Within the past several decades, we have seen an
increase in research on the infiltration of leukocyte
sub-populations (LSPs), being drawn, sequestered and embedded
within solid tumor tissue corresponding to elevated concentrations
of chemokines such as CCL2 (1). Both
animal and in vitro studies have shown CCL2 can sequester
macrophages and other immune components such as myeloid-derived
suppressor cells or regulatory T cells all of which promote immune
evasion, epithelial-to-mesenchymal transition, tumor growth,
metastasis, and immune evasion. High concentrations of
pro-inflammatory proteins such as CCL2, TNFα, matrix
metalloproteinase 9, interleukin-6 (IL-6), chemokine (C-X-C motif)
ligands (e.g., CXCL) (1–4), granulocyte-macrophage
colony-stimulating factor and other chemokine ligands (e.g., CCLs)
(5–9)
are commonly reported as tumor promoting proteins in diverse
cancers such as thyroid, brain, gastric, lung, glioblastoma
multiforme and breast (2–7,10–15).
What is evidently a critical situation is that these
inflammatory proteins, in particular, the CCL2 and IL-6 are brought
about by the actual cancer treatments themselves (e.g.,
radiotherapy (16) chemotherapy
(8), which in turn are then
associated with tumor recurrence (17) and chemo-resistance (18,19),
Inflammatory events in general, whether it be from other parts of
the body such as the liver (9,20)
adipose tissue in obesity or arising from viral origin tend to
elevate TNF-a, IL −6 and CCL2 then becoming risk factors for the
development of diverse cancers (21)
aggressive tumors with advanced stage tumor grade and greater rates
of mortality (22,23). Meanwhile, it is believed that drugs
or natural compounds that can attenuate CCL2 and IL-6 would slow
the aggressive nature of advanced cancers (24–26) to
the inclusion of triple negative breast cancer (TNBC) and hormone
positive breast cancers (27,28). It
is believed that utilizing synthetic or natural small molecules as
CCR2 inhibitors (CCR2i) can increase overall survival odds
(29,30).
In our previous work, we found that apigenin, a
pigment naturally found in parsley, can modulate TNFα triggered
release of chemokines in a TNBC model using MDA-MB-231 cells
(31). In the present study, we
carried out a similar experiment using a TNBC cell line derived
from an African American woman (MDA-MB-468, MDA-MB-468 cells),
which express enormously high levels of CCL2 upon impact by TNFα as
demonstrated by the current work.
Triple-negative human breast tumor (MDA-MB-468)
cells were obtained from the American Type Culture Collection
(Rockville, MD, USA). Dulbecco's modified Eagle's medium (DMEM),
fetal bovine serum (FBS), and penicillin/streptomycin were all
obtained from Invitrogen. Recombinant human TNFα and CCL2 ELISA
kits were purchased from RayBiotech (RayBiotech Inc.).
MDA-MB-468 cells were grown in high-glucose DMEM
(w/phenol red and glutamine) supplemented with 10% FBS and 1%
[10,000 U/ml] penicillin G sodium + [10,000 µg/ml] streptomycin
sulfate. Cells were grown at 37°C with humidified 95% air and 5%
CO2 and sub-cultured every 3–5 days.
Viable cell count was determined by Alamar blue.
Briefly, 96-well plates were seeded with MDA-MB-468 cells at a
density of 5×104 cells/100 µl/well with various
treatments. After 24 h, Alamar blue (0.1 mg/ml in HBSS) was added
at 15% v/v to each well and incubated for 6–8 h. Quantitative
analysis of dye conversion was measured using a Biotek Synergy
multi-mode detection reader equipped with Gen5 software 550/580
(excitation/emission). Data were expressed as a percentage of the
untreated control groups.
Supernatants from experimental treatments were
collected, centrifuged at 1,000 × g for 5 min at 4°C and evaluated
for MCP-1/CCL2 using Human MCP1 ELISA from RaybioRayBiotech Life,
following the manufacturer's instructions. Briefly, a dilution from
10–50% of supernatants was made with assay buffer (final working
volume = 100 µl), and standards was added to 96-well plates
pre-coated with the capture antibody. Samples were washed 4×
between steps, and after adding the HRP-conjugate, the
substrate/stopping solutions were added, and plates were read at
450 nm using a Biotek Synergy multi-mode detection reader equipped
with Gen5 software. All data were expressed as concentration
derived from a standard curve in pg/ml.
Cells were collected by a 3X wash in ice-cold HBSS,
then a rapid freeze with storage at −80°C. Total RNA was isolated
and purified using the TRIzol/chloroform method, the quality was
assessed, and concentration was equalized to 82 ng/µl in
nuclease-free water. Whole transcriptome analysis was conducted
according to the GeneChipTM WT PLUS Reagent Manual for Whole
Transcript (WT) Expression Arrays for human 2.1 Array Strips
(32). Briefly, RNA was synthesized
to first strand cDNA, second-strand cDNA and followed by
transcription to cRNA. cRNA was purified and assessed for yield,
before 2nd cycle single-stranded cDNA synthesis, hydrolysis of RNA
and purification of 2nd cycle single-stranded cDNA. cDNA was then
quantified for yield and equalized to 176 ng/ml. Subsequently, cDNA
was fragmented, labeled and hybridized on to the arrays before
being subject to fluidics and imaging using the Gene Atlas
(Affymetrix- Thermo Fisher Scientific, Inc.).
A Kruskal-Wallis test, followed by a Dunn's multiple
comparison test was used to evaluate statistical differences from
controls and a one-way ANOVA followed by a Tukey's multiple
comparisons test to evaluate statistical differences between two
cell lines both using GraphPad prism software (GraphPad Software).
The array data quality control and initial processing from CEL to
CHP files were conducted using expression console, followed by data
analysis using the Affymetrix transcriptome analysis console
(Affymetrix-Thermo Fisher Scientific, Inc.). The data have been
deposited into the Gene Expression Omnibus for public analysis at
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE133968.
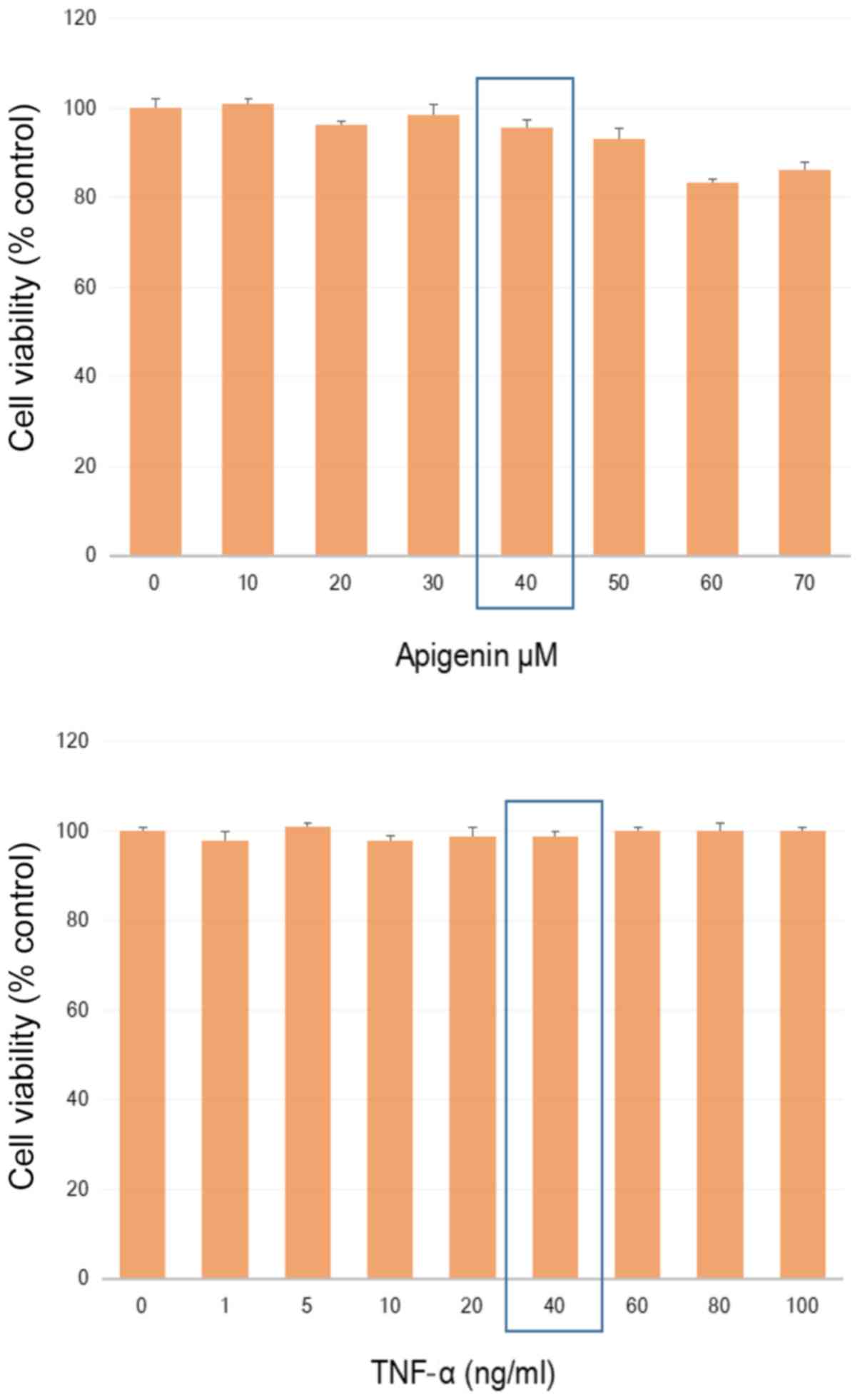
A non-lethal working concentration was established
in MDA-MB-468 cells for TNFα and apigenin (Fig. 1) to where sub-lethal values were
determined by a dose response using apigenin [40 uM], and TNFα [40
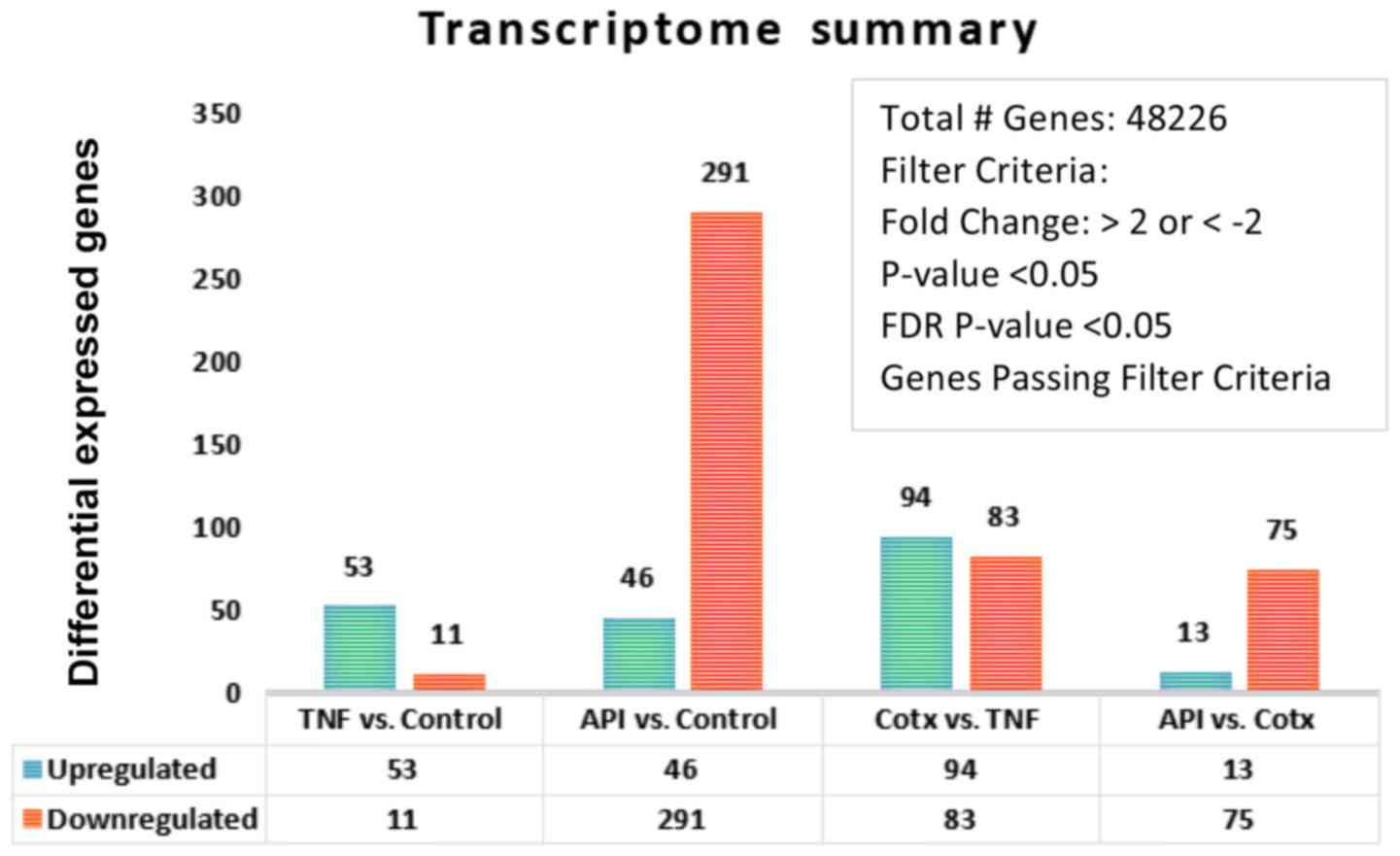
ng/ml]. Whole transcriptomic differential changes between untreated
controls, TNFα (40 ng/ml), apigenin (40 uM) and co-treatment (CoTx)
[TNFα (40 ng/ml) + apigenin (40 uM)] were acquired and the summary
by a number of deferentially expressed genes shown in Fig. 2. Comparing the Control vs. TNFα only,
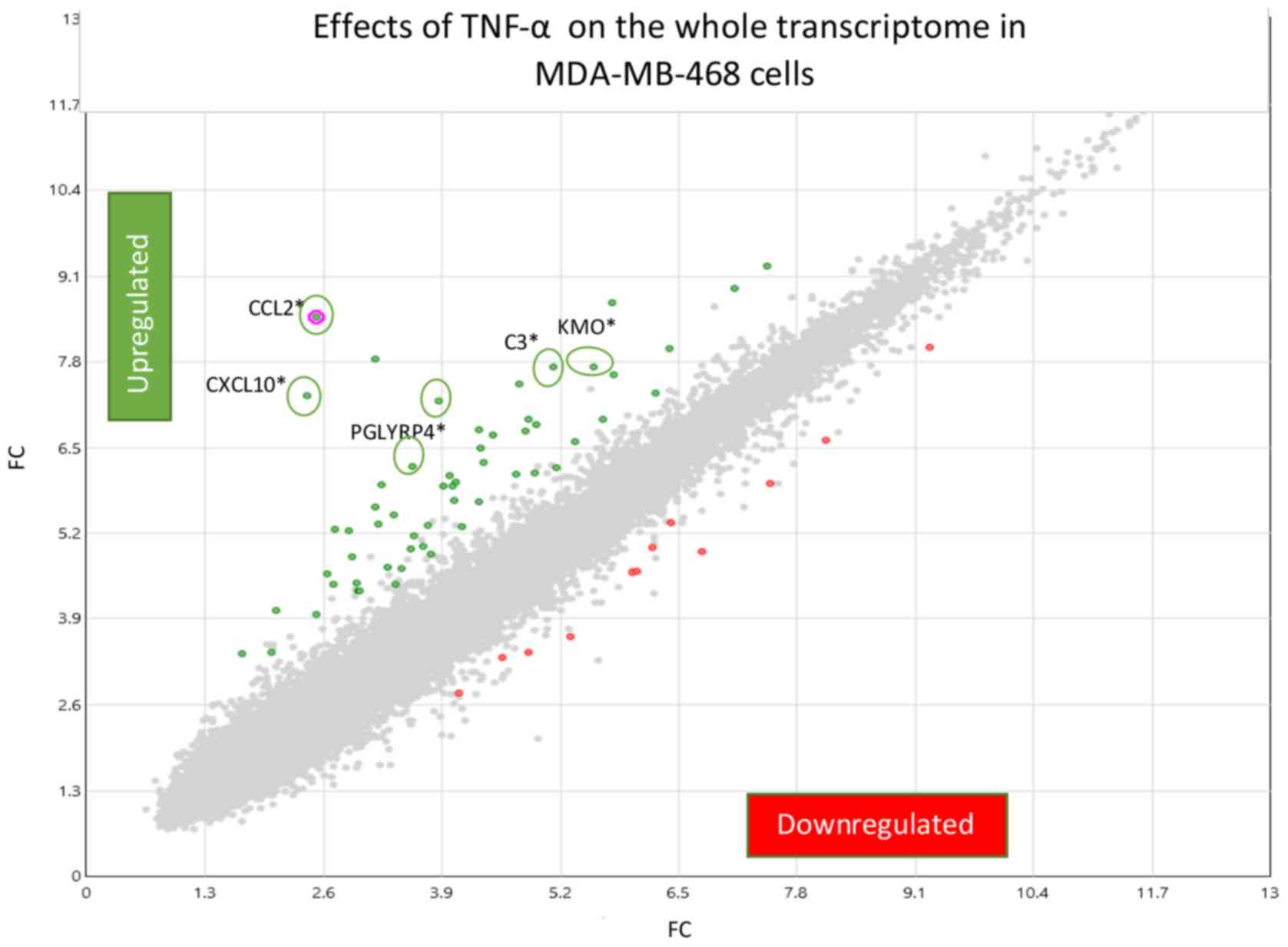
we provide a fold change (FC) scatter plot (Fig. 3) corresponding to signal and
processed data presented in Table I.
Gene level differential expression analysis was conducted on 48,226
genes where TNFα caused significant up-regulation of 53 transcripts
and down-regulation of 11 transcripts.
Limited therapeutic options are available for TNBC
patients and consequently can result in aggressive metastatic
disease, with greater mortality rates in African American (AA)
women, relative to Caucasian-American (33,34).
This health disparity may arise due to diagnosis at later stages of
the disease (35) or a predisposed
racially distinct genetic or epigenetic profile (36,37) with
a propensity toward an overactive oncogenic p38 MAPK,
Wnt/β-catenin, IGF2/ERbeta signaling axis (38–40).
Additional factors to a health disparity arising in AA women
regarding TNBC include vitamin D deficiencies (41) socioeconomic factors, later stage
diagnosis, obesity, or even breast feeding patterns (42–44).
As with all human cancers, late stage diagnosis is
associated with greater mortality rates to which the immune system
can play a critical role. In the case with solid tumors such as
breast cancer, inflammatory like secretion of cytokines to the
tumor microenvironment can drive infiltration of tumor-associated
macrophages (TAMs) and neutrophils (TANs) which promote tumor
survival, metastasis, invasion, angiogenesis, resistance and turn
off host immune surveillance, all equating to poor survival rates
(2,45–47). It
is believed that use of drugs or natural compounds that can
suppress oncogenic cytokines (e.g., CXCL1, CCL18, CCL8, CCL2, IL-4,
IL-8, IL-6, etc.) (17,48–53) such
as apigenin, EGCG or butein can curtail these biochemical driven
events and provide therapeutic advantages against aggressive
inflammatory breast cancers (54,55).
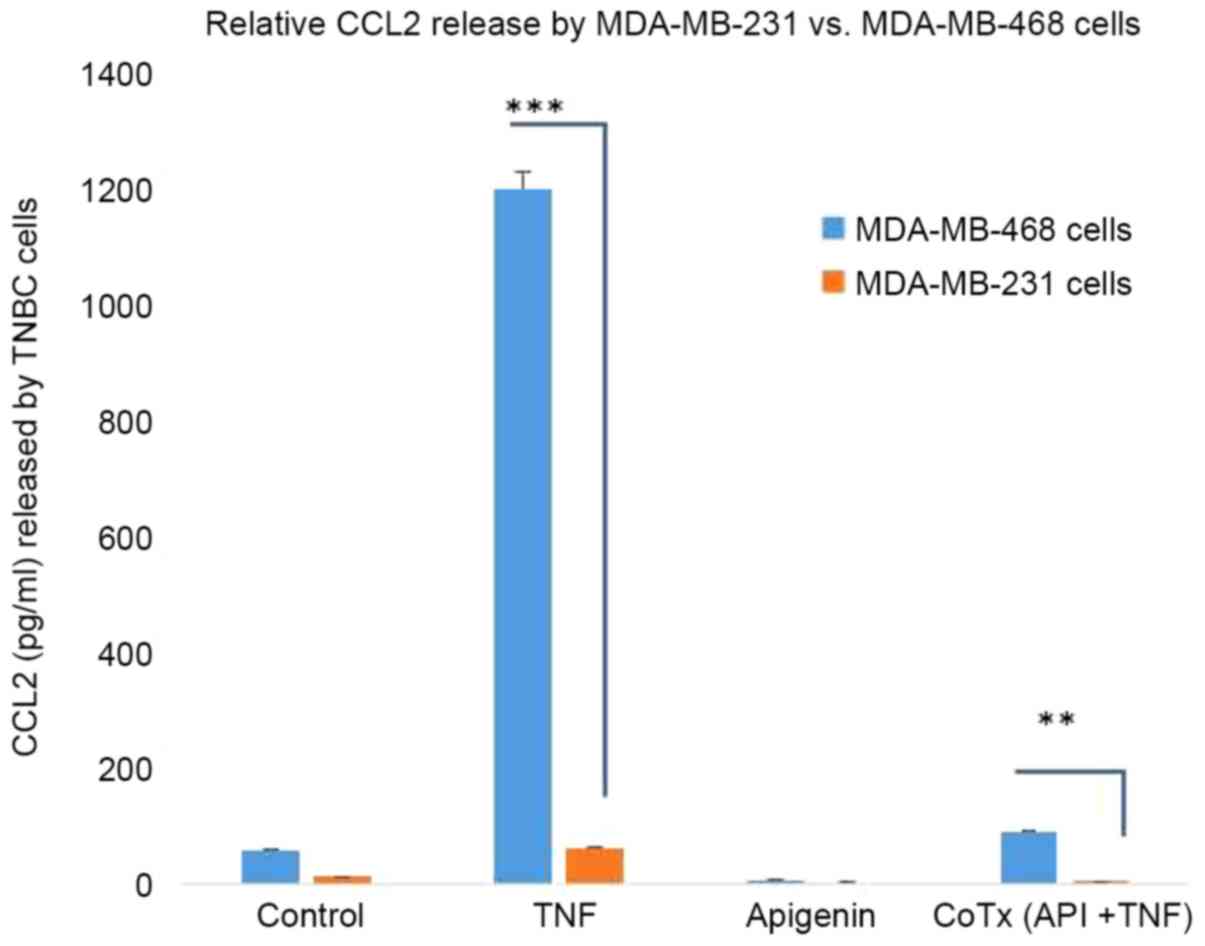
In the present study, an inflammatory profile was
evoked by TNFα, where the highest induced transcript in MDA-MB-468
cells was CCL2, confirmed at both the mRNA and protein level. The
rise in CCL2 is reported throughout the literature, where it serves
to drive tumor invasion, metastasis, and recurrence (2,17). CCl2
expression is also fairly consistent among breast cancer
subcategories: (luminal: ER+ and/or PR+) (56), HER2+ (27) or basal like TNBC cell lines (6,57,58).
Given that our studies suggest a possible disparity with higher
levels of CCL2 in the African American cell line MDA-MB-468 vs.
MM-231, we reviewed oncomine.org
Oncomine™ for CCL2 difference among races, finding no obvious
difference between African American vs. Caucasian in this aspect.
Similarly, in our work-we find no difference in baseline CCL2
levels in the two cell lines, with the disparity arising only with
the treatment of TNFα which is an experimental model of
inflammatory breast cancer. Future studies will be required to
evaluate the inflammatory response across racially divergent breast
cancer cell lines or tissues.
What we do know, however, is that compounds like
apigenin that attenuate the CCL2/CCR2 axis would slow the
aggressive nature of TNBC and hormone positive breast cancers
(27,28) by attenuating invasion, metastasis,
EMT and the development of drug resistance (59–62).
CCL2 inhibitors have been tested in various tumors, tumor cells and
xenograft models with CCL2 lowering effects brought about by
losartan (63) anlotinib (64) imatinib (65) zoledronic acid (66) oroxylin A (67) aspirin (68) natural compounds in coffee (kahweol
acetate, cafestol) (69) or
conophylline from Ervatamia microphylla (70) which can reduce invasive inflammatory
tumor infiltration. The mechanism of action for CCL2 reducing
agents may center around the modification of upstream or downstream
targets such as PLEK2/EGRF (71)
HER2-EGF/HRG, PI3K-NF-kB axis (27)
SRC, PKC (58) the neddylation
pathway (72) or the well-known
mitogen-activated protein kinases and phosphatidylinositol
3-kinase/Akt cell signaling pathways (73). While others have reported apigenin to
have an effect on NF-kappaB/Snail pathway (74), pSTAT3, pERK or PI3K/pAkt (75), our previous studies suggest the
effects of TNFα in TNBC cell lines, as it relates to CCL2 are
driven through the higher expression of IKBK epsilon (31).
It is important to note that when studying the
effects of natural compounds such as apigenin on the entire
transcriptome of cancer cells, there will most always likely be
changes in both directions for oncogenes and tumor suppressors,
some of these changes would not be advantageous. In this work, for
example, we show that apigenin suppressed the TNFα mediated rise in
a potent tumor suppressor: CXCL10. While previous studies
consistently that CXCL10 is up-regulated in normal vs. tumor tissue
(76,77) this particular protein acts as the
major tumor suppressor, evoked by IFN-γ treatment and somehow plays
a role in the re-expression of MHC-1, PD-L1, the infiltration of
anti-tumoral CD4(+) and CD8(+) T cells (78,79), NK
cells, cytotoxic lymphocytes (CTLs) to the tumor to turn on immune
surveillance and heighted survival odds in diverse human cancers
(80–82). While the beneficial effects of
apigenin in cancer are consistently reported, any compound that
would turn off the CXCL9, −10, −11/CXCR3 axis could harm the host
immunes system to destroy self-malignant tumor tissue (83).
In contrast, the current study shows that apigenin
turns on host immune surveillance by its effect on reducing-TNFα
induced SLITRK6. SKITRK 6 is a membrane receptor, which is elevated
in many cancers [e.g., epithelial tumors, bladder, lung, breast,
and glioblastoma (84,85)] and has been deemed an immune
checkpoint for target amongst a relatively new class of drugs
approved by the FDA (86). SLITRK6
is the target of an antibody drug conjugate AGS15E currently in
phase I clinical trials, believed to reactivate the hosts immune
surveillance against self-malignant cells (87). While it is outside the scope of
discussion to elaborate on every transcript change, this work
serves as a general framework for public genomic data evaluation.
Re: Gene Expression Omnibus for public analysis at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE133968.
Previously reported data clearly indicate the
existence of disparity in the mortality rates associated with TNBC
in African Americans, and there a need for initiatives to establish
novel and effective therapies to target aggressive tumors marked by
a propelling inflammatory component. Overall, we believe there is
enough support to warrant clinical trials for the use of apigenin,
as there is a growing body of researching showing its antitumor
effects from multiple stand points from blocking mutagenic induced
cancers [e.g., methyl-nitrosourea,
methyl-n-nitro-N-nitrosoguanidine, benzo(a)pyrene or
2-aminoanthracene] (88) to
inhibition of ornithine decarboxylase (89) and its overall antioxidant,
anti-inflammatory effects (90,91).
Data on the clinical efficacy of substances like apigenin for human
use to reduce CLL2 will also need to be confirmed, as well as
establishing its bioavailability, absorption, therapeutic
concentration and application (prevention, treatment or for
chemotherapy drug augmentation) (55,91–94).
Not applicable.
The present study was supported by the National
Institute of Minority Health and Health Disparities of the National
Institutes of Health (grant nos. U54 MD007582 and P20
MD006738).
DB was involved in the conceptualization of the
manuscript, conducted the primary research, methodology and data
analysis. EM carried out the transcriptomic microarray study, data
analysis, and wrote and critically edited the manuscript. KFAS was
involved in the conceptualization, experimental design and data
analysis, and manuscript preparation and critical revision of the
manuscript. In addition, KFAS provided oversight management of the
project, including consultation, research direction and was
responsible for funds acquisition, and the provision of the
resources. AH was involved in the conceptualization of the
manuscript, the methodology, and involved in writing and critically
editing the manuscript. ETO was involved in the conceptualization
of the manuscript, involved in the methodology, data analysis, and
was involved in writing and critically editing the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Yoshimura T: The production of monocyte
chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments.
Cytokine. 98:71–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES,
Koh M, Lim HK, Jung J, Park SY and Moon A: Tumor-associated
macrophages secrete CCL2 and induce the invasive phenotype of human
breast epithelial cells through upregulation of ERO1-α and MMP-9.
Cancer Lett. 437:25–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Porrello A, Leslie PL, Harrison EB,
Gorentla BK, Kattula S, Ghosh SK, Azam SH, Holtzhausen A, Chao YL,
Hayward MC, et al: Factor XIIIA-expressing inflammatory monocytes
promote lung squamous cancer through fibrin cross-linking. Nat
Commun. 9:19882018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandal PK, Biswas S, Mandal G, Purohit S,
Gupta A, Majumdar Giri A, Roy Chowdhury S and Bhattacharyya A: CCL2
conditionally determines CCL22-dependent Th2-accumulation during
TGF-beta-induced breast cancer progression. Immunobiology.
223:151–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshimura T: The chemokine MCP-1 (CCL2) in
the host interaction with cancer: A foe or ally? Cell Mol Immunol.
15:335–345. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liubomirski Y, Lerrer S, Meshel T,
Rubinstein-Achiasaf L, Morein D, Wiemann S, Körner C and Ben-Baruch
A: Tumor-stroma-inflammation networks promote pro-metastatic
chemokines and aggressiveness characteristics in triple-negative
breast cancer. Front Immunol. 10:7572019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rotondi M, Coperchini F, Latrofa F and
Chiovato L: Role of Chemokines in Thyroid Cancer Microenvironment:
Is CXCL8 the Main Player? Front Endocrinol (Lausanne). 9:3142018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Mazidi S, Alotaibi M, Nedjadi T,
Chaudhary A, Alzoghaibi M and Djouhri L: Blocking of cytokines
signalling attenuates evoked and spontaneous neuropathic pain
behaviours in the paclitaxel rat model of chemotherapy-induced
neuropathy. Eur J Pain. 22:810–821. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khazali AS, Clark AM and Wells A:
Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from
hepatocyte-induced dormancy. Br J Cancer. 118:566–576. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J and Wang X, Wang Y, Li S and Wang
X: Krüppel like factor 6 splice variant 1 (KLF6-SV1) overexpression
recruits macrophages to participate in lung cancer metastasis by
up-regulating TWIST1. Cancer Biol Ther. 20:680–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Kitajima S, Kohno S, Yoshida A,
Tange S, Sasaki S, Okada N, Nishimoto Y, Muranaka H, Nagatani N, et
al: Retinoblastoma inactivation induces a protumoral
microenvironment via enhanced CCL2 secretion. Cancer Res.
79:3903–3915. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Y, Li H, Deng Y, Tai Y, Zeng K,
Zhang Y, Liu W, Zhang Q and Yang Y: Cancer-associated fibroblasts
induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster
immune suppression in hepatocellular carcinoma. Cell Death Dis.
9:4222018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Yu Y, Zhou L, Ma J, Tang K, Xu P,
Ji T, Liang X, Lv J, Dong W, et al: Circulating tumor
microparticles promote lung metastasis by reprogramming
inflammatory and mechanical niches via a macrophage-dependent
pathway. Cancer Immunol Res. 6:1046–1056. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Jin G, Qian J, Yang H, Tang H, Meng
X and Li Y: Digital gene expression profiling analysis and its
application in the identification of genes associated with improved
response to neoadjuvant chemotherapy in breast cancer. World J Surg
Oncol. 16:822018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mano Y, Yoshio S, Shoji H, Tomonari S,
Aoki Y, Aoyanagi N, Okamoto T, Matsuura Y, Osawa Y, Kimura K, et
al: Bone morphogenetic protein 4 provides cancer-supportive
phenotypes to liver fibroblasts in patients with hepatocellular
carcinoma. J Gastroenterol. 54:1007–1018. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bedini N, Cicchetti A, Palorini F, Magnani
T, Zuco V, Pennati M, Campi E, Allavena P, Pesce S, Villa S, et al:
Evaluation of mediators associated with the inflammatory response
in prostate cancer patients undergoing radiotherapy. Dis Markers.
2018:91281282018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heiskala M, Leidenius M, Joensuu K and
Heikkila P: High expression of CCL2 in tumor cells and abundant
infiltration with CD14 positive macrophages predict early relapse
in breast cancer. Virchows Arch. 474:3–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izumi K and Mizokami A: Suppressive role
of androgen/androgen receptor signaling via chemokines on prostate
cancer cells. J Clin Med. 8:E3542019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Natsagdorj A, Izumi K, Hiratsuka K,
Machioka K, Iwamoto H, Naito R, Makino T, Kadomoto S, Shigehara K,
Kadono Y, et al: CCL2 induces resistance to the antiproliferative
effect of cabazitaxel in prostate cancer cells. Cancer Sci.
110:279–288. 2019.PubMed/NCBI
|
|
20
|
Wang X, Yang X, Tsai Y, Yang L, Chuang KH,
Keng PC, Lee SO and Chen Y: IL-6 mediates macrophage infiltration
after irradiation via up-regulation of CCL2/CCL5 in non-small cell
lung cancer. Radiat Res. 187:50–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He S and Zhang X: The rs1024611 in the
CCL2 gene and risk of gynecological cancer in Asians: A
meta-analysis. World J Surg Oncol. 16:342018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan X, Liu Z, Zhang J and Jin X:
Myeloid-derived suppressor cell accumulation in renal cell
carcinoma is correlated with CCL2, IL-17 and IL-18 expression in
blood and tumors. Adv Clin Exp Med. 27:947–953. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amann B, Perabo FG, Wirger A, Hugenschmidt
H and Schultze-Seemann W: Urinary levels of monocyte
chemo-attractant protein-1 correlate with tumour stage and grade in
patients with bladder cancer. Br J Urol. 82:118–121. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saik OV, Nimaev VV, Usmonov DB, Demenkov
PS, Ivanisenko TV, Lavrik IN and Ivanisenko VA: Prioritization of
genes involved in endothelial cell apoptosis by their implication
in lymphedema using an analysis of associative gene networks with
ANDSystem. BMC Med Genomics. 12 (Suppl 2):S472019. View Article : Google Scholar
|
|
25
|
Roblek M, Protsyuk D, Becker PF,
Stefanescu C, Gorzelanny C, Glaus Garzon JF, Knopfova L,
Heikenwalder M, Luckow B, Schneider SW and Borsig L: CCL2 is a
vascular permeability factor inducing CCR2-dependent endothelial
retraction during lung metastasis. Mol Cancer Res. 17:783–793.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yumimoto K, Sugiyama S, Mimori K and
Nakayama KI: Potentials of C-C motif chemokine 2-C-C chemokine
receptor type 2 blockers including propagermanium as anticancer
agents. Cancer Sci. 110:2090–2099. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Triulzi T, Forte L, Regondi V, Di Modica
M, Ghirelli C, Carcangiu ML, Sfondrini L, Balsari A and Tagliabue
E: HER2 signaling regulates the tumor immune microenvironment and
trastuzumab efficacy. Oncoimmunology. 8:e15129422019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song M, Sasazuki S, Camargo MC, Shimazu T,
Charvat H, Yamaji T, Sawada N, Kemp TJ, Pfeiffer RM, Hildesheim A,
et al: Circulating inflammatory markers and colorectal cancer risk:
A prospective case-cohort study in Japan. Int J Cancer.
143:2767–2776. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grossman JG, Nywening TM, Belt BA, Panni
RZ, Krasnick BA, DeNardo DG, Hawkins WG, Goedegebuure SP, Linehan
DC and Fields RC: Recruitment of CCR2+ tumor associated
macrophage to sites of liver metastasis confers a poor prognosis in
human colorectal cancer. Oncoimmunology. 7:e14707292018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coniglio SJ: Role of tumor-derived
chemokines in osteolytic bone metastasis. Front Endocrinol
(Lausanne). 9:3132018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bauer D, Redmon N, Mazzio E and Soliman
KF: Apigenin inhibits TNFalpha/IL-1alpha-induced CCL2 release
through IKBK-epsilon signaling in MDA-MB-231 human breast cancer
cells. PLoS One. 12:e01755582017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mazzio EA, Lewis CA and Soliman KFA:
Transcriptomic profiling of MDA-MB-231 cells exposed to boswellia
serrata and 3-O-acetyl-B-boswellic acid; ER/UPR mediated programmed
cell death. cancer genomics proteomics. 14:409–425. 2017.PubMed/NCBI
|
|
33
|
Garlapati C, Joshi S, Sahoo B, Kapoor S
and Aneja R: The persisting puzzle of racial disparity in triple
negative breast cancer: Looking through a new lens. Front Biosci
(Schol Ed). 11:75–88. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newman LA, Jenkins B, Chen Y, Oppong JK,
Adjei E, Jibril AS, Hoda S, Cheng E, Chitale D, Bensenhaver JM, et
al: Hereditary susceptibility for triple negative breast cancer
associated with western sub-saharan african ancestry: Results from
an international surgical breast cancer collaborative. Ann Surg.
270:484–492. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hossain F, Danos D, Prakash O, Gilliland
A, Ferguson TF, Simonsen N, Leonardi C, Yu Q, Wu XC, Miele L and
Scribner R: Neighborhood social determinants of triple negative
breast cancer. Front Public Health. 7:182019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiagge E, Jibril AS, Davis M,
Murga-Zamalloa C, Kleer CG, Gyan K, Divine G, Hoenerhoff M,
Bensenhave J, Awuah B, et al: Androgen receptor and ALDH1
expression among internationally diverse patient populations. J
Glob Oncol. 4:1–8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bassey-Archibong BI, Hercules SM, Rayner
LGA, Skeete DHA, Smith Connell SP, Brain I, Daramola A, Banjo AAF,
Byun JS, Gardner K, et al: Kaiso is highly expressed in TNBC
tissues of women of African ancestry compared to Caucasian women.
Cancer Causes Control. 28:1295–1304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Telonis AG and Rigoutsos I: Race
disparities in the contribution of miRNA isoforms and tRNA-derived
fragments to triple-negative breast cancer. Cancer Res.
78:1140–1154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torres-Luquis O, Madden K, N'Dri N M, Berg
R, Olopade OF, Ngwa W, Abuidris D, Mittal S, Lyn-Cook B and
Mohammed SI: LXR/RXR pathway signaling associated with
triple-negative breast cancer in African American women. Breast
Cancer (Dove Med Press). 11:1–12. 2018.PubMed/NCBI
|
|
40
|
Austin D, Hamilton N, Elshimali Y, Pietras
R, Wu Y and Vadgama J: Estrogen receptor-beta is a potential target
for triple negative breast cancer treatment. Oncotarget.
9:33912–33930. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Y, Sarkissyan M, Clayton S, Chlebowski
R and Vadgama JV: Association of vitamin D3 Level with breast
cancer risk and prognosis in African-American and hispanic women.
Cancers (Basel). 9:E1442017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma H, Ursin G, Xu X, Lee E, Togawa K, Duan
L, Lu Y, Malone KE, Marchbanks PA, McDonald JA, et al: Reproductive
factors and the risk of triple-negative breast cancer in white
women and African-American women: A pooled analysis. Breast Cancer
Res. 19:62017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Siddharth S and Sharma D: Racial disparity
and triple-negative breast cancer in African-American women: A
multifaceted affair between obesity, biology, and socioeconomic
determinants. Cancers (Basel). 10:E5142018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dietze EC, Chavez TA and Seewaldt VL:
Obesity and triple-negative breast cancer: Disparities,
controversies, and biology. Am J Pathol. 188:280–290. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao X, Qu J, Sun Y, Wang J, Liu X, Wang
F, Zhang H, Wang W, Ma X, Gao X and Zhang S: Prognostic
significance of tumor-associated macrophages in breast cancer: A
meta-analysis of the literature. Oncotarget. 8:30576–30586.
2017.PubMed/NCBI
|
|
46
|
Jeong H, Hwang I, Kang SH, Shin HC and
Kwon SY: Tumor-associated macrophages as potential prognostic
biomarkers of invasive breast cancer. J Breast Cancer. 22:38–51.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Valeta-Magara A, Gadi A, Volta V, Walters
B, Arju R, Giashuddin S, Zhong H and Schneider RJ: Inflammatory
breast cancer promotes development of M2 tumor-associated
macrophages and cancer mesenchymal cells through a complex
chemokine network. Cancer Res. 79:3360–3371. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang N, Liu W, Zheng Y, Wang S, Yang B, Li
M, Song J, Zhang F, Zhang X, Wang Q and Wang Z: CXCL1 derived from
tumor-associated macrophages promotes breast cancer metastasis via
activating NF-κB/SOX4 signaling. Cell Death Dis. 9:8802018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu Y, Zheng H, Li Q, Li S, Lai H, Song E,
Li D and Chen J: Discovery of CCL18 antagonist blocking breast
cancer metastasis. Clin Exp Metastasis. 36:243–255. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Little AC, Pathanjeli P, Wu Z, Bao L, Goo
LE, Yates JA, Oliver CR, Soellner MB and Merajver SD: IL-4/IL-13
stimulated macrophages enhance breast cancer invasion via
Rho-GTPase regulation of synergistic VEGF/CCL-18 signaling. Front
Oncol. 9:4562019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cassetta L, Fragkogianni S, Sims AH,
Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P,
Lin EY, et al: Human tumor-associated macrophage and monocyte
transcriptional landscapes reveal cancer-specific reprogramming,
biomarkers, and therapeutic targets. Cancer Cell. 35:588–602.e510.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gupta S, Jain A, Syed SN, Pflüger-Müller
B, Leisegang MS, Weigert A, Brandes RP, Ebersberger I, Brüne B and
Namgaladze D: IL-6 augments IL-4-induced polarization of primary
human macrophages through synergy of STAT3, STAT6 and BATF
transcription factors. Oncoimmunology. 7:e14941102018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu X, Ye J, Huang C, Yan Y and Li J: M2
macrophage-derived IL6 mediates resistance of breast cancer cells
to hedgehog inhibition. Toxicol Appl Pharmacol. 364:77–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tandon I and Sharma NK: Macrophage
flipping from foe to friend: A matter of interest in breast
carcinoma heterogeneity driving drug resistance. Curr Cancer Drug
Targets. 19:189–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sudhakaran M, Sardesai S and Doseff AI:
Flavonoids: New frontier for immuno-regulation and breast cancer
control. Antioxidants (Basel). 8:E1032019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Han R, Gu S, Zhang Y, Luo A, Jing X, Zhao
L, Zhao X and Zhang L: Estrogen promotes progression of
hormone-dependent breast cancer through CCL2-CCR2 axis by
upregulation of Twist via PI3K/AKT/NF-κB signaling. Sci Rep.
8:95752018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dutta P, Sarkissyan M, Paico K, Wu Y and
Vadgama JV: MCP-1 is overexpressed in triple-negative breast
cancers and drives cancer invasiveness and metastasis. Breast
Cancer Res Treat. 170:477–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yao M, Fang W, Smart C, Hu Q, Huang S,
Alvarez N, Fields P and Cheng N: CCR2 chemokine receptors enhance
growth and cell-cycle progression of breast cancer cells through
SRC and PKC Activation. Mol Cancer Res. 17:604–617. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ling Z, Yang X, Chen X, Xia J, Cheng B and
Tao X: CCL2 promotes cell migration by inducing
epithelial-mesenchymal transition in oral squamous cell carcinoma.
J Oral Pathol Med. 48:477–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu W, Wei Q, Han M, Zhou B, Wang H, Zhang
J, Wang Q, Sun J, Feng L, Wang S, et al: CCL2-SQSTM1 positive
feedback loop suppresses autophagy to promote chemoresistance in
gastric cancer. Int J Biol Sci. 14:1054–1066. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang T, Zhan Q, Peng X, Qiu Z and Zhao T:
CCL2 influences the sensitivity of lung cancer A549 cells to
docetaxel. Oncol Lett. 16:1267–1274. 2018.PubMed/NCBI
|
|
62
|
Sarin N, Engel F, Rothweiler F, Cinatl J,
Michaelis M, Frötschl R, Fröhlich H and Kalayda GV: Key players of
cisplatin resistance: towards a systems pharmacology approach. Int
J Mol Sci. 19:E7672018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Regan DP, Coy JW, Chahal KK, Chow L,
Kurihara JN, Guth AM, Kufareva I and Dow SW: The angiotensin
receptor blocker losartan suppresses growth of pulmonary metastases
via AT1R-independent inhibition of CCR2 signaling and monocyte
recruitment. J Immunol. 202:3087–3102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lu J, Zhong H, Chu T, Zhang X, Li R, Sun
J, Zhong R, Yang Y, Alam MS, Lou Y, et al: Role of
anlotinib-induced CCL2 decrease in anti-angiogenesis and response
prediction for nonsmall cell lung cancer therapy. Eur Respir J.
53:18015622019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yao Z, Zhang J, Zhang B, Liang G, Chen X,
Yao F, Xu X, Wu H, He Q, Ding L and Yang B: Imatinib prevents lung
cancer metastasis by inhibiting M2-like polarization of
macrophages. Pharmacol Res. 133:121–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liu H, Wang SH, Chen SC, Chen CY and Lin
TM: Zoledronic acid blocks the interaction between breast cancer
cells and regulatory T-cells. BMC Cancer. 19:1762019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ku WT, Tung JJ, Lee TJ and Lai KC:
Long-term exposure to oroxylin a inhibits metastasis by suppressing
CCL2 in oral squamous cell carcinoma Cells. Cancers (Basel).
11:E3532019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang D, Yue DL, Wang D, Chen XF, Yin XY,
Wang YP, Yang L and Zhang Y: Aspirin inhibits cell stemness of
esophageal cancer by downregulation of chemokine CCL2. Zhonghua
Zhong Liu Za Zhi. 40:744–749. 2018.(In Chinese). PubMed/NCBI
|
|
69
|
Iwamoto H, Izumi K, Natsagdorj A, Naito R,
Makino T, Kadomoto S, Hiratsuka K, Shigehara K, Kadono Y, Narimoto
K, et al: Coffee diterpenes kahweol acetate and cafestol
synergistically inhibit the proliferation and migration of prostate
cancer cells. Prostate. 79:468–479. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ishii N, Araki K, Yokobori T, Hagiwara K,
Gantumur D, Yamanaka T, Handa T, Tsukagoshi M, Igarashi T, Watanabe
A, et al: Conophylline suppresses pancreatic cancer desmoplasia and
cancer-promoting cytokines produced by cancer-associated
fibroblasts. Cancer Sci. 110:334–344. 2019.PubMed/NCBI
|
|
71
|
Shen H, He M, Lin R, Zhan M, Xu S, Huang
X, Xu C, Chen W, Yao Y, Mohan M and Wang J: PLEK2 promotes
gallbladder cancer invasion and metastasis through EGFR/CCL2
pathway. J Exp Clin Cancer Res. 38:2472019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhou L, Jiang Y, Liu X, Li L, Yang X, Dong
C, Liu X, Lin Y, Li Y, Yu J, et al: Promotion of tumor-associated
macrophages infiltration by elevated neddylation pathway via
NF-κB-CCL2 signaling in lung cancer. Oncogene. 38:5792–5804. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ding M, He SJ and Yang J: MCP-1/CCL2
Mediated by autocrine loop of PDGF-BB promotes invasion of lung
cancer cell by recruitment of macrophages via CCL2-CCR2 axis. J
Interferon Cytokine Res. 39:224–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tong J, Shen Y, Zhang Z, Hu Y, Zhang X and
Han L: Apigenin inhibits epithelial-mesenchymal transition of human
colon cancer cells through NF-κB/Snail signaling pathway. Biosci
Rep. 39:BSR201904522019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee HH, Jung J, Moon A, Kang H and Cho H:
Antitumor and anti-invasive effect of apigenin on human breast
carcinoma through suppression of IL-6 expression. Int J Mol Sci.
20:E31432019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu M, Li D, Yang C and Ji JS: MicroRNA-34a
inhibition of the TLR signaling pathway Via CXCL10 suppresses
breast cancer cell invasion and migration. Cell Physiol Biochem.
46:1286–1304. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Chen J, Guan GW, Zhang T, Lu FM
and Chen XM: Expression and clinical significance of chemokine
CXCL10 and its receptor CXCR3 in hepatocellular carcinoma. Beijing
Da Xue Xue Bao Yi Xue Ban. 51:402–408. 2019.(In Chinese).
PubMed/NCBI
|
|
78
|
Guo J, Xiao Y, Iyer R, Lu X, Lake M,
Ladror U, Harlan J, Samanta T, Tomlinson M, Bukofzer G, et al:
Empowering therapeutic antibodies with IFN-α for cancer
immunotherapy. PLoS One. 14:e02198292019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu Y, Yuan L, Lu Q, Xu H and He X:
Distinctive profiles of tumor-infiltrating immune cells and
association with intensity of infiltration in colorectal cancer.
Oncol Lett. 15:3876–3882. 2018.PubMed/NCBI
|
|
80
|
Fang S, Xu T, Xiong M, Zhou X, Wang Y,
Haydu LE, Ross MI, Gershenwald JE, Prieto VG, Cormier JN, et al:
Role of immune response, inflammation, and tumor immune
response-Related cytokines/chemokines in melanoma progression. J
Invest Dermatol. 139:2352–2358.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nieto JC, Zamora C, Porcel JM, Mulet M,
Pajares V, Muñoz-Fernandez AM, Calvo N, Espinosa I, Pascual-García
M, Bielsa S and Vidal S: Migrated T lymphocytes into malignant
pleural effusions: An indicator of good prognosis in lung
adenocarcinoma patients. Sci Rep. 9:29962019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kang SH, Keam B, Ahn YO, Park HR, Kim M,
Kim TM, Kim DW and Heo DS: Inhibition of MEK with trametinib
enhances the efficacy of anti-PD-L1 inhibitor by regulating
anti-tumor immunity in head and neck squamous cell carcinoma.
Oncoimmunology. 8:e15150572019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tokunaga R, Zhang W, Naseem M, Puccini A,
Berger MD, Soni S, McSkane M, Baba H and Lenz HJ: CXCL9, CXCL10,
CXCL11/CXCR3 axis for immune activation-A target for novel cancer
therapy. Cancer Treat Rev. 63:40–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Morrison K, Challita-Eid PM, Raitano A, An
Z, Yang P, Abad JD, Liu W, Lortie DR, Snyder JT, Capo L, et al:
Development of ASG-15ME, a novel antibody-drug conjugate targeting
SLITRK6, a new urothelial cancer biomarker. Mol Cancer Ther.
15:1301–1310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lin JK, Chen YC, Huang YT and Lin-Shiau
SY: Suppression of protein kinase C and nuclear oncogene expression
as possible molecular mechanisms of cancer chemoprevention by
apigenin and curcumin. J Cell Biochem Suppl. 28-29:39–48. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Alhalabi O, Rafei H, Shah A,
Siefker-Radtke A, Campbell M and Gao J: Targeting advanced
urothelial carcinoma-developing strategies. Curr Opin Oncol.
31:207–215. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sanford T, Porten S and Meng MV: Molecular
analysis of upper tract and bladder urothelial carcinoma: Results
from a microarray comparison. PLoS One. 10:e01371412015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Birt DF, Walker B, Tibbels MG and Bresnick
E: Anti-mutagenesis and anti-promotion by apigenin, robinetin and
indole-3-carbinol. Carcinogenesis. 7:959–963. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Birt DF, Mitchell D, Gold B, Pour P and
Pinch HC: Inhibition of ultraviolet light induced skin
carcinogenesis in SKH-1 mice by apigenin, a plant flavonoid.
Anticancer Res. 17:85–91. 1997.PubMed/NCBI
|
|
90
|
Sharma A, Ghani A, Sak K, Tuli HS, Sharma
AK, Setzer WN, Sharma S and Das AK: Probing into therapeutic
anti-cancer potential of apigenin: Recent trends and future
directions. recent pat inflamm Allergy Drug Discov. Aug
16–2019.doi: 10.2174/1872213X13666190816160240 (Epub ahead of
print). View Article : Google Scholar
|
|
91
|
Ginwala R, Bhavsar R, Chigbu DI, Jain P
and Khan ZK: Potential role of flavonoids in treating chronic
inflammatory diseases with a special focus on the anti-inflammatory
activity of apigenin. Antioxidants (Basel). 8:E352019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Chen Z, Tian D, Liao X, Zhang Y, Xiao J,
Chen W, Liu Q, Chen Y, Li D, Zhu L and Cai S: Apigenin combined
with gefitinib blocks autophagy flux and induces apoptotic cell
death through inhibition of HIF-1α, c-Myc, p-EGFR, and glucose
metabolism in EGFR L858R+T790M-mutated H1975 cells. Front
Pharmacol. 10:2602019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen X, Xu H, Yu X, Wang X, Zhu X and Xu
X: Apigenin inhibits in vitro and in vivo tumorigenesis in
cisplatin-resistant colon cancer cells by inducing autophagy,
programmed cell death and targeting m-TOR/PI3K/Akt signalling
pathway. J BUON. 24:488–493. 2019.PubMed/NCBI
|
|
94
|
Gao AM, Zhang XY, Hu JN and Ke ZP:
Apigenin sensitizes hepatocellular carcinoma cells to doxorubic
through regulating miR-520b/ATG7 axis. Chem Biol Interact.
280:45–50. 2018. View Article : Google Scholar : PubMed/NCBI
|