Introduction
Lymph node metastasis (LNM) is an independent risk
factor affecting the prognosis of patients with cervical cancer
(CC). Accurate determination of LNM in patients with early CC is of
great significance for guiding individualized adjuvant therapy and
improving the prognosis (1).
Although the LNM rate of early stage CC (IA1-IB1) is only 0.0–29.3%
(2), the recurrence rate remains of
15% in patients without LNM (3).
Lentz et al (3) used
immunohistochemistry (IHC) to assess 3,106 lymph nodes from 132
patients with pathologically negative lymph nodes, and reported
that micrometastases can be found in 15% patients with early stage
cervical cancer who were considered as lymph node negative by
conventional histologic analysis. This is due to the failure of
traditional pathological methods to diagnose the micro-metastasis
of lymph nodes (4), and the fact
that detection of micro-metastasis of lymph nodes requires highly
specific biomarkers, such as cytokeratin, squamous cell carcinoma
antigen (SCC) and human papilloma virus (HPV) (5). However, IHC or cytokeratin are
considered less specific (6,7). The high-risk human papilloma virus
(HR-HPV) DNA is considered a useful biological tool for detecting
the lymph node micrometastases in patients with CC (8,9).
Previous studies have reported the prevalence of HPV-DNA in both
pelvic lymph nodes and para-aortic lymph nodes; however, these
studies have used different laboratory methods, clinical
environments and measurements (10–12).
Dürst et al (13)
demonstrated, via reverse transcription-quantitative PCR (RT-qPCR),
that HPV16-E6-E7-mRNA is more sensitive and specific than
cytokeratin-(CK)19-mRNA for the detection of disseminated tumor
cells in sentinel lymph nodes (SLNs). The present study
demonstrated that HPV16-E6-E7-mRNA improved the diagnostic rate of
micrometastasis and thus concluded it to be a valuable tool for
evaluating prognosis (13). RNA can
be easily degraded without RT; however, its stability increases
following RT into cDNA. DNA is the product sequence amplified, and
RT fluorescence qPCR methods can improve the sensitivity and
specificity (9). The present study
used RT-qPCR in order to search for HPV16/18 DNA sequences in SLNs
and to evaluate the association between HPV and lymph node
micrometastasis of CC, as well as to investigate its clinical
significance.
Materials and methods
Patient information
The SLNs were collected from 100 patients at the
Department of Gynecologic Oncology at the Affiliated Tumor Hospital
of Guangxi Medical University (Nanning, China) between August 2017
and August 2018. The inclusion criteria met by all patients were as
follows: i) Preoperative cervical biopsy pathological diagnosis was
confirmed as invasive carcinoma (squamous, adenocarcinoma or
adenosquamous carcinoma); ii) International Federation of
Gynecology and Obstetrics (FIGO) stage (14) was IIA2-IIA; iii) cervical fluid-based
cytology HPV test was performed prior to surgery (HC-2 method),
including tests for HR HPV type 17 (HPV16, −18, −31, −33, −35, −39,
−45, −51, −52, −53, −56, −58, −59, −66, −68, −82 and −83); and iv)
the complete data of clinical cases were collected. The exclusion
criteria were as follows: i) Patients who underwent previous
cervical treatment (Loop Electrosurgical Excision Procedure and
cone excision); ii) the imaging findings indicated marked
retroperitoneal lymph node metastasis or distant metastasis; and
iii) patients who received preoperative radiotherapy. The present
study was approved by The Medical Ethics Committee of Guangxi
Medical University Affiliated Tumor Hospital (registration number,
LW2018028). Written consent was obtained from all patients prior to
sample collection.
Identification of SLN
Prior to surgery, the patients were placed in the
lithotomy position and were anesthetized. The normal cervix around
the tumor was injected with 1 ml of carbon nanoparticles (CNP) at 3
and 9 o'clock, with a deep (0.3±0.5 cm) cervical injection. The
injection process lasted for at least 3 min, following which
pressure was applied locally to prevent the CNP extravasation after
the injection. During surgery, lymph nodes staining in the drainage
field of the pelvic lymph system were identified. The lymph nodes
that stained within 15 min of injection were defined as SLNs,
whilst those remaining were classed as non-SLNs (nSLNs).
Subsequently, the SLNs were removed and the tumor position was
recorded and counted. Following detection of the SLNs, patients
underwent laparoscopic radical hysterectomy + pelvic
lymphadenectomy (with or without para-aortic lymph node sampling).
The same surgical group of physicians performed the surgery. These
physicians were gynecologists of the affiliated tumor hospital of
Guangxi medical university, and were from the same operation group
of the surgeon who operated.
Specimen collection
Following collection of the SLNs, the fresh tissue
was cut into 3 mm thick sections. The samples were then transferred
into tagged sterile EP tubes without RNA enzymes, and stored at
−80°C. Other specimens were labeled and sent for routine
pathological examination.
Reagents and primers
The following reagents were purchased from Takara
Biotechnology Co., Ltd. Tissue RNA extraction kits (Takara MiniBEST
Universal RNA Extraction Kit PrimeScript™), transcriptional Master
Mix (Perfect Real Time, SYBR®) and RT-PCR reagents
(Premix Ex Taq TM II Perfect Real Time).
The following primer pairs were used for the
RT-qPCR: HPV16-mRNA: Forward: 5′-AAGGGCGTAACCGAAATCGGT-3′ and
reverse: 5′-GTTTGCAGCTCTGTGCATA-3′; amplified fragments 141 bp.
HPV18-mRNA: Forward, 5′-CATTTTGTGAACAGGCAGAGC-3′ and reverse:
5′-ACTTGTGCATCATTGTGGACC-3′; and GAPDH: Forward:
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse:
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
RNA extraction
HPV-DNA was selected for extraction from SLNs under
sterile RNase-free conditions to prevent RNA degradation, according
to the manufacturer's protocol. The total RNA was measured using a
nucleic acid protein analyzer (NanoDropND-2000 micrometer; NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). Specimens with
A260/280 values between 1.8–2.1 were of good purity.
Specimens with high integrity and purity were subjected to RT in
order to synthesize cDNA. cDNA reaction system for reverse
transcription synthesis was as follows: 5× PrimeScript RTMaster Mix
×1(for RealTime) 4.0 µl + RNA 4.0–12.0 µl (depending on RNA
concentration) + RNaseFree dH2O to 20.0 µl at 37°C for
15 min (reverse transcription reaction), 85°C for 5 sec
(deactivation reaction of reverse transcriptase), at 4°C for 4–5
min, and finally stored at −20°C.
RT-qPCR
The total reaction system was 20 µl, which included
2.0 µl cDNA, 0.8 µl sense and anti-sense primers, 10.0 µl
SYBR®Premix Ex Taq™ II, 0.4 µl ROX Reference Dye (50×)
and 20.0 µl distilled H2O. The follow PCR cycle was
applied: Stage 1, predenaturation at 95°C for 30 sec and for 1
cycle; stage 2, PCR reaction at 95°C for 5 sec, 60°C for 30–34 sec,
and for 40 cycles in total. The target genome, the internal
reference genome and the 2-tube negative control (with sterile
distilled water instead of cDNA) were set for each amplification.
Each experiment was performed in triplicate.
Agarose gel electrophoresis
Agarose (1 g) was mixed with 50 ml 0.5X TBE buffer
to make 5% agarose gel. DNA marker and PCR products were loaded
into the wells and electrophoresis was performed at 100V for 50
min. Following electrophoresis, the gel was removed and the results
were recorded using a color analyzer. Each experiment was performed
in triplicate. The expression of HPV-DNA in SLNs was analyzed,
using 50–500 bp DNA markers as a reference standard. The DNA
sequence amplified by PCR was compared with the HPV16 and HPV18 DNA
in the gene bank (HPV 16 sequence, https://www.ncbi.nlm.nih.gov/nucleotide/KY994539.1?report=genbank&log$=nuclalign&blast_rank=94&RID=XRTDTSNB016;
HPV18 sequence, http://www.ncbi.nlm.nih.gov/nucleotide/MF288722.1?report=genbank&log$=nuclalign&blast_rank=3&RID=XRS4T07H014)
for Blast sequence alignment.
Judgement and relative quantitative
analysis of positive results
The reaction system and reaction conditions were as
follows: 20 µl of total reaction system containing 1.0 µl of cDNA
template, 0.8 µl HPV16 and HPV18 gene sense and anti-sense primers
each, 10.0 µl of SYBR®Premix Ex Taq™ II, 0.4 µl of ROX
Reference Dye (50×) and 20.0 µl of distilled H2O. The
reaction conditions were as follows: Stage 1, predenaturation at
95°C for 30 sec and for 1 cycle; stage 2, PCR reaction at 95°C for
5 sec, 60°C for 30 sec, and for 40 cycles in total; stage 3, 95°C
for 15 sec and for 1 cycle; predenaturation at 94°C for 5 min,
followed by 30 sec at 94°C, 1 min at 55°C, and 1 min at 72°C for a
total of 40 cycles, and finally extended at 72°C for 10 min.
Samples were stored at 4°C. Following the reaction, the
fluorescence reaction curve was automatically obtained by software
TOWER3G 3.4 (Analytik Jena AG), and the amplification efficiency
and cycle threshold value (15) of
each reaction system were calculated. Each experiment was performed
in triplicate.
Pathological examination
SLNs were fixed in 10% formaldehyde for 24 h at room
temperature, washed and dehydrated with increasing gradient of
ethanol (70% for 2–4 h, 80% for 2–4 h, 95% for 2–4 h and 100% for
1–2 h), and washed with xylene for 30 min to 2 h. Samples (3 µm
thick) were then embedded in paraffin and the lymph node slices
were stained with hematoxylin and eosin (H&E) for 50 min at
room temperature prior to IHC analysis. SLNs were incubated with
the wide-spectrum horn cytokeratin AEl/AE3 (no. M351501-2; Dako;
Agilent Technologies, Inc.) for expression in the cytoplasm, which
was detected using the two-step anti-rabbit/mouse universal IHC kit
(no. GK500705; Dako Agilent Technologies, Inc.). The criteria for
the tumor cells were as follows: Large nucleus, deep staining, an
imbalance of nucleo-cytoplasm ratio and AE1/AE3 is positive. The
SLNs that were negative in pathology but HPV-DNA positive were
further examined using an ultrastaging protocol (16). Two adjacent 5-µm sections were cut at
each of two levels 200 µm apart. At each level, one slide was
stained with H&E (20 min at room temperature) and the other was
used for immunohistochemistry (IHC) using the anti-cytokeratin
AE1:AE3 (1 h at room temperature). Sections were imaged using an
optical microscope (OlympusBX45; Olympus Corporation;
magnification, ×100).
Statistical analysis
Statistical analysis was performed using SPSS
software (IBM Corp.; version 22.0;). Standard summary statistics
were used to describe primary data: Data were presented by the
median values. The positive rate of lymph node metastasis was
compared by χ2 test. The Mann-Whitney rank sum test of
non-parametric data was used to compare the mean independent values
of continuous variables. The association between discrete variables
was determined by Fisher's exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient basic information
The present study included 100 patients with CC. The
median age was 52 years (range 33–68 years) and the median body
mass index was 22.42 kg/m2 (range 15.60–27.64
kg/m2). There were a total of 78 cases of squamous cell
carcinoma, 20 cases of adenocarcinoma, two cases of adenosquamous
carcinoma, 51 cases of grade 3 (G3), 27 cases of moderate
differentiation, eight cases of high differentiation and 14
unclassified cases. Additionally, there were zero cases with IA2
stage, 32 cases with IB1 stage, 24 cases with IB2 stage, 21 cases
with stage IIA1 and 23 cases with stage IIA2 CC. The tumor diameter
was ≤2 cm in 19 cases, 2–4 cm in 45 cases and >4 cm in 36 cases.
In the 36 cases where the tumor lesions were >4 cm, paclitaxel +
oxaliplatin neoadjuvant chemotherapy was administrated over 1–2
courses. Furthermore, there were 28 cases of cervical stromal
invasion <1/3, 30 cases of 1/3-2/3, 42 cases of >2/3, 30
cases of Lymphovascular Space Invasion (LVSI) positive, 92 cases of
HPV positive and 8 cases of HPV negative. There were 1,680 lymph
node dissections, including 345 SLNs and 1,335 nSLNs (Table I).
 | Table I.Demographics and tumor factors. |
Table I.
Demographics and tumor factors.
|
Characteristics | No. of cases,
n |
|---|
| Histology |
|
|
Squamous cell carcinoma | 78 |
|
Adenocarcinoma | 20 |
|
Adenosquamous carcinoma | 2 |
| Tumor grading |
|
| G1 | 8 |
| G2 | 27 |
| G3 | 51 |
|
Uncategorized | 14 |
| Stage |
|
|
IA2 | 0 |
|
IB1 | 32 |
|
IB2 | 24 |
|
IIA1 | 21 |
|
IIA2 | 23 |
| Tumor size |
|
| <2
cm | 19 |
| 2–4
cm | 45 |
| >4
cm | 36 |
| Depth of
invasion |
|
|
<1/3 | 28 |
|
1/3-2/3 | 30 |
|
>2/3 | 42 |
| Vaginal margin
positive | 0 |
| Lymphovascular
space invasion | 30 |
| Neoadjuvant
chemotherapy | 18 |
| HPV infection |
|
|
Yes | 92 |
| No | 8 |
Electrophoresis and amplification
curves of fluorescence qPCR amplification products
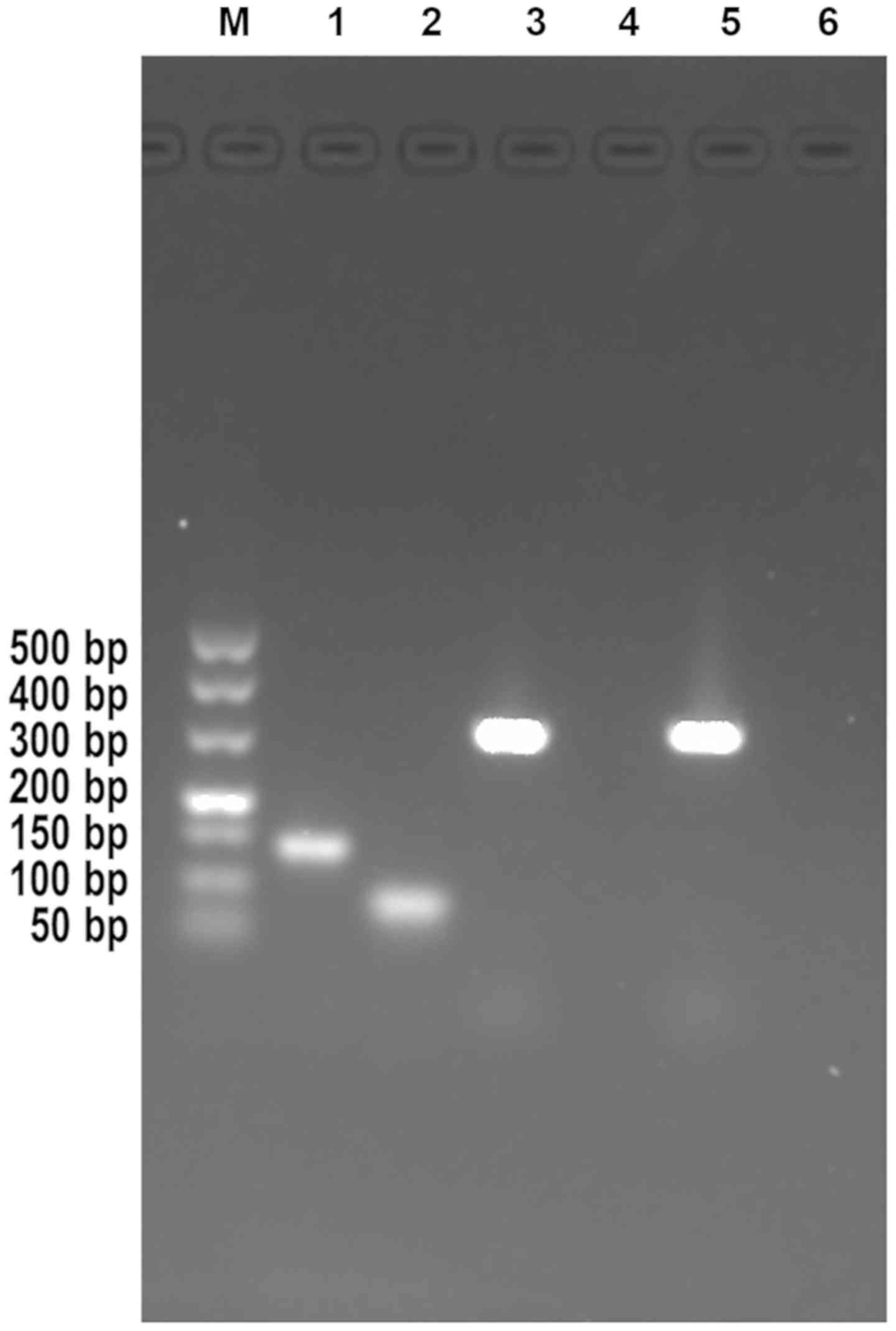
The results of agarose gel electrophoresis in the
present study demonstrated that the HPV16 and HPV18 genes had a
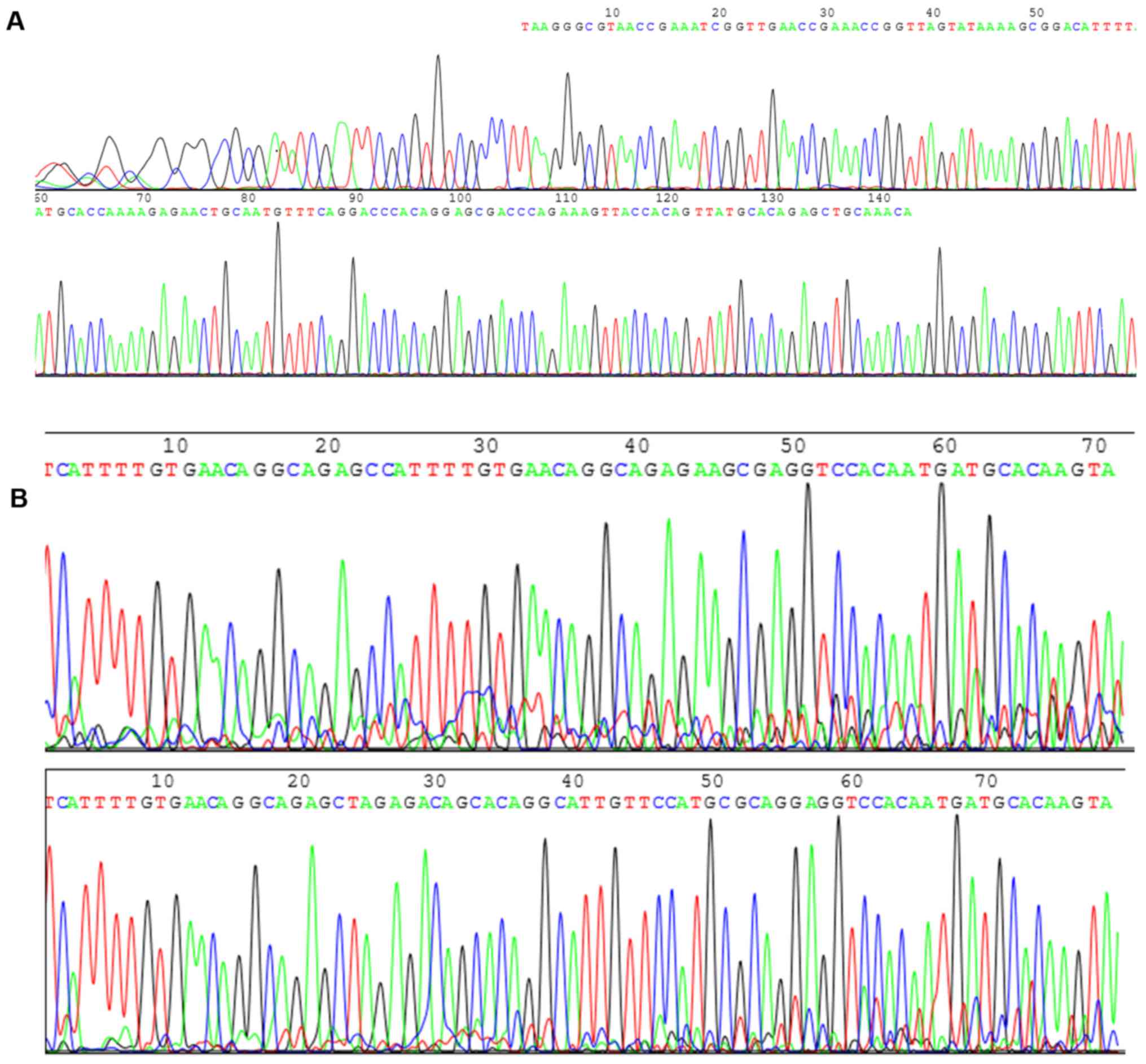
size of 141 and 80 bp, respectively (Fig. 1). The sequencing of HPV16 and HPV18
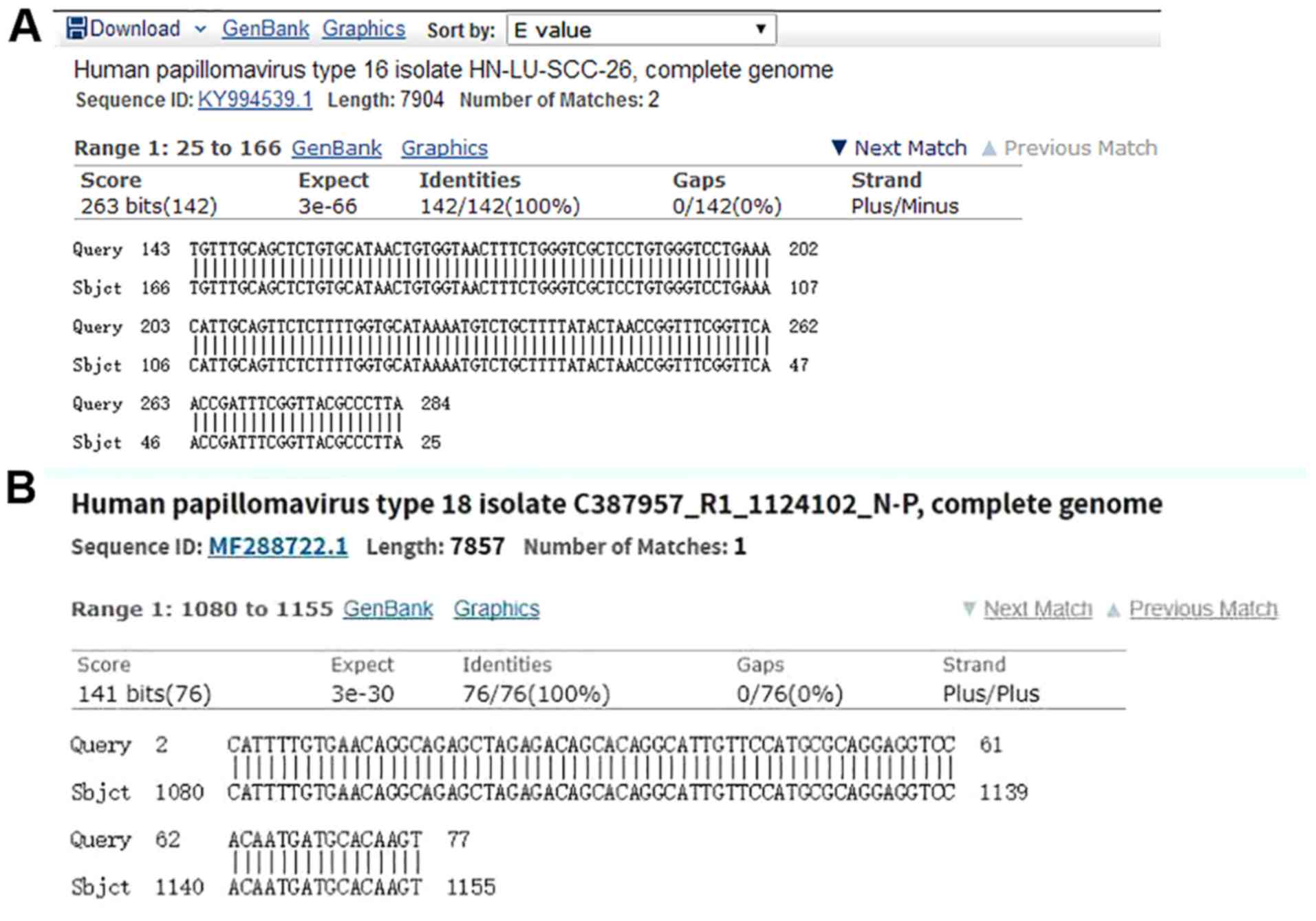
was a part of the whole sequencing presented in Fig. 2. The results of the present study
demonstrated that the amplified DNA sequence was the same when
compared with GenBank HPV16 and HPV18. Sequencing was conducted by
Sangon Biotech Co., Ltd. (Fig. 3),
which further confirmed that the obtained target gene sequence was
correct.
HPV-DNA detection of primary tumor and
SLN
Of the 100 patients, 92 cases were diagnosed HPV-DNA
positive by cervical liquid-based cytology, whereby the positive
rate was 92%. HPV16 was the most common type, which was detected in
71 cases. Of these, one case was mixed with HPV66, one case was
mixed with HPV35 and one case was mixed with both HPV52 and HPV42.
HPV18, HPV51, HPV33 and HPV59-DNA were detected in 13, four, two
and two cases, respectively. Furthermore, two cases were mixed with
HPV16 and HPV18. The lymph node metastasis was detected in 14
patients via routine pathological examination. In addition, the
RT-qPCR demonstrated that 28 patients had HPV-DNA expression in
SLNs, whereas 72 patients did not. Of those positive for HPV-DNA
expression, 13 were HPV16 positive, nine were HPV18 positive and
six were other positive (HPV66, 35, 52 and 45; Table II).
 | Table II.Status of HPV-DNA in SLNs. |
Table II.
Status of HPV-DNA in SLNs.
| HPV type | SLN positive | SLN negative | Total |
|---|
| HPV16 | 13 | 45 | 58 |
| HPV18 | 9 | 13 | 22 |
| Other | 6 | 14 | 20 |
SLN histopathology
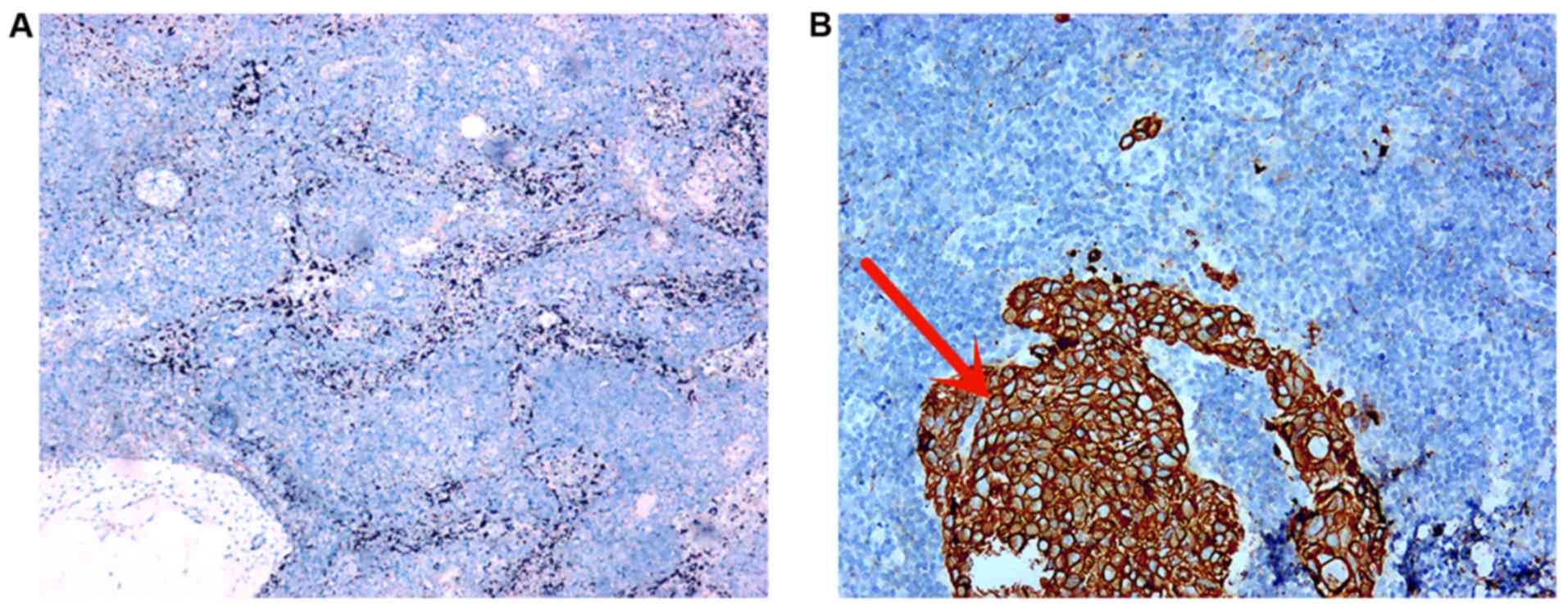
IHC analysis demonstrated positive staining of
AEl/AE3 in the cytoplasm of cancer cells (Fig. 4B), which presented with a
characteristic yellow-brown color, while no signal was observed in
the SLN lymph nodes (Fig. 4A).
Expression of HPV-DNA in SLN and
comparison with histopathological examination
The expression of HPV16/18 was positive in 109 SLNs
in 28 patients; [positive rate was 31.6% (109/345)]. The
histopathological examination demonstrated that there were 44 SLNs
with metastasis in 14 cases [positive rate was 12.8% (44/345)]; and
the pathologically positive lymph nodes were also all positive for
HPV16/18. Of the 301 negative lymph nodes confirmed by pathological
histology, the expression of HPV16/18 was positive in 65 SLNs
[positive rate was 21.6% (65/301)] (P<0.001). Of the 14 patients
with positive histopathology, the detection rate of HPV16/18 DNA
was 100% (14/14), of which, HPV16 accounted for 71.4% (10/14) and
HPV18 for 28.6% (4/14). In the 86 patients (301 lymph nodes) with
negative histopathology, the detection rate of HPV16/18 DNA was
21.6% (65/301), while the detection rates of HPV16 and HPV18 were
20.6% (62/301) and 1.7% (5/301), respectively. HPV16 and HPV18 were
simultaneously positive in five (1.7%) lymph nodes. In addition,
ultrastaging was performed on all SLNs negative with H&E
staining, which detected micrometastases in 13 of the 14 patients
(Table III).
 | Table III.Comparison of positive rate of lymph
node detection by RT-qPCR and pathology. |
Table III.
Comparison of positive rate of lymph
node detection by RT-qPCR and pathology.
|
| SLN |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Method | + | − | Total | χ2 | P-value |
|---|
| Pathology | 44 | 301 | 345 | 35.482 | <0.001 |
| RT-PCR | 109 | 236 | 345 |
|
|
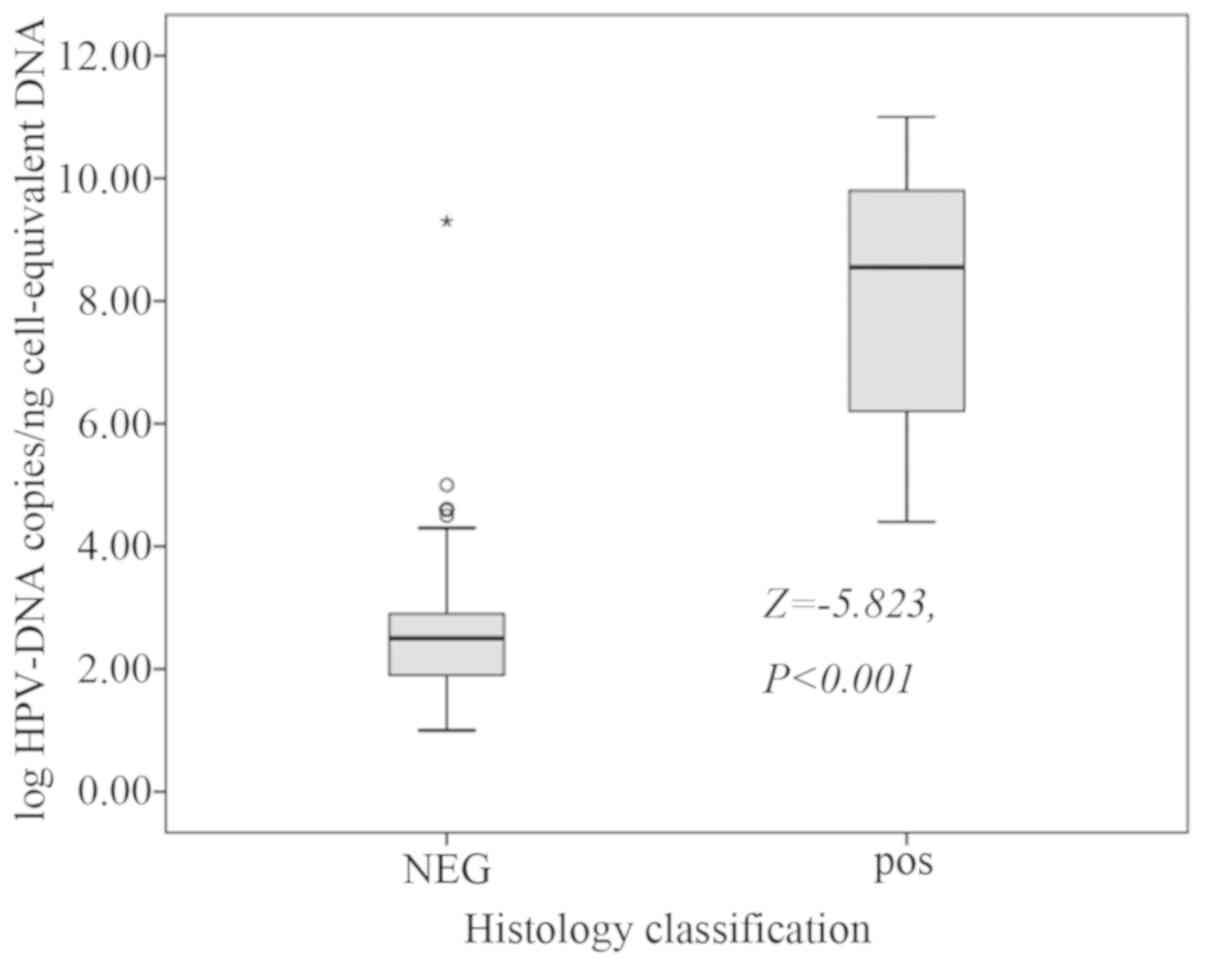
A total of 345 SLNs were surgically removed, of
which 44 were positive and 301 were negative. The median detection
of HPV-DNA of the negative SLNs was 2.50 (1.90–2.90) copies/ng,
while the median examination of HPV-DNA of the positive SLNs were
8.55 (6.20–9.80) copies/ng (Fig. 5;
Z=−5.823, P<0.001).
Association between the positive
expression of HPV-DNA in SLN and clinical pathological factors
According to the clinicopathological features of the
patients, numerous factors were selected to evaluate the
association between positive expression of HPV-DNA and SLNs. The
results of the present study demonstrated that the positive
expression of HPV-DNA in SLN was significantly associated with
clinical stage (P<0.001). Progression in the clinical stage was
associated with an increase in tumor size and a greater possibility
of metastasis (P=0.010). Nevertheless, the positive expression of
HPV-DNA in SLN was not associated with age of patient, depth of
cervical invasion, histological grade, lymphatic or LVSI, SCCAg
(P>0.05; Table IV).
 | Table IV.Association between positive
expressions of HPV-DNA and the clinical pathological factors in
SLNs. |
Table IV.
Association between positive
expressions of HPV-DNA and the clinical pathological factors in
SLNs.
| Clinicopathological
features | No. of cases,
n | Positive | Positive rate,
% | P-value |
|---|
| Age |
|
|
| 0.524 |
| ≤40
years | 6 | 1 | 16.67 |
|
| >40
years | 94 | 27 | 28.72 |
|
| Clinical stage |
|
|
| <0.001 |
|
IA2 | 0 | 0 | 0 |
|
|
IB1 | 32 | 1 | 3.12 |
|
|
IB2 | 24 | 1 | 4.17 |
|
|
IIA1 | 21 | 8 | 19.05 |
|
|
IIA2 | 23 | 18 | 78.26 |
|
| Tumor size |
|
|
| 0.010 |
| <2
cm | 19 | 9 | 47.37 |
|
| 2–4
cm | 45 | 15 | 33.33 |
|
| >4
cm | 36 | 4 | 11.11 |
|
| Histological
grade |
|
|
| 0.148 |
| G1 | 8 | 1 | 12.50 |
|
| G2 | 27 | 8 | 29.63 |
|
| G3 | 51 | 18 | 35.29 |
|
| NA | 14 | 1 | 7.14 |
|
| Depth of cervical
stromal invasion |
|
|
| 0.141 |
|
<1/3 | 28 | 4 | 14.28 |
|
|
1/3-2/3 | 30 | 9 | 30.00 |
|
|
>2/3 | 42 | 15 | 35.71 |
|
| LVSI |
|
|
| 0.080 |
|
Positive | 30 | 12 | 40.00 |
|
|
Negative | 70 | 16 | 22.85 |
|
| SCCAg |
|
|
| 0.233 |
| ≤1.5
µg/l | 27 | 6 | 22.22 |
|
| >1.5
µg/l | 63 | 22 | 34.92 |
|
Discussion
The metastasis of tumor cells through lymphatic
vessels to distant lymph nodes is considered the main adverse
factor affecting the prognosis of CC (17). Nevertheless, local or distant
recurrence may occur decades after treatment of the primary lesion
in up to 15% of patients with early stage CC [patients without
lymph node metastasis (pN0)]. This event may occur due to the
proliferation of occult tumor cells and the existence of lymph node
micrometastasis that are not readily detected by conventional
pathology. The detection of micrometastasis of lymph nodes requires
highly specific biomarkers, such as cytokeratin, SCC and HPV.
However, IHC or cytokeratin are considered less specific (3–6). Dürst
et al (13) demonstrated that
HPV16-E6-E7-mRNA was more sensitive and specific than
cytokeratin-(CK)19-mRNA for the detection of disseminated tumor
cells in SLNs. HR-HPV persistent infection is one of the most
important risk factors for CC, but also for several other types of
cancer, including head and neck, vaginal, vulva, penile and anal
cancer (18). In addition, previous
studies have demonstrated that the presence of HPV is associated
with the prognosis of patients after radiotherapy or concurrent
radiochemotherapy, while the pelvic lymph node of HPV is associated
with an increased risk of recurrence (19–21). HPV
DNA was chosen as a molecular marker for the detection of tumor
cells in SLN shown to be negative by conventional histopathology,
by using specific primers of HPV16 and HPV18.
The Cancer Genome Atlas Research Network (22) has suggested that 95% of primary CC
are HPV positive. In addition, <10% of invasive CC cases are
classified as ‘HPV negative’, using the standard test for HR
subtypes (23–25). HPV screening is more sensitive than
cytological screening in detecting high-grade neoplasia (26). Based on this evidence, the new
guidelines in Europe, Australia and the USA have now recommended
HPV screening for all women aged ≥30 (27–29). In
addition, the HPV-DNA has been proposed as a potential biomarker
for tumor metastasis associated with HPV (4). To date, a number of studies (9,21,30) have
attempted to investigate the association between HR-HPV DNA
detection in lymph nodes, lymph node metastasis and the prognosis
of patients with CC. In a previous study, the safety of SLN mapping
was demonstrated as well as how SLN can represent the status of
pelvic lymph nodes (31). It is
known that in early stage CC, if SLN do not exhibit metastasis,
then neither do other lymph nodes (31). Therefore, the detection of HPV-DNA
expression in SLN also suggests the expression status of pelvic
lymph nodes.
The detection of HPV-DNA in CC has been reported in
both ThinPrep cytology test and plasma (32). Lancaster et al (33) first proposed the use of HPV-DNA for
the detection of CC metastasis. They revealed that these metastatic
tumors carry the same HR-HPV type as the primary tumor. But the
results of published data are not the same (34). Considering that HPV-DNA is usually
integrated into the genome of host cancer cells, it can be used as
a sensitive biomarker for the detection of micrometastasis
(34). Furthermore, it can be used
to investigate the potential spread of single tumor cells,
particularly when pathological indications for the surgical margin
and lymph nodes are negative (34).
Tortora et al (35) examined
20 patients with primary CC and demonstrated that seven out of 20
(35%) women had metastatic spread in peri-tumor tissues and/or
lymph nodes, as determined by histology. In addition, the HPV-DNA
was detected in all histological positive samples as well as in 16
and 25% of histological negative peri-tumor tissues and lymph
nodes, respectively. Furthermore, an evidence-based study (34), which included 1,333 patients with
early stage CC who underwent pathological examination and HPV
testing, demonstrated that expression of HPV-DNA is detected in
~75% of the 488 patients with at least one lymph node metastasis.
In the present study, the most common HPV type was HPV16, which was
observed in 71 cases (77.2%), followed by: Mixed HPV66 in one case,
mixed HPV35 in one case, and mixed HPV52 and HPV45 in one case. In
addition, there were 13 cases of HPV18 type (14.1%), two cases of
HPV16 and HPV18 mixed types (2.1%, four cases of HPV51 type (4.3%),
two cases of HPV33 type (2.1%) and two cases of HPV59 type (2.1%).
The expression of HPV16 and HPV18 in lymph nodes was observed in 13
cases (46.4%) and nine cases (32.1%), respectively. HPV-DNA was
detected in all patients with positive pathology. Dürst et
al (13) reported that 60% of
primary tumors were positive for HPV16 and 22% for HPV18, and were
also positive for HPV35, HPV45 and HPV73. HPV-mRNA expression in
SLN was 27.5% positive, 63.0% negative and 9.5% possible negative
was detected by PCR. To the best of our knowledge, Lukaszuk et
al (8) performed the first
prospective study on frozen fresh tissue of cervical lesions and
pelvic lymph nodes, and demonstrated that HPV-DNA in lymph nodes
was an independent tumor risk factor associated with survival rate
and mortality. Furthermore, Dürst et al (13) evaluated the expression of HPV E6/E7
mRNA as a molecular biomarker in negative sentinel lymph nodes and
demonstrated that HPV-mRNA was detected in 27.5% of patients with
SLN, while 22 patients exhibited recurrence. In addition, patients
that were HPV-mRNA negative in SLN had significantly longer
tumor-free survival (P=0.002). Furthermore, the Cox regression
analysis hazard ratio (95% CI) for disease-recurrence was 3.8
(1.5–9.3; P=0.004) for HPV-mRNA-positive compared with
HPV-mRNA-negative patients. Dürst et al (13) suggested that HPV-mRNA positive SLN
has prognostic value independent of tumor size, particularly for
tumors >20 mm in diameter. Further risk stratification using
HPV-mRNA as a molecular biomarker may benefit patients. The present
study further evaluated the expression of HPV E6/E7 mRNA in lymph
nodes (not in SLNs) and analyzed the prognosis (13). In addition to evaluating the
expression of HPV-DNA in SLNs, the present study also compared
pathological results to verify whether there was micrometastasis in
lymph nodes with negative pathology but positive HPV-DNA
expression. Furthermore, the sensitivity of HPV-DNA expression in
SLN was compared with routine pathology (13). Köhler et al (36) demonstrated that histologically
confirmed metastatic lymph nodes were also HPV E6/E7 mRNA positive,
resulting in a sensitivity of 100%. A total of four histologically
free sentinel nodes were positive for HPV E6/E7 mRNA, resulting in
a specificity of 96.4%. The HPV E6/E7 mRNA assay in the SLNs of
patients with CC was feasible and highly accurate. The detection of
HPV mRNA with negative SLNs may denote a shift from microscopic
identification of metastasis to the molecular level. In the present
study, a total of 28 cases of 109 SLNs, detected by RT-qPCR, were
HPV16/18 positive, with a positive rate of 31.6% (109/345).
Nevertheless, only 14 patients with a total of 44 SLNs metastases
were detected by traditional histopathological methods, with a
positive rate of 12.8% (44/345) (P<0.001). HPV16/18 were all
positive in the histologically positive nodes. Among the 301 SLNs
with pathological negative, 65 SLNs were positive for HPV16/18
(21.6%). The results of the present study suggest that
pathologically negative, but high levels of HPV-DNA expression, may
indicate an early and significant risk of lymphatic tumor
metastasis. In terms of post-operative treatment, pathologically
negative lymph nodes with high load HPV-DNA positive may need to be
treated with adjuvant treatment to decrease the risk of distant
disease recurrence (37). In
addition, the present study demonstrated that the HPV-DNA in SLNs
was associated with clinical stage and tumor size (P<0.05), but
not with the age of the patient, depth of cervical invasion,
histological grade, LVSI and SCCAg (P>0.05). To the best of our
knowledge, there are currently no data published.
The main limitation of the present study was the
short follow-up time. In addition, the associations between
recurrence rate, mortality and HPV-DNA positive expression in SLN
were not evaluated.
Overall, the results of the present study suggest
that detection of HPV-DNA expression in SLN has a higher positive
rate than histopathology. The high load of HPV-DNA in lymph nodes
can provide additional evidence of metastasis, while its
quantification may be used as a useful clinical biomarker. For
patients with negative lymph nodes, HPV-DNA detection may have the
potential to guide surveillance. In addition, HPV-DNA positive
patients will require closer surveillance than HPV-DNA negative
patients.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Development and
Application of Appropriate Medical and Health Technology in GuangXi
(grant no. S2017100) and GuangXi Key Research and Development Plan
Fund (grant no. AB17292092). The funding body had no further role
in the collection, review or publication of the data presented in
this article.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and DY conceived and designed the study. YL, XX
and XN wrote the manuscript. YL, XX and XN performed HPV expression
analysis, and prepared figures and tables. YL, XX and DY
interpreted the results. YL and XX revised the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Medical Ethics
Committee of Guangxi Medical University Affiliated Tumor Hospital
(registration number: LW2018028). Written consent was obtained from
all patients prior to sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delgado G, Bundy BN, Fowler WC Jr, Stehman
FB, Sevin B, Creasman WT, Major F, DiSaia P and Zaino R: A
prospective surgical pathological study of stage I squamous
carcinoma of the cervix: A gynecologic oncology group study.
Gynecol Oncol. 35:314–320. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slama J, Dundr P, Dusek L and Cibula D:
High false negative rate of frozen section examination of sentinel
lymph nodes in patients with cervical cancer. Gynecol Oncol.
129:384–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lentz SE, Muderspach LI, Felix JC, Ye W,
Groshen S and Amezcua CA: Identification of micrometastases in
histologically negative lymph nodes of early-stage cervical cancer
patients. Obstet Gynecol. 103:1204–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okamoto S, Niikura H, Nakabayashi K,
Hiyama K, Matoda M, Takeshima N, Watanabe M, Nagase S, Otsuki T and
Yaegashi N: Detection of sentinel lymph node metastases in cervical
cancer: Assessment of KRT19 mRNA in the one-step nucleic acid
amplification (OSNA) method. Gynecol Oncol. 130:530–536. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang BX and Fang F: Progress in the study
of lymph node metastasis in early-stage cervical cancer. Curr Med
Sci. 38:567–574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bostick PJ, Chatterjee S, Chi DD, Huynh
KT, Giuliano AE, Cote R and Hoon DS: Limitations of specific
reverse-transcriptase polymerase chain reaction markers in the
detection of metastases in the lymph nodes and blood of breast
cancer patients. J Clin Oncol. 16:2632–2640. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Trappen PO, Gyselman VG, Lowe DG, Ryan
A, Oram DH, Bosze P, Weekes AR, Shepherd JH, Dorudi S, Bustin SA
and Jacobs IJ: Molecular quantification and mapping of lymph-node
micrometastases in cervical cancer. Lancet. 357:15–20. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lukaszuk K, Liss J, Gulczynski J, Nowaczyk
M, Nakonieczny M, Piatkowski M, Sliwinski W, Baay M, Wozniak I, Maj
B and Lukaszuk M: Predictive value of HPV DNA in lymph nodes in
surgically treated cervical carcinoma patients-a prospective study.
Gynecol Oncol. 104:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carow K, Read C, Häfner N, Runnebaum IB,
Corner A and Dürst M: A comparative study of digital PCR and
real-time qPCR for the detection and quantification of HPV mRNA in
sentinel lymph nodes of cervical cancer patients. BMC Res Notes.
10:5322017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petry KU, Liebrich C, Luyten A, Zander M
and Iftner T: Surgical staging identified false HPV-negative cases
in a large series of invasive cervical cancers. Papillomavirus Res.
4:85–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coutant C, Barranger E, Cortez A, Dabit D,
Uzan S, Bernaudin JF and Darai E: Frequency and prognostic
significance of HPV DNA in sentinel lymph nodes of patients with
cervical cancer. Ann Oncol. 18:1513–1517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slama J, Drazdakova M, Dundr P, Fischerova
D, Zikan M, Pinkavova I, Freitag P, Fanta M, Kuzel D, Zima T and
Cibula D: High-risk human papillomavirus DNA in paraaortic lymph
nodes in advanced stages of cervical carcinoma. J Clin Virol.
50:46–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dürst M, Hoyer H, Altgassen C, Greinke C,
Häfner N, Fishta A, Gajda M, Mahnert U, Hillemanns P, Dimpfl T, et
al: Prognostic value of HPV-mRNA in sentinel lymph nodes of
cervical cancer patients with pN0-status. Oncotarget.
6:23015–23025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: FIGO Cancer Report 2018. Int J Gynecol Obstet.
143 (Suppl 2):S22–S36. 2018. View Article : Google Scholar
|
|
15
|
Kawase J, Asakura H, Kurosaki M, Oshiro H,
Etoh Y, Ikeda T, Watahiki M, Kameyama M, Hayashi F, Kawakami Y, et
al: Rapid and accurate diagnosis based on real-time PCR cycle
threshold value for the identification of campylobacter jejuni,
astA gene-positive escherichia coli, and eae gene-positive E. coli.
Jpn J Infect Dis. 71:79–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cibula D, Zikana M, Slama J, Fischerova D,
Kocian R, Germanova A, Burgetova A, Dusek L, Dundr P, Gregova M and
Nemejcova K: Risk of micrometastases in non-sentinel pelvic lymph
nodes in cervical cancer. Gynecol Oncol. 143:83–86. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wiebe E, Denny L and Thomas G: Cancer of
the cervix uteri. Int J Gynaecol Obstet. 119 (Suppl 2):S100–S109.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 (Suppl 1):S2–S23. 2015. View Article : Google Scholar
|
|
19
|
Biesaga B, Janecka A, Mucha-Małecka A,
Adamczyk A, Szostek S, Słonina D, Halaszka K and Przewoźnik M:
HPV16 detection by qPCR method in relation to quantity and quality
of DNA extracted from archival formalin fixed and paraffin embedded
head and neck cancer tissues by three commercially available kits.
J Virol Methods. 236:157–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schroeder L, Boscolo-Rizzo P, Dal Cin E,
Romeo S, Baboci L, Dyckhoff G, Hess J, Lucena-Porcel C, Byl A,
Becker N, et al: Human papillomavirus as prognostic marker with
rising prevalence in neck squamous cell carcinoma of unknown
primary: A retrospective multicentre study. Eur J Cancer. 74:73–81.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuglsang K, Blaakaer J, Petersen LK,
Mejlgaard E, Hammer A and Steiniche T: Detection of high-risk human
papillomavirus DNA in tissue from primary cervical cancer tumor,
pelvic lymph nodes and recurrent disease. Papillomavirus Res.
7:15–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cancer Genome Atlas Research Network;
Albert Einstein College of Medicine; Analytical Biological
Services; Barretos Cancer Hospital; Baylor College of Medicine;
Beckman Research Institute of City of Hope; Buck Institute for
Research on Aging; Canada's Michael Smith Genome Sciences Centre;
Harvard Medical School; Helen F. Graham Cancer Center &Research
Institute at Christiana Care Health Services et al, . Integrated
genomic and molecular characterization of cervical cancer. Nature.
543:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arbyn M, Tommasino M, Depuydt C and
Dillner J: Are 20 human papillomavirus types causing cervical
cancer? J Pathol. 234:431–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hopenhayn C, Christian A, Christian WJ,
Watson M, Unger ER, Lynch CF, Peters ES, Wilkinson EJ, Huang Y,
Copeland G, et al: Prevalence of human papillomavirus types in
invasive cervical cancers from 7 US cancer registries before
vaccine introduction. J Low Genit Tract Dis. 18:182–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mbatani N, Adams T, Wijk LV, Behrens C,
Tam T, Wright T Jr, Stoler M and Denny L: Performance of an human
papillomavirus test in samples from women with
histolopathologically confirmed invasive cervical cancer. J Low
Genit Tract Dis. 20:151–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ronco G, Dillner J, Elfström KM, Tunesi S,
Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi
P, et al: Efficacy of HPV-based screening for prevention of
invasive cervical cancer: Follow-up of four European randomised
controlled trials. Lancet. 383:524–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Armaroli P, Villain P, Suonio E, Almonte
M, Anttila A, Atkin WS, Dean PB, de Koning HJ, Dillner L, Herrero
R, et al: European code against cancer, 4th edition: Cancer
screening. Cancer Epidemiol. 39 (Suppl 1):S139–S152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carter J, Hammond I and Smith M: The
renewal of the national cervical screening program. Med J Aust.
206:2742017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawaya GF, Kulasingam S, Denberg TD and
Qaseem A; Clinical Guidelines Committee of American College of
Physicians, : Cervical cancer screening in average-risk women: Best
practice advice from the clinical guidelines committee of the
American college of physicians. Ann Intern Med. 162:851–859. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Liu D, Lin B, Hao Y, Zhou D, Qi Y
and Zhang S: Expression of high-risk HPV DNA and CK19 in pelvic
lymph nodes in stage Ia-IIa cervical cancer and their clinical
value. Oncol Rep. 27:1801–1806. 2012.PubMed/NCBI
|
|
31
|
Lu Y, Wei JY, Yao DS, Pan ZM and Yao Y:
Application of carbon nanoparticles in laparoscopic sentinel lymph
node detection in patients with early-stage cervical cancer. PLoS
One. 12:e01838342017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duvlis S, Popovska-Jankovic K, Arsova ZS,
Memeti S, Popeska Z and Plaseska-Karanfilska D: HPV E6/E7 mRNA
versus HPV DNA biomarker in cervical cancer screening of a group of
Macedonian women. J Med Virol. 87:1578–1586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lancaster WD, Castellano C, Santos C,
Delgado G, Kurman RJ and Jenson AB: Human papillomavirus
deoxyribonucleic acid in cervical carcinoma from primary and
metastatic sites. Am J Obstet Gynecol. 154:115–119. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noventa M, Ancona E, Cosmi E, Saccardi C,
Litta P, D'Antona D, Nardelli GB and Gizzo S: Usefulness, methods
and rationale of lymph nodes HPV-DNA investigation in estimating
risk of early stage cervical cancer recurrence: A systematic
literature review. Clin Exp Metastasis. 31:853–867. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tortora M, Annunziata C, Liguori G, Losito
S, Botti G, Greggi S, Buonaguro L, Buonaguro FM and Tornesello ML:
Detection of human papillomavirus DNA in peri-tumor tissues and
pelvic lymph nodes as potential molecular marker of micrometastasis
in cervical cancer. Infect Agent Cancer. 11:222016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Köhler C, Le X, Dogan NU, Pfiffer T,
Schneider A, Marnitz S, Bertolini J and Favero G: Molecular
diagnosis for nodal metastasis in endoscopically managed cervical
cancer: The accuracy of the APTIMA test to detect high-risk human
papillomavirus messenger RNA in sentinel lymph nodes. J Minim
Invasive Gynecol. 23:748–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lillo F, Galli L, Lodini S, Taccagni G,
Ferrari A and Origoni M: Extralesional detection and load of human
papillomavirus DNA: A possible marker of preclinical tumor spread
in cervical cancer. J Low Genit Tract Dis. 12:204–209. 2008.
View Article : Google Scholar : PubMed/NCBI
|