Introduction
Colorectal cancer is the second most common cancer
in women (9.5%) and the third in men (10.2%) and ranked as the
second most common cause of cancer death (9.2%) worldwide (1). Therefore, sensitive and noninvasive
diagnoses are important to improve its treatment outcomes. However,
conventionally used biomarkers in blood samples, such as
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9), do not always indicate the pathological aspects of
malignancy (2).
The use of circulating tumor cells (CTCs) as the
next-generation cancer marker has been an active research topic in
the field of oncology for the past two decades (3–6). The
peripheral blood CTC concentration is reported to be extremely low:
<10 cells/ml in patients with metastatic cancer (3,7).
However, CTCs are not present or scarcely detected in the blood of
healthy individuals nor in those with nonmalignant diseases
(8,9). Nucleic acid evaluation in CTCs and
direct enumeration of CTCs are typical methods used to detect CTCs
requiring highly sensitive techniques. To evaluate nucleic acid
levels in blood CTCs, related gene expressions are examined using
reverse transcription-quantitative PCR (RT-qPCR), or DNA arrays
(9–13). Cell capturing methods based on size
or surface antibodies (6,10), such as the CellSearch™ System
(Veridex LLC) (CellSearch) and other microfluidic devices, are used
for direct enumeration (14–16). CellSearch is a semi-automated system
for CTC quantification as a diagnostic method for metastatic
breast, colorectal, and prostate cancers approved by the US Food
and Drug Administration (FDA) (17,18).
Moreover, the efficacy of this system has been reported in
metastatic breast, prostate, esophageal, and colorectal cancers
(19–25). However, the use of CellSearch is
limited due to its high cost (26–28) and
low sensitivity to some cancer types, such as hepatocellular
carcinoma (28,29). Approximately 18% of non-metastatic
and 41% of metastatic patients with colon cancer are positive with
CTCs in the CellSearch system (30).
Nagrath et al (15) developed a microfluidic device known
as the ‘CTC-chip’ to overcome these limitations. The ‘CTC-chip’
facilitates efficient and selective separation of CTCs from whole
blood samples, mediated by the interaction of target CTCs with
antibody-coated microposts under precisely controlled laminar flow
conditions (15,31). Subsequently, a novel ‘polymeric
CTC-chip’ was developed to isolate CTCs, with lower cost, high
transparency that facilitates observation through the chip, and
convertibility of antibodies to coat the surface to arrest cancer
cells than that of the existing CTC-chips (32–36).
In the present study, the capture efficiency of the
polymeric CTC-chip was measured using colorectal cancer cells
spiked in phosphate-buffered saline (PBS) or healthy whole blood at
first. Next, CTCs in clinical blood samples were detected in
patients with colorectal cancer. The sensitivity of CTC detection
in the blood samples of patients with colorectal cancer was
compared with that of the CEA and CA19-9 tests.
Materials and methods
Preparation of cancer cells
HCT116 (ATCC® CCL-247™) colorectal cancer
cells were cultured and exhibited a high expression of epithelial
cell-adhesion molecule (EpCAM), in McCoy's 5A medium (cat. no.
16600082; Invitrogen) with 10% fetal bovine serum and 1%
penicillin-streptomycin at 37°C in a humidified 5% CO2
atmosphere. Then, the EpCAM expression in HCT116 cells was
evaluated with a flow cytometer (FACSVerse; BD Biosciences) using a
PE/Cy7-conjugated anti-human CD326 (EpCAM) antibody (cat. no.
324221; BioLegend) and FlowJo software (ver.9; FlowJo LCC). To
determine the EpCAM localization in the cells, Alexa
Fluor® 594-conjugated anti-human CD326 (EpCAM)
antibodies (cat. no. 324228; BioLegend) at 5 µg/ml was added to the
HCT116 cell suspension; the mixture was allowed to sit for 2 h at
room temperature and examined using a fluorescence microscope
system (BZ-X710; Keyence) in a 24-well plastic dish (a cell culture
plate with a lid; Sigma-Aldrich).
Preparation of cancer cell
suspensions
To measure the capture efficiency, HCT116 cells were
fluorescently labeled using the Cell Explorer™ Live Cell Tracking
kit (cat. no. 22621; AAT Bioquest). The cells were spiked in PBS
containing 5% bovine serum albumin (BSA; PBS suspension) or the
whole blood obtained from a healthy donor and stored in a vacuum
blood collection tube containing ethylenediaminetetraacetic acid
(EDTA; VP-DK052K; Terumo; blood suspension) at 4°C. All cell
suspensions were prepared at approximately 1,000 cells/ml
concentration, and the precise concentration of each suspension was
determined.
Antibody coating on the chip
surface
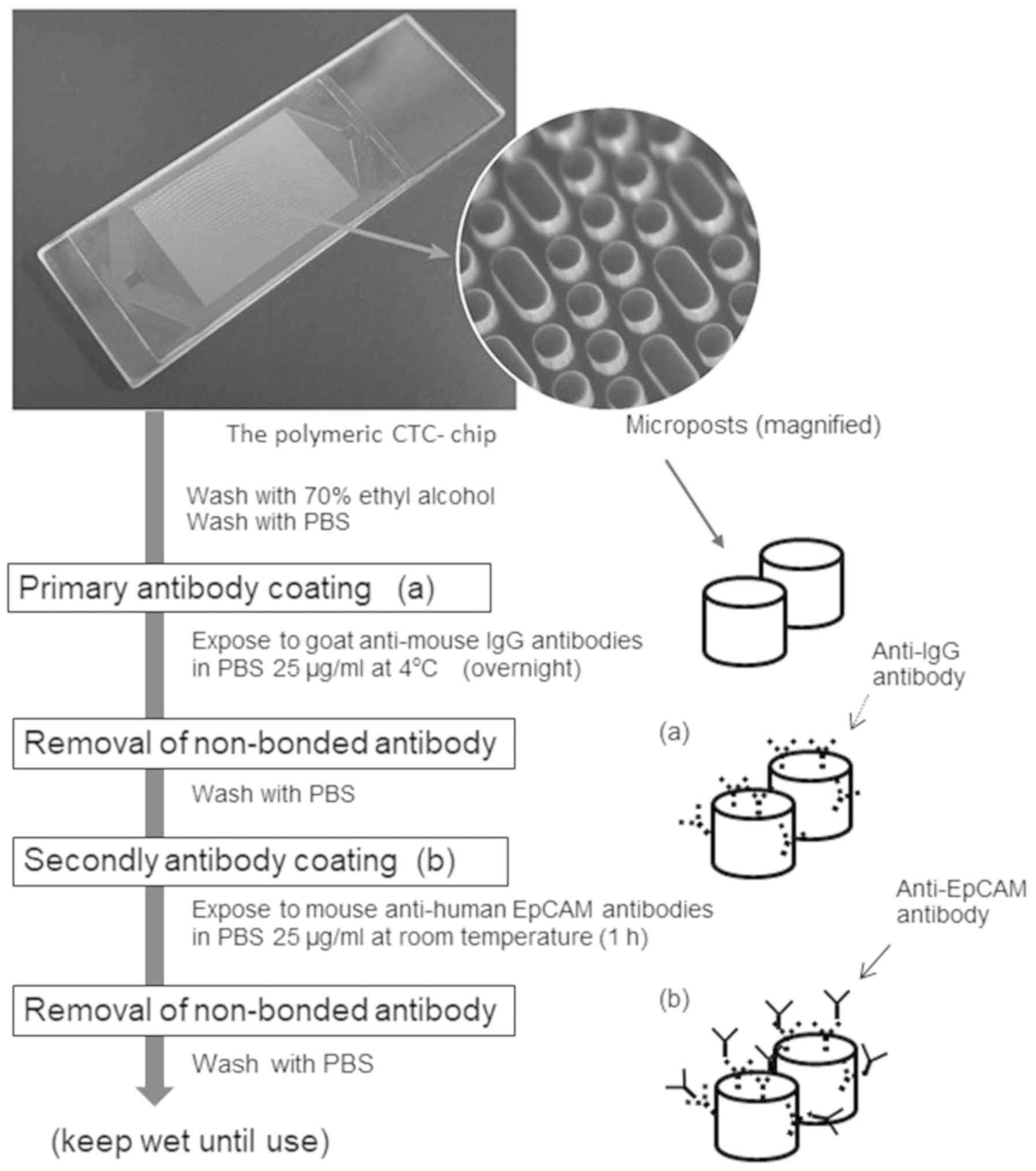
An antibody coating of the polymeric CTC-chip
surface was determined using the method described by Ohnaga et
al (32), with the process
outline illustrated in Fig. 1. The
chip was washed with 70% ethyl alcohol once for hydrophilization
and then exposed to goat anti-mouse IgG antibodies overnight (cat.
no. 1032-01; Southern Biotech) in PBS at a 25 µg/ml concentration
at 4°C. Then, the chip surface was washed with PBS once to remove
any non-bonded anti-IgG antibodies and kept wet. Next, the chip
surface was coated with mouse anti-human EpCAM antibodies (cat. no.
sc-59906; Santa Cruz Biotechnology) in PBS at a 25 µg/ml
concentration and stored at room temperature for 1 h. The chip was
washed with PBS again after the antibody treatment.
CTC capturing system and evaluation of
the cell-capture efficiency
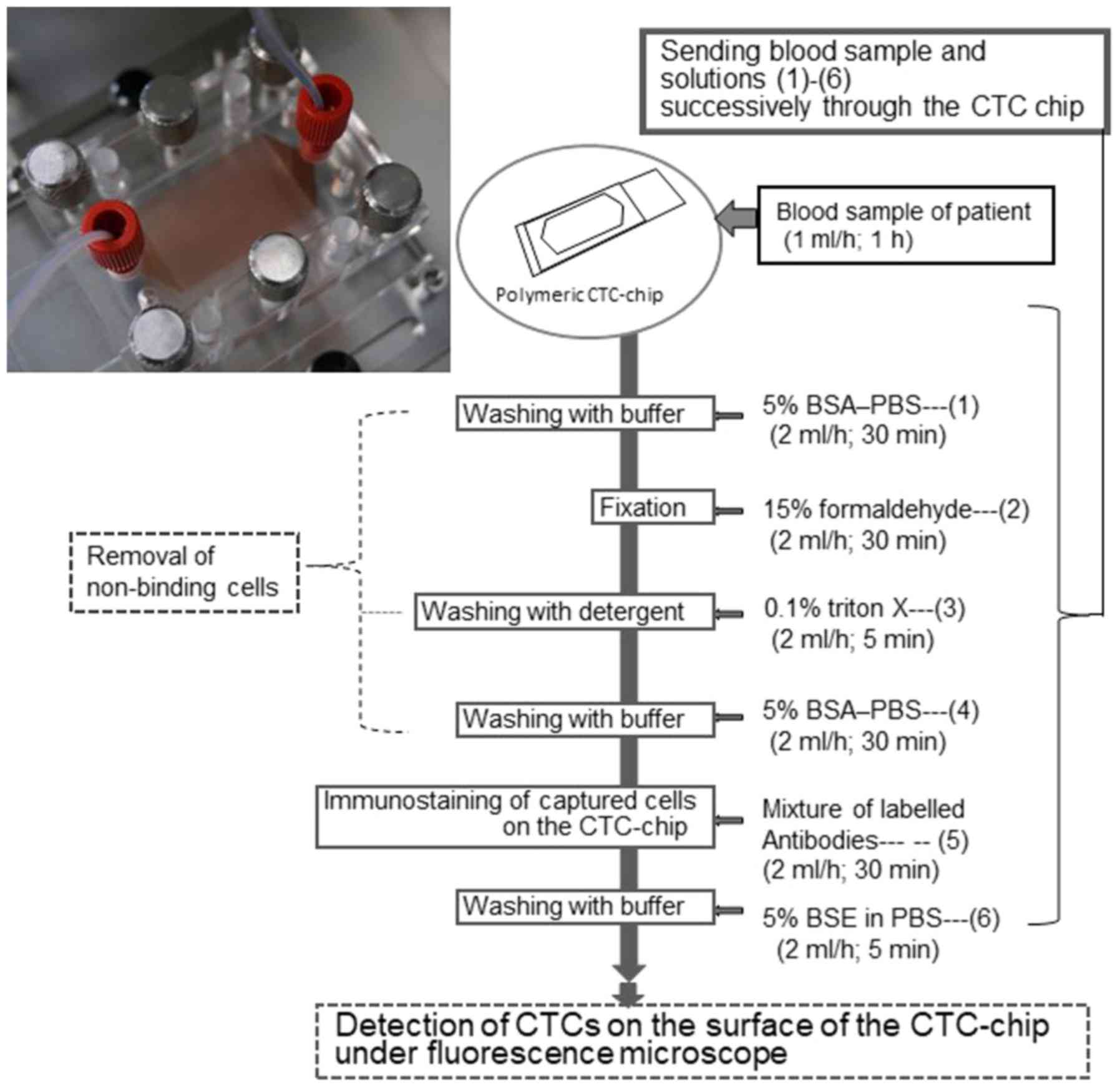
The sample flow and CTC capturing were performed
using the method described by Ohnaga et al (32). The workflow of CTC detection with the
polymeric CTC-chip is outlined in Fig.
2. Briefly, the polymeric CTC-chip coated with antibodies was
set in a holder and fixed on an inverted fluorescence microscope
stage (CKX41; Olympus). The size of the polymeric CTC-chip was
75×25 mm, and surface microstructures comprised two types of
micropost arrays in a wide channel (32). The two ports of the holder were then
connected to a syringe or sample tube with tubing and fittings,
which allowed the liquid sample to flow through the channel
(32). Then, the sample tube was
shaken to prevent cell precipitation and adhesion. Each sample was
sent to the chip using a syringe pump at a constant flow rate of 1
ml/h. After allowing the samples to flow through the polymeric
CTC-chip, the tube was washed with PBS containing 5% BSA once at 1
ml/h for 15 min to remove the suspended cells. The chip surface was
then examined using an inverted fluorescent microscope (CKX41)
equipped with a digital video camera (HDR-CX535; Sony) during the
flow test. In this study, three types of polymeric CTC-chips were
used to assess the capture efficiency with PBS and blood
suspensions: i) No antibody treatment (non-treated chip); ii) a
goat anti-mouse IgG antibody solitary coating (IgG-chip); and iii)
a primary coating of goat anti-mouse IgG antibody and a secondary
coating of mouse anti-human EpCAM antibody (EpCAM-chip). Notably,
the flow test was repeated three to four times for the two
suspensions using each of the three types of chips.
Thus, the number of cells remaining in the chip
after the flow test (Nr) was determined. The number of cells that
passed through the chip inlet (Np) had been evaluated before the
test based on the cell concentration in ea43ch suspension. The
cell-capture efficiency, defined as Nr/Np, was evaluated (32) for each test.
Characteristics of patients and
volunteers
We enrolled 13 patients (age range, 59–77 years;
men, 9; women, 4) with stages II–IV (UICC classification)
colorectal cancer (37) and 2
healthy volunteers (control group), without detectable cancer or
serious diseases, in the Department of Coloproctological Surgery,
Juntendo University Hospital (Tokyo), from August 2015 to March
2016 (Table I). Histological
features and differentiation grades of cancer tissue samples were
evaluated in the Department of Diagnostic Pathology of the Juntendo
University Hospital, according to JSCCR classification (38): Each patient was classified into the
following categories: well-differentiated tubular adenocarcinoma
(tub1), intermediately differentiated tubular adenocarcinoma
(tub2), or poorly differentiated adenocarcinoma (por). In addition,
CEA and CA19-9 levels in blood samples drawn from the participants
before receiving any cancer treatment were evaluated. In this
study, a value was considered ‘positive’ when higher than the set
conventional cut-off values (5.0 ng/ml for CEA and 37.0 U/ml for
CA19-9) (39–41). This study was conducted in accordance
with the Declaration of Helsinki and was approved by the Ethics
Committee of Juntendo University (cat. no. 2015036). Furthermore,
written informed consent was obtained from all participants after
adequate counseling using written documents that described the
research aim and any possible risks involved.
 | Table I.Patient characteristics with detected
number of CTCs and CEA and CA19-9 values. |
Table I.
Patient characteristics with detected
number of CTCs and CEA and CA19-9 values.
|
|
|
|
|
|
|
| CTCs
(number/ml) |
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Patent no. | Age | Sex | Site | Histologic
features | TNM
classification | Stage | Before surgery | After
surgerya | CEA (ng/ml) | CA 19-9 (U/ml) |
|---|
| 1 | 74 | M | A | tub2 | T3 N0 M0 | II A | 1 | 2 | 7.9 | 37 |
| 2 | 77 | F | D | tub2 | T4a N0 M0 | II B | 6 | 2 | 6.8 | 7 |
| 3 | 76 | M | Rb | tub2 | T3 N0 M0 | II A | 2 | 0 | 2.9 | 23 |
| 4 | 77 | M | S | tub1 | T4b N0 M0 | II C | 4 | 1 | 4.9 | 34 |
| 5 | 66 | F | C | tub2 | T3 N1b M0 | III B | 1 | 0 | 23.6 | 24 |
| 6 | 59 | M | Rb | tub2 | T3 N2b M0 | III C | 6 | 3 | 3.5 | 8 |
| 7 | 70 | M | RS | tub2 | T3 N1b M1a
(H2) | IVA | 12 |
| 387.7 | 94 |
| 8 | 72 | M | A | tub2 | T3 N0 M1a (H1) | IVA | 7 |
| 14.3 | 21 |
| 9 | 74 | M | RS | tub1 | T3 N2b M1a
(LYM) | IVA | 18 |
| 12.8 | 27 |
| 10 | 67 | M | S | tub1 | T4b N2a M1a
(LYM) | IVA | 5 |
| 34.8 | 6 |
| 11 | 77 | F | A | tub2 | T4a N2a M1a
(H2) | IVA | 2 |
| 15.8 | 20 |
| 12 | 61 | F | A | tub2 | T3 N2b M1b (H1
PUL1) | IVB | 5 |
| 7.6 | 6 |
| 13 | 71 | M | Ra | por | T3 N1a M1a
(H1) | IVA | 0 |
| 1.4 | 6 |
Detection of CTCs from blood
samples
Using a vacuum blood collection tube containing
EDTA, 2 ml of blood samples were drawn from all patients one day
preoperatively. In patients with stages II and III cancer, blood
samples were obtained again 6 days postoperatively. The blood
samples were then placed in the polymeric CTC-chip coated with both
goat anti-mouse IgG antibodies and mouse anti-human EpCAM
antibodies at a flow rate of 1 ml/h for 1 h, as previously
described. After setting the blood samples, the chip was washed
with PBS containing 5% BSA at a flow rate of 2 ml/h for 30 min to
remove the blood cell constituents that were nonspecifically
combined with the chip. After using a 15% formaldehyde to anchor
the cells, the chip was washed with 0.1% Triton X-100. Then, the
aqueous antibody solution was successively introduced into the
chip.
The FDA-approved technique to detect CTCs relies on
the use of antibodies that target EpCAM, followed by cytokeratin
(CK) and CD45 staining, to confirm the epithelial phenotype
(42). In the present study,
fluorescein isothiocyanate (FITC)-conjugated anti-mouse CK 8 + 18
(207) antibodies (ab190366; Abcam Plc, Cambridge, UK; 5 µl: 50
µg/ml) and Alexa Fluor® 594-conjugated anti-mouse CD45
antibodies (cat. no. 103144; BioLegend; 5 µl: 50 µg/ml) were used
to differentiate epithelial adenocarcinoma cells and leukocytes.
Diamidino-2-phenylindole dihydrochloride (DAPI; 20 µl: 1.5 µg/ml),
a nuclear staining reagent, was also used to evaluate the
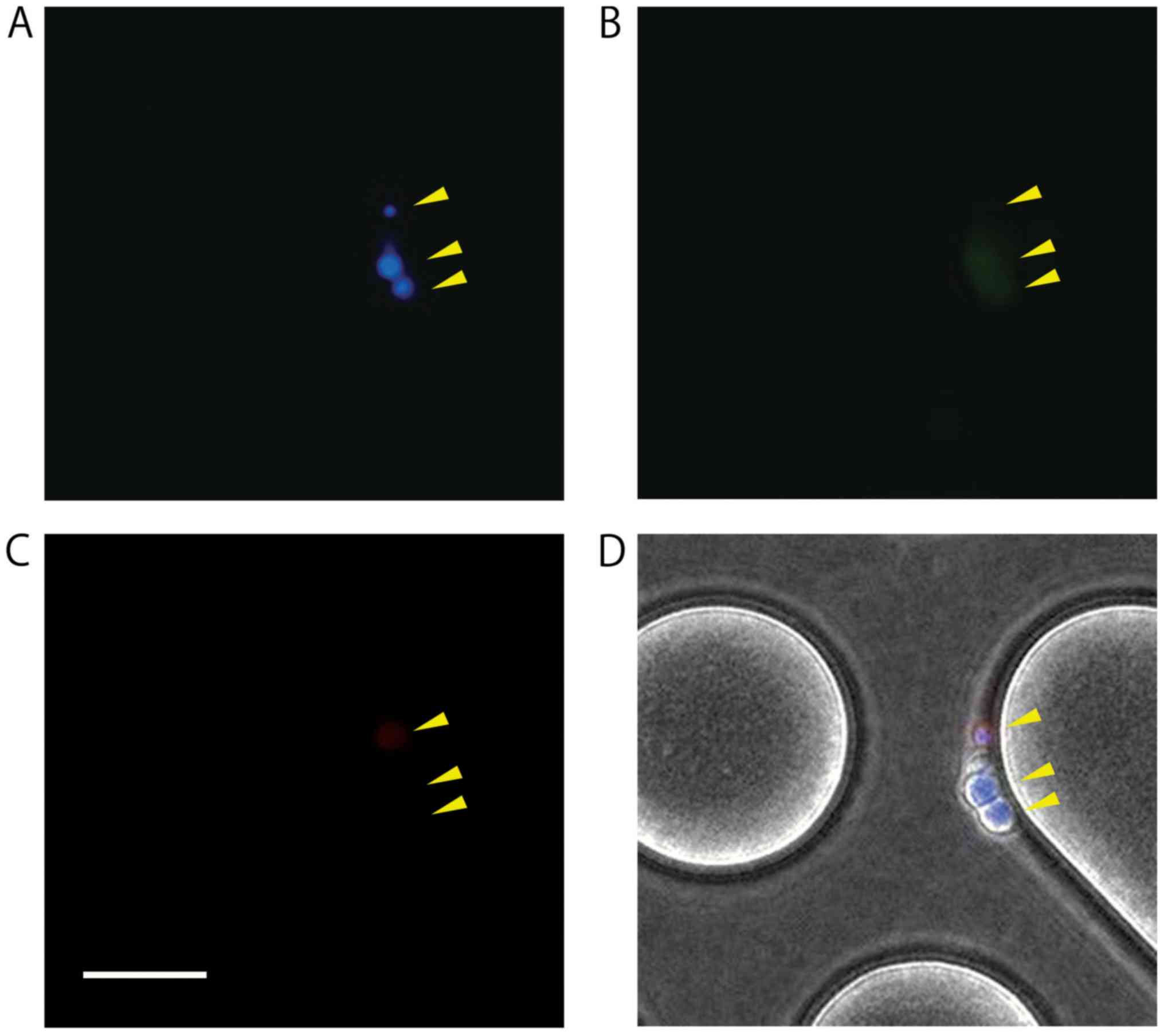
presence/absence of cell nuclei. A cell was defined as to contain
CTC when it was positive for both DAPI and CK but negative for
CD45. Furthermore, CTCs remaining on the chip were observed and
counted with images obtained using the inverted fluorescence
microscope (CKX41).
Statistical analyses
The effects of antibody treatments (none, IgG, and
IgG + EpCAM) of the polymeric CTC-chips on the cell-capture
efficiencies were examined using an analysis of variance (ANOVA)
after the angular transformation on the cell-capture efficiency
defined as Nr/Np (the captured and numbers of cells that passed
through the chip), as previously described in both PBS and blood
suspensions. Using Welch's two-sample t-test, effects of the
polymeric CTC-chip treatment on capture efficiency were assessed
based on the difference between the average cell-capture efficiency
values and the angular transformation between two different
treatments: No treatment, IgG-chip; no treatment, EpCAM-chip; and
IgG-chip, EpCAM-chip (in both PBS and blood suspensions). Welch's
t-test was also used to evaluate differences in the number of CTCs
detected in blood samples obtained from patients with stages II–III
and IV cancer. The paired t-test was used to determine the
difference in numbers of CTC/ml between blood samples pre- and
postoperatively in patients with stages II–III cancer. Moreover,
the Fisher exact test was used to compare the rates of patients
detected with CTCs and those positive for CEA and CA19-9 tests. All
statistical analyses were performed using the R software (v.3.2.5)
(43).
Results
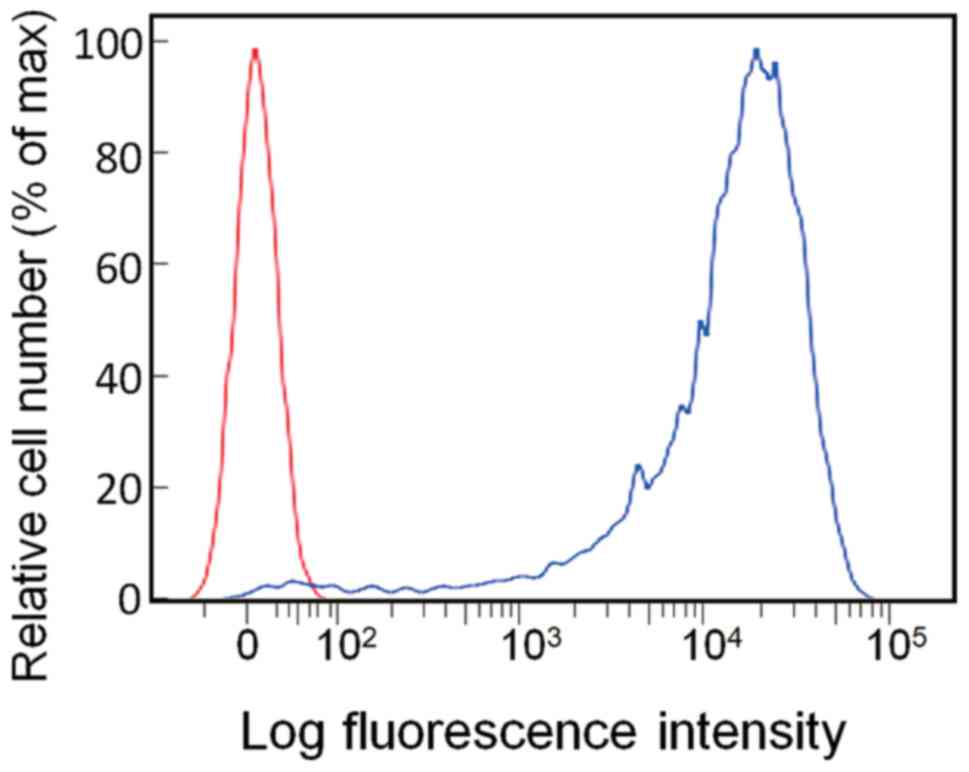
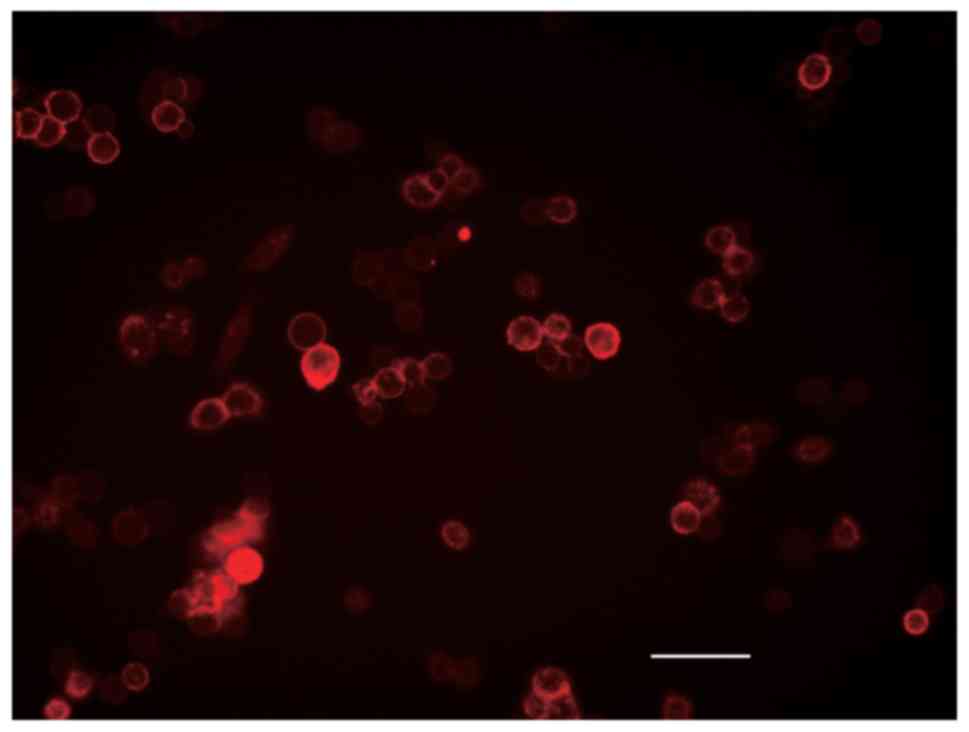
EpCAM expression of HCT116
The EpCAM expression in HCT116 cells was evaluated
with flow cytometry using anti-EpCAM antibodies, showing that 97.3%
of HCT116 cells were positive for EpCAM (Fig. 3). The immunofluorescence staining
suggested that EpCAM was localized on the cell surface (Fig. 4).
Capture efficiency of the polymeric
CTC-chip covered with antibodies
Capturing efficiencies of colorectal cancer cells
spiked in PBS and blood suspensions were tested using the polymeric
CTC-chip by conducting three different treatments: i) Primarily
coated with goat anti-mouse IgG antibodies and secondarily coated
with mouse anti-human EpCAM antibodies, ‘EpCAM-chip’; ii) coated
only with goat anti-mouse IgG antibodies, ‘IgG-chip’; or iii) no
antibody treatment, ‘non-treated chip.’ Each suspension sample was
set on the chip using a syringe pump at 1 ml/h for 1 h. We
determined that 1,109.4±392.8 (average number ± SD) cells in the
PBS suspension and 1,160.8±119.9 cells in the blood suspension
passed through the chip inlet during the suspension sending period.
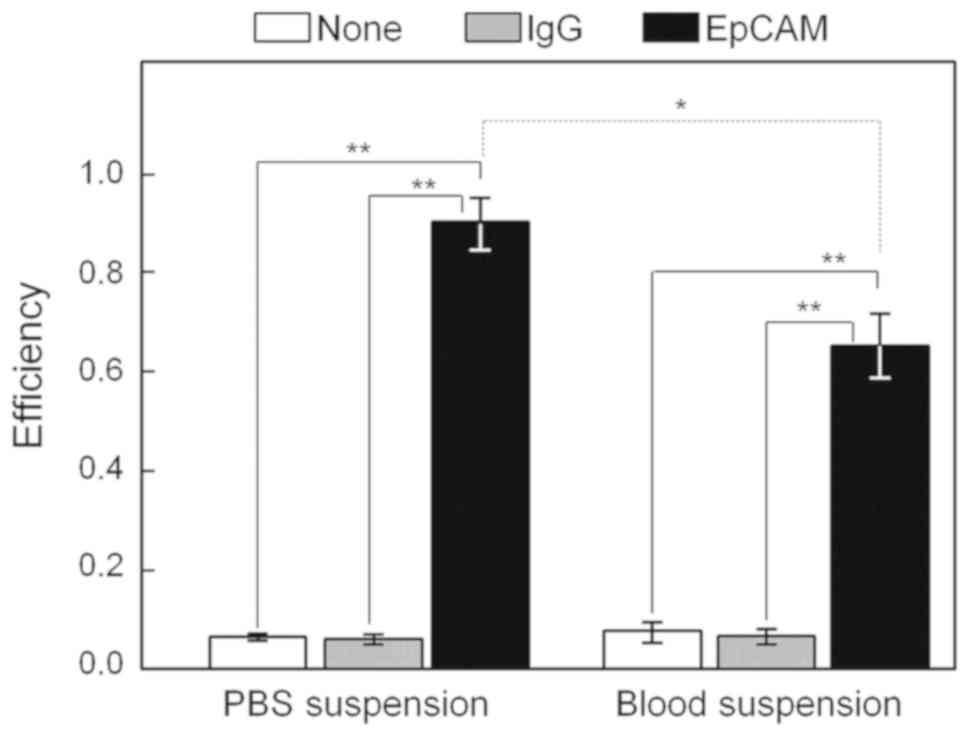
In the PBS suspension, capture efficiencies were 0.909±0.053
(average rate ± SD) for the EpCAM-chip (n=4), 0.060±0.010
for the IgG-chip (n=3), and 0.066±0.007 for the non-treated
chip (n=3; Fig. 5), whereas
in the blood suspension, the efficiencies were 0.650±0.064 for the
EpCAM-chip (n=4), 0.066±0.016 for the IgG-chip (n=3),
and 0.077±0.002 for the non-treated chip (n=3; Fig. 5).
Significant differences in the cell-capture
efficiency were determined after the angular transformation among
the three chip treatments in the PBS (P=7.610×10−8) and
blood (P=3.336×10−7) suspensions using ANOVA. In the PBS
suspension, the capture efficiency values after an angular
transformation assessed using Welch's t-test were significantly
higher in the EpCAM-chip than those in the non-treated chip
(P=1.077×10−4, df=3.220) and the IgG-chip
(P=6.994×10−5, df=3.426). In addition, values in
the non-treated chip did not differ from those in the IgG-chip
(P=0.4384, df=3.617). A similar tendency in the blood
suspension was observed, showing that capture efficiency values
after an angular transformation two-tailed Welch's t-test were
significantly higher in the EpCAM-chip than those in the
non-treated chip (P=2.647×10−4, df=3.028) and
IgG-chip (P=2.180×10−5, df=4.561). Values in the
non-treated chip did not differ from those in the IgG-chip
(P=0.3794, df=2.057). Furthermore, the capture efficiency of
the EpCAM-chip was significantly higher in the PBS suspension than
that in the blood suspension (P=0.001071, df=5.643).
Detection of CTCs in blood samples of
patients with colorectal cancer and healthy volunteers
CTCs in the preoperative peripheral blood samples of
13 patients with stage II–IV colorectal cancer were enumerated on
the chip (Figs. 6 and 7, Table I).
The mean number of CTCs/ml in patients with stages II and III
cancers (3.3±2.3, n=6) tended to be lower than those with
stage IV, with near-marginal significance (7.0±6.2, n=7;
t=1.4563, P=0.0919, one-tailed Welch's t-test). No CTCs were
detected in one patient with stage IV cancer, with histological
features defined as poorly differentiated adenocarcinoma (por),
which was different from all other cases of well or moderately
differentiated tubular adenocarcinoma (tub1 or tub2).
After excluding this patient, the mean number of
CTCs/ml associated with stage IV cancer (8.2±5.8, n=6)
tended to be higher than those with stages II and III cancer
(3.3±2.3, n=6), with difference that is close to being
statistically significant (t=1.8806, P=0.0524, one-tailed
Welch's t-test).
The number of CTC/ml in blood samples
postoperatively (mean ± SD: 1.3±1.2) was significantly lower than
that preoperatively (3.3±2.3) in patients with stages II and III
cancers (n=6; t=2.7386, P=0.0204, one-tailed paired
t-test). In addition, no CTCs were detected in two healthy
volunteer blood samples on polymeric CTC-chips.
The sensitivity of CTC detection using
the polymeric CTC-chip compared with conventional biomarkers
The detection rate of CTCs was compared with that of
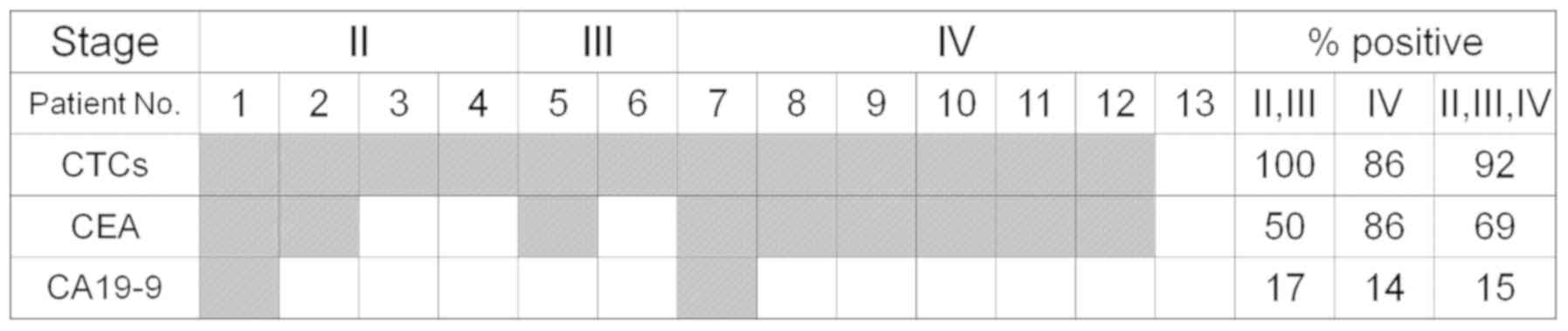
positive rates detected using cancer markers. Nine patients had CEA
or CA19-9 levels of higher than the cut-off values (CEA, 5.0 ng/ml;
CA19-9, 37.0 U/ml) (Table I;
Fig. 7). At least one CTC (per ml)
was detected in 12 of 13 patients, whereas the CEA and CA19-9
levels were positive, i.e., above the cut-off values, in nine and
two patients, respectively. For all cancer stages (II–IV), a
significant difference was observed between the positivity rate
from CTC detection and that from two biomarkers (P=0.0002251
according to the 3×2 two-sided Fisher's exact test). In addition,
the detection rate of CTCs in all patients was higher than that of
CA19-9 (P=0.0002127, Fisher's exact test) but not significantly
higher than that of CEA (P=0.3217). These two conventional markers
exhibited negative levels in the patient (no. 13 in Fig. 7) in whom no CTCs were detected. In
all six patients with stage IV cancer (except for no. 13), both CTC
and CEA values were positive, whereas the CA19-9 value was positive
in only one patient. Although CTCs were detected in all six
patients with stages II and III cancer, CEA and CA19-9 values were
positive in only two and one, respectively, of the four patients
with stage II cancer, and in one and no patient, respectively, in
two patients with stage III cancer.
Discussion
Capturing device development is expected to make
CTCs a sensitive clinical biomarker to diagnose and predict the
prognosis and treatment effects in cancers (6,10,16).
Using one of the new microfluidic devices to capture CTCs, the
polymeric CTC-chip, CTCs were detected in the blood samples of most
patients with colorectal cancer (12 of 13) who participated in this
study. The CTC detection was confirmed as more sensitive than the
two most common conventional marker tests for the diagnosis of
colorectal cancer.
Newly developed devices for CTC detection are
typically examined based on their capture efficiency of the target
cancer cells (15,16,44,45). In
this study, the efficiency of the polymeric CTC-chip was first
assessed on the colorectal cancer cell line, HCT116. The polymeric
CTC-chip secondarily treated with anti-EpCAM antibodies exhibited a
considerably high capture rate for HCT116 cells: 90% in the PBS
suspension and 65% in the whole blood suspension, which were
comparable to or exceeded those reported in previous studies
(15,16,44,45). The
efficiency seemed to exceed with those in existing devices. Nagrath
et al (15) reported that the
capture efficiency of the silicon microchip was 65–80% in the PBS
suspension for several cell lines, which fit the prediction that
HCT116, an EpCAM-positive cell line, could be captured with the
polymeric CTC-chip treated with the anti-EpCAM antibody. However,
5–7% of CTCs were captured using the chip without the anti-EpCAM
antibody treatment, demonstrating that the chip trapped some cells
with nonspecific bonds.
The number of CTCs/ml tended to be higher in
patients with stage IV than those with stages II and III cancers.
Thus, CTC concentration seemed to increase with cancer state
progression. Moreover, the number of CTCs/ml in patients with
stages II and III cancers postoperatively became lower than that
preoperatively. These results suggested that the polymeric CTC-chip
system could potentially be used to monitor disease progression and
the treatment effects in patients with colorectal cancer.
CTCs are considered specific to cancer and are not
detected in the peripheral blood of healthy individuals (8,9). No CTCs
were detected on the polymeric CTC-chip in the blood samples of two
healthy volunteers. In this study, each patient was classified as
‘CTC positive’ when more than one CTCs were detected in the sample.
The CTC positive rate in all stages tended to be higher than the
positive rate obtained by blood tests with two conventional
markers. CTCs were detected in all patients with stages II and III
cancers (six patients), although three of them tested negative for
CEA and five tested negative for CA19-9, suggesting that CTCs could
be an effective cancer marker because of they are potentially
detected in earlier stages than existing tumor markers.
The anti-EpCAM antibody binds with the EpCAM, which
is typically expressed on epithelial cells (3,5,11). However, the use of an anti-EpCAM
antibody might not adequately capture EpCAM-negative cancer cells
(14,29,42,45). In
this study, CTCs were not detected in the blood sample of one
patient with stage IV cancer. In this case, CEA and CA19-9 values
did not exceed the cut-off values despite cancer progression and
distant metastasis. Histologically, the cancer tissue sample of
this case was classified as poorly differentiated adenocarcinoma
(por), whereas those of all other cases were classified as
differentiated adenocarcinoma (tub1, 2). The cause of this failure
of CTC detection remains unknown, although it may be related to the
characteristics of the cancer tissue.
Other microfluidic devices treated with anti-PSMA,
HER2, or epidermal growth factor receptor (EGFR) antibodies have
detected CTCs in the blood samples of patients with prostate,
breast, and lung cancers (45–47).
Antibodies used in the secondary treatment on the polymeric
CTC-chip are easily changeable. To take advantage of this feature,
EpCAM-negative mesothelioma cells were captured using this
polymeric CTC-chip treated with anti-podoplanin antibodies
(34,35). Using anti-EGFR antibodies on the
surface of the polymeric CTC-chip, Ohnaga et al (36) recently reported a high capturing
efficiency against several cell lines expressing EGFR.
Using alive CTCs alone, the expression level of
oncogenes that are assumed to be related to colorectal cancer of a
single cell or its offspring cells was analyzed. In circulating
lung cancer cells collected from a microfluidic device, gene
mutations in EGFR were detected (47). Future advancements of the CTC
isolation technique in the polymeric CTC-chip will facilitate the
evaluation of molecular markers.
This study validated the usefulness of CTC detection
in blood samples obtained from patients with colorectal cancer
using the polymeric CTC-chip; nevertheless, the number of
participants was limited. Further studies should be conducted with
more patients, including those in earlier stages of the disease, to
assess the sensitivity of the polymeric CTC-chip in diagnosing
colorectal cancer.
Acknowledgements
The authors would like to thank Dr Yu Okazawa and Dr
Kosuke Mizukoshi (Department of Coloproctological Surgery, Juntendo
University, Tokyo, Japan), for providing the colorectal cancer cell
line.
Funding
The present study was supported by JSPS KAKENHI
(grant nos. 16K08974 and 25460700) to YT, TO and HK and by the
Takeda Science Foundation in 2014 for HK.
Availability of data and materials
The datasets used and/analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK designed the study and provided the overall
guidance with the advice of KS. KK analyzed the clinical data with
help and advice from KS and YT. TO prepared the polymeric CTC-chip
and antibody treatment. KK and MH conducted the detection of CTCs
with the assistance of TU. KK, MH, HK and MF performed statistical
data analyses and preparation of figures. The manuscript was
written by KK and MF with critical revision by MH, TO, KS and HK.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Juntendo University, School of Medicine (approval
no. 2016047). All participants (patients and healthy volunteers)
provided written informed consent before participation.
Patient consent for publication
All participants in this work (patients and healthy
volunteers) provided written informed consent for the publication
of this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Locker GY, Hamilton S, Harris J, Jessup
JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF and Bast RC Jr;
ASCO, : ASCO 2006 update of recommendations for the use of tumor
markers in gastrointestinal cancer. J Clin Oncol. 24:5313–5327.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Racila E, Euhus D, Weiss AJ, Rao C,
McConnell J, Terstappen LW and Uhr JW: Detection and
characterization of carcinoma cells in the blood. Proc Natl Acad
Sci USA. 95:4589–4594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacob K, Sollier C and Jabado N:
Circulating tumor cells: Detection, molecular profiling and future
prospects. Expert Rev Proteomics. 4:741–756. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paterlini-Brechot P and Benali NL:
Circulating tumor cells (CTC) detection: Clinical impact and future
directions. Cancer Lett. 253:180–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ignatiadis M, Lee M and Jeffrey SS:
Circulating tumor cells and circulating tumor DNA: Challenges and
opportunities on the path to clinical utility. Clin Cancer Res.
21:4786–4800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krivacic RT, Ladanyi A, Curry DN, Hsieh
HB, Kuhn P, Bergsrud DE, Kepros JF, Barbera T, Ho MY, Chen LB, et
al: A rare-cell detector for cancer. Proc Natl Acad Sci USA.
101:10501–10504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato N, Hayashi N, Imamura Y, Tanaka Y,
Kinoshita K, Kurashige J, Saito S, Karashima R, Hirashima K, Nagai
Y, et al: Usefulness of transcription-reverse transcription
concerted reaction method for detecting circulating tumor cells in
patients with colorectal cancer. Ann Surg Oncol. 19:2060–2065.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giannopoulou L, Kasimir-Bauer S and
Lianidou ES: Liquid biopsy in ovarian cancer: Recent advances on
circulating tumor cells and circulating tumor DNA. Clin Chem Lab
Med. 56:186–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasimir-Bauer S, Hoffmann O, Wallwiener D,
Wallwiener D, Kimmig R and Fehm T: Expression of stem cell and
epithelial-mesenchymal transition markers in primary breast cancer
patients with circulating tumor cells. Breast Cancer Res.
14:R152012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iinuma H, Okinaga K, Egami H, Mimori K,
Hayashi N, Nishida K, Adachi M, Mori M and Sasako M: Usefulness and
clinical significance of quantitative real-time RT-PCR to detect
isolated tumor cells in the peripheral blood and tumor drainage
blood of patients with colorectal cancer. Int J Oncol. 28:297–306.
2006.PubMed/NCBI
|
|
13
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and Ahsen O: Circulating tumour cells escape
from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nagrath S, Sequist LV, Maheswaran S, Bell
DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky
A, et al: Isolation of rare circulating tumour cells in cancer
patients by microchip technology. Nature. 450:1235–1239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ankeny JS, Court CM, Hou S, Li Q, Song M,
Wu D, Chen JF, Lee T, Lin M, Sho S, et al: Circulating tumour cells
as a biomarker for diagnosis and staging in pancreatic cancer. Brit
J Cancer. 114:1367–1375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veridex, LLC: 510(k) Summary, .
CellSearch™ circulating tumor cell kit, premarket
notification-expanded indications for UseColorectal. Nov 20–2007,
https://www.accessdata.fda.gov/cdrh_docs/pdf7/k071729.pdfNovember
12–2018
|
|
18
|
Veridex, LLC: 510(k) Summary, .
CellSearch™ circulating tumor cell kit premarket
notification-expanded indications for use-metastatic prostate
cancer. Feb 27–2008, https://www.accessdata.fda.gov/cdrh_docs/pdf7/K073338.pdfNovember
13–2008
|
|
19
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cristofanilli M, Hayes DF, Budd GT, Ellis
MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC,
et al: Circulating tumor cells: A novel prognostic factor for newly
diagnosed metastatic breast cancer. J Clin Oncol. 23:1420–1430.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cohen SJ, Punt CJ, Iannotti N, Saidman BH,
Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC,
et al: Relationship of circulating tumor cells to tumor response,
progression-free survival, and overall survival in patients with
metastatic colorectal cancer. J Clin Oncol. 26:3213–3221. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Bono JS, Scher HI, Montgomery RB,
Parker C, Miller MC, Tissing H, Doyle GV, Terstappen LW, Pienta KJ
and Raghavan D: Circulating tumor cells predict survival benefit
from treatment in metastatic castration-resistant prostate cancer.
Clin Cancer Res. 14:6302–6309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller MC, Doyle GV and Terstappen LW:
Significance of circulating tumor cells detected by the CellSearch
system in patients with metastatic breast colorectal and prostate
cancer. J Oncol. 2010:6174212010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lowes LE, Hedley BD, Keeney M and Allan
AL: User-defined protein marker assay development for
characterization of circulating tumor cells using the
CellSearch® system. Cytometry A. 81:983–995. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reeh M, Effenberger KE, Koenig AM,
Riethdorf S, Eichstädt D, Vettorazzi E, Uzunoglu FG, Vashist YK,
Izbicki JR, Pantel K and Bockhorn M: Circulating tumor cells as a
biomarker for preoperative prognostic staging in patients with
esophageal cancer. Ann Surg. 261:1124–1130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato N, Hayashi N, Imamura Yu, Tanaka Y,
Kinoshita K, Kurashige J, Saito S, Karashima R, Hirashima K, Nagai
Y, et al: Usefulness of transcription-reverse transcription
concerted reaction method for detecting circulating tumor cells in
patients with colorectal cancer. Ann Surg Oncol. 19:2060–2065.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Viswanath B, Kim S and Lee K: Recent
insights into nanotechnology development for detection and
treatment of colorectal cancer. Int J Nanomedicine. 11:2491–2504.
2016.PubMed/NCBI
|
|
28
|
Guo W, Yang X, Sun YF, Shen MN, Ma XL, Wu
J, Zhang CY, Zhou Y, Xu Y, Hu B, et al: Clinical significance of
EpCAM mRNA-positive circulating tumor cells in hepatocellular
carcinoma by an optimized negative enrichment and qRT-PCR-based
platform. Clin Cancer Res. 20:4794–4805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kelley RK, Magbanua MJ, Butler TM,
Collisson EA, Hwang J, Sidiropoulos N, Evason K, McWhirter RM,
Hameed B, Wayne EM, et al: Circulating tumor cells in
hepatocellular carcinoma: A pilot study of detection, enumeration,
and next-generation sequencing in cases and controls. BMC Cancer.
15:2062015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa
Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S, et
al: Clinical significance of circulating tumor cells in blood from
patients with gastrointestinal cancers. Ann Surg Oncol.
15:3092–3100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sequist LV, Nagrath S, Toner M, Haber DA
and Lynch TJ: The CTC-chip: An exciting new tool to detect
circulating tumor cells in lung cancer patients. J Thorac Oncol.
4:281–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ohnaga T, Shimada Y, Moriyama M, Kishi H,
Obata T, Takata K, Okumura T, Nagata T, Muraguchi A and Tsukada K:
Polymeric microfluidic devices exhibiting sufficient capture of
cancer cell line for isolation of circulating tumor cells. Biomed
Microdevices. 15:611–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohnaga T, Shimada Y, Takata K, Obata T,
Okumura T, Nagata T, Kishi H, Muraguchi A and Tsukada K: Capture of
esophageal and breast cancer cells with polymeric microfluidic
devices for CTC isolation. Mol Clin Oncol. 4:599–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chikaishi Y, Yoneda K, Ohnaga T and Tanaka
F: EpCAM-independent capture of circulating tumor cells with a
‘universal CTC-chip’. Oncol Rep. 37:77–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoneda K, Chikaishi Y, Kuwata T, Ohnaga T
and Tanaka F: Capture of mesothelioma cells with ‘universal’
CTC-chip. Oncol Lett. 15:2635–2640. 2018.PubMed/NCBI
|
|
36
|
Ohnaga T, Takei Y, Nagata T and Shimada Y:
Highly efficient capture of cancer cells expressing EGFR by
microfluidic methods based on antigen-antibody association. Sci
Rep. 8:120052018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: TNM classification of malignant tumours. 7th. Oxford, UK:
Wiley-Blackwell; 2009
|
|
38
|
Japanese Society for Cancer of the Colon
and Rectum (JSCCR): Japanese classification of colorectal
carcinoma. 2nd English. Kanehara and Co. Ltd; Tokyo: 2009
|
|
39
|
Thomsen M, Skovlund E, Sorbye H, Bolstad
N, Nustad KJ, Glimelius B, Pfeiffer P, Kure EH, Johansen JS, Tveit
KM, et al: Prognostic role of carcinoembryonic antigen and
carbohydrate antigen 19-9 in metastatic colorectal cancer: A
BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J
Cancer. 118:1609–1616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stojkovic Lalosevic M, Stankovic S,
Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J,
Brankovic M, Pavlovic Markovic A and Krivokapic Z: Can preoperative
CEA and CA19-9 serum concentrations suggest metastatic disease in
colorectal cancer patients? Hell J Nuc Med. 20:41–45. 2017.
|
|
41
|
Watanabe M, Takemasa I, Kaneko N, Yokoyama
Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M and Nishimura O:
Clinical significance of circulating galectins as colorectal cancer
markers. Oncol Rep. 25:1217–1226. 2011.PubMed/NCBI
|
|
42
|
Pecot CV, Bischoff FZ, Mayer JA, Wong KL,
Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW,
et al: A novel platform for detection of CK+ and CK- CTCs. Cancer
Discov. 1:580–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
R Core Team, . R: A language and
environment for statistical computing. R foundation for statistical
computing; Vienna, Austria: https://www.R-project.org/2016
|
|
44
|
Lu YT, Zhao L, Shen Q, Garcia MA, Wu D,
Hou S, Song M, Xu X, Ouyang WH, Ouyang WW, et al: NanoVelcro Chip
for CTC enumeration in prostate cancer patients. Methods.
64:144–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Galletti G, Sung MS, Vahdat LT, Shah MA,
Santana SM, Altavilla G, Kirby BJ and Giannakakou P: Isolation of
breast cancer and gastric cancer circulating tumor cells by use of
an anti HER2-based microfluidic device. Lab Chip. 14:147–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gleghorn JP, Pratt ED, Denning D, Liu H,
Bander NH, Tagawa ST, Nanus DM, Giannakakou PA and Kirby BJ:
Capture of circulating tumor cells from whole blood of prostate
cancer patients using geometrically enhanced differential
immunocapture (GEDI) and a prostate-specific antibody. Lab Chip.
10:27–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maheswaran S, Sequist LV, Nagrath S, Ulkus
L, Brannigan B, Collura CV, Inserra E, Diederichs S, Iafrate AJ,
Bell DW, et al: Detection of mutations in EGFR in circulating
lung-cancer cells. N Engl J Med. 359:366–377. 2008. View Article : Google Scholar : PubMed/NCBI
|