Introduction
Melanoma is an aggressive cancer with a rapid
increase in incidence rate worldwide. The number of new melanoma
cases diagnosed is estimated to increase by 7.7% in 2019 (1). Less than half of patients diagnosed
with metastatic melanoma survived longer than 1 year, and only 20%
of them were alive after 3 years (2). Current therapies for melanoma,
including chemotherapy, immunotherapy and targeted therapy, pose
various limitations, such as low response rates, severe side
effects, toxicity and high tendency to develop tolerance (3,4). Thus,
novel therapies for melanoma are required.
A number of signaling pathways are involved in
melanoma progression and many molecular targets have been
identified for melanoma treatment (5). Signal transducer and activator of
transcription 3 (STAT3) has been proposed as one of the therapeutic
targets for melanoma as it is constitutively activated with high
frequency in melanoma (6).
Activation of STAT3 promotes cell proliferation, inhibits cell
apoptosis and facilitates cell migration in melanoma (7). Overexpression of a dominant-negative
STAT3 variant has been demonstrated to result in growth inhibition
and regression, and prevents metastasis of melanoma cells in
vivo (8). Furthermore, STAT3
knockdown with siRNAs in melanoma cells has been demonstrated to
induce apoptosis, and inhibit proliferation and migration (9). Clinical trials on a number of STAT3
inhibitors, including WP1066, AZD9150, STAT3 DECOY, OPB-31121 and
OPB-51602), have been approved for the treatment of melanoma.
However, some trials have been discontinued due to the severity of
adverse effects (6). Thus, future
studies are required in order to develop safe and effective STAT3
inhibitors for the treatment of melanoma.
Dioscin is a natural steroid saponin that is present
in several herbs, including the rhizome of Dioscorea
opposita Thunb, and as such, has a long history of consumption
as a part of the normal human diet (10). Several pharmacological activities of
dioscin, such as lipid-lowering, anti-virus, anti-inflammatory and
anti-cancer activities have been indicated to have a beneficial
effect on human health (11).
Previous studies have demonstrated that dioscin has anti-melanoma
effects in murine B16 cells and allograft mouse models (12–14).
Dioscin has been reported to inhibit STAT3 phosphorylation in a
cerebral ischemia-reperfusion injury rat model (15) and suppresses Src (an upstream kinase
of STAT3) in different types of colon tumor (16). Therefore, it is speculated that
dioscin is a safe anti-melanoma phytocompound that inhibits
Src/STAT3 signaling.
The present study investigated the anti-melanoma
effects of dioscin in both murine and human cell models and
assessed the involvement of the Src/STAT3 signaling pathway in
these effects.
Materials and methods
Reagents and cell lines
Dioscin (purity >98% as determined by
high-performance liquid chromatography) was obtained from Shanghai
Yuanye Bio-Technology Co., Ltd. Human A375 melanoma cells, murine
B16F10 melanoma cells and murine L929 fibroblasts were obtained
from the American Type Culture Collection (ATCC). The
B16STAT3C (overexpressing a constitutively active STAT3
mutant, STAT3C Flag pRc/CMV) and B16NC (expressing the
empty vector, pcDNA 3.0) cell lines were previously established
(8). The plasmids were obtained from
AddGene Inc. (cat. no. 8722). Cells were cultured in DMEM
supplemented with 5% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.),
and maintained at 37°C and in a humidified atmosphere of 5%
CO2.
CCK-8 assay
A Cell Counting Kit-8 (CCK-8) assay was performed
according to the manufacturer's protocol to determine the
cytotoxicity of dioscin towards A375 cells, B16F10 cells and L929
cells (17). Cells were seeded in
96-well plates at a density of 5,000 cells/well and were treated
with dioscin at various concentrations (0, 1, 2, 4 and 8 µM) at
37°C in a humidified atmosphere of 5% CO2 for 24 and 48
h. A total of 10 µl of CCK-8 (Dojindo Molecular Technologies, Inc.)
solution was added to each well and incubated at 37°C in a
humidified atmosphere of 5% CO2 for an additional 2 h.
The absorbance was measured at 450 nm using a microplate
spectrophotometer (BD Biosciences).
Crystal violet staining
Crystal violet staining was performed to visualize
the effects of dioscin on the viability of A375 cells and B16F10
cells, according to the manufacturer's protocol (7). Cells were seeded in 6-well plates at a
density of 100,000 cells/well and were treated with dioscin at
various concentrations (0, 2, 4 and 8 µM) under 37°C in a
humidified atmosphere of 5% CO2 for 48 h. Treated cells
were fixed with 10% formalin at room temperature for 5 min,
followed by staining with 0.05% crystal violet solution in
distilled water at room temperature for 30 min. Cells were then
washed twice with PBS and scanned using an Epson V370 Scanner
(Epson Co., Ltd.). Subsequently, the purple formazan crystals were
dissolved in 1 ml of 33% glacial acetic acid (Sigma-Aldrich; Merck
KGaA) and viability was assessed at a wavelength of 570 nm using a
microplate spectrophotometer (BD Biosciences).
Cell apoptosis assay
The apoptotic effect of dioscin in A375 cells and
B16F10 cells was quantified using Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) double staining assays
(18), with the Apoptosis Detection
kit (Abcam), according to the manufacturer's protocol. Following
treatment with dioscin (0, 1 and 2 µM) for 24 h, both the detached
and adherent cells were harvested and subsequently incubated in 500
µl of labeling solution (5 µl of AnnexinV-FITC, 5 µl of PI and 490
µl of binding buffer) in darkness at room temperature for 15 min.
Flow cytometric analyses were performed using a C6 flow cytometer
(BD Biosciences) and CellQuest™ Pro software version 5.1 (BD
Biosciences).
Wound heal assay
The effect of dioscin on cell migration was assessed
via a wound healing assay (7). A375
cells and B16F10 cells were seeded at a density of 1,000,000
cells/well and grown to 100% confluence in 6-well plates. The
confluent cell monolayer was scratched with a sterile 10 µl pipette
tip across the center of each well in order to produce a clean,
straight wound area. Cells were subsequently washed twice with PBS
in order to remove the detached cells, and incubated with dioscin
(0.00, 0.25 and 0.50 µM) in serum-free DMEM medium at 37°C in a
humidified atmosphere of 5% CO2 for 12 h. Cell migration
was captured at the 0 and 12 h time points using a digital camera
installed on a Leica DM3000 inverted fluorescence microscope (Leica
Microsystems Ltd.) with a ×5 objective lens under the bright field
mode. A total of five images were captured for each well. Migration
rate was calculated using the following equation:
(A-B)/A ×100%, where A represents the width of
wound at 0 h and B represents the width of wound at 12
h.
Western blot analysis
Whole cell lysates were prepared from cultured
B16F10 and A375 cells using RIPA lysis buffer [50 mM Tris (pH 7.4),
150 mM NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS],
with the addition of 1 mM PMSF, 2 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4
and 0.5 µg/ml leupeptin. Protein concentrations were measured using
Quick Start Bradford 1X Dye reagent (Bio-Rad Laboratories, Inc.)
according to the manufacturer's protocol. Each protein sample was
loaded at a concentration of 10 µg/µl on 8% SDS/PAGE gels.
Subsequently, the proteins were transferred from the gels to
nitrocellulose membranes. After that, the membranes were blocked in
5% skimmed milk (Devondale, Saputo Dairy Austrlia Pty Ltd.) in
Tris-buffered saline Tween-20 (TBST) (Sigma-Aldrich Inc.; Merck
KGaA) at room temperature for 1 h. Next, the membranes were
incubated in diluents of primary antibodies at 4°C for 12 h. After
incubation, the membranes were washed with TBST for three times.
Then the membranes were incubated in diluents of secondary
antibodies at room temperature for 1 h and washed with TBST for
three times. Standard western blotting assay was performed as
previously described (19). Protein
bands were visualized using the enhanced chemiluminescence
detection system (Invitrogen; Thermo Fisher Scientific, Inc.). The
primary antibodies STAT3 (cat. no. 12640), Src (cat. no. 2108),
phospho-STAT3 (Tyr705; cat. no. 9145), phospho-Src (Tyr416; cat.
no. 6943) and GAPDH (cat. no. 5174) were obtained from Cell
Signaling Technology, Inc., and diluted with 5% bovine serum
albumin (BSA) (Sigma-Aldrich Inc.; Merck KGaA) in TBST at a ratio
of 1:1,000. The secondary antibody anti-rabbit IgG, HRP-linked
antibody (cat. no. 7074) was obtained from Cell Signaling
Technology, Inc. and diluted with 5% BSA in TBST at a 1:10,000
ratio.
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was extracted from B16F10 cells and A375
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, and
reverse transcribed into cDNA using the PrimeScript™ RT reagent kit
(Takara Bio, Inc.) according to the manufacturer's protocol. The
reverse transcription parameters were 37°C for 15 min followed by
85°C for 5 sec and 4°C for 15 min. RT-qPCR (20) was performed in triplicate in a total
volume of 10 µl consisting of 5 µl of 2X SYBR green PCR Master Mix,
1 µl of forward primer (10 µM), 1 µl of reverse primer (10 µM) and
3 µl of template in sterile distilled water, using iTaq™ Universal
SYBR Green Supermix (Bio-Rad Laboratories, Inc.) with a ViiA 7
Real-time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR parameters were as follows: Initial denaturation at
95°C for 10 min; 40 cycles of 95°C for 15 sec; and a final
extension at 60°C for 1 min. The mRNA levels were quantified using
the comparative CT method (21) and
normalized to the internal reference gene GAPDH. The primers used
in the present study are presented in Table I.
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
| Gene | Forward | Reverse |
|---|
| Human GAPDH |
5′-ACCCATCACCATCTTCCAGGAG-3′ |
5′-GAAGGGGCGGAGATGATGAC-3′ |
| Human cyclin
D1 |
5′-GAACGAATCTACCCATTACCAG-3′ |
5′-GAGATGCCCGTGATGAACC-3′ |
| Human Bcl-2 |
5′-GCCATATGGCGCACGCTGGGAGAA-3′ |
5′-GCGCTCGAGTCACTTGTGGCCCAGATAG-3′ |
| Human MMP-2 |
5′-AGTGGTCCGTGTGAAGTATG-3′ |
5′-GTATCAGTGCAGCTGTTGTA-3′ |
| Mouse GAPDH |
5′-CCATGGAGAAGGCCGGGG-3′ |
5′-CAAAGTTGTCATGGATGACC-3′ |
| Mouse cyclin
D1 |
5′-GTCATCAAGTGTGACCCG-3′ |
5′-GCACAGTCTGCCTGATGC-3′ |
| Mouse Bcl-2 |
5′-TGTAAGGACGAAACGGGACT-3′ |
5′-AAAGCCAGCAGCACATTTCT-3′ |
| Mouse MMP-2 |
5′-CTGGAATGCCATCCCTGATAA-3′ |
5′-GGTTCTCCAGCTTCAGGTAATAA-3′ |
Statistical analysis
All data are presented as mean ± standard deviation.
Comparisons among groups were performed using one-way ANOVA
followed by Dunnett's multiple comparisons using the statistical
GraphPad Prism software (version 6.0; GraphPad Software Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
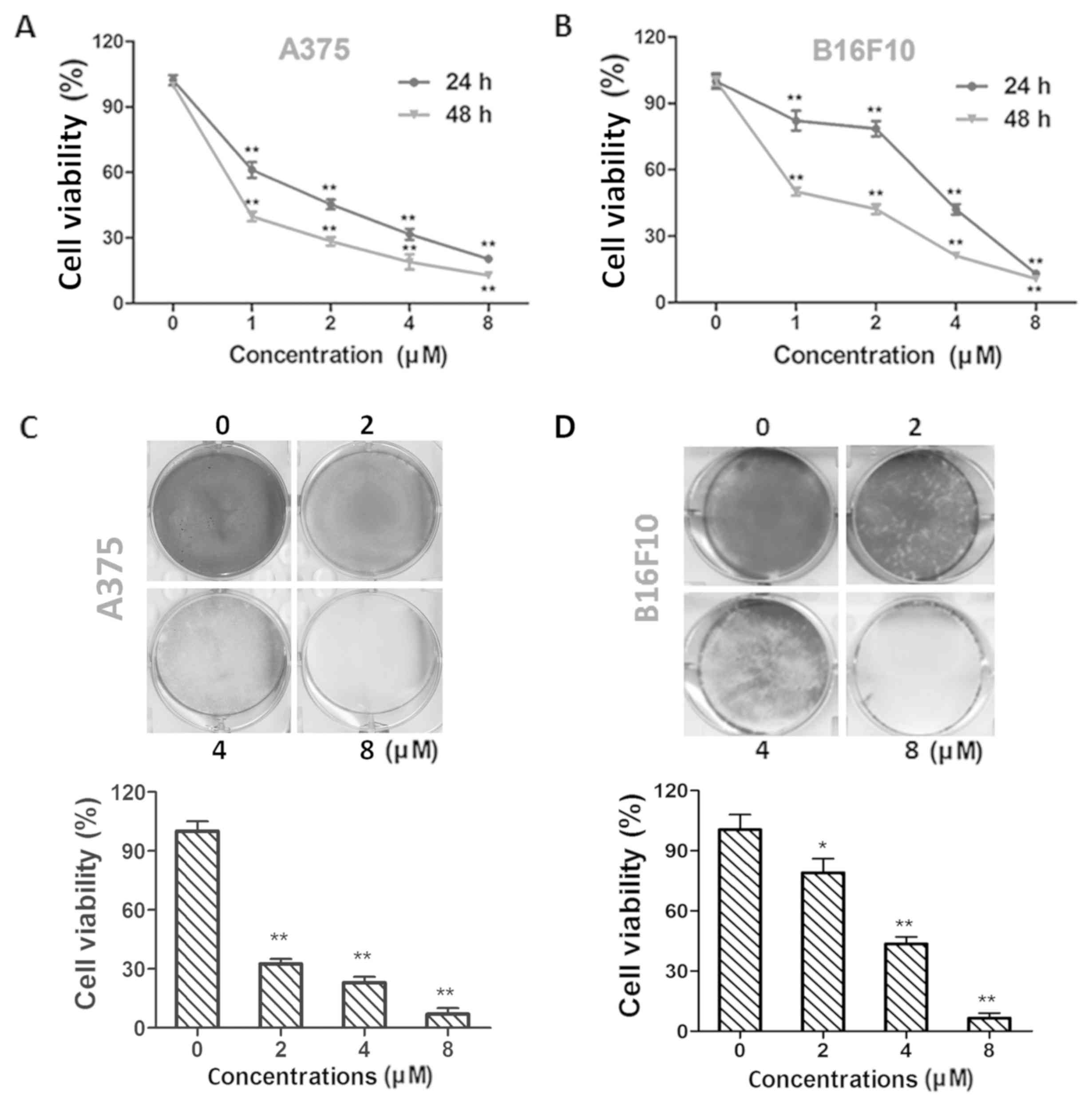
Dioscin exhibits higher cytotoxicity
in melanoma cells than in normal cells
Treatment with dioscin (1, 2, 4 and 8 µM) at 24 and
48 h decreased the viability of A375 cells (P<0.01; Fig. 1A) and B16F10 cells (P<0.01;
Fig. 1B) in a time- and
dose-dependent manner. The half maximal inhibitory concentration
(IC50) values in A375 cells were 1.54±0.32 and 0.57±0.18
µM for 24 and 48 h treatments, respectively. The IC50
values in B16F10 cells were 3.14±0.14 and 1.16±0.17 µM for 24 and
48 h treatments, respectively. The results of crystal violet
staining further validate the effects of dioscin on A375
(P<0.01; Fig. 1C) and B16F10
(P<0.05; Fig. 1D) cell viability.
In addition, viability decreasing effects of 1, 2, 4 and 8 µM of
dioscin were less potent in L929 fibroblasts than in B16F10 and
A375 melanoma cells (P<0.01; Fig.
S1).
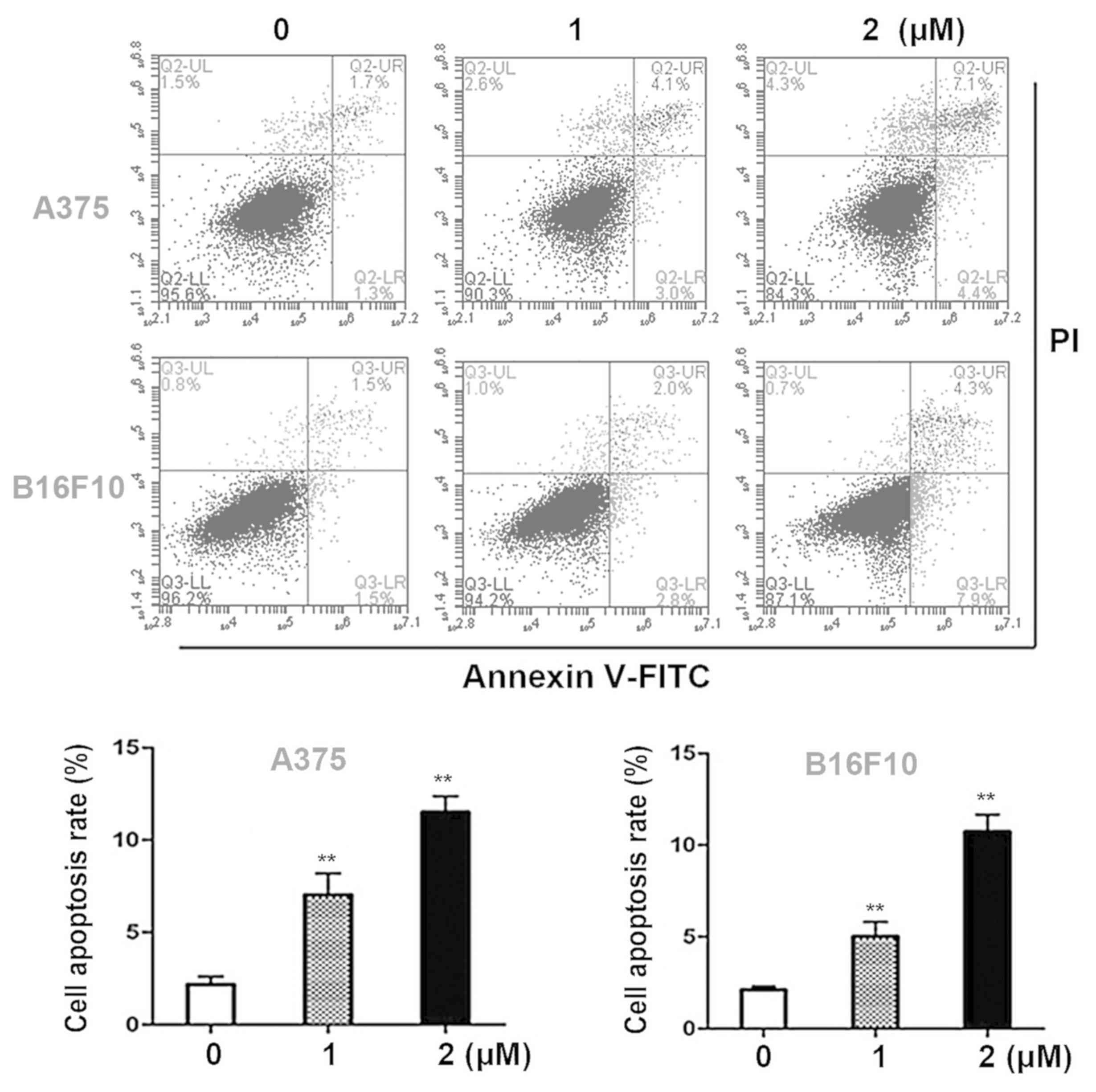
Dioscin induces apoptosis in melanoma
cells
Annexin V- FITC/PI double staining assays were
performed in order to quantify the apoptotic effects of dioscin in
melanoma cells. Dioscin treatments (1, 2 µM) for 24 h significantly
induced apoptosis in A375 cells and B16F10 cells in a
dose-dependent manner (Fig. 2A). The
results of the present study demonstrated that in A375 cells, 1 and
2 µM of dioscin at 24 h significantly increased the apoptotic cell
ratios to 6.6±1.1% (P<0.01) and 11.6±0.9% (P<0.01), compared
with the control cells (2.5±0.5%). In B16F10 cells, 1 and 2 µM of
dioscin at 24 h increased the apoptotic cell ratios to 5.0±0.8%
(P<0.01) and 10.9±0.8% (P<0.01), compared with the control
cells (2.4±0.2%) (Fig. 2B).
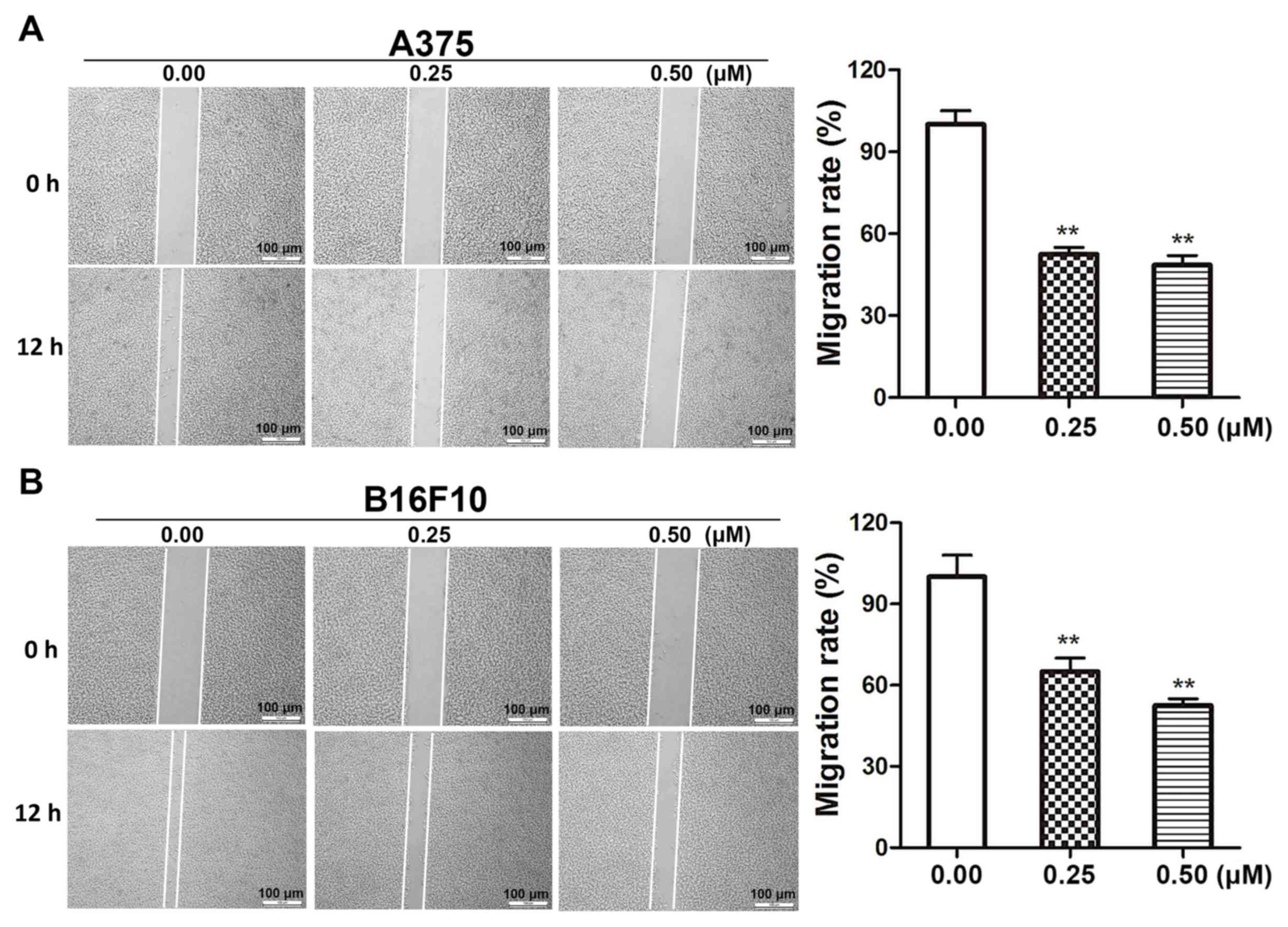
Dioscin decreases the migratory
ability of melanoma cells
Wound healing assays were performed in order to
determine the effects of dioscin on melanoma cell migration.
Dioscin treatments (0.25 and 0.50 µM) for 12 h significantly
inhibited the migration of A375 cells (Fig. 3A) and B16F10 cells (Fig. 3B). The results of the present study
demonstrated that 0.25 and 0.50 µM of dioscin significantly
decreased cell migration by 48.5% (P<0.01) and 52.4%
(P<0.01), respectively, in A375 cells, and by 37.3% (P<0.01)
and 46.5% (P<0.01), respectively, in B16F10 cells. However,
dioscin at 0.25 and 0.50 µM did not significantly affect the
viability of A375 cells and B16F10 cells after a 12-h treatment
(Fig. S2).
Dioscin inhibits the activation of Src
and STAT3 in melanoma cells
Western blotting was performed in order to determine
the effects of dioscin on the activation of Src and STAT3 in
melanoma cells. The results of the present study demonstrated that
treatment with dioscin (0.25, 0.50, 1.00 and 2.00 µM) for 12
(Fig. 4A), 24 (Fig. 4B) and 48 h (Fig. 4C) dose-dependently suppressed the
phosphorylation of Src (Tyr 416) and STAT3 (Tyr 705), but did not
affect protein levels of total STAT3 and total Src, in A375 cells
and B16F10 cells. Treatments with dioscin (0.25, 0.50, 1.00 and
2.00 µM) for 12, 24 and 48 h also dose-dependently lowered the
ratios of p-Src/Src and p-STAT3/STAT3 (Fig. S3).
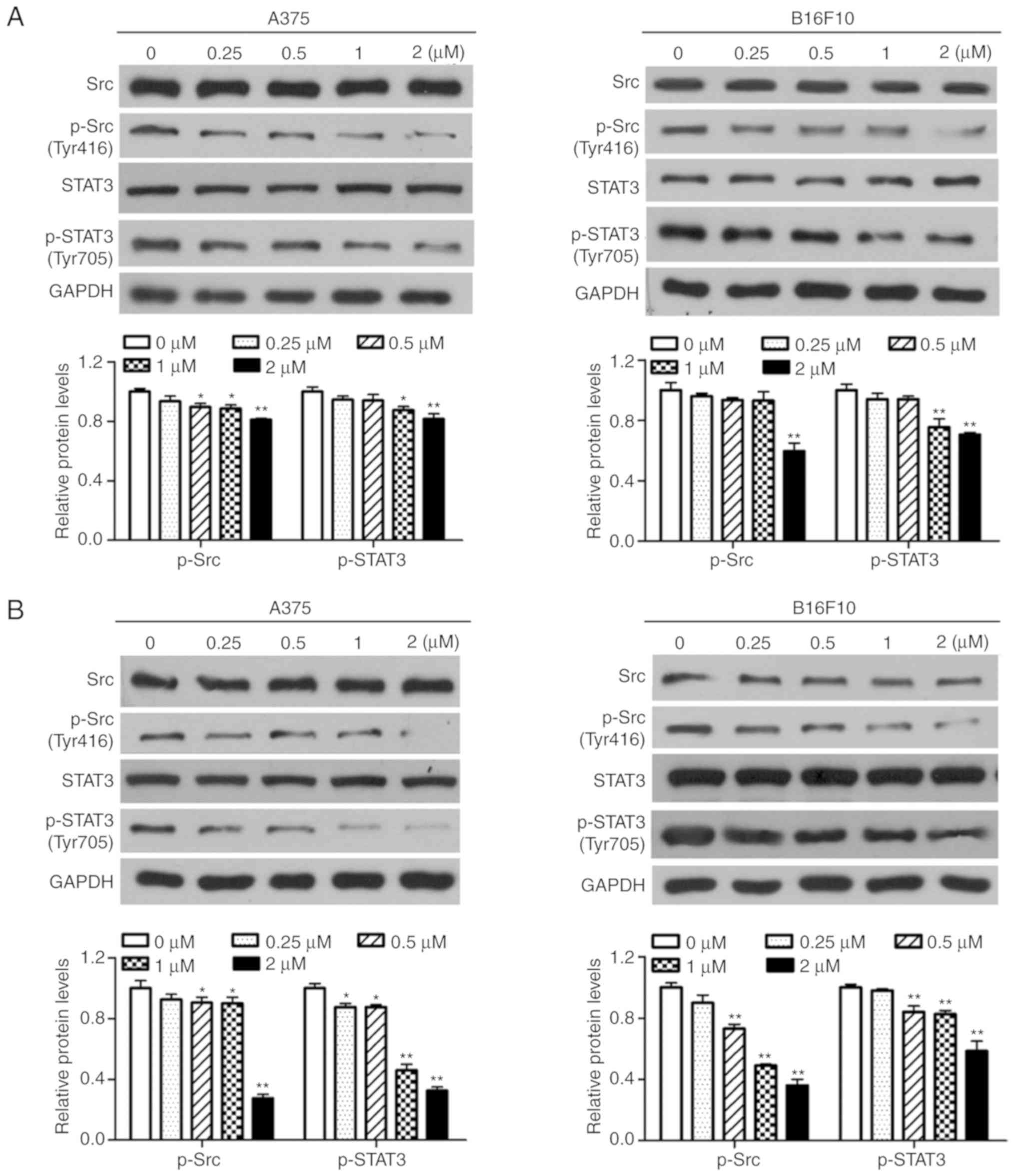 | Figure 4.Dioscin inhibits phosphorylation of
Src and STAT3 in melanoma cells. (A) Western blot analyses of A375
cells and B16F10 cells treated with dioscin (0.00, 0.25, 0.50, 1.00
and 2.00 µM) for 12 h. (B) Western blot analyses of A375 cells and
B16F10 cells treated with dioscin (0.00, 0.25, 0.50, 1.00 and 2.00
µM) for 24 h. (C) Western blot analyses of A375 cells and B16F10
cells treated with dioscin (0.00, 0.25, 0.50, 1.00 and 2.00 µM) for
48 h. Protein levels of total and phosphorylated Src (Tyr416,
p-Src), and total and phosphorylated STAT3 (Tyr705, p-STAT3) were
determined by western blotting. GAPDH was used as the endogenous
control. Representative bands from each group are presented in the
upper panels. Band intensity was analyzed using ImageJ software and
are presented in the lower panels. The relative protein levels of
the control group were normalized to 1. Data presented in bar
charts are the mean ± standard deviation of three independent
experiments. *P<0.05; **P<0.01 vs. control. STAT3, signal
transducer and activator of transcription 3; FBS, fetal bovine
serum. |
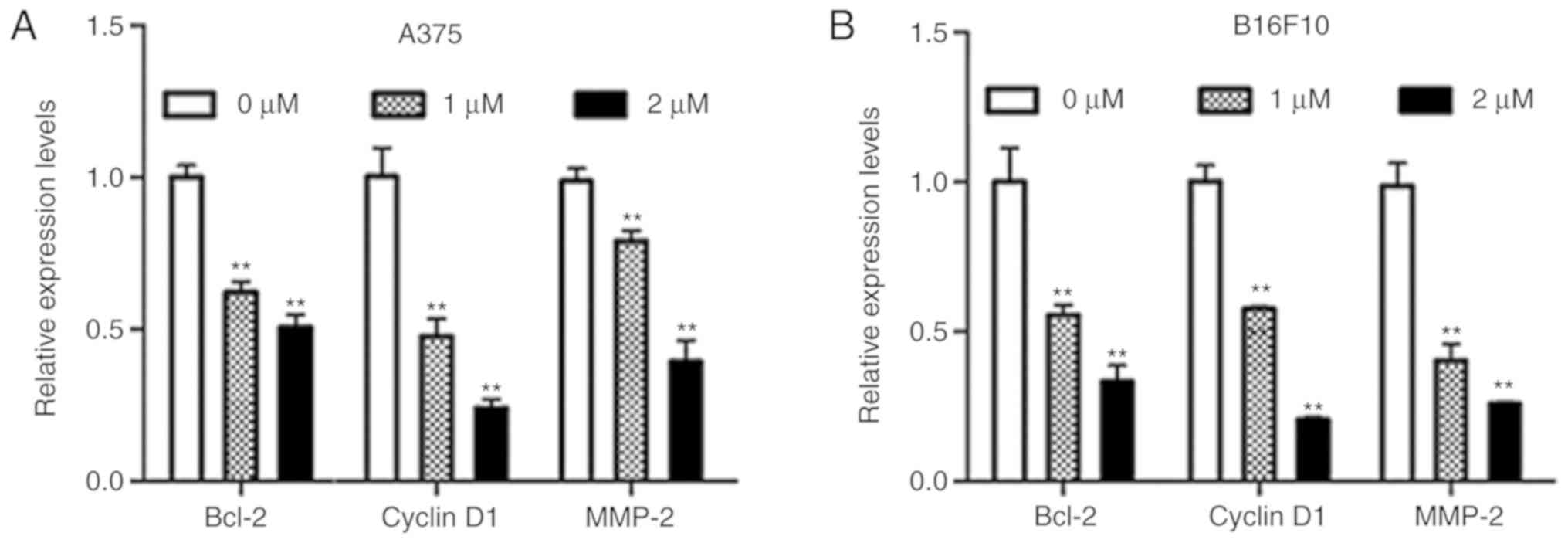
Dioscin decreases the expression of
STAT3 target genes in melanoma cells
B-cell lymphoma-2 (Bcl-2) and cyclin D1 are
STAT3-target genes that are involved in cell survival, and matrix
metallopeptidase-2 (MMP-2) is a STAT3-target gene that is involved
in cell migration (9). The results
of the present study demonstrated that dioscin significantly
downregulated mRNA levels of Bcl-2, cyclin D1 and MMP-2 in A375
cells (P<0.01; Fig. 5A) and
B16F10 cells (P<0.01; Fig. 5B).
These results further indicate that inhibiting STAT3 signaling is
involved in the anti-melanoma action of dioscin.
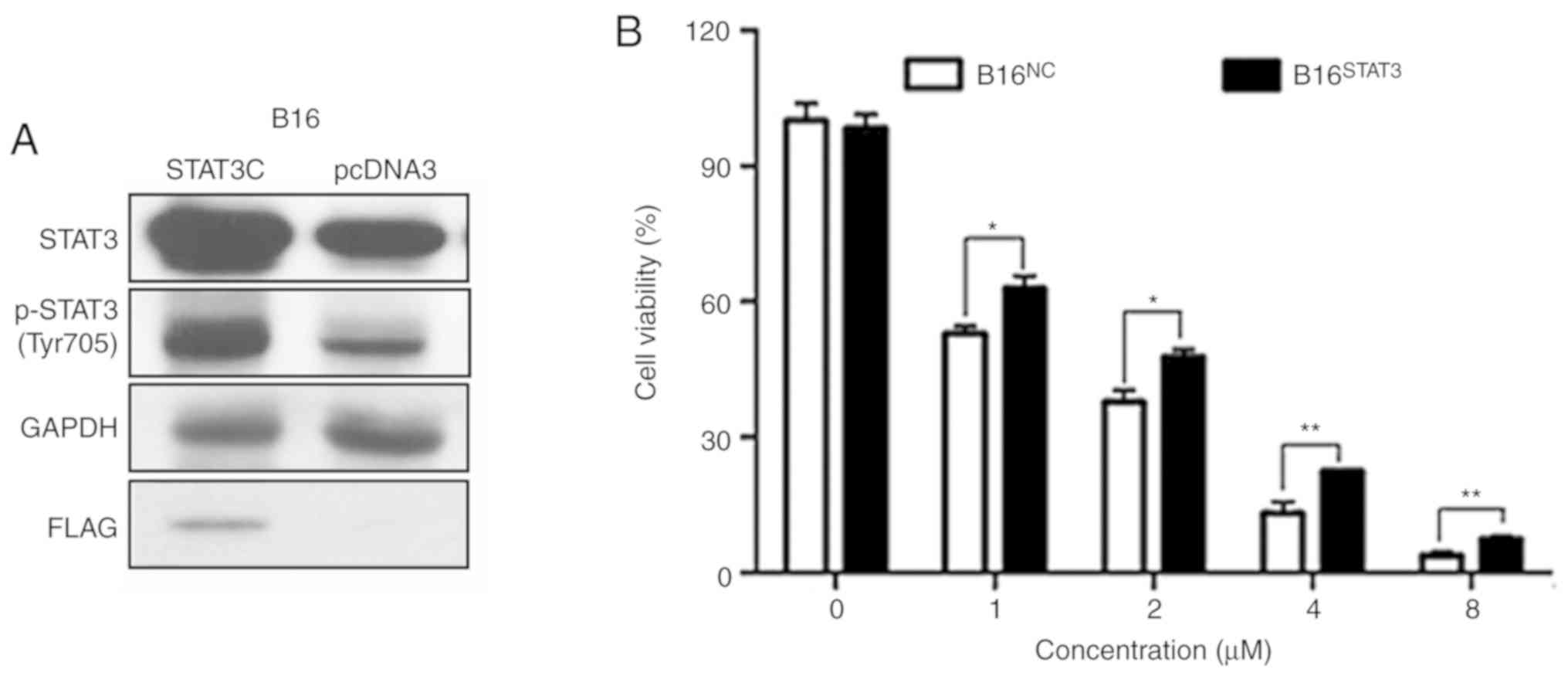
Overexpression of STAT3 diminishes the
effects of dioscin on cell viability
Stable B16STAT3C (overexpressing a
constitutively active STAT3 mutant) and B16NC cell lines
(expressing the empty vector) were used in order to validate the
involvement of STAT3 signaling in the anti-melanoma effects of
dioscin. The western blot results of the present study demonstrated
that protein levels of total STAT3 and phospho-STAT3 (Tyr705) were
higher in B16STAT3C cells than in B16NC
cells, indicating that STAT3 was overexpressed in
B16STAT3C cells (Fig. 6A,
the same as in Reference 8). The effects of dioscin on the
viability of B16NC cells and B16STAT3C cells
were compared. Following treatment for 48 h, the cytotoxic effects
of different concentrations of dioscin (1, 2, 4 and 8 µM) were
demonstrated to be less potent toward B16STAT3C cells
than B16NC cells (P<0.05; Fig. 6B), indicating that over-activation of
STAT3 weakened the anti-proliferative effects of dioscin.
Discussion
The present study investigated the anti-melanoma
effects of dioscin in human A375 cells and murine B16F10 melanoma
cells. In agreement with previous studies (13,14,22), the
results of the present study demonstrated that dioscin decreased
melanoma cell viability (Fig. 1).
Furthermore, the results of the present study indicated that
dioscin induced apoptosis (Fig. 2),
and restrained migration in melanoma cells (Fig. 3). A previous study demonstrated that
dioscin inhibits B16 allograft melanoma growth and metastasis
(13). The results of the present
and previous studies suggest that dioscin has the potential to be
developed as an anti-melanoma agent.
To the best of our knowledge, the present study was
the first to demonstrate that inhibiting STAT3 signaling is, at
least partially, responsible for the anti-melanoma effects of
dioscin. Over-activation of STAT3 could not completely reverse the
effect of dioscin on cell viability (Fig. 6), indicating that inhibition of STAT3
is not the sole molecular mechanism of action of dioscin. A number
of previous studies have demonstrated that upregulation of connexin
26 and connexin 43, and downregulation of phospho-CREB and MITF
contribute to the anti-melanoma molecular mechanisms of dioscin
(13,14,22).
Whether additional molecular mechanisms are involved in the
anti-melanoma effects of dioscin remains to be elucidated.
STAT3 is activated in several types of cancer,
including breast, gastric and lung cancer (20,23–25).
Dioscin has been reported to inhibit the growth of these different
types of cancer in mice (11).
Whether dioscin suppresses these types of cancer by inhibiting
STAT3 signaling needs to be further studied.
Apart from promoting cell proliferation, inhibiting
cell apoptosis, and facilitating cell migration and invasion,
activation of STAT3 signaling can also promote angiogenesis and
immune evasion in melanoma (26).
Whether dioscin inhibits melanoma angiogenesis and immune evasion,
however, remains to be elucidated.
Overall, the results of the present study confirmed
the anti-melanoma effects of dioscin in more cell models and with
more parameters, and demonstrated that inhibiting the Src/STAT3
signaling pathway contributes to the anti-melanoma molecular
mechanisms of dioscin. The results of the present study provide
further pharmacological groundwork for developing dioscin as a
novel anti-melanoma agent.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science,
Technology and Innovation Commission of Shenzhen (grant nos.
JCYJ20160229210327924 and JCYJ20170817173608483); the Department of
Science and Technology of Guangdong Province (grant nos.
2016A030313007 and 2017B050506003); the Innovation and Technology
Commission of Hong Kong (grant no. GHX/002/17GD); National Natural
Science Foundation of China (grant nos. 81673649, 8187141799 and
81803788); the Food and Health Bureau of Hong Kong (grant nos.
14150571 and 15163441); the Research Grants Council of Hong Kong
(grant nos. 12125116 and 12102918); and the Hong Kong Baptist
University (grant nos. FRG1/16-17/048 and FRG2/17-18/032).
Availability of data and materials
The datasets used and analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
YXL, BWX performed the majority of the experiments
and interpreted the data. YXL drafted the initial manuscript. YJC,
XQF, PLZ, JXB, JYC, CLY, JKL, YPW, JW, YW and KKC participated in
several experiments and analyzed the data. CL designed the present
study. ZLY designed the present study and finalized the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MMP-2
|
matrix metalloproteinase-2
|
References
|
1
|
Skin cancer facts & statistics 2019.
https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/
|
|
2
|
Rogiers A, Boekhout A, Schwarze JK, Awada
G, Blank CU and Neyns B: Long-term survival, quality of life, and
psychosocial outcomes in advanced melanoma patients treated with
immune checkpoint inhibitors. J Oncol. 2019:52690622019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garbe C, Peris K, Hauschild A, Saiag P,
Middleton M, Spatz A, Grob JJ, Malvehy J, Newton-Bishop J,
Stratigos A, et al: Diagnosis and treatment of melanoma. European
consensus-based interdisciplinary guideline-update 2012. Eur J
Cancer. 48:2375–2390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palumbo G, Lorenzo GD, Ottaviano M and
Damiano V: The future of melanoma therapy: Developing new drugs and
improving the use of old ones. Future Oncol. 12:2531–2534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kortylewski M, Jove R and Yu H: Targeting
STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev.
24:315–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siveen KS, Sikka S, Surana R, Dai X, Zhang
J, Kumar AP, Tan BK, Sethi G and Bishayee A: Targeting the STAT3
signaling pathway in cancer: Role of synthetic and natural
inhibitors. Biochim Biophys Acta. 1845:136–154. 2014.PubMed/NCBI
|
|
7
|
Li T, Fu X, Tse AK, Guo H, Lee KW, Liu B,
Su T, Wang X and Yu Z: Inhibiting STAT3 signaling is involved in
the anti-melanoma effects of a herbal formula comprising Sophorae
Flos and Lonicerae Japonicae Flos. Sci Rep. 7:30972017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YX, Bai JX, Li T, Fu XQ, Guo H, Zhu
PL, Chan YC, Chou JY, Yin CL, Li JK, et al: A TCM formula
comprising Sophorae Flos and Lonicerae Japonicae Flos alters
compositions of immune cells and molecules of the STAT3 pathway in
melanoma microenvironment. Pharmacol Res. 142:115–126. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Ren S, Xu F, Ma Z, Liu X and Wang
L: Recent advances in the pharmacological activities of dioscin.
Biomed Res Int. 2019:57636022019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu LN, Wei YL and Peng JY: Advances in
study of dioscin-a natural product. Zhongguo Zhong Yao Za Zhi.
40:36–41. 2015.(In Chinese). PubMed/NCBI
|
|
12
|
Wei Y, Xu Y, Han X, Qi Y, Xu L, Xu Y, Yin
L, Sun H, Liu K and Peng J: Anti-cancer effects of dioscin on three
kinds of human lung cancer cell lines through inducing DNA damage
and activating mitochondrial signal pathway. Food Chem Toxicol.
59:118–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kou Y, Ji L, Wang H, Wang W, Zheng H, Zou
J, Liu L, Qi X, Liu Z, Du B and Lu L: Connexin 43 upregulation by
dioscin inhibits melanoma progression via suppressing malignancy
and inducing M1 polarization. Int J Cancer. 141:1690–1703. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao J, Zhang G, Li B, Wu Y, Liu X, Tan Y
and Du B: Dioscin augments HSV-tk-mediated suicide gene therapy for
melanoma by promoting connexin-based intercellular communication.
Oncotarget. 8:798–807. 2017.PubMed/NCBI
|
|
15
|
Tao X, Sun X, Yin L, Han X, Xu L, Qi Y, Xu
Y, Li H, Lin Y, Liu K and Peng J: Dioscin ameliorates cerebral
ischemia/reperfusion injury through the downregulation of TLR4
signaling via HMGB-1 inhibition. Free Radic Biol Med. 84:103–115.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong Q, Qing Y, Wu Y, Hu X, Jiang L and Wu
X: Dioscin inhibits colon tumor growth and tumor angiogenesis
through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol
Appl Pharmacol. 281:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Chen Q, Liu W and Lin JM: A simple
and versatile microfluidic cell density gradient generator for
quantum dot cytotoxicity assay. Lab Chip. 13:1948–1954. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su T, Bai JX, Chen YJ, Wang XN, Fu XQ, Li
T, Guo H, Zhu PL, Wang Y and Yu ZL: An ethanolic extract of
Ampelopsis Radix exerts anti-colorectal cancer effects and potently
inhibits STAT3 signaling in vitro. Front Pharmacol. 8:2272017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng BC, Ma XQ, Kwan HY, Tse KW, Cao HH,
Su T, Shu X, Wu ZZ and Yu ZL: A herbal formula consisting of rosae
multiflorae fructus and Lonicerae Japonicae Flos inhibits
inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J
Ethnopharmacol. 153:922–927. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eriksson E, Milenova I, Wenthe J, Dimberg
A, Moreno R, Ullenhag G, Alemany R and Loskog A: Abstract 3662:
Activation of CD40 while inhibiting IL6/STAT3 using oncolytic
viruses induces mature DCs with high cytokine production but blocks
PDL1 expression. Cancer Res. 77:36622017.
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishina A, Ebina K, Ukiya M, Fukatsu M,
Koketsu M, Ninomiya M, Sato D and Kimura H: Dioscin derived from
Solanum melongena L.‘Usukawamarunasu’ attenuates α-MSH-Induced
melanogenesis in B16 murine melanoma cells via downregulation of
phospho-CREB and MITF. J Food Sci. 80:H2354–H2359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jing N and Tweardy DJ: Targeting Stat3 in
cancer therapy. Anticancer Drugs. 16:601–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xing E, Guo Y, Feng G, Song H, An G, Zhao
X and Wang M: Effects of dioscin on T helper 17 and regulatory
T-cell subsets in chicken collagen type II-induced arthritis mice.
J Chin Med Assoc. 82:202–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao T, Jia H, Cheng Q, Xiao Y, Li M, Ren
W, Li C, Feng Y, Feng Z, Wang H and Zheng J: Nifuroxazide prompts
antitumor immune response of TCL-loaded DC in mice with
orthotopically- implanted hepatocarcinoma. Oncol Rep. 37:3405–3414.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Groner B, Lucks P and Borghouts C: The
function of Stat3 in tumor cells and their microenvironment. Semin
Cell Dev Biol. 19:341–350. 2008. View Article : Google Scholar : PubMed/NCBI
|