Introduction
As the most common type of cancer that is developed
from the nuclear layer of the retina, retinoblastoma (Rb) mainly
affects children before the age of 5-years-old (1). In numerous regions of the world,
especially developing countries, most Rb cases are diagnosed at
advanced stages and the mortality rate is high (2). It has been estimated that even more Rb
patients will die due to this disease in developing countries after
treatment (2,3). At present, external-beam radiotherapy
and enucleation are the main therapeutic approaches for Rb
(4). In addition, chemotherapy has
also been developed to treat extraocular and metastatic Rb
(5,6). However, treatment outcomes are
generally poor.
PTEN signaling is a well-known tumor-suppressive
pathway in different types of cancer (7). The PTEN pathway is usually inactivated
during cancer progression and the re-activation of this signaling
is considered to be a promising approach for cancer therapies
(8). In cancer biology, PTEN
suppresses cancer progression mainly by inhibiting the phosphatidyl
inositide 3 kinase (PI3K)-protein kinase B (Akt) pathway, which is
a major activated survival pathway in cancer cells (9). However, the upstream regulator of PTEN
has not been well studied. In a recent study, Hu et al
(10) reported a novel long
noncoding (lnc)RNA named placenta-specific 2 (PLAC2) as a novel
inhibitor of cell cycle progression in glioma. PLAC2 participates
in glioma by interacting with signal transducer and activator of
transcription 1 (STAT1), which has crosstalk with PTEN (11). However, the interaction between PLAC2
and PTEN has not been reported. Therefore, this study was carried
out to investigate the involvement of PLAC2 in Rb, as well as its
possible interaction with PTEN.
Materials and methods
Study subjects
A total of 89 Rb patients were admitted by Shanghai
Ninth People's Hospital between June 2016 and December 2018. The
present study selected 60 Rb cases (sex: 33 males and 27 females;
age: 11 months to 4.2 years, 2.2±0.4 years) based on strict
criteria. Inclusion criteria: i) Newly diagnosed Rb cases; ii) no
initiated therapies were observed. Exclusion criteria: i) Therapies
were carried out before this study; ii) recurrent Rb; iii) other
clinical disorders were diagnosed; iv) histories of previous
malignancies. Based on clinical findings, there were 12, 11, 15, 10
and 12 cases at group A-E (International Classification for
Intraocular Retinoblastoma), respectively. Group A, tumors within
the retina <3 mm; Group B, tumors within the retina >3 mm;
Group C, minor tumor spread within the back of the eye; Group D,
tumor spread throughout the back of the eye; Group E, tumor spread
to lens, or causes increased eye pressure, or causes bleeding from
the eye. All patients' guardians were informed with the
experimental details and they all signed informed consent. The
aforementioned hospital Ethics Committee approved this study.
Tissue specimens and cells
Non-tumor (within 2 cm around the tumor site) and Rb
tissues were obtained from each patient by biopsy. All the tissues
were checked by at least 3 pathologists to make sure all the
specimens were correct (cancer cell percentage in non-tumor tissues
should be below 1%).
For in vitro experiments, human Rb cell lines
Y79 and WERI-Rb-1 (American Type Culture Collection) were used.
Cells culture conditions were 5% CO2 and 37°C. The cell
culture medium was RPMI-1640 Medium (20% fetal bovine serum).
Transient transfections
PLAC2 and PTEN expression vectors were constructed
using pcDNA3 (Sangon Biotech Co., Ltd.). PTEN small interfering
(si)RNA (5′-UAGCAGAAACAAAAGGAGAUAUC-3′) and negative control siRNA
(5′-GUCGUCAAAGUCAGGUACACCGA-3′) were from Shanghai GenePharma Co.,
Ltd. Y79 and WERI-Rb-1 cells were collected at the confluence of
70–80%. Nucleofector™ Technology (Lonza Group, Ltd.) was used to
transfect 10 nM PLAC2 and PTEN expression vector, 10 empty pcDNA3
vectors negative control (NC), 35 nM PTEN siRNA, or 35 nM NC siRNA
were transfected into 105 cells. The control group
included cells with no transfections. Subsequent experiments were
performed at 24 h post-transfections.
Reverse transcription-quantitative
(RT-q)PCR
Ribozol (Thermo Fisher Scientific., lnc.) was mixed
with Y79 and WERI-Rb-1 cells (1 ml per 105 cells) and
tissues (0.5 ml per 0.02 g tissue) to extract total RNAs. All RNA
samples were digested with DNase I. AMV Reverse Transcriptase XL
(Clontech Laboratories, Inc.) was used to perform reverse
transcription by incubating at 25°C for 10 min, 55°C for 20 min and
80°C for 10 min. SYBR Green Master Mix (Bio-Rad Laboratories, Inc.)
was used to prepare qPCR reaction mixtures. The expression of PLAC2
and PTEN was detected using 18S rRNA or GAPDH as endogenous
control, respectively. Reaction conditions were: 95°C for 1 min,
followed by 40 cycles of 95°C for 10 sec and 60°C for the 50 sec.
It is worth noting that multiple primers were used and similar
results were obtained. Primer sequences were:
5′-CGGCTACTAGCGGTTTTAC-3′ (forward) and 5′-AAGAAGATGCGGCTGACTG-3′
(reverse) for GAPDH; 5′-TGTGGCCCAAACTCAGGGATACA-3′ (forward) and
5′-GATGACAGTGGCTGGAGTTGTC-3′ for PLAC2 (reverse);
5′-GAGTTCCCTCAGCCGTTACCT-3′ (forward) and
5′-AGGTTTCCTCTGGTCCTGGTA-3′ for (reverse) PTEN mRNA;
5′-GCTTAATTTGACTCAACACGGG-3′ (forward) and
5′-GCTATCAATCTGTCAATCCTGTC-3′ for (reverse) 18S rRNA. All
experiments were repeated 3 times and data were processed using the
2−ΔΔCq method (12). The
sample with the highest ΔCq value was set to ‘1’, all other samples
were normalized to this sample.
Western blotting
Y79 and WERI-Rb-1 cells were collected at 24 h
post-transfections and 1 ml RIPA solution (Thermo Fisher
Scientific, Inc.) was used to mix with 105 cells to
extract total proteins. BCA assay (Thermo Fisher Scientific, Inc.)
was used to measure protein concentration. Protein samples were
incubated at 100°C for 10 min and electrophoresis was performed
using 10% SDS-PAGE gel with 30 µg protein per well. Following
protein transfer to PVDF membranes, blocking was performed in
non-fat milk (5%) for 2 h at room temperature. Primary antibodies
of rabbit polyclonal PTEN (cat. no. ab31392; 1:900; Abcam) and
rabbit polyclonal GAPDH (cat. no. ab9485; 1:900; Abcam) for at
least 12 h at 4°C. IgG-horseradish peroxidase secondary antibody
(1:800; goat anti-rabbit; cat. no. MBS435036; MyBioSource, Inc.)
was used to further incubate with PVDF membranes at room
temperature for 2 h. Signals were developed using
Immobilon® Western Chemiluminescent HRP Substrate (cat.
no. WBKLS0050; Sigma-Aldrich; Merck KGaA) and signals were
processed using Image J v1.46 software (National Institute of
Health). The gray value of the control group was set to 1, all
other groups were normalized to this group.
Cell apoptosis assay
Y79 and WERI-Rb-1 cells were collected at 24 h
post-transfection and 5×104 cells were mixed with 1 ml
RPMI-1640 medium (no serum) to prepare single-cell suspensions. A
6-well plate was used to cultivate cells (2 ml per well) under
conditions of 5% CO2 and 37°C for 48 h. Following
digestion using 0.25% trypsin, cells were stained with propidium
iodide and Annexin V-FITC (Dojindo Molecular Technologies, Inc.)
for 30 min in dark at 4°C. Finally, apoptotic cells were separated
by flow cytometry using NovoCyte Benchtop Flow Cytometer. Data were
processed using FCSalyzer v.0.9.12 software (free available from:
http://sourceforge.net/projects/fcsalyzer/).
Statistical analysis
All experiments were performed with at least 3
biological replicates. Mean ± SEM values were calculated and were
used for all comparisons GraphPad Prism 6 (GraphPad Software,
Inc.). Differences between non-tumor and Rb tissues were analyzed
by performing a paired t-test. Differences among different cell
transfection groups were explored by performing analysis of
variance (ANOVA; one-way) and Tukey test. Correlations were
analyzed by Pearson's correlation coefficient. P<0.05 was
considered to indicate statistically significant.
Results
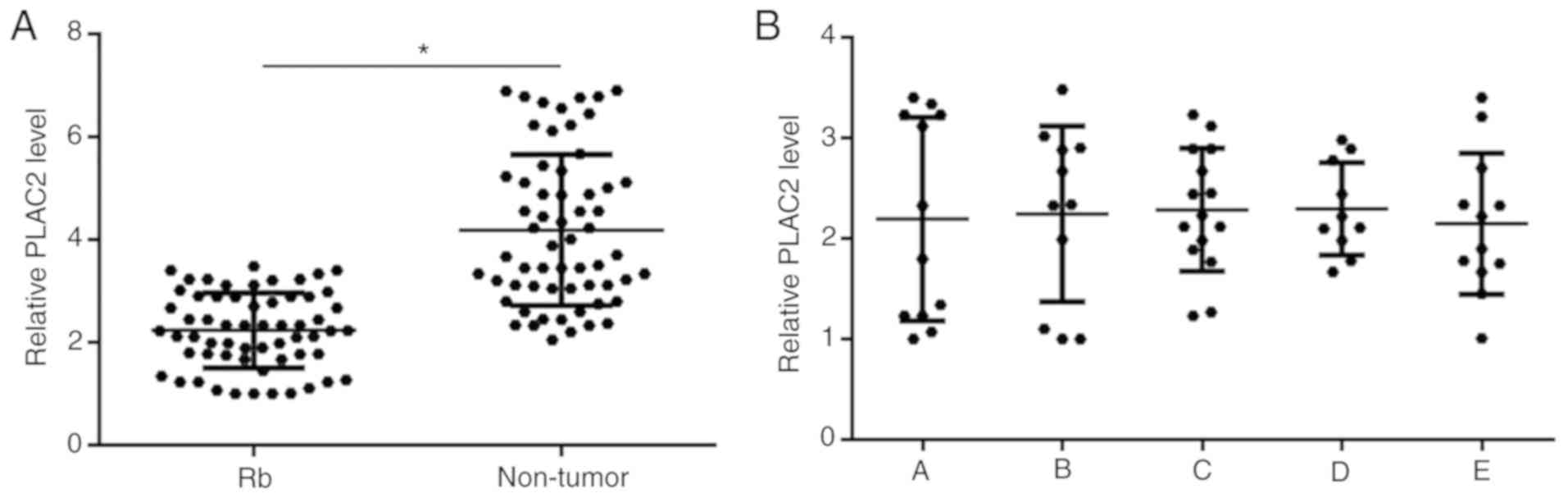
PLAC2 is downregulated in Rb tissues
but not affected by cancer development
PLAC2 in two types of tissues of Rb patients (n=60)
was detected by performing RT-qPCR. Expression data were compared
between two types of tissues by performing a paired t-test. It was
observed that expression levels of PLAC2 were significantly
decreased in Rb tissues compared with non-tumor tissues (P<0.05;
Fig. 1A). Based on clinical
findings, there were 12, 11, 15, 10 and 12 cases at group A-E
(International Classification for Intraocular Retinoblastoma),
respectively. Expression levels of PLAC2 in Rb tissues were
compared among 5 groups of patients by performing an ANOVA
(one-way) and Tukey test. It was observed that expression levels of
PLAC2 in Rb tissues were not significantly different among the 5
groups (Fig. 1B).
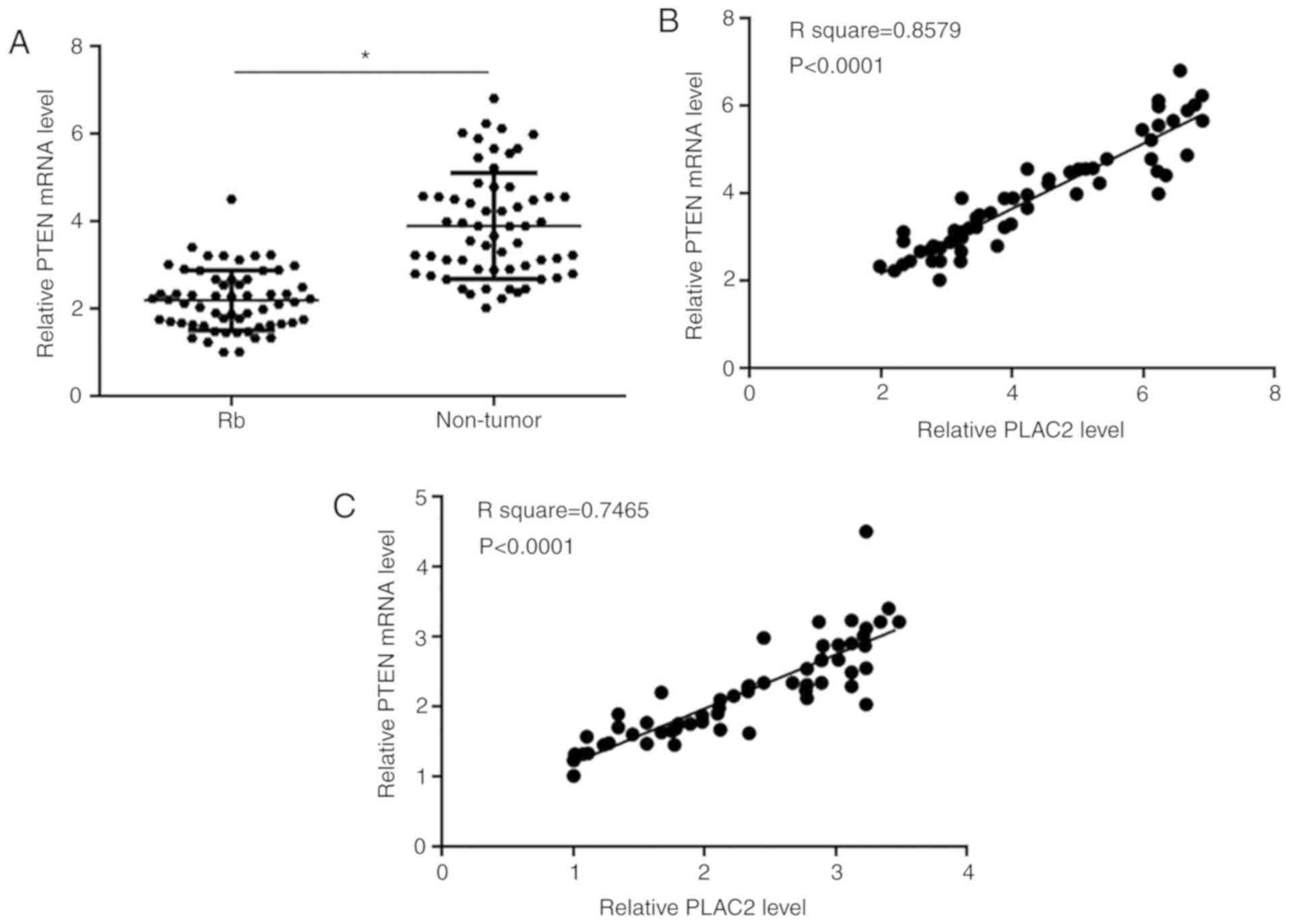
PTEN was positively correlated with
PLAC2
PTEN mRNA in two types of tissues of Rb patients
(n=60) was detected by performing RT-qPCR. Paired t-test analysis
showed that expression levels of PTEN mRNA were significantly
decreased in Rb tissues compared with non-tumor tissues (P<0.05;
Fig. 2A). Correlations between PTEN
mRNA and PLAC2 were analyzed by performing Pearson's correlation
coefficient. It was observed that PTEN mRNA and PLAC2 were
positively correlated both in non-tumor tissues (Fig. 2B) and Rb tissues (Fig. 2C).
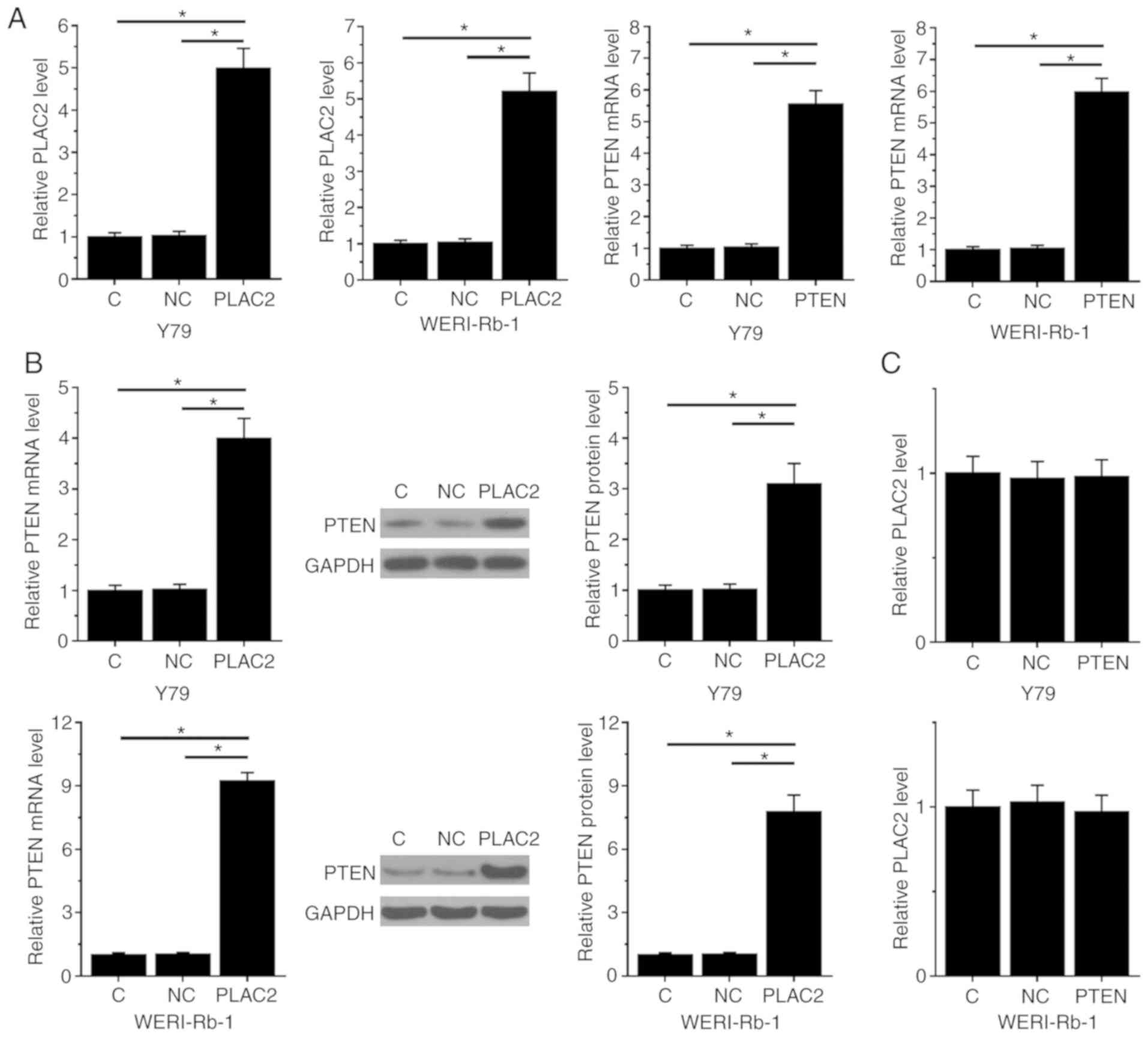
PLAC2 upregulates PTEN in Rb
cells
Y79 and WERI-Rb-1 cells were transfected with PTEN
and PLAC2 expression vectors. Expression levels of PTEN mRNA and
PLAC2 were measured by qPCR at 24 h post-transfection. Compared
with NC and C the control groups, expression levels of PTEN and
PLAC2 were significantly increased at 24 h post-transfections in
cells of both Y79 and WERI-Rb-1 cell lines (P<0.05; Fig. 3A). Moreover, compared with the two
controls, PLAC2 over-expression resulted in significantly
upregulated PTEN in cells of both Y79 and WERI-Rb-1 cell lines
(left, mRNA expression detected by qPCR; middle, representative
western blot image; right, normalized western blot data; P<0.05;
Fig. 3B), while PLAC2 expression was
not significantly affected by PTEN over-expression in cells both
Y79 and WERI-Rb-1 cell lines (Fig.
3C).
PLAC2 promotes Rb cell apoptosis
through PTEN
Compared with NC and C the two control groups PLAC2
and PTEN over-expression caused an increased apoptotic rate of Rb
cells. PTEN siRNA silencing led to a significantly decreased
apoptotic rate and reduced effects of PLAC2 over-expression on
cells of both Y79 (Fig. 4A) and
WERI-Rb-1 (Fig. 4B) cell lines.
(P<0.05). In addition, the cell apoptotic rates of Y79 and
WERI-Rb-1 cells were consistent with the expression levels of PTEN
mRNA in both Y79 (Fig. S1A) and
WERI-Rb-1 (Fig. S1B) cells
(P<0.05).
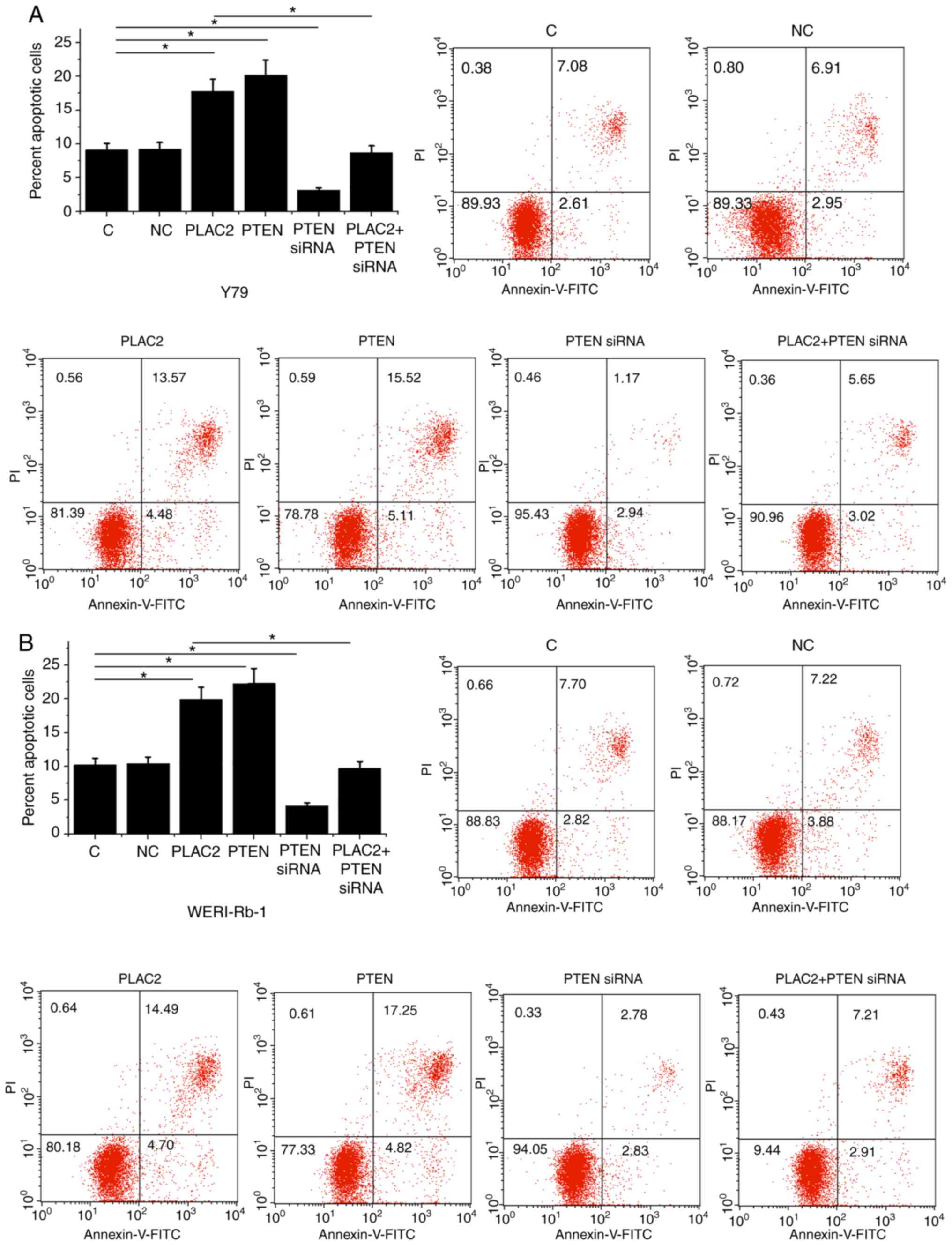 | Figure 4.PLAC2 promotes Rb cell apoptosis
through PTEN. Compared with NC and C the two control groups, PLAC2,
and PTEN over-expression caused an increased apoptotic rate of Rb
cells of both (A) Y79 and (B) WERI-Rb-1cell lines. PTEN siRNA
silencing led to the decreased apoptotic rate and reduced effects
of PLAC2 over-expression. *P<0.05. C, control, untransfected
cell; NC, negative control, cells transfected with empty vector;
Rb, retinoblastoma; PLAC2, placenta-specific 2; siRNA, small
interfering; FITC, fluorescein isothiocyanate; PI, propidium
iodide. |
Discussion
To the best of our knowledge, the functionality of
PLAC2 has only been reported in glioma (10). The present study investigated the
involvement of PLAC2 in Rb. It was found that PLAC2 was
downregulated in Rb and may promote cancer cell apoptosis by
downregulating PTEN.
PTEN signaling is a well-characterized
tumor-suppressive pathway (12). The
development and progression of cancer requires a complex network
between tumor suppressors and oncogenes, and PTEN is the main
player in the inhibitory network by serving as the brake of the
PI3K-Akt cancer cell survival pathway (9). The activation of PTEN dephosphorylates
PIP3, thereby inhibiting the activity of Akt (13). Therefore, activation of PTEN is a
promising approach to induce cancer cell apoptosis, thereby
inhibiting tumor growth and progression. Consistently, the present
study also observed the downregulation of PTEN in Rb tissues. The
present study also observed inhibited Rb cell apoptosis after PTEN
silencing and promoted Rb cell apoptosis after PTEN
over-expression. The current study further confirmed the
tumor-suppressive role of PTEN in Rb.
More and more studies have shown that the expression
of PTEN in cancer cells can be regulated by certain lncRNAs
(14–16). Guo et al (14) reported that GAS5 plays a
tumor-suppressive role in endometrial cancer by inducing the
expression of PTEN. LncRNA FER1L4 can also upregulate PTEN
endometrial cancer to suppresses cancer cell proliferation and
inhibit cell cycle progression (15). In another study, Li et al
(16) reported that lncRNA UCA1
expression induced by hypoxia inducible factor-1α can inactivate
PTEN signaling to accelerate cell proliferation. It has been
reported that STAT1 can inhibit the expression of microRNA-18a in
colorectal cancer by upregulating PTEN (11). It is also known that in glioma cells,
PLAC2 upregulates STAT1 to inhibit cancer cell progression by
inhibiting cell cycle progression (10). In the present study, PLAC2 was shown
to be a likely upstream activator of PTEN in Rb. Therefore, it is
possible that PLAC2 can indirectly upregulate PTEN through the
upregulation of STAT1. However, the present study failed to include
STAT1, which will be included in the authors' future studies to
further verify the present hypothesis.
In conclusion, PLAC2 was downregulated in Rb and
PLAC2 over-expression may induce the apoptosis of Rb cells by
upregulating PTEN.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS performed the experimental work, data analysis,
clinical research and manuscript writing. YQ performed the data
collection and some experimental work. ML performed the research
design, literature research and manuscript review. All authors read
and approved the final manuscript.
Ethics approval and informed consent
Ethical approval was obtained from the Ethics
Committee of Shanghai Ninth People's Hospital. Informed consent was
obtained from all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests and all authors confirm their accuracy.
References
|
1
|
Dimaras H, Dimba EA and Gallie BL:
Challenging the global retinoblastoma survival disparity through a
collaborative research effort. Br J Ophthalmol. 94:1415–1416. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shields CL and Shields JA: Retinoblastoma
management: Advances in enucleation, intravenous chemoreduction,
and intra-arterial chemotherapy. Curr Opin Ophthalmol. 21:203–212.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Villegas VM, Hess DJ, Wildner A, Gold AS
and Murray TG: Retinoblastoma. Curr Opin Ophthalmol. 24:581–588.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abramson DH: Retinoblastoma in the 20th
century: Past success and future challenges the Weisenfeld lecture.
Invest Ophthalmol Vis Sci. 46:2683–2691. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varan A, Kiratli H, Aydin B, Tarlan B,
Poyraz CB, Akyüz C and Büyükpamukçu M: The treatment of
retinoblastoma with four-drug regimen including cisplatin,
etoposide, vincristine, and cyclophosphamide. Pediatr Hematol
Oncol. 29:529–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chawla B, Jain A and Azad R: Conservative
treatment modalities in retinoblastoma. Indian J Ophthalmol.
61:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Cristofano A and Pandolfi PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parsons R: Human cancer, PTEN and the PI-3
kinase pathway. Semin Cell Dev Biol. 15:171–176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu YW, Kang CM, Zhao JJ, Nie Y, Zheng L,
Li HX, Li X, Wang Q and Qiu YR: LncRNA PLAC2 down-regulates RPL36
expression and blocks cell cycle progression in glioma through a
mechanism involving STAT1. J Cell Mol Med. 22:497–510. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Li X, Tan F, Yu N and Pei H:
STAT1 inhibits MiR-181a expression to suppress colorectal cancer
cell proliferation through PTEN/Akt. J Cell Biochem. 118:3435–3443.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamada KM and Araki M: Tumor suppressor
PTEN: Modulator of cell signaling, growth, migration and apoptosis.
J Cell Sci. 114:2375–2382. 2001.PubMed/NCBI
|
|
13
|
Simpson L and Parsons R: PTEN: Life as a
tumor suppressor. Exp Cell Res. 264:29–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo C, Song WQ, Sun P, Jin L and Dai HY:
LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in
endometrial cancer cells. J Biomed Sci. 22:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiao Q and Li H: LncRNA FER1L4 suppresses
cancer cell proliferation and cycle by regulating PTEN expression
in endometrial carcinoma. Biochem Biophys Res Commun. 478:507–512.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Xiao Y and Huang T: HIF-1α-induced
upregulation of lncRNA UCA1 promotes cell growth in osteosarcoma by
inactivating the PTEN/AKT signaling pathway. Oncol Rep.
39:1072–1080. 2018.PubMed/NCBI
|