Introduction
Leukemia is a malignancy affecting leukocytes and
can be subdivided into myeloid and lymphocytic leukemia. The
mortality rate of chronic myeloid leukemia has reduced since
imatinib methylate was approved as the treatment. However, total
acute myeloid leukemia-associated mortalities have continued to
rise over the past 20 years (1).
Numerous furoquinoline alkaloids have been identified and studied,
including dictamnine, confusameline, skimmianine and kokusaginine,
all of which belong to the Rutaceae family (2). The majority of furoquinoline alkaloids
influence multiple biological processes, such as antifungal
activity (3), antiplatelet
aggregation (4) and Ca2+
influx suppression (5). Acrophylline
and acrophyllidine have structures that are similar to
furoquinolone, and can be isolated from Acronychia, Dictamnus,
Ptelea, Glycosmis and Ruta plants (6), which exhibit antimicrobial and
anticancer activities (7,8). Acrophyllidine and its synthetic
derivatives also exhibit significant anti-allergic activity via
suppressing mast cell degranulation (9). Moreover, ethyl
2-(3-hydroxyanilino)-4-oxo-4,5-dihydrofuran-3-carboxylate, an
intermediate in furoquinolone synthesis, exhibits anti-inflammatory
activity (10) and ethyl
2-[N-p-chlorobenzyl-(2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01007) has also
been revealed to induce apoptosis in mouse leukemia (WEHI-3) and
human cervical cancer (CaSki) cell lines (11,12).
Notably, JOT01006 activates BCL2 antagonist/killer 1,
poly(ADP-ribose) polymerase 1 and caspase-3, resulting in apoptosis
and inhibiting the migration of human cervical cancer HeLa cells
(13). However, anticancer activity
of the intermediates in furoquinolone synthesis is rarely reported
in treating acute myeloid leukemia.
The present study aimed to characterize the
anti-proliferative and apoptotic activity of ethyl
2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (compound 131) in
acute promyelocytic leukemia HL-60 cells. The current results
indicate that compound 131 induces apoptosis in HL-60 cells, and
this was associated with increased intracellular Ca2+,
increased reactive oxygen species (ROS), activation of caspase-3
and a decrease in mitochondrial membrane potential. Hence, compound
131 may represent a novel target for treating acute promyelocytic
leukemia.
Materials and methods
Cells
Human promyelocytic leukemia HL-60 and plasma cell
leukemia ARH-77 cells (Bioresource Collection and Research Centre)
were cultured in RPMI-1640 medium plus 10% fetal bovine serum (FBS)
(Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C and 5% CO2. Vero cells (a monkey
kidney epithelial cell line) (Bioresource Collection and Research
Centre) were cultivated in Eagle's Minimum Essential Medium (Thermo
Fisher Scientific, Inc.) containing 10% FBS, 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C, 5% CO2.
Reagents
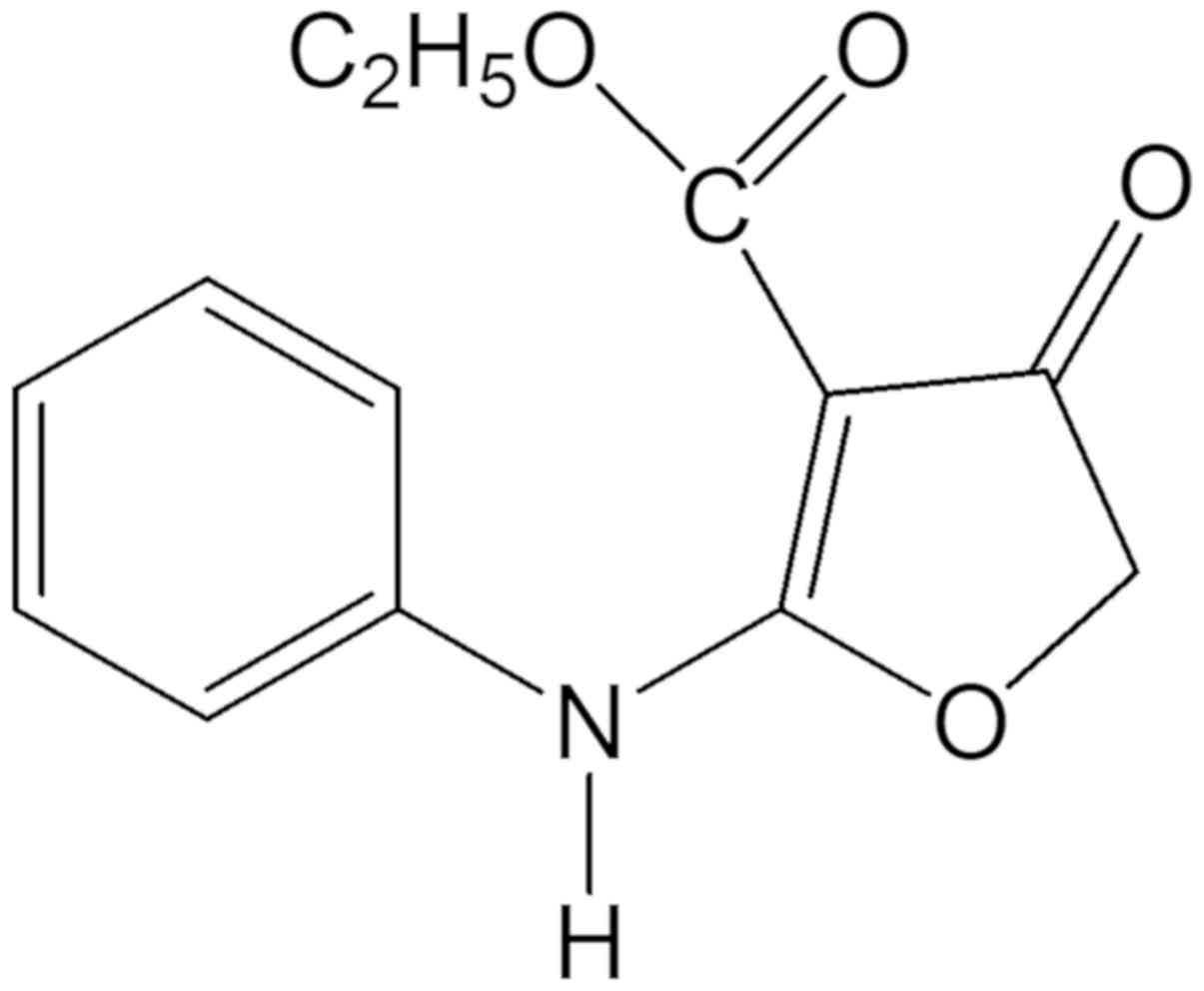
Ethyl 2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate
(compound 131) was manufactured and refined using high-performance
liquid chromatography as described in a previous study (10). Briefly, a mixture of diethyl malonate
(32.0 g, 0.2 mol) in 50 ml tetrahydrofuran (THF) with chloroacetyl
chloride (11.3 g, 0.1 mol) in 100 ml THF was incubated at 10–12°C
for 1 h followed by 40–45°C for 1 h; ethyl
2-ethoxy-4-oxo-4,5-dihydrofuran-3-carboxylate was produced post
cooling. Finally, the ethoxy group in the compound was substituted
with aniline after stirring at room temperature for 1 h and heating
on a water bath at 80°C for 3 h to yield ethyl
2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (compound 131).
After the product of the reaction had been confirmed by performing
via thin layer chromatography on silica gel-protected aluminum
sheets (Type 60 F254; Merck KGaA) in which the spots were detected
using a UV-lamp, the reaction was further mixed with 100 cc of ice
water to form a precipitate; white crystals of compound 131 (18.29
g; yield, 74%; melting point, 115–116°C) were generated after the
precipitate was recrystallized from 90% ethanol at room temperature
°C for 1–2 days. The structure of compound 131 (Fig. 1) was confirmed via mass spectrometry
(m/z) as follows: 3267.87 (-NH-), 1695.26 (C4=O), 1672.59
(C3-CO-OEt); UV λmax nm (MeOH) (log ε): 297 (4.523); 1H-NMR (200
MHz, CDCl3) δ: 1.24 (3H, t, J=7 Hz, H-2″), 4.20
(2H, q, J=7 Hz, H-1″), 4.67 (2H, s, H-5), 7.25 (5H,
m, H-2′, H-3′, H-4′, H-5′, H-6′), 10.264 (1H,
s,-NH-); 13C-NMR (200 MHz, DMSO-d6) δ: 14.67 (C-2″), 59.37
(C-1″), 75.30 (C-5), 86.99 (C-3), 123.48 (C-2′, C-6′), 126.35
(C-4′), 129.27 (C-3′, C-5′), 135.24 (C-1′), 164.18 (C-2), 177.34
(C-3″), 188.84 (C-4).
MTT assay
HL-60, ARH-77 or Vero cells (1×104/well)
were cultured in 96-well plates in the presence or absence of
compound 131 (0, 5, 25 and 50 µM) at 37°C for 2 days followed by
the addition of 20 µl 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) per
well for another 4-h incubation at 37°C. After removing the
cultured media, the reduced form of MTT in the cells was
solubilized using 150 µl dimethyl sulfoxide for 15 min. The
survival rate of treated cells was measured as the ratio of the
optical density (OD)(570–630 nm) of treated cells to
that of mock cells, similar to the calculation of the 50% cytotoxic
concentration (CC50).
Cell cycle analysis and caspase-3
fluorimetric assay
A total of 2×105 HL-60 cells were treated
with compound 131 (0, 5, 25 or 50 µM) for 48 h at 37°C.
Subsequently, cells were collected after centrifugation at 100 × g
(Kubota Corporation) for 5 min at room temperature and cell cycle
profile analysis was performed using propidium iodide (PI) staining
and caspase-3 activity analysis using BD ApoAlert Caspase
Fluorescent assay kit (cat. no. 51-6632AK and 51-6632BK; BD
Biosciences) as described in our previous report (14,15).
PI-stained cells were analyzed at a wavelength of 488 nm by flow
cytometry (BD Biosciences). The supernatants of treated cell
lysates were added to the wells of the caspase-3 assay with BD
ApoAlert Caspase Fluorescent assay kit for a 2-h incubation at 37°C
according to the manufacturer's protocol. Subsequently, caspase-3
activity was examined using a fluorescent substrate and a
fluorescent plate reader (BioTek China) using an excitation
wavelength of 380 nm and an emission wavelength of 460 nm.
Western blotting assay
The cells treated with compound 131 were harvested
and washed with ice-cold PBS by centrifuging at 100 × g (Kubota
Corporation) for 5 min at 4°C, and then mixed with the RIPA lysis
buffer (cat. no. R0278, Sigma-Aldrich; Merck KGaA) in microfuge
tubes for 30 min at 4°C. The lysate of treated cells was collected
after centrifugation at 12000 × g for 20 min at 4°C, and the
protein concentration was determined using coomassie protein assay
reagent (cat. no. 27813, Sigma-Aldrich; Merck KgaA) at an
absorbance of 280 nm. A total of 20 µg of lysate per lane from the
cells treated with compound 131 was dissolved in 2X SDS-PAGE sample
buffer (Sigma-Aldrich; Merck KGaA), boiled at 95°C for 5 min,
loaded in 12% SDS-PAGE gels and then analyzed using a vertical
electrophoresis system (Bio-Rad Laboratories, Inc.). The separated
proteins in the gels were transferred to nitrocellulose papers, and
were blocked with 5% skim milk at room temperature overnight. The
immune reaction on the blots was performed using a 1:2,000 dilution
of anti-caspase-3 (cat. no. 9662), anti-Bax (cat. no. 2772),
anti-Bcl-2 (cat. no. 3498) and anti-β-actin (cat. no. 4970)
antibodies in at room temperature overnight, and 1:3,000 dilution
of horseradish peroxidase-conjugated goat anti-mouse IgG antibodies
(cat. no. 7076) (Cell Signaling Technology, Inc.) at room
temperature for 2 h. The immune-reactive bands were developed using
enhanced chemiluminescence solution (Amersham Pharmacia Biotech
Ltd.), as previously described (14,15).
Cytoplasmic-free Ca2+,
intracellular ROS and mitochondrial membrane potential
(ΔΨm) assays using flow cytometry
To detect intracellular calcium levels, treated
cells were collected and incubated with 10 µM Fluo-3/AM for 30 min
at 37°C in the dark and analyzed at 526 nm by flow cytometry
(16). In the intracellular ROS
assay, cells stained with 2,7-dichlorodihydrofluorescein diacetate
(DCFH-DA) were examined using flow cytometry as described in our
previous study (17). To measure the
ΔΨm, compound 131-treated cells were analyzed with
DiOC6 staining at 37°C for 30 min and examined using
flow cytometry (17).
Statistical analysis
Data from three independent experiments of mock
cells and compound 131-treated cells were analyzed by one-way ANOVA
followed by Scheffe's post hoc test using SPSS 12.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with compound 131 results in
cell growth inhibition and apoptosis in HL-60 cells
Initially, 23 synthesized intermediates of
furoquinolone at 50 µM were screened for anti-proliferative
activity against HL-60 and Vero cells; only compound 131 (ethyl
2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate) exhibited a
significant inhibitory effect on the proliferation of HL-60 (but
not Vero cells) (Fig. S1).
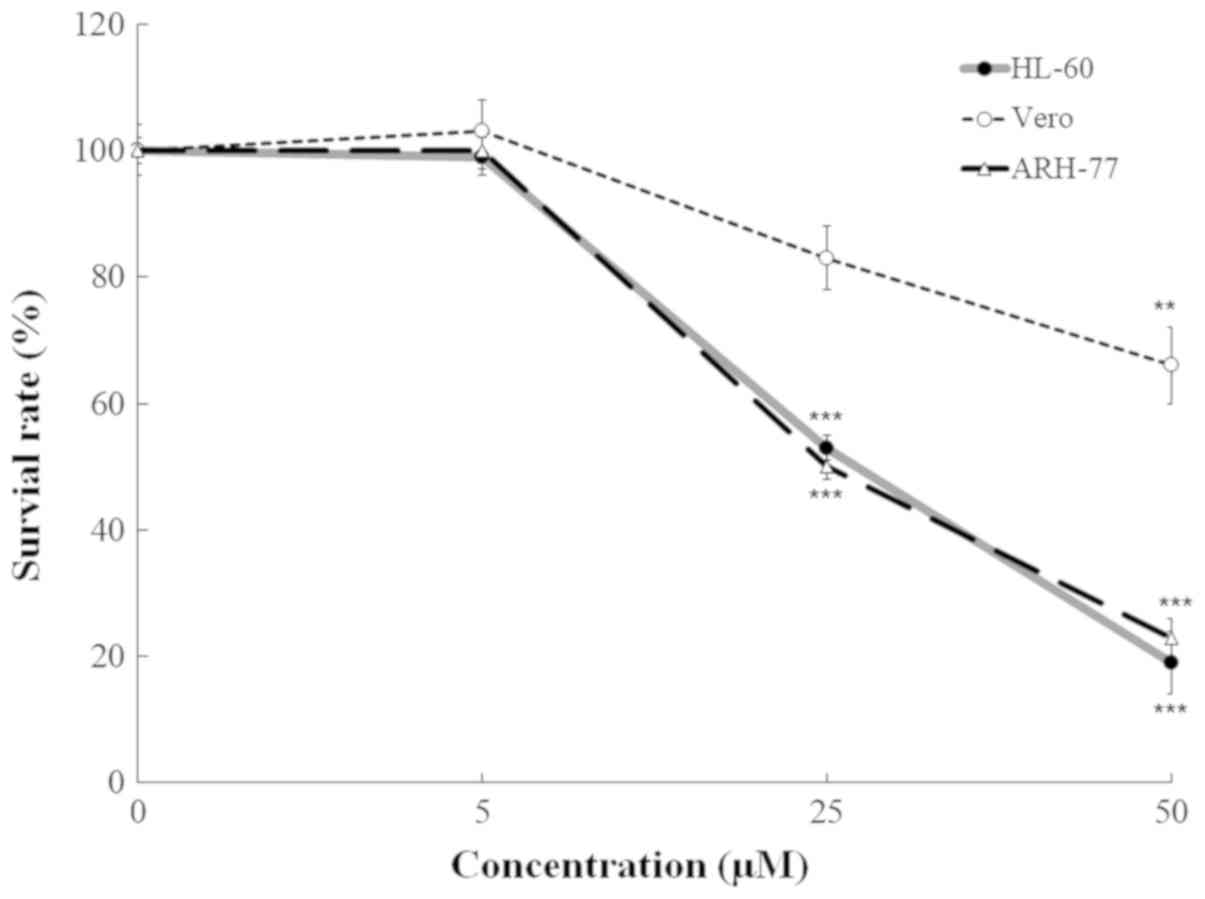
Subsequently, the cells were treated with compound 131 at 0, 5, 25
and 50 µM for 48 h at 37°C; the cell viability was tested using the
MTT assay to determine the CC50 values of compound 131
on the proliferation of HL-60, ARH-77 and Vero cells. The survival
rates of HL-60 and ARH-77 cells treated with compound 131 were
significantly lower compared with treated Vero cells (Fig. 2). The CC50 values of
compound 131 were 23.5 µM for HL-60, 24.2 µM for ARH-77 and 87.0 µM
for Vero cells. These results demonstrated that compound 131
exhibits significant anti-proliferative effects against HL60 and
ARH-77 cells.
Activation of caspase-3 in HL-60 cells
following treatment with compound 131
To determine whether compound 131 initiated
apoptosis, the cell cycle and caspase-3 activity of treated cells
were further analyzed using flow cytometry, western blotting and
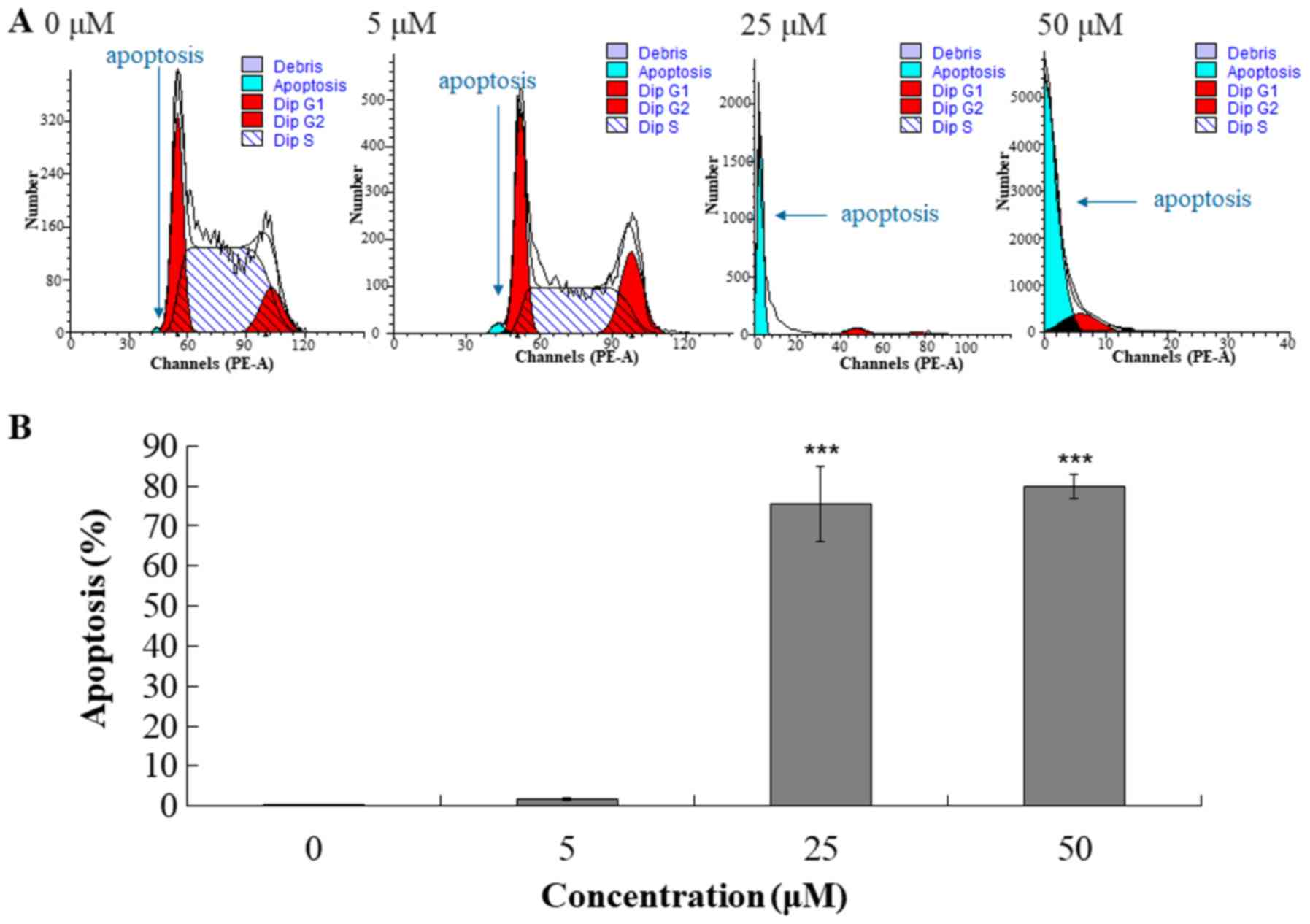
caspase-3 enzymatic activity assays (Figs. 3 and 4). Cell cycle analysis indicated that
compound 131 increased the percentage of cells in the
sub-G1 phase (apoptotic fraction) in a
concentration-dependent manner in HL-60 cells (Fig. 3A). The percentage of apoptotic cells
(sub-G1 fraction) was 0.2, 1.6, 75.4 and 80.0% in cells
treated with compound 131 at 0, 5, 25 and 50 µM, respectively
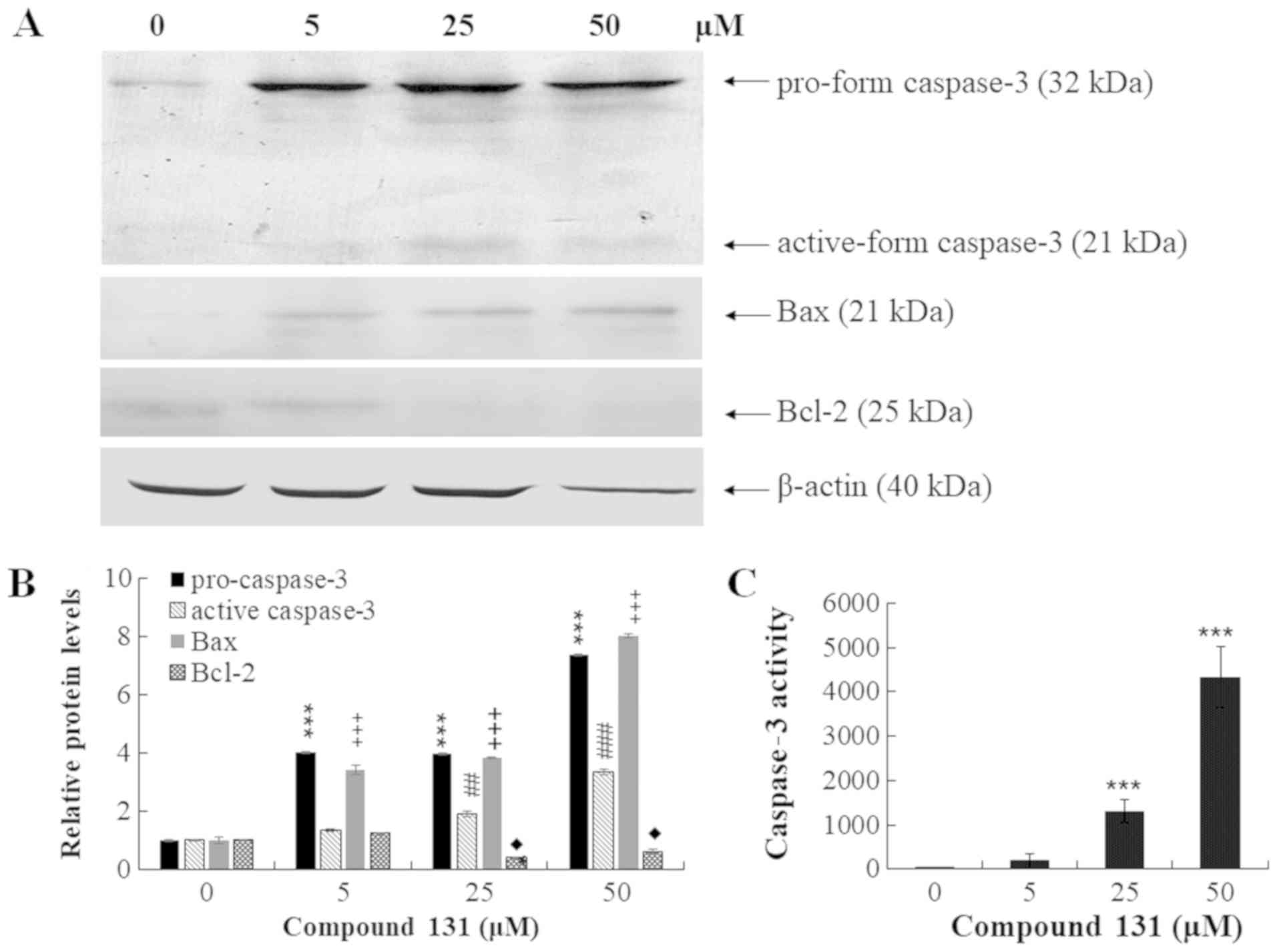
(Fig. 3B). Moreover, western blot
analysis of cell lysates indicated that compound 131 treatment
increased the protein levels of pro- and active forms of caspase-3,
and also upregulated Bax and downregulated Bcl-2 in a
concentration-dependent manner (Fig. 4A
and B). Fluorescence assays of caspase-3 enzymatic activity
revealed that caspase-3 enzymatic activity in compound 131-treated
cells was higher compared with mock cells by 6-, 43- and 140-fold
for 5, 25 and 50 µM, respectively (Fig.
4C). The current results indicate that compound 131
significantly promotes caspase-3 and Bax activation, but suppresses
Bcl-2 expression, in apoptotic cells.
Treatment with compound 131 results in
increases of intracellular calcium and ROS in HL-60 cells
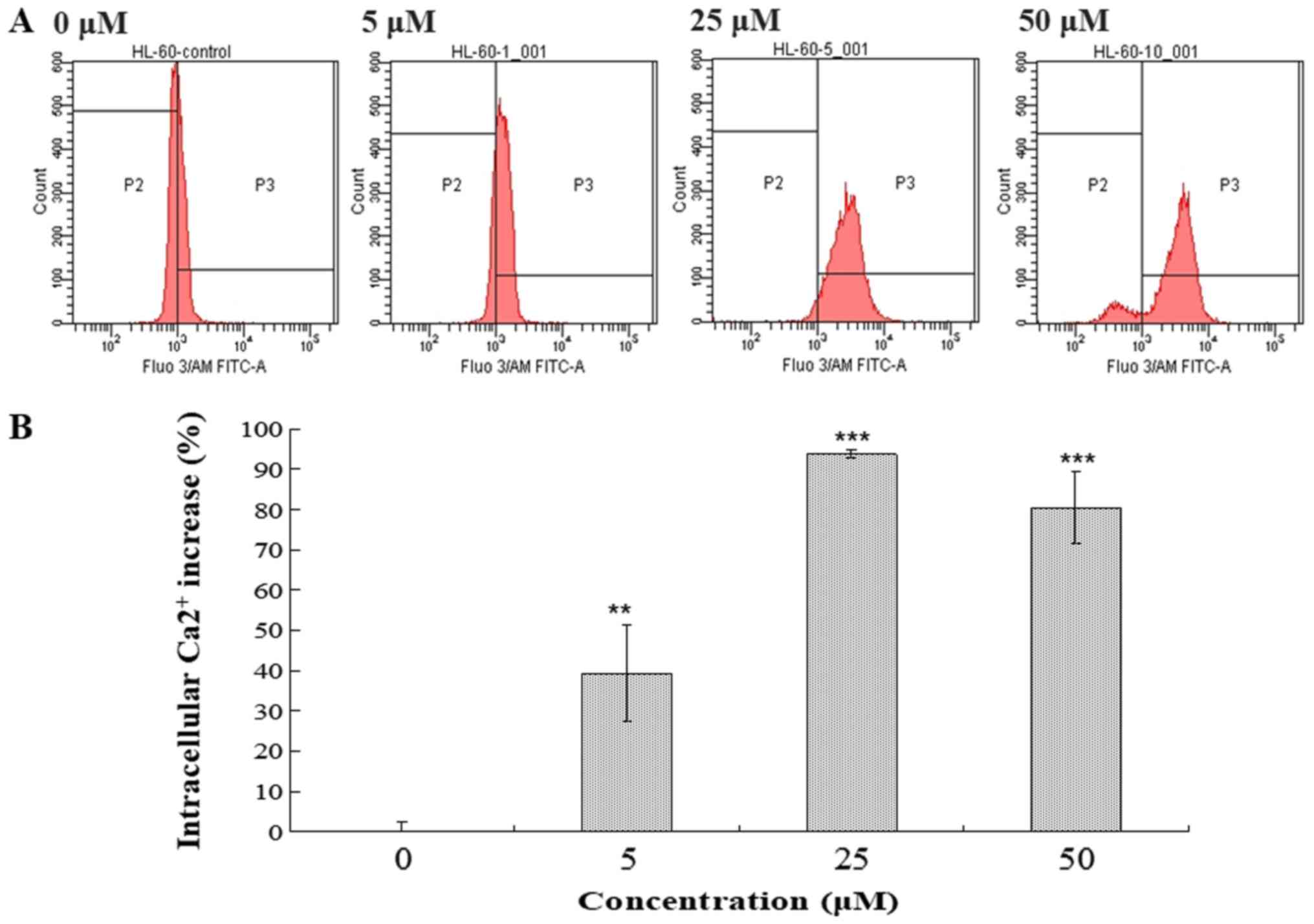
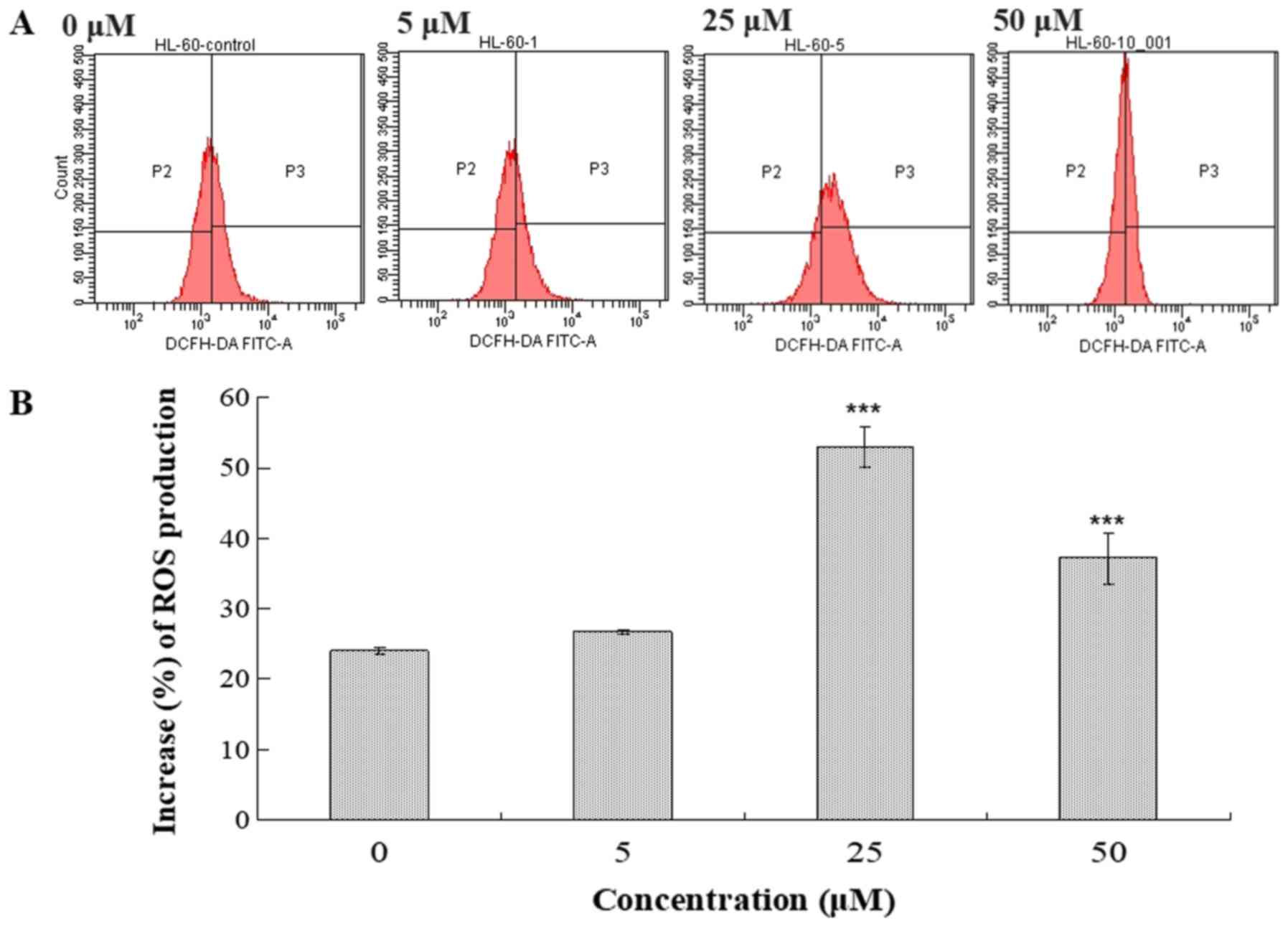
Intracellular Ca2+ accumulation and ROS
generation serve a critical role in apoptosis (17–20). The
effects of compound 131 on intracellular Ca2+ and ROS
levels in HL-60 cells were examined (Figs. 5 and 6). Following treatment with compound 131
(0, 5, 25 or 50 µM) at 37°C for 48 h, cells were harvested, stained
with Fluo-3/AM or DCFH-DA and analyzed using flow cytometry.
Compound 131 treatment resulted in the increase of intracellular
Ca2+ release from the ER in HL-60 cells. A 39.2%
increase resulted from treatment with 5 µM, a 93.8% increase from
treatment with 25 µM and an 80.4% increase following treatment with
50 µM compared with PBS-treated cells, respectively (Fig. 5). In addition, compound 131
significantly increased the production of intracellular ROS
compared with mock cells (Fig. 6).
The results indicate that compound 131 treatment significantly
stimulates an increase in intracellular Ca2+ and ROS
levels in HL-60 cells.
Treatment with compound 131 results in
a decrease of mitochondrial membrane potential in HL-60 cells
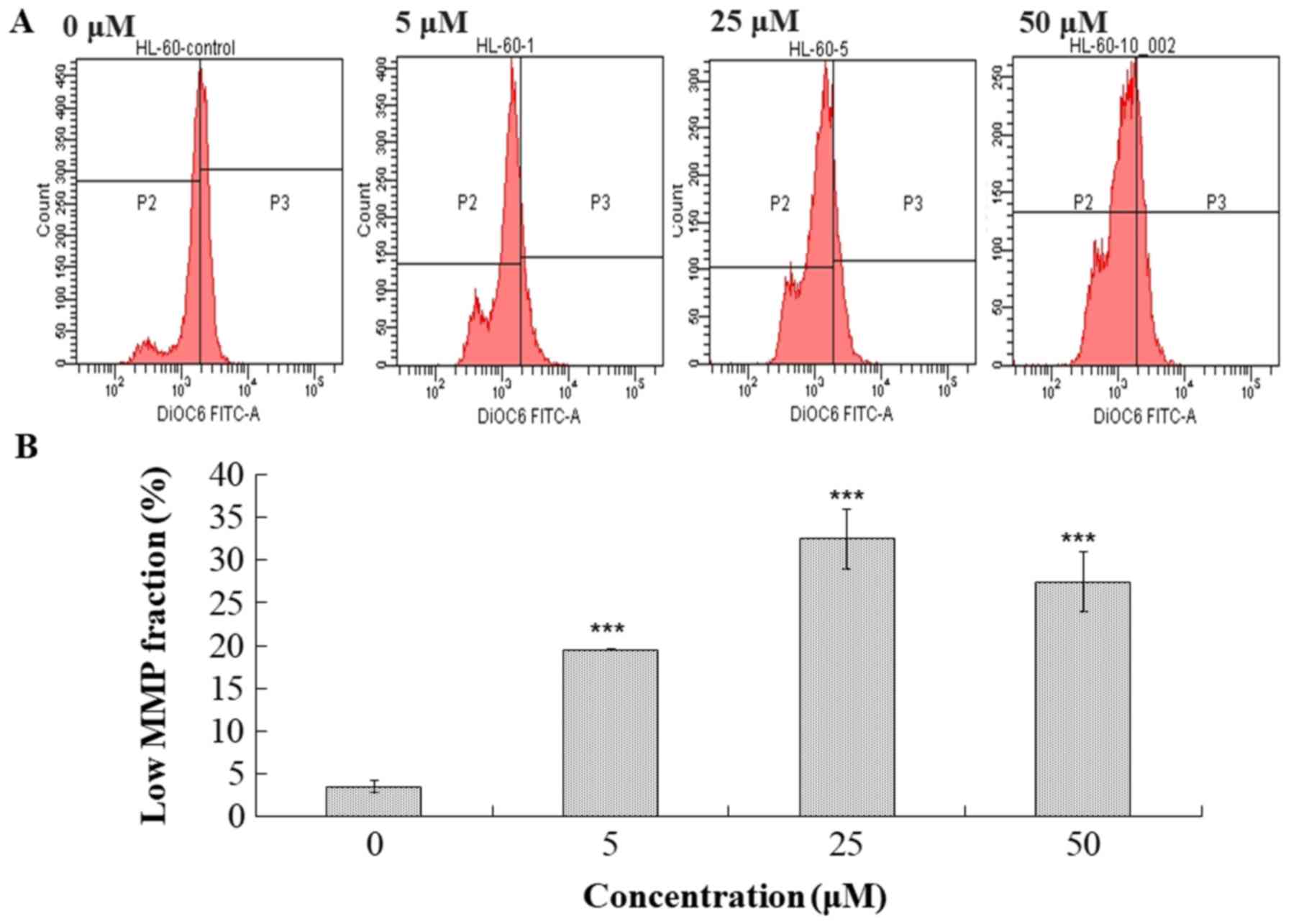
The disruption of mitochondrial membrane potential
(MMP) has been reported to correlate with ROS generation (21–23).
Treated cells were tested to determine the MMP levels by staining
with DiOC6 and analysis using flow cytometry (Fig. 7). Cells treated with compound 131
exhibited a significant decrease in DiOC6 intensity by
19.6% for 5 µM, 32.5% for 25 µM and 27.45% for 50 µM. Thus, it was
revealed that treatment with compound 131 also resulted in a
decrease in MMP in HL-60 cells.
Discussion
To the best of our knowledge, this is the first
report to demonstrate that ethyl
2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (compound 131), an
intermediate of furoquinoline synthesis, exerts anti-leukemic
effects and induces apoptosis via the upregulation of caspase-3 and
Bax in HL-60 cells. These effects are also associated with an
increase in intracellular calcium, an increase in ROS levels and a
decrease in MMP expression. The mechanism of compound 131-induced
apoptosis was similar to previous reports of apoptosis induced by
4,5-dihydrofuran-3-carboxylate derivatives (11,12). In
addition, a structure-function association study demonstrated that
ethyl-2-(3-methoxyanilino)-4-oxo-4,5-dihyfrofuran-3-carboxylate and
ethyl-2-(3-oxyanilino)-4-oxo-4,5-dihyfrofuran-3-carboxylate
exhibited lower anti-proliferative properties than compound 131
(ethyl 2-anilino-4-oxo-4,5-dihydrofuran-3-carboxylate) (data not
shown). The aforementioned result indicates that the aniline group
in compound 131 serves a key role in its anti-proliferative
activity against HL60 cells. Ethyl 2-[N-p-chlorobenzyl-(2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01007) triggered
the mitochondria-dependent pathway, which was significantly
correlated with cytoplasmic Ca2+ levels in human
cervical CaSki cancer cell apoptosis (11). Moreover, ethyl
2-[N-m-chlorobenzyl-(2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01006) induced the
increase in ROS production that resulted in the caspase-dependent
apoptosis of human cervical cancer cells (13). In addition to anticancer activity,
the intermediates of furoquinoline synthesis and furoquinoline
derivatives have been revealed to exhibit diverse pharmacological
effects, such as 5-HT2 receptor antagonist activity (24), vasorelaxation via the suppression of
calcium influx (25), anti-allergic
effects (9) and the blocking of
outward K+ current and Na+ channels (5). A previous study revealed that the
intermediates of furoquinoline synthesis exhibited moderate
inhibitory effects on the growth of human ovarian cancer A2780
cells (26).
In summary, the present results indicated that
compound 131 inhibits the proliferation and induces apoptosis in
HL-60 cells; moreover, it was associated with the production of
intracellular Ca2+ and ROS, and reduced the
mitochondrial membrane potential. Compound 131, a novel
4,5-dihydrofuran-3-carboxylate, represents a promising compound
that may inform the development of new anti-leukemia agents. The
current results also revealed the biological properties of compound
131 and indicated the mechanisms underlying its anti-leukemia
effects.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding was received from the China Medical
University (Taichung, Taiwan) under the Featured Areas Research
Center Program within the framework of the Higher Education Sprout
Project by the Ministry of Education (grant nos. CHM106-6-2 and
CMRC-CHM-2). The present study was also sponsored by grants from
the Ministry of Science and Technology, Taiwan (grant nos.
MOST107-2923-B-039-001-MY3 and MOST108-2320-B-039-039-MY3) and
China Medical University (grant nos. CMU106-BC-1, CMU106-ASIA-06,
CMU107-S-14 and CMU107-ASIA-12).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
ACH, CSL, JCL, HCL, WHL and CWL designed and
conducted the experiments. ACH, CSL, and JCL performed data
analysis. ACH and CWL wrote the first draft of the manuscript. All
authors reviewed and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao T, Li-Talley M, Buck A and Chen W: An
emerging trend of rapid increase of leukemia but not all cancers in
the aging population in the United States. Sci Rep. 9:120702019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tarus PK, Coombes PH, Crouch NR,
Mulholland DA and Moodley B: Furoquinoline alkaloids from the
southern African Rutaceae Teclea natalensis. Phytochemistry.
66:703–706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao W, Wolfender JL, Hostettmann K, Xu R
and Qin G: Antifungal alkaloids and limonoid derivatives from
Dictamnus dasycarpus. Phytochemistry. 47:7–11. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen KS, Chang YL, Teng CM, Chen CF and Wu
YC: Furoquinolines with antiplatelet aggregation activity from
leaves of Melicope confusa. Planta Med. 66:80–81. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su MJ, Chang GJ, Wu MH and Kuo SC:
Electrophysiological basis for the antiarrhythmic action and
positive inotropy of HA-7, a furoquinoline alkaloid derivative, in
rat heart. Br J Pharmacol. 122:1285–1298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lahey FN and McCamish M: Acrophylline and
acrophyllidine. Two new alkaloids from Acronychia
haplophylla. Tetrahedron Lett. 12:1525–1527. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao YL, Chen YL, Sheu JY, Chen IL, Wang
TC and Tzeng CC: Synthesis and antimycobacterial evaluation of
certain fluoroquinolone derivatives. Bioorg Med Chem. 13:3921–3926.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sheu JY, Chen YL, Tzeng CC, Hsu SL, Fang
KC and Wang TC: Synthesis, and antimycobacterial and cytotoxic
evaluation of certain fluoroquinolone derivatives. Helv Chim Acta.
86:2481–2489. 2003. View Article : Google Scholar
|
|
9
|
Huang AC, Lin TP, Kuo SC and Wang JP: The
antiallergic activities of synthetic acrophylline and
acrophyllidine. J Nat Prod. 58:117–120. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang JP, Tsao LT, Raung SL, Hsu MF and Kuo
SC: Inhibition by HAJ11 of respiratory burst in neutrophils and the
involvement of protein tyrosine phosphorylation and phospholipase D
activation. Br J Pharmacol. 120:79–87. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang AC, Chung JG, Kuo SC, Lu HF and Lin
TP: Synthesis and cytotoxic activity of certain
2,3,4,9-tetrahydrofuro [2,3-b] quinolin-3,4-dione and ethyl
2-(substituted aniline) −4-oxo-4,5-dihydrofuran-3-carboxylate
derivatives in murine leukemia WEHI-3 cells. In Vivo. 21:227–236.
2007.PubMed/NCBI
|
|
12
|
Lin TP, Huang AC, Wei HC, Lin JG and Chung
JG: Ethyl 2- [N-p-chlorobenzyl- (2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01007) induces
apoptosis in human cervical cancer Ca Ski cells. In Vivo.
21:397–406. 2007.PubMed/NCBI
|
|
13
|
Huang AC, Lin TP, Weng YS, Ho YT, Lin HJ,
Huang LJ, Kuo SC and Chung JG: Ethyl
2-[N-m-chlorobenzyl-(2′-methyl)]
anilino-4-oxo-4,5-dihydrofuran-3-carboxylate (JOT01006) induces
apoptosis in human cervical cancer HeLa cells. Anticancer Res.
27:2505–2514. 2007.PubMed/NCBI
|
|
14
|
Yang TC, Shiu SL, Chuang PH, Lin YJ, Wan
L, Lan YC and Lin CW: Japanese encephalitis virus NS2B-NS3 protease
induces caspase 3 activation and mitochondria-mediated apoptosis in
human medulloblastoma cells. Virus Res. 143:77–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang TC, Lai CC, Shiu SL, Chuang PH, Tzou
BC, Lin YY, Tsai FJ and Lin CW: Japanese encephalitis virus
down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38
MAPK activation in human promonocyte cells. Microbes Infect.
12:643–651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Viarengo A, Cenesi L, Moore MN and Orunesu
M: Effects of Hg2+ and Cu2+ on the cytosolic
Ca2+ level in molluscan blood cells evaluated by
confocal microscopy and spectrofluorimetry. Marine Biol.
119:557–564. 1994. View Article : Google Scholar
|
|
17
|
Lin SS, Huang HP, Yang JS, Wu JY, Hsai TC,
Lin CC, Lin CW, Kuo CL, Gibson Wood W and Chung JG: DNA damage and
endoplasmic reticulum stress mediated curcumin-induced cell cycle
arrest and apoptosis in human lung carcinoma A-549 cells through
the activation caspases cascade- and mitochondrial-dependent
pathway. Cancer Lett. 272:77–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattson MP and Chan SL: Neuronal and glial
calcium signaling in Alzheimer's disease. Cell Calcium. 34:385–397.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benali-Furet NL, Chami M, Houel L, De
Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R,
Ichas F, Rizzuto R and Paterlini-Bréchot P: Hepatitis C virus core
triggers apoptosis in liver cells by inducing ER stress and ER
calcium depletion. Oncogene. 24:4921–4933. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haynes CM, Titus EA and Cooper AA:
Degradation of misfolded proteins prevents ER-derived oxidative
stress and cell death. Mol Cell. 15:767–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue X, Piao JH, Nakajima A, Sakon-Komazawa
S, Kojima Y, Mori K, Yagita H, Okumura K, Harding H and Nakano H:
Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein
response (UPR) in a reactive oxygen species (ROS)-dependent
fashion, and the UPR counteracts ROS accumulation by TNFalpha. J
Biol Chem. 280:33917–33925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kadenbach B: Intrinsic and extrinsic
uncoupling of oxidative phosphorylation. Biochim Biophys Acta.
1604:77–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rego AC and Oliveira CR: Mitochondrial
dysfunction and reactive oxygen species in excitotoxicity and
apoptosis: Implications for the pathogenesis of neurodegenerative
diseases. Neurochem Res. 28:1563–1574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng JT, Chang TK and Chen IS:
Skimmianine and related furoquinolines function as antagonists of
5-hydroxytryptamine receptors in animals. J Auton Pharmacol.
14:365–374. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu SM, Ko FN, Su MJ, Wu TS, Wang ML, Huang
TF and Teng CM: Vasorelaxing effect in rat thoracic aorta caused by
fraxinellone and dictamine isolated from the Chinese herb
Dictamus dasycarpus Turcz: Comparison with cromakalim and
Ca2+ channel blockers. Naunyn Schmiedebergs Arch
Pharmacol. 345:349–355. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao S, Al-Rehaily AJ, Brodie P, Wisse JH,
Moniz E, Malone S and Kingston DG: Furquinoline alkaloids of
Ertela (Monnieria) trifolia (L.) Kuntze from the Suriname
rainforest. Phytochemistry. 69:553–557. 2008. View Article : Google Scholar : PubMed/NCBI
|