Introduction
The occurrence of cutaneous squamous cell carcinoma
(cSCC), which is the second most common type of non-melanoma skin
cancer in Korea, has markedly increased in numerous countries. The
age-standardized incidence rate of squamous cell carcinoma during
1999–2014 in Korea was 1.34 per 100,000 people for men, and 1.04
per 100,000 for women. The average annual percentage change (AAPC)
of cSCC has increased both in women [AAPC, 6.8 (95% CI, 5.3 to
8.4)] and men [AAPC, 3.3 (95% CI, 2.6 to 4.0)] (1,2).
Surgical treatment is curative in most cases of cSCC; in
particular, Mohs micrographic surgery (MMS) has become an common
treatment option for various types of cutaneous neoplasm, including
cSCC (3). As a standard form of
tissue-conservative skin cancer surgery, MMS ensures clearance of
pathological margins via intraoperative histopathologic
interpretation using the fresh-frozen tissue technique; therefore,
MMS leads to a lower recurrence rate compared with other therapies
that use conventional wide excision (3). However, certain patients that
experience recurrence following MMS require adjuvant therapy
(3–5). Since adjuvant therapy can cause
numerous side effects, it is crucial to identify a reliable method
for assessing the risk of recurrence in patients with cSCC
following surgery.
The clinical risk factors for cSCC recurrence
include tumor invasion depth, size, differentiation status,
presence of perineural invasion and location (6). In addition, certain molecular
biomarkers, including tumor protein 53, cyclin-dependent kinase
inhibitor 2A, telomerase reverse transcriptase gene (TERT) and
programmed cell death ligand 1 (PD-L1) have been considered as
potential factors involved in cSCC progression. In particular, TERT
promoter mutations and increased PD-L1 expression have been
considered as molecular risk factors for cSCC recurrence (7–9).
However, these predictive risk factors are inadequate to properly
assess the recurrence risk of cSCC with high reproducibility and
reliability (5,6).
Epithelial-to-mesenchymal transition (EMT) is a
crucial process for cancer cell local invasion and metastasis that
acts through the loss of epithelial properties and the acquisition
of a mesenchymal phenotype (10).
SNAIL, which is a zinc finger transcriptional repressor that
functions as a crucial EMT regulator by repressing E-cadherin, is
associated with poor prognosis in various types of cancer, such as
breast cancer, ovarian cancer, and colorectal cancer (11–13). In
cancer cells, activated canonical Wnt signaling inhibits glycogen
synthase kinase 3 (GSK-3)-dependent phosphorylation of SNAIL, which
subsequently leads to the inhibition of SNAIL degradation,
resulting in increased SNAIL protein expression (14). Axis inhibition protein 2 (AXIN2),
which is a GSK-3 scaffolding protein and a downstream target of the
Wnt signaling pathway, can stabilize nuclear SNAIL expression
through the regulation of a nucleocytoplasmic shuttle for GSK-3
(10). Furthermore, it has been
reported that AXIN2 expression is positively correlated with SNAIL
expression in breast and colon cancer (10,15);
however, the predictive value of the expression of these proteins
in cSCC recurrence remains unclear.
This retrospective cohort study aimed to evaluate
the predictive value of AXIN2 and SNAIL expression in the
recurrence of cSCC and to determine an accurate risk prediction
model for cSCC recurrence.
Materials and methods
Clinical materials
A total of 111 patients with primary cSCC who had
undergone MMS between January 2000 and December 2010 at the Yonsei
University Health System, Seoul, Korea, were included in the
present study. Patients who had undergone MMS for recurrent cSCC
were excluded from this study. Inclusion criteria were listed as
follows: Recurrence of cSCC was first clinically diagnosed and
confirmed histologically and histological pattern of cSCC tissue
samples were confirmed independently by two pathologists in a
blinded manner. All tissue samples were obtained from the
Department of Pathology, Yonsei University Health System, Seoul,
Korea. The tissue samples were fixed with 4% formalin, embedded
with paraffin, and stored at room temperature (RT) prior to use.
This study was approved by the Institutional Review Board for
Bioethics of Yonsei University Health System, Severance Hospital
(approval no. IRB 4-2018-0331).
IHC
Formalin-fixed paraffin-embedded tissue samples were
cut into 4-µm tissue sections, deparaffinized with 98.5% xylene and
rehydrated with an ethanol gradient series (99.9, 80, and 75%
ethanol). Antigen retrieval and blocking of endogenous peroxidase
activity were performed using antigen retrieval buffer at 100°C for
2 min (Dako; Agilent Technologies, Inc.) and a mixture of methanol
and hydrogen peroxide in a 40:1 ratio at RT for 20 min,
respectively. Tissue sections were incubated with the primary
antibodies rabbit monoclonal anti-human AXIN2 (Abcam; cat. no.
ab109307; 1:250) and polyclonal rabbit anti-human SNAIL (Abcam;
cat. no. ab53519; 1:250) at RT for 1 h, as well as REAL EnVision
HRP Rabbit/Mouse Detection System at RT for 30 min (Dako; Agilent
Technologies, Inc.; cat. no. K5007; prediluted; Agilent
Technologies, Inc.) as a secondary antibody, according to the
manufacturer's protocols. Visualization and counterstaining were
performed using chromogen 3,3′-diaminobenzidine (Dako; Agilent
Technologies, Inc.) and hematoxylin at RT for 2 min, respectively.
Rabbit IgG (Dako; Agilent Technologies, Inc.) was used as a
negative control.
AXIN2 and SNAIL expression levels were evaluated
using the weighted histoscore method (16). Briefly, the total histoscore was
determined based on tissue staining intensity and percentage of
positively stained cells. Staining intensity was scored as 0, 1, 2
and 3 for negative, light brown, brown and dark brown staining,
respectively. The histoscore was calculated as follows: Total
histoscore = (0 × percentage of negative cells) + (1 × percentage
of light-brown cells) + (2 × percentage of brown cells) + (3 ×
percentage of dark brown cells). For subsequent analysis, patients
were subdivided into low and high expression groups with
histoscores of 0–100 and 101–300, respectively. The percent of
patients with high or low protein expression in each group was
calculated and presented in Table
I.
 | Table I.Clinicopathological significance of
AXIN2 and SNAIL expression in 111 patients with cutaneous squamous
cell carcinoma. |
Table I.
Clinicopathological significance of
AXIN2 and SNAIL expression in 111 patients with cutaneous squamous
cell carcinoma.
|
|
| AXIN2, n (%) | SNAIL, n (%) |
|---|
|
|
|
|
|
|---|
| Variable | Case number, n
(%) | Low | High | P-value | Low | High | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
| ≤74 | 56 (50.5) | 43 (76.8) | 13 (23.2) | 0.057 | 35 (62.5) | 21 (37.5) | 0.642 |
|
>74 | 55 (49.5) | 33 (60.0) | 22 (40.0) |
| 32 (58.2) | 23 (41.8) |
|
| Sex |
|
|
|
|
|
|
|
| Male | 50 (45.0) | 35 (70.0) | 15 (30.0) | 0.753 | 28 (56.0) | 22 (44.0) | 0.395 |
|
Female | 61 (55.0) | 41 (67.2) | 20 (32.8) |
| 39 (63.9) | 22 (36.1) |
|
| Site |
|
|
|
|
|
|
|
|
Scalp | 14 (12.6) | 7 (50.0) | 7 (50.0) | 0.317 | 9 (64.3) | 5 (35.7) | 0.773 |
|
Face | 54 (48.6) | 36 (66.7) | 18 (33.3) |
| 32 (59.3) | 22 (40.7) |
|
|
Ear | 10 (9.0) | 7 (70.0) | 3 (30.0) |
| 5 (50.0) | 5 (50.0) |
|
|
Lip | 15 (13.5) | 13 (86.7) | 2 (13.3) |
| 11 (73.3) | 4 (26.7) |
|
|
Acral | 18 (16.2) | 13 (72.2) | 5 (27.8) |
| 10 (55.6) | 8 (44.4) |
|
| Size, cm |
|
|
|
|
|
|
|
|
≤1.7 | 56 (50.5) | 44 (78.6) | 12 (21.4) | 0.021a | 39 (69.6) | 17 (30.4) | 0.044a |
|
>1.7 | 55 (49.5) | 32 (58.2) | 23 (41.8) |
| 28 (50.9) | 27 (49.1) |
|
|
Differentiation |
|
|
|
|
|
|
|
| WD | 41 (36.9) | 27 (65.9) | 14 (34.1) | 0.099 | 25 (61.0) | 16 (39.0) | 0.824 |
| MD | 62 (55.9) | 46 (74.2) | 16 (25.8) |
| 38 (61.3) | 24 (38.7) |
|
| PD | 8 (7.2) | 3 (37.5) | 5 (62.5) |
| 4 (50.0) | 4 (50.0) |
|
| Recurrence |
|
|
|
|
|
|
|
|
Yes | 18 (16.2) | 8 (44.4) | 10 (55.6) | 0.017a | 7 (38.9) | 11 (61.1) | 0.042a |
| No | 93 (83.8) | 68 (73.1) | 25 (26.9) |
| 60 (64.5) | 33 (35.5) |
|
Statistical analysis
χ2 and Fisher's exact tests were used to
analyze the associations between AXIN2 and SNAIL expression and
patients' clinicopathological characteristics. Kaplan-Meier
survival curves were plotted based on various clinical factors and
protein expression, and the significance was analyzed using the
log-rank test to determine the recurrence-free survival of patients
with cSCC. Furthermore, the strength of associations among various
clinical factors and cSCC recurrence was analyzed using the Cox
proportional hazards model. The nomogram for assessment of cSCC
recurrence risk was constructed using AXIN2 and Snail expression,
as well as various clinicopathological characteristics such as sex,
age, lesion site, tumor size, and differentiation, and evaluated
with the concordance index (c-index) and a calibration plot
(17). R package version 3.1.1 (The
R Foundation for Statistical Computing; http://www.r-project.org) with the rms (3.5.0) and eha
(2.7.6) packages was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient clinicopathological
characteristics
In the present cohort, 18 (16.2%) patients presented
recurrence (interval of recurrence, 1–91 months), whereas 93
(83.8%) patients did not experience recurrence following MMS during
the follow-up period of 156 months. Tissue samples were confirmed
independently by two pathologists in a blinded manner. The age of
patients at diagnosis ranged between 30 and 98 years, with a median
age of 74 years. The patient cohort comprised 50 (45.0%) men and 61
(55.0%) women, and the tumor size ranged from 0.3 cm to 4.5 cm,
with a median tumor size of 1.7 cm. cSCC lesions were located on
the face (54/111; 48.6%), extremities (18/111; 16.2%), lip (15/111;
13.5%), scalp (14/111; 12.6%) and ear (10/111; 9.0%). In addition,
the results of the histological grading (18) demonstrated that 41 cases (36.9%) were
well differentiated (WD), 62 cases (55.9%) were moderately
differentiated (MD) and 8 cases (7.2%) were poorly differentiated
(PD). In addition, none of the patients included in this study
presented lymph node metastasis or distant metastasis.
AXIN2 and SNAIL expression in cSCC
tissue samples
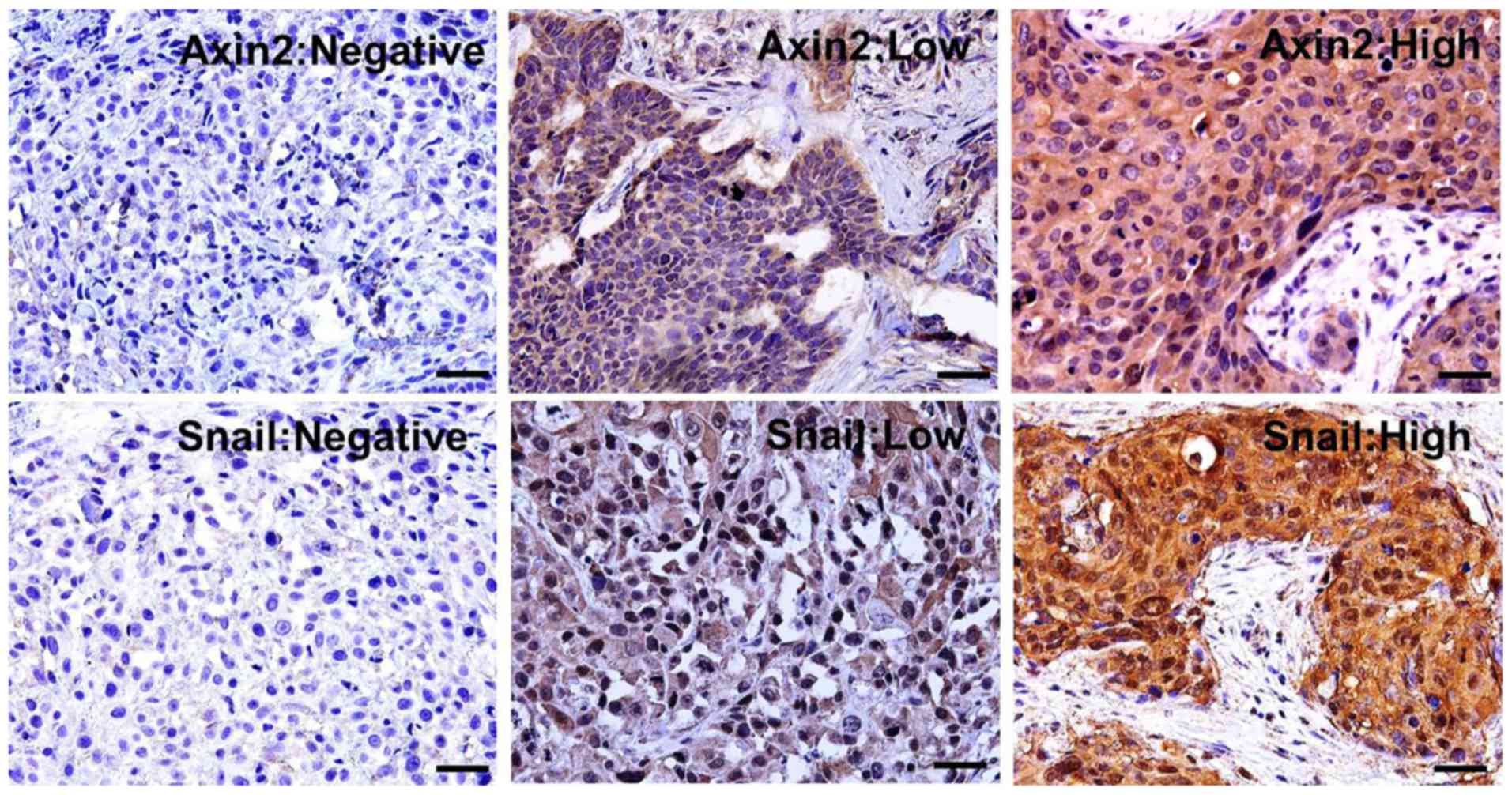
AXIN2, which is a scaffolding protein, was mostly
found in the cytoplasm of cancer cells in cSCC tissues. In
addition, the results demonstrated that AXIN2 expression was low in
76 (68.5%) and high in 35 (31.5%) cSCC tissues. SNAIL, which is a
transcription factor, was found in the cytoplasm and the nucleus of
cSCC cells. Furthermore, SNAIL expression was low in 67 (60.4%) and
high in 44 (39.6%) cSCC tissues (Table
I). Representative expression patterns for AXIN2 and SNAIL IHC
are presented in Fig. 1.
A significant association was identified between
AXIN2 and SNAIL expression in cSCC tissues. In addition, high SNAIL
expression was detected in 62.9% cSCC tissues with high AXIN2
expression. By contrast, in cSCC tissues with low AXIN2 expression,
only 28.9% exhibited high SNAIL expression (Fig. 2A; P=0.001).
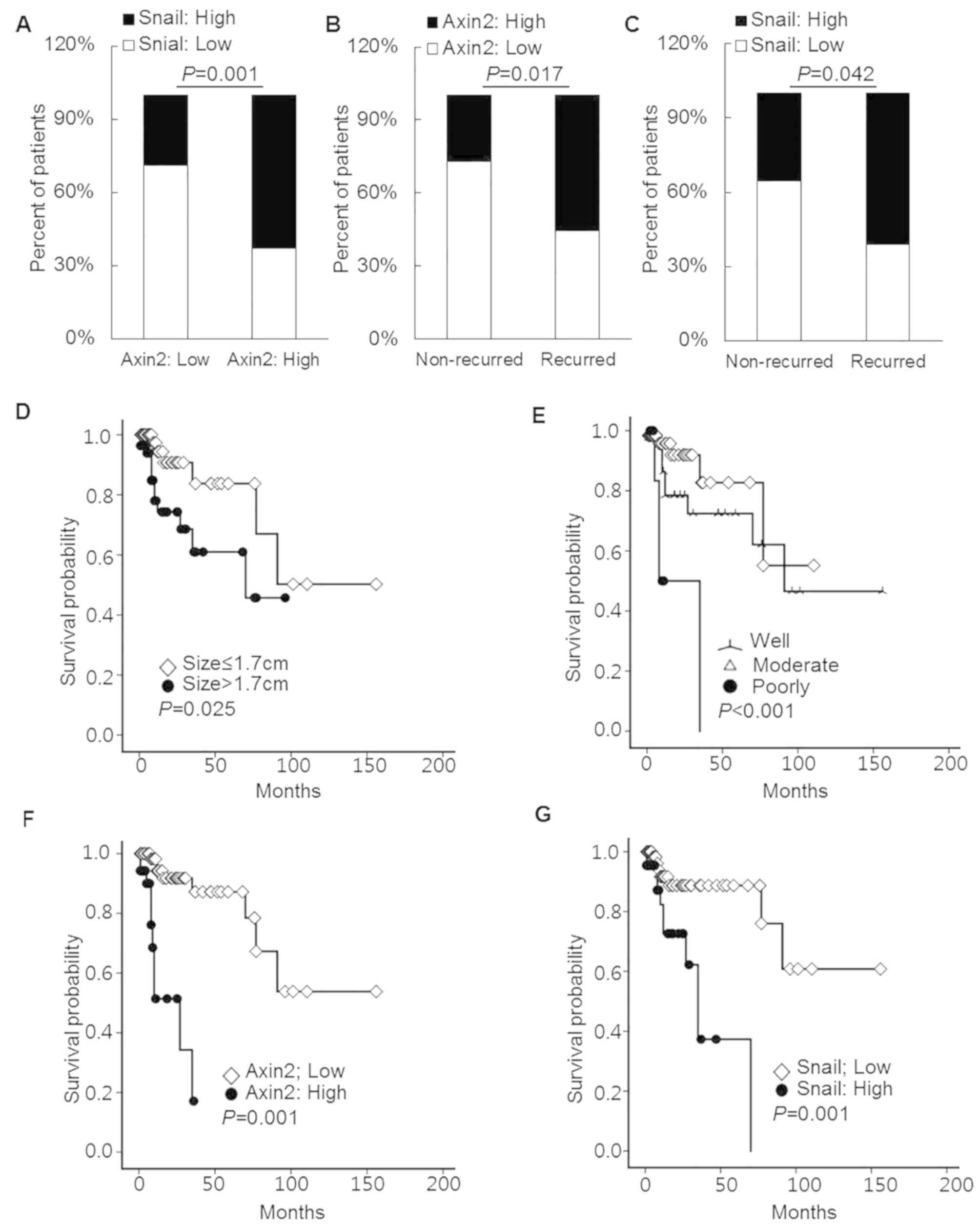 | Figure 2.AXIN2 and SNAIL expression in cSCC
tissue samples. (A) AXIN2 expression was significantly associated
with SNAIL expression in cSCC tissues (χ2 test,
P=0.001). Recurrence occurred more frequently in patients with (B)
high AXIN2 or (C) SNAIL expression compared with patients with low
AXIN2 or SNAIL expression (Fisher's exact test, P=0.017 and
P=0.042, respectively). Patients with (D) larger tumors, (E) poorly
differentiated histological grade, (F) high AXIN2 expression and
(G) high SNAIL expression presented decreased recurrence-free
survival rates (log-rank test, P=0.025, P<0.001, P=0.001 and
P=0.001, respectively). AXIN2, axis inhibition protein 2; cSCC,
cutaneous squamous cell carcinoma. |
Clinicopathological significance of
AXIN2 and SNAIL expression in patients with cSCC
High expression levels of AXIN2 and SNAIL were more
frequently detected in patients with recurrence (55.6 and 61.1%,
respectively) compared with patients without recurrence (26.9 and
35.5%, respectively; P=0.017 and P=0.042, respectively; Fig. 2B and C). Furthermore, tumor size and
AXIN2 and SNAIL expression levels were significantly associated in
cSCC tissues (Table I). High
expression levels of AXIN2 and SNAIL were more frequently detected
in patients with larger tumor size (41.8 and 49.1%, respectively)
compared with patients with smaller tumor size (21.4 and 30.4%,
respectively; P=0.021 and P=0.044, respectively; Table I). No significant association was
identified between AXIN2 and SNAIL protein expression levels and
other clinicopathological characteristics, including age, sex,
lesion site and differentiation (Table
I).
Risk factors for recurrence in
patients with cSCC
The results of the Kaplan-Meier curve analysis
demonstrated that recurrence-free survival was significantly
associated with tumor size (P=0.025), differentiation status
(P<0.001) and expression of AXIN2 (P=0.001) and SNAIL (P=0.001;
Fig. 2D-G). In addition, poor
recurrence-free survival was observed in patients with tumors of
larger size (median survival duration of 13 months for tumor size
≤1.7 cm vs. 8 months for tumor size >1.7 cm), PD histological
grade (median survival duration of 12.0 and 11.0 months for WD and
MD histological grades, respectively, and of 8.0 months for
patients with PD histological grade), high AXIN2 expression (median
survival duration of 16.0 months for low AXIN2 expression vs. 6.0
months for high AXIN2 expression) or high SNAIL expression (median
survival duration of 13.0 months for low SNAIL expression vs. 8.0
months for high SNAIL expression). According to the American Joint
Committee on Cancer (8th edition) (19), tumor diameter >2 cm is the main
cutoff value for tumor size (19,20). In
the present study, no association was identified between tumor size
and recurrence-free survival when using the 2 cm tumor size cut-off
value. However, the median tumor size cutoff of 1.7 cm was more
predictive of cumulative disease-free survival rate (Fig. 2D; P=0.025). This difference in the
cutoff value of tumor size may be due to the difference between MMS
and other surgical methods.
The results of the Cox-regression analysis
demonstrated that age [hazard ratio (HR), 0.955; 95% confidence
interval (CI), 0.914–0.999; P=0.043], high AXIN2 expression (HR,
15.169; 95% CI, 3.149–73.072; P=0.001) and high SNAIL expression
(HR, 4.795; 95% CI, 1.329–17.296; P=0.045) were independent risk
factors for recurrence-free survival in patients with cSCC
(Table II).
 | Table II.Risk factors for recurrence in
patients with cutaneous squamous cell carcinoma. |
Table II.
Risk factors for recurrence in
patients with cutaneous squamous cell carcinoma.
| Variable | Hazard ratio (95%
CI) | P-value |
|---|
| Sex | 1.650
(0.492–5.533) | 0.417 |
| Age | 0.955
(0.914–0.999) | 0.043a |
| Lesion site |
|
|
|
Scalp | 1 |
|
|
Face | 0.467
(0.104–2.101) | 0.321 |
|
Ear | 0.802
(0.103–6.231) | 0.833 |
|
Lip | 0.409
(0.069–2.425) | 0.325 |
|
Acral | 0.451
(0.081–2.541) | 0.364 |
|
Size | 1.119
(0.297–4.213) | 0.868 |
|
Differentiation |
|
|
| WD | 1 |
|
| MD | 0.817
(0.241–2.769) | 0.746 |
| PD | 1.516
(0.303–7.582) | 0.613 |
|
SNAIL | 4.795
(1.329–17.296) | 0.045a |
|
AXIN2 | 15.169
(3.149–73.072) | 0.001a |
Construction of a predictive
nomogram
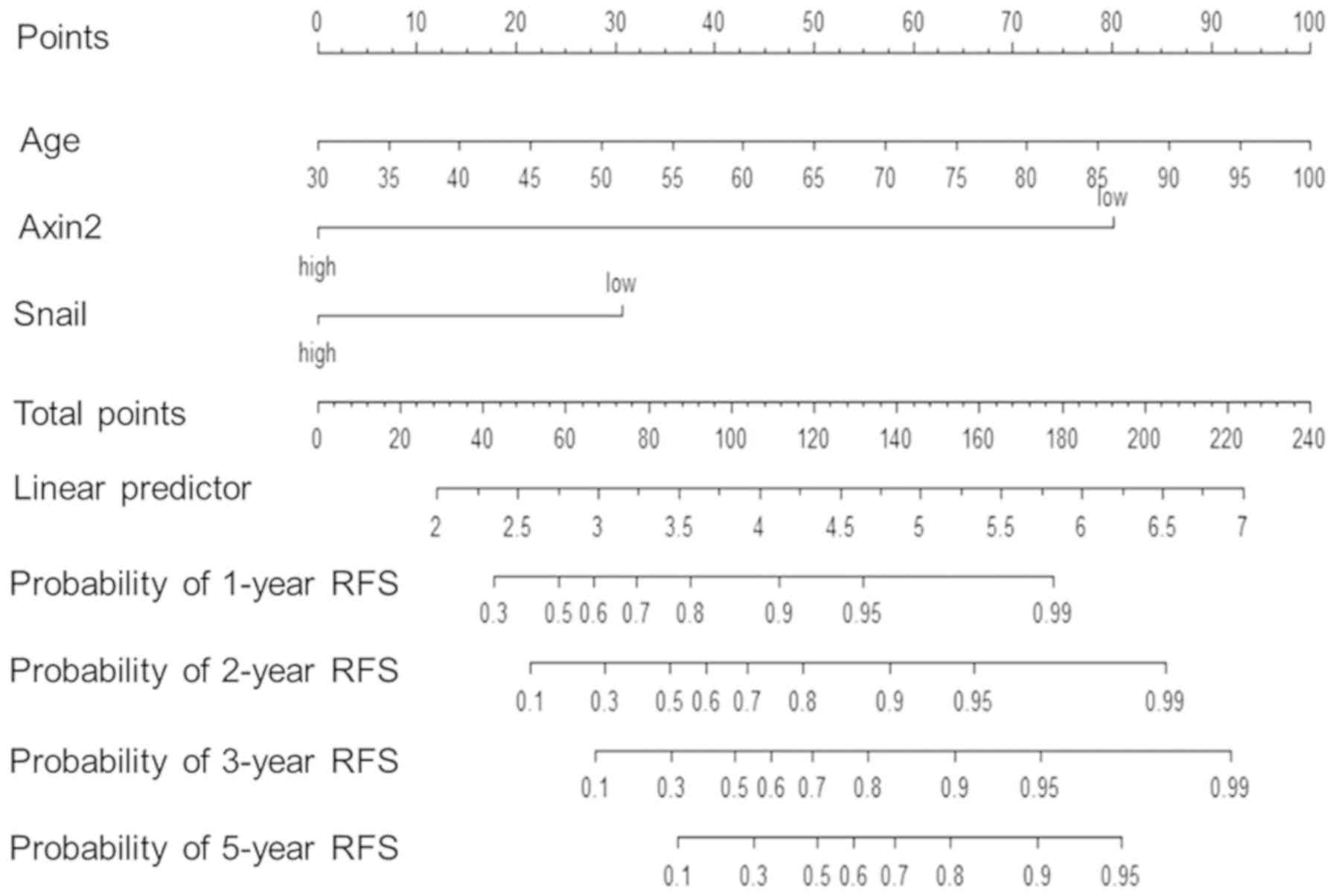
Predictive nomograms were constructed using
patients' clinicopathological characteristics and/or AXIN2 and
SNAIL protein expression to analyze the 1-, 2-, 3- and 5-year
recurrence-free survival in patients with cSCC. The c-index of the
nomogram constructed with a combination of clinicopathological
characteristics, including sex, age, lesion site, tumor size and
differentiation, was ~0.40. When the nomogram was constructed using
AXIN2 and SNAIL protein expression, the c-index was ~0.69. When all
clinicopathological characteristics and AXIN2 and SNAIL protein
expression were combined, the c-index of the nomogram was ~0.61.
Furthermore, the c-index increased to ~0.75 when the nomogram was
constructed with independent risk factors such as age and
expression of AXIN2 and SNAIL (Fig.
3).
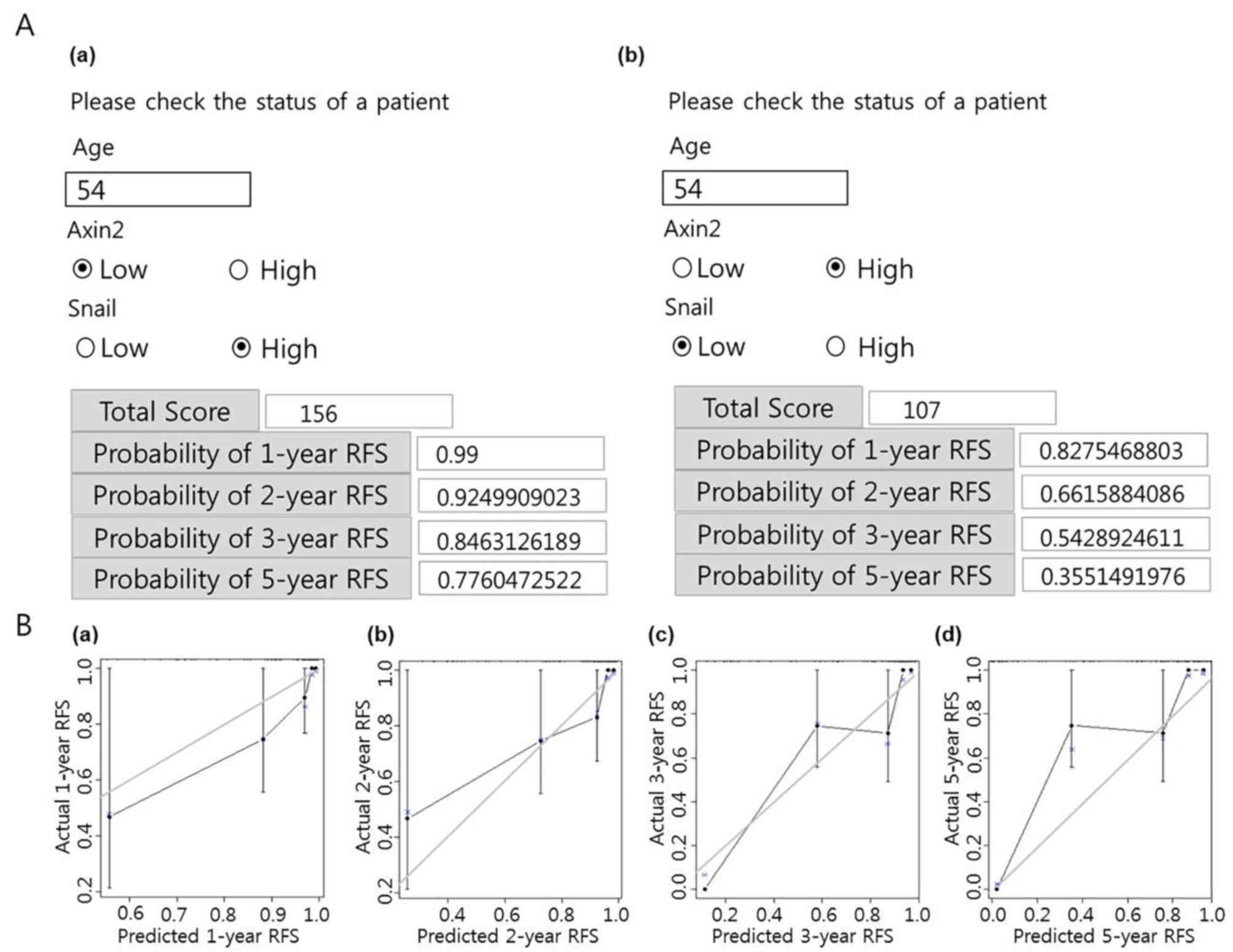
To demonstrate the practical usage of the nomogram,
a nomogram in Hypertext Markup Language (HTML) format that included
predictive factors, total score and probabilities of
recurrence-free survival was constructed (Fig. 4A). As presented in Fig. 4Aa, in a patient aged 54 years with
low AXIN2 and high SNAIL expression, the probability of
recurrence-free survival calculated using the nomogram was 99,
92.5, 84.6 and 77.6% after 1, 2, 3 and 5 years, respectively. By
contrast, as presented in Fig. 4Ab,
a 54-year-old patient with high AXIN2 expression and low SNAIL
expression presented a probability of recurrence-free survival of
82.8, 66.2, 54.3 and 35.5% after 1, 2, 3 and 5 years, respectively.
The constructed nomogram with the highest c-index was calibrated to
further evaluate the association between the predicted probability
and the real outcomes of patients with cSCC. The predicted outcomes
are presented on the x-axis, and the actual data for
recurrence-free survival of each patient with cSCC are presented on
the y-axis. An ideal nomogram would demonstrate perfect consensus
(x=y) between data predicted by the nomogram and actual
recurrence-free survival for each patient in the cohort. The
results of the present study demonstrated a relatively solid line,
especially for the prediction of recurrence-free survival after 1
and 2 years (Fig. 4B).
Discussion
Aberrant activation of the Wnt signaling pathway and
subsequent initiation of EMT have been demonstrated to be
associated with poor prognosis in various types of cancer,
including cSCC (10,14,21). In
the present study, the predictive value of the protein expression
of two EMT genes, AXIN2 and SNAIL, in the recurrence of cSCC was
evaluated over a long-term follow-up period.
Wnt signaling is activated by binding of the Wnt
ligand to its co-receptors, including seven-transmembrane-domain
Frizzled receptors and a low-density lipoprotein (LDL)
receptor-related protein (LRP5/LRP6). In the absence of Wnt
ligands, GSK3 assembles a β-catenin destruction complex and
subsequently mediates β-catenin degradation (22). Previous studies reported that AXIN2
is a molecular component of the β-catenin destruction complex that
can repress various downstream target genes of β-catenin,
indicating its role as a tumor suppressor. However, when aberrant
activation of the Wnt signaling pathway occurs, AXIN2 protein is
translocated from the β-catenin destruction complex to the LRP5
receptor via the phosphorylated Dishevelled, leading to the
inactivation of the β-catenin destruction complex. β-catenin is
subsequently protected from the degradation process and further
involved in the transcription of Wnt target genes (23,24).
Previous studies suggested that AXIN2 may serve
oncogenic roles. As one of the T-cell factor/lymphoid enhancer
factor downstream targets of canonical Wnt signaling pathway, AXIN2
has been demonstrated to be upregulated in various types of cancer,
including hepatoblastoma, cervical, breast and colorectal cancer
(10,25,26).
Furthermore, a study has reported that AXIN2 upregulation mediates
the increased nuclear SNAIL and β-catenin expression by supporting
the nuclear export function of GSK-3 in breast cancer cells
(10). In addition, the present
study demonstrated that AXIN2 and SNAIL expression levels were
significantly associated in cSCC tissues. A previous study reported
that AXIN2 knockdown led to decreased invasive ability of
colorectal cancer cells (15),
whereas another study demonstrated that AXIN2 can induce genomic
instability by influencing centrosome cohesion at the mitotic
checkpoint (27). These findings
suggested that AXIN2 may act as an oncogene protein during cancer
progression, but not as a tumor suppressor.
Consistent with observations in breast cancer
(10), the results of the present
study reported that SNAIL expression was more frequently detected
in cSCC tissues with high AXIN2 expression compared with cSCC
tissues with low AXIN2 expression. Furthermore, the results of the
Cox regression analysis demonstrated that the expression of AXIN2
and SNAIL was an independent risk factor for recurrence-free
survival in patients with cSCC. In particular, patients with high
AXIN2 and SNAIL expression presented a higher risk of recurrence
compared with patients with low AXIN2 and SNAIL expression or
patients with high expression of AXIN2 or SNAIL only. These results
suggested that AXIN2-mediated SNAIL stabilization and aberrant
activation of the Wnt signaling pathway may serve crucial roles in
cSCC recurrence.
In the present study, certain clinicopathological
characteristics, including large tumor size and poor tumor
differentiation, were also identified as risk factors for
recurrence in patients with cSCC according to the Kaplan-Meier
analysis. Furthermore, age was considered as an independent risk
factor for recurrence in this study. However, when the nomogram was
constructed using only clinicopathological characteristics, the
prediction model accuracy was very low, the c-index of the nomogram
was ~0.40. The accuracy was improved by considering the molecular
markers as predictive factors. The c-index increased to ~0.75 when
the clinicopathological characteristics were combined with AXIN2
and SNAIL expression. In addition, the results of the calibration
plot also demonstrated that the nomogram exhibited a high level of
predictive accuracy in patients with cSCC. Further identification
of novel biomarkers related to cSCC pathogenesis are required in
order to increase the prediction accuracy of the nomogram.
Since AXIN2 is a target of the canonical Wnt
signaling pathway, the results of the present study suggested that
activation of the canonical Wnt signaling pathway and EMT
progression may be involved in cSCC recurrence. In addition, AXIN2
and SNAIL expression may be considered as potential predictive
biomarkers for assessing the risk of recurrence in patients with
cSCC.
The present study had limitations. Certain important
clinical characteristics, including previous history of organ
transplantation, diabetes mellitus and other cancers, were excluded
from analysis as they were missing in >50% patients. The
predictive nomogram constructed in the present study may therefore
not be fully performant. However, to the best of our knowledge, the
present study described the first prediction model that may be used
to investigate the risk of recurrence in patients with cSCC
following MMS.
Acknowledgements
The authors would like to thank Professor Haiyue
Chen and Dr Meici Piao for histological evaluation.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81560503) and the National
Research Foundation of Korea (grant no. 2017R1C1B2005574 and
2019R1A2C1003028).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
Histological analysis was performed by GZ and ZZ.
GZ, ZZ, and YO performed immunohistochemistry for AXIN2 and SNAIL.
KK, ML and DY analyzed the data. KC, MR and ZJ conceived the study
and wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board for Bioethics of Yonsei University Health System, Severance
Hospital (approval no. IRB 2018-0874-001). Patient consent for
using the stored tissue was exempted by approval of IRB.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh CM, Cho H, Won YJ, Kong HJ, Roh YH,
Jeong KH and Jung KW: Nationwide trends in the incidence of
melanoma and non-melanoma skin cancers from 1999 to 2014 in South
Korea. Cancer Res Treat. 50:729–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lobeck A, Weiss C, Orouji A, Koch PS, Heck
M, Utikal J, Koenen W, Faulhaber J, Klemke CD and Felcht M: Single
center analysis of the dermatosurgical patient cohort of a tumor
center in Germany. Hautarzt. 68:377–384. 2017.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le ST, Kamal HY and Khachemoune A: Mohs
micrographic surgery for cutaneous malignancies: A focus review of
its indications in pediatric age groups. Pediatr Dermatol.
35:434–440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmults CD, Karia PS, Carter JB, Han J
and Qureshi AA: Factors predictive of recurrence and death from
cutaneous squamous cell carcinoma: A 10-year, single-institution
cohort study. JAMA Dermatol. 149:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson AK, Kelley BF, Prokop LJ, Murad
MH and Baum CL: Risk factors for cutaneous squamous cell carcinoma
recurrence, metastasis, and disease-specific death: A systematic
review and meta-analysis. JAMA Dermatol. 152:419–428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roscher I, Falk RS, Vos L, Clausen OPF,
Helsing P, Gjersvik P and Robsahm TE: Validating 4 staging systems
for cutaneous squamous cell carcinoma using population-based data:
A nested case-control study. JAMA Dermatol. 154:428–434. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campos MA, Macedo S, Fernandes M, Pestana
A, Pardal J, Batista R, Vinagre J, Sanches A, Baptista A, Lopes JM
and Soares P: TERT promoter mutations are associated with poor
prognosis in cutaneous squamous cell carcinoma. J Am Acad Dermatol.
80:660–669.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Diez I, Hernandez-Ruiz E, Andrades
E, Gimeno J, Ferrándiz-Pulido C, Yébenes M, García-Patos V, Pujol
RM, Hernández-Muñoz I and Toll A: PD-L1 expression is increased in
metastasizing squamous cell carcinomas and their metastases. Am J
Dermatopathol. 40:647–654. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim
NH, Cha SY, Ryu JK, Choi YJ, Kim J, et al: A Wnt-Axin2-GSK3beta
cascade regulates Snail1 activity in breast cancer cells. Nat Cell
Biol. 8:1398–1406. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muenst S, Daster S, Obermann EC, Droeser
RA, Weber WP, von Holzen U, Gao F, Viehl C, Oertli D and Soysal SD:
Nuclear expression of snail is an independent negative prognostic
factor in human breast cancer. Dis Markers. 35:337–344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YL, Zhao XM, Shuai ZF, Li CY, Bai QY,
Yu XW and Wen QT: Snail promotes epithelial-mesenchymal transition
and invasiveness in human ovarian cancer cells. Int J Clin Exp Med.
8:7388–7393. 2015.PubMed/NCBI
|
|
13
|
Brzozowa M, Michalski M, Wyrobiec G,
Piecuch A, Dittfeld A, Harabin-Słowińska M, Boroń D and Wojnicz R:
The role of Snail1 transcription factor in colorectal cancer
progression and metastasis. Contemp Oncol (Pozn). 19:265–270.
2015.PubMed/NCBI
|
|
14
|
Yook JI, Li XY, Ota I, Fearon ER and Weiss
SJ: Wnt-dependent regulation of the E-cadherin repressor snail. J
Biol Chem. 280:11740–11748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu
CY, Li XY and Weiss SJ: Canonical Wnt suppressor, Axin2, promotes
colon carcinoma oncogenic activity. Proc Natl Acad Sci USA.
109:11312–11317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Zheng Z, Shin YK, Kim KY, Rha SY,
Noh SH, Chung HC and Jeung HC: Angiogenic factor thymidine
phosphorylase associates with angiogenesis and lymphangiogenesis in
the intestinal-type gastric cancer. Pathology. 46:316–324. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen F, Lin L, Yan L, Liu F, Qiu Y, Wang
J, Hu Z, Wu J, Bao X, Lin L, et al: Nomograms and risk scores for
predicting the risk of oral cancer in different sexes: A
large-scale case-control study. J Cancer. 9:2543–2548. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous squamous cell carcinoma: A review of high-risk and
metastatic disease. Am J Clin Dermatol. 17:491–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th. New York:
Springer; 2017, View Article : Google Scholar
|
|
20
|
Mullen JT, Feng L, Xing Y, Mansfield PF,
Gershenwald JE, Lee JE, Ross MI and Cormier JN: Invasive squamous
cell carcinoma of the skin: Defining a high-risk group. Ann Surg
Oncol. 13:902–909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halifu Y, Liang JQ, Zeng XW, Ding Y, Zhang
XY, Jin TB, Yakeya B, Abudu D, Zhou YM, Liu XM, et al: Wnt1 and
SFRP1 as potential prognostic factors and therapeutic targets in
cutaneous squamous cell carcinoma. Genet Mol Res. 15:2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taketo MM: Shutting down Wnt
signal-activated cancer. Nat Genet. 36:320–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mao J, Wang J, Liu B, Pan W, Farr GH III,
Flynn C, Yuan H, Takada S, Kimelman D, Li L and Wu D: Low-density
lipoprotein receptor-related protein-5 binds to Axin and regulates
the canonical Wnt signaling pathway. Mol Cell. 7:801–809. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cadigan KM and Liu YI: Wnt signaling:
Complexity at the surface. J Cell Sci. 119:395–402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hughes TA and Brady HJ: Regulation of
axin2 expression at the levels of transcription, translation and
protein stability in lung and colon cancer. Cancer Lett.
233:338–347. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lustig B, Jerchow B, Sachs M, Weiler S,
Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM,
Birchmeier W and Behrens J: Negative feedback loop of Wnt signaling
through upregulation of conductin/axin2 in colorectal and liver
tumors. Mol Cell Biol. 22:1184–1193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hadjihannas MV, Bruckner M and Behrens J:
Conductin/axin2 and Wnt signalling regulates centrosome cohesion.
EMBO Rep. 11:317–324. 2010. View Article : Google Scholar : PubMed/NCBI
|