2472. 2016. View Article : Google Scholar
|
|
25
|
Wang Y, Zhao C, Chang L, Jia R, Liu R,
Zhang Y, Gao X, Li J, Chen R, Xia X, Bulbul A, et al: Circulating
tumor DNA analyses predict progressive disease and indicate
trastuzumab-resistant mechanism in advanced gastric cancer.
EBioMedicine. 43:261–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu J, Wu WK, Li X, He J, Li XX, Ng SS, Yu
C, Gao Z, Yang J, Li M, et al: Novel recurrently mutated genes and
a prognostic mutation signature in colorectal cancer. Gut.
64:636–645. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voss MH, Hakimi AA, Pham CG, Brannon AR,
Chen YB, Cunha LF, Akin O, Liu H, Takeda S, Scott SN, et al: Tumor
genetic analyses of patients with metastatic renal cell carcinoma
and extended benefit from mTOR inhibitor therapy. Clin Cancer Res.
20:1955–1964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drilon A, Wang L, Arcila ME,
Balasubramanian S, Greenbowe JR, Ross JS, Stephens P, Lipson D,
Miller VA, Kris MG, et al: Broad, Hybrid Capture-based
next-generation sequencing identifies actionable genomic
alterations in lung adenocarcinomas otherwise negative for such
alterations by other genomic testing approaches. Clin Cancer Res.
21:3631–3639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murtaza M, Dawson SJ, Pogrebniak K, Rueda
OM, Provenzano E, Grant J, Chin SF, Tsui DWY, Marass F, Gale D, et
al: Multifocal clonal evolution characterized using circulating
tumour DNA in a case of metastatic breast cancer. Nat Commun.
6:87602015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacoby MA, Duncavage EJ and Walter MJ:
Implications of tumor clonal heterogeneity in the era of
next-generation sequencing. Trends Cancer. 1:231–241. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abbosh C, Birkbak NJ, Wilson GA,
Jamal-Hanjani M, Constantin T, Salari R, Le Quesne J, Moore DA,
Veeriah S, Rosenthal R, et al: Phylogenetic ctDNA analysis depicts
early-stage lung cancer evolution. Nature. 545:446–451. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wan JCM, Massie C, Garcia-Corbacho J,
Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R and Rosenfeld N:
Liquid biopsies come of age: Towards implementation of circulating
tumour DNA. Nat Rev Cancer. 17:223–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Phallen J, Sausen M, Adleff V, Leal A,
Hruban C, White J, Anagnostou V, Fiksel J, Cristiano S, Papp E, et
al: Direct detection of early-stage cancers using circulating tumor
DNA. Sci Transl Med. 9(pii): eaan24152017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zehir A, Benayed R, Shah RH, Syed A,
Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM, et
al: Mutational landscape of metastatic cancer revealed from
prospective clinical sequencing of 10,000 patients. Nat Med.
23:703–713. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Müller V, Clemens M, Jassem J, Al-Sakaff
N, Auclair P, Nüesch E, Holloway D, Shing M and Bang YJ: Long-term
trastuzumab (Herceptin®) treatment in a continuation
study of patients with HER2-positive breast cancer or HER2-positive
gastric cancer. BMC Cancer. 18:2952018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oyama K, Fushida S, Tsukada T, Kinoshita
J, Watanabe T, Shoji M, Nakanuma S, Okamoto K, Sakai S, Makino I,
et al: Evaluation of serum HER2-ECD levels in patients with gastric
cancer. J Gastroenterol. 50:41–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi HZ, Wang YN, Huang XH, Zhang KC, Xi
HQ, Cui JX, Liu GX, Liang WT, Wei B and Chen L: Serum HER2 as a
predictive biomarker for tissue HER2 status and prognosis in
patients with gastric cancer. World J Gastroenterol. 23:1836–1842,
2017.30. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dawson SJ, Tsui DW, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tie J, Wang Y, Tomasetti C, Li L, Springer
S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al:
Circulating tumor DNA analysis detects minimal residual disease and
predicts recurrence in patients with stage II colon cancer. Sci
Transl Med. 8:346ra3922016. View Article : Google Scholar
|