Introduction
Exercise is recommended for patients with various
diseases including cancer (1,2). Walking
as well as resistance training has been reported to improve
physical function and skeletal muscle mass in patients with cancer
(3,4). In accordance with the American College
of Sports Medicine guidelines, exercise training is a key
recommendation to maintain activity even in cancer patients
(5). Cancer rehabilitation (CR), a
new multidisciplinary intervention for cancer patients, consists of
nutritional and physical therapy. CR improves fatigue, pain,
physical function, and quality of life in patients with cancer
(6).
Sarcopenia is defined as loss of skeletal muscle
mass and function. Sarcopenia frequently occurs in patients with
chronic liver disease (CLD) regardless of its etiology (7,8).
Sarcopenia in patients with CLD is associated with a decline in
their quality of life and physical activity (9). Sarcopenia is also significantly
associated with hepatic fibrosis (10). Moreover, sarcopenia is an independent
prognostic factor for hepatocellular carcinoma (HCC) (11). Taken together, it is important to pay
attention to sarcopenia in patients with CLD.
In patients with cirrhosis and esophageal varices,
moderate-intensity exercise increases portal pressure and may,
therefore, increase the risk of variceal bleeding (12). In addition, moderate-intensity
exercise stimulates the renal vasoconstrictor system, markedly
impairing the renal function in patients with cirrhosis and ascites
(13). In this way, exercise may
exacerbate the general condition of patients of cirrhosis with
complications such as esophageal varices and ascites. However,
Hiraoka et al (14) showed
that branched-chain amino acid (BCAA) supplementation and
low-intensity exercise are effective for improving muscle volume
and strength in patients with CLD. In addition, Locklear et
al (15) demonstrated that, even
in patients with end-stage liver disease, exercise has potential
benefits in terms of endurance and functional outcome measures
without adverse effects. We also previously reported that CR
improved not only physical ability but also muscle mass without
worsening liver function during hospitalization for cancer
treatment in patients with CLD and advanced HCC (16,17).
Thus, CR has beneficial effects on sarcopenia in
patients with CLD and HCC; however, the effects of CR on prognosis
remain unclear in patients with CLD and advanced HCC. This study
aimed to investigate the effects of CR on the prognosis of patients
with CLD and advanced HCC.
Patients and methods
Study design
CR for in-hospital patients with cancer is an
approved health care service covered by health insurance from the
Ministry of Health, Labour and Welfare in Japan. Therefore,
intentionally using a non-exercise control group is contrary to
medical ethics. Thus, we performed a prospective cohort study to
evaluate effects of CR on skeletal muscle mass during
hospitalization for patients with HCC and CLD who underwent
TACE.
Ethics
The study was conducted in accordance with the
ethical guidelines of the Declaration of Helsinki, as reflected in
the prior approval given by the institutional review board of
Kurume University (approval no. 15072). Informed consent was
obtained from an opt-out approach, and personal information was
protected during data collection.
Subjects
A total of 152 consecutive patients with HCC and CLD
from February 2013 to October 2016 participated in this study.
Inclusion criteria were hospitalized patients with HCC and CLD who
(1) were 20 years of age or older,
(2) had a performance status of
grade 0 to 2 as defined by the Eastern Cooperative Oncology Group
(18), and (3) had undergone treatment with TACE.
Exclusion criteria were patients with (1) risk of HCC rupture, (2) history or presence of grade 2–4 hepatic
encephalopathy according to the West Haven Criteria (19), (3)
risk of esophageal or gastric varices rupture, (4) heart failure, or (5) respiratory failure. The exclusion
criteria were assessed upon study enrollment. All patients
underwent upper gastrointestinal endoscopy by gastroenterologists
every 6 to 12 months, and the risk of esophageal or gastric varices
rupture was assessed according to the 2015 evidence-based clinical
practice guidelines for liver cirrhosis from the Japanese Society
of Gastroenterology (20). The
definition of heart failure was based on guidelines from the
American College of Cardiology Foundation/American Heart
Association Task Force and Heart Failure Society of America
(21,22). The definition of respiratory failure
was based on guidelines from the European Respiratory
Society/American Thoracic Society (23,24).
Outpatient doctors and nurses recommended exercise to all patients.
Patients who agreed to exercise were classified into the CR group
(n=85), and those who did not agree to exercise were classified
into the control group (n=67). We classified enrolled patients into
the High SMI or Low SMI group according to the JSH criteria for
sarcopenia (7), and the High VFA or
Low VFA group as previously described (25). We also divided the patients into the
High or Low LDH group and the High total protein group or Low total
protein group based on the reference values of serum LDH and total
protein levels, respectively.
Exercise regimen
To maintain physical ability and prevent sarcopenia,
patients in the CR group were treated with exercise, instructed by
physical therapists certified in the rehabilitation of cancer
patients. The rehabilitation was started on the day following TACE
unless the patient had a fever of 38°C or greater as previously
described (16). The frequency and
duration of exercise performed were recorded during the
hospitalization. After discharge, patients in the CR group were
instructed to continue the exercises by themselves during the
observation period.
According to the guidelines of American College of
Sports Medicine (26), the exercise
consisted of the following 4 types of training (median 2.5
metabolic equivalents/20–40 min/day): 1) stretching, 2) strength
training, 3) balance training, and 4) endurance training (16,17). A
static stretching was conducted for 3–5 min, targeting the
quadriceps femoris muscles, hamstrings, hip adductor muscles, and
gastrocnemius. The static stretch was held to each muscle for 10–20
seconds at the point of feeling tightness (16,25).
Strength training was conducted for 10 min, targeting the
quadriceps femoris muscles, gastrocnemius, and tibialis anterior
muscles. Quadriceps femoris muscle and iliopsoas strength training
were conducted at moderate to high intensity by a hand-held
dynamometer. Gastrocnemius and tibialis anterior muscles were
conducted trained by the patient's weight. One set contained of 10
repetitions, and a maximum of 3 sets were performed (16,25).
Balance training contain the patients practicing a one-leg stance
and tandem stance for 5–10 min. The patient stood on a straight
line and maintain posture in tandem stance training (16,25).
Endurance training contained either ergometer or walking for 10–15
min. The intensity of endurance training was 11–13 points on the
Borg scale, and the target heart rate was set by using the Karvonen
Formula (16,25).
Diagnosis, the Barcelona Clinic Liver
Cancer (BCLC) staging system, tumor node metastasis (TNM) staging,
and treatment of HCC
HCC was diagnosed by a combination of tests for
serum tumor makers, such as alpha-fetoprotein and des-γ-carboxy
prothrombin, and imaging procedures, such as ultrasonography,
computed tomography (CT), magnetic resonance imaging (MRI), and
angiography, or a tumor biopsy (27). We evaluated the clinical stage of HCC
using the Barcelona Clinic Liver Cancer (BCLC) staging system
(27) and TNM staging based on the
Liver Cancer Study Group of Japan criteria (28). We treated the patients according to
clinical practice guidelines for HCC of The Japan Society of
Hepatology (29).
Additional treatment for recurrence of
HCC after initial TACE
When HCC recurred, additional treatment for HCC was
selected based on The Japan Society of Hepatology Consensus-Based
Treatment Algorithm for HCC (28).
Moreover, additional HCC treatment with hepatic arterial infusion
chemotherapy/tyrosine kinase inhibitors was selected when HCC
showed TACE failure/refractoriness defined by any of following
criteria: i) an ineffective response after two or more consecutive
TACE procedures that is evident on response evaluation CT or MRI
after 1–3 months, even after chemotherapeutic agents are changed
and/or the feeding artery is reanalyzed; ii) two or more
consecutive progressions in the liver (including an increase in the
number of tumors compared to that before the previous TACE
procedure), even after changing the chemotherapeutic agents and/or
reanalysis of feeding artery, on response evaluation CT/MRI after
1–3 months following adequately performed selective TACE; iii)
continuous elevation of tumor markers right after TACE even though
transient minor reduction is observed; iv) appearance of vascular
invasion, or v) appearance of extrahepatic spread (28).
Measurement of skeletal muscle index
(SMI) and visceral fat area (VFA)
SMI and VFA were evaluated using CT images obtained
before and after TACE. The first CT images were obtained to
evaluate HCC before TACE. The second CT images were obtained to
evaluate the effectiveness of TACE against HCC. VFA was measure at
slices at the umbilical level (29).
We measured skeletal muscle mass and VFA by using manual tracings
on CT images, and their sum was calculated using Image-J software
(National Institutes of Health, Bethesda, MD, USA) (30). Skeletal muscle mass was normalized by
the square of the height, and the data was expressed as SMI.
Measurement of physical function
Grip strength, 10-meter walking speed, and 6-min
walking test were evaluated by qualified physical therapists.
Handgrip was measured on the non-dominant hand using a dynamometer
(TKK5401; Takei Scientific Instruments Co., Ltd.) according to
Japan Society of Hepatology guidelines (7). The 10-meter walking speed was measured
to evaluate gait speed. To evaluate the 10-meter maximal gait time,
2 meters were added to allow for acceleration before and
deceleration after the 10-meter gait test, respectively (31). The 6-min walking test was measured by
evaluating the total ambulated distance according to the American
Thoracic Society guidelines (32).
Biochemical tests
We measured following blood biochemical tests;
aspartate aminotransferase, alanine aminotransferase, alkaline
phosphatase, gamma-glutamyl transpeptidase, albumin, total
bilirubin, creatinine, estimated glomerular filtration rate,
creatine kinase, hemoglobin A1c, prothrombin activity, and platelet
count. We also measured serum levels of α-fetoprotein,
des-γ-carboxy prothrombin.
Changes in variables before and after
TACE
Changes in SMI and VFA between pre- and post-TACE
were evaluated by the ΔSMI, and ΔVFA. In addition, ΔSMI and ΔVFA
were evaluated by a stratification analysis according to sex.
Similarly, changes in each physical function were evaluated by the
Δ variables. All Δvariables were evaluated by change in the
variable (Δ variable = variable after TACE-variable before
TACE).
Definition of event and follow-up
An event was defined as death from any cause in this
study. After discharge, patients were followed up until death or
the study censor date by routine physical examinations, biochemical
tests, and ultrasonography, CT, or magnetic resonance imaging
according to the HCC guidelines of the Japan Society of Hepatology
(29). The median observation period
was 511 days (range, 13–1180 days).
Statistical analysis
Data are expressed as the median (interquartile
range [IQR]), range, or number. The differences between the control
and CR groups were analyzed by using Wilcoxon rank sum tests. The
difference ΔSMI in CR group among the etiologies of liver disease
(multiple groups) was examined by analysis of variance followed by
Scheffe's post hoc test. The difference ΔSMI between female and
male or Child-Pugh class A and B/C were analyzed by using Wilcoxon
rank sum tests. The level of statistical significance was set at
P<0.05.
A multivariate Cox regression analysis with a
stepwise variable selection was used to identify independent
variables associated with the prognosis of patients with HCC. In
the present study, we did not conduct a univariate analysis, and
the explanatory variables were selected in a stepwise manner,
minimizing the Bayesian information criterion as previously
described (33). Overall survival
analysis was performed using the Kaplan-Meier method followed by
the log-rank test.
Propensity scores for all the patients were
estimated by a logistic regression model using the following
baseline characteristics as covariates: Age, sex, BCLC stage,
Child-Pugh score, and BCAA supplementation as previously described
(2). To generate 88 pairs of
patients, we employed a one-to-one nearest-neighbor matching
algorithm with an optimal caliper of 0.2 without replacement. The
c-statistic in this study was 0.603, and 88 CLD patients with HCC
(control [n=44] and CR [n=44]) were analyzed.
All the statistical analyses were performed using
JMP®13 software (SAS Institute Inc.) or the R software
package (R Foundation for Statistical Computing, 2012).
Results
Patient characteristics
Patient characteristics data were obtained before
TACE. The characteristics, the CR and control group are shown in
Table I. Patients in the CR group
were significantly older than those in the control group. The VFA
in the CR group was significantly higher than that in the control
group. However, there was no significant difference in the
female-to-male ratio, body mass index, or SMI between the CR and
control groups. No significant difference was seen in TNM stage,
BCLC classification, or levels of α-fetoprotein and des-γ-carboxy
prothrombin. There was no significant difference in Child-Pugh
classification, hemoglobin A1c value, or estimated glomerular
filtration rate between the CR and control groups (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| Control group | Cancer
rehabilitation group |
|
|---|
|
|
|
|
|
|
|---|
| Factors | Reference
value | Median (IQR) or
percentage (n) | Range
(min-max) | Median (IQR) or
percentage (n) | Range
(min-max) | P-value |
|---|
| Observation
period | N/A | 424 (233–734) | 13–1180 | 522
(345.5–835) | 56-1038.2 | 0.0299 |
| Number, n | N/A | 67 |
| 85 |
|
|
| Age, years | N/A | 74.0
(68.0–78.0) | 41.0–85.0 | 75.0
(71.0–80.0) | 63.0–91.0 | 0.0371 |
| Sex,
female/male | N/A | 30%/70%
(20/47) | N/A | 40%/60%
(34/51) | N/A | 0.1943 |
| Body mass index,
kg/m2 | 18.5–24.9 | 22.3
(20.4–23.9) | 16.0–32.7 | 23.0
(21.0–25.7) | 16.7–37.8 | 0.0597 |
| Etiology of liver
disease, AIH/ Alcohol/HBV/HCV/NASH/others | N/A |
0%/11.9%/6%/76.1%/4.5%/1.5%
(0/8/4/51/3/1) | N/A |
3.5%/10.6%/9.4%/63.5%/1.2%/11.8%
(3/11/8/54/1/8) | N/A | 0.0901 |
| Viremia of HCV,
presence/absence | N/A | 44/7 | N/A | 41/13 | N/A | 0.1771 |
| HCC stage,
I/II/III/IV | N/A |
4.5%/23.9%/44.8%/26.8% (3/16/30/18) | N/A |
10.6%/38.8%/31.8%/18.8% (9/33/27/16) | N/A | 0.0675 |
| BCLC
classification, stage A/B/C | N/A | 4.5%/82.1%/13.4%
(3/55/9) | N/A | 10.6%/75.3%/14.1%
(9/64/12) | N/A | 0.3668 |
| Number of TACE
sessions at baseline | N/A | 1 (0–2) | 0–9 | 1 (0–2) | 0–8 | 0.5497 |
| Number of TACE
sessions during the observation period | N/A | 3 (1–5) | 1–12 | 3 (1–4) | 1–9 | 0.2033 |
| Additional HCC
treatment with HAIC/TKIs during the observation period, Yes/No | N/A | 32.8%/67.2%
(22/45) | N/A | 32.9%/67.1%
(28/57) | N/A | 0.9890 |
| AFP, ng/ml | ≤10.0 | 50.9
(11.4–348.5) | 1.3–22385.0 | 36.2
(6.9–229.1) | 1.3–67036.0 | 0.2632 |
| DCP, mAU/ml | ≤40.0 | 257.0
(44.5–4523.5) | 10.0–745283.0 | 101.0
(33.5–777.5) | 12.0–104513.0 | 0.1692 |
| Child-Pugh class,
A/B/C | N/A | 52.2%/47.8% /0%
(35/32/0) | N/A | 62.4%/37.7% /0%
(53/32/0) | N/A | 0.2099 |
| AST, IU/l | 13–30 | 48(32–68) | 13–177 | 43 (30.5–54) | 18–183 | 0.0804 |
| ALT, IU/l | 10–30 | 35 (23–55) | 7–101 | 29 (22–39.5) | 7–186 | 0.1518 |
| Lactate
dehydrogenase, IU/l | 120–240 | 238 (201–277) | 118–498 | 211
(181–251.5) | 129–624 | 0.0215 |
| ALP, IU/l | 115–359 | 381 (291–522) | 144–1170 | 351 (288–500) | 180–1467 | 0.5330 |
| GGT, IU/l | 13–64 | 47
(28.75–96.25) | 17–395 | 44 (25.5–75.5) | 9–551 | 0.3759 |
| Cholinesterase,
U/l | 201–421 | 130
(93.5–174.75) | 47–314 | 155 (117–200) | 53–360 | 0.0723 |
| Total protein,
g/dl | 6.6–8.1 | 7.21
(6.94–7.59) | 5.65–8.52 | 7.16
(6.66–7.62) | 5.86–8.89 | 0.5160 |
| Albumin, g/dl | 4.1–5.1 | 3.33
(2.97–3.63) | 2.31–4.40 | 3.37
(3.05–3.71) | 2.27–4.39 | 0.4058 |
| Total bilirubin,
mg/dl | 0.40–1.20 | 0.99
(0.76–1.27) | 0.33–2.31 | 0.82
(0.64–1.22) | 0.31–2.78 | 0.0764 |
| BUN, mg/dl | 8.6–22.9 | 16.1
(13.2–20.8) | 8.8–36.9 | 16.9
(14–19.75) | 5.9–47.6 | 0.9084 |
| Creatinine,
mg/dl | 0.65–1.07 | 0.74
(0.60–0.95) | 0.35–8.50 | 0.73
(0.61–0.91) | 0.43–1.91 | 0.5513 |
| eGFR, ml/min/1.73
m2 | >90.0 | 74.5 (55–92.2) | 5.5–129.5 | 71.7
(55.8–86.8) | 27.3–119.5 | 0.5330 |
| Total cholesterol,
mg/dl | 150–199 | 146
(127–172.5) | 81–254 | 136 (125–153) | 79–233 | 0.1781 |
| Creatine kinase,
U/l | 59–248 | 100.5
(67.5–169.3) | 34–386 | 87 (55–127.25) | 10–374 | 0.0611 |
| HbA1c, % | 4.3–5.8 | 5.8 (5.2–6.35) | 4.6–8.0 | 5.8 (5.5–6.4) | 4.3–13.4 | 0.4915 |
| Prothrombin
activity, % | 80–120 | 74.5
(63–88.75) | 14.6–118 | 79(67.5–89.5) | 14.5–117 | 0.5247 |
| Red blood cell
count, ×104/µl | 435–555 | 377 (341–411) | 186–498 | 389 (352–420) | 249–615 | 0.1877 |
| Hemoglobin,
g/dl | 13.7–16.8 | 11.7
(10.2–12.8) | 7.0–16.4 | 11.7
(10.45–12.75) | 7.3–15.6 | 0.7807 |
| White blood cell
count, /µl | 3,300-8,600 | 3,500
(2,700–4,700) | 1700–9700 | 3,800
(3,150–5,150) | 1,800-21,100 | 0.1808 |
| Platelet count,
×103/mm3 | 15.8–34.8 | 9 (6.9–14.5) | 3.4–24.4 | 10.8
(8.4–13.95) | 3.2–46.6 | 0.1809 |
| BCAA
supplementation, yes/no | N/A | 67.2%/32.8%
45/22 | N/A | 61.1%/38.8%
52/33 | N/A | 0.4456 |
| Hospitalization,
days | N/A | 15 (13–20) | 8–38 | 15 (11–21) | 7–55 | 0.4862 |
| Evaluation period
for CT, days | N/A | 53 (34–84) | 7–309 | 50 (35–80) | 7–312 | 0.8324 |
| Period between TACE
and CT, days | N/A | 11 (9–15) | 6–26 | 9 (7–20) | 2–125 | 0.3958 |
| Exercise days
during hospitalization, days | N/A | N/A | N/A | 6.5 (5–9) | 2–26 | N/A |
| Total duration of
exercise, h | N/A | N/A | N/A | 3.3 (2.7–4.4) | 1-8.7 | N/A |
| Metabolic
equivalents | N/A | N/A | N/A | 2.5 (2–3) | 2–4 | N/A |
| Grip strength,
kg | N/A | N/A | N/A | 24.1
(19.2–28.9) | 10.8–42.8 | N/A |
| 10-meter walk test,
sec | N/A | N/A | N/A | 7.33
(6.10–9.81) | 5.41–13.55 | N/A |
| 6-min walk test,
meter | N/A | N/A | N/A | 379.81
(288.85–421.52) | 26.2–574.1 | N/A |
| SMI,
cm2/m2 | N/A | 31.5
(22.4–39.1) | 13.8–57.0 | 29.7
(23.7–34.6) | 11.9–51.2 | 0.3632 |
| VFA,
cm2/m2 | N/A | 19.23
(10.10–30.96) | 2.13–71.95 |
26.55(15.75–36.41) | 2.13–92.65 | 0.012 |
No significant difference was observed between the
CR and control groups regarding the prevalence of patients treated
with BCAA supplementation, hospitalization period, or the
evaluation interval for CT (Table
I). There was no significant difference in the number of TACE
sessions at the baseline, number of TACE sessions during the
observation period, or additional HCC treatment with hepatic
arterial infusion of chemotherapy/tyrosine kinase inhibitors during
the observation period (Table I).
There were no patients who underwent liver transplants during the
observation periods.
During the hospitalization, the median length of
therapeutic exercise was 6.5 days (IQR, 5–9 days; range, 2–26 days)
in the CR group with a median of 3.33 h of exercise performed (IQR,
2.67–4.42 h; range, 1–8.67 h).
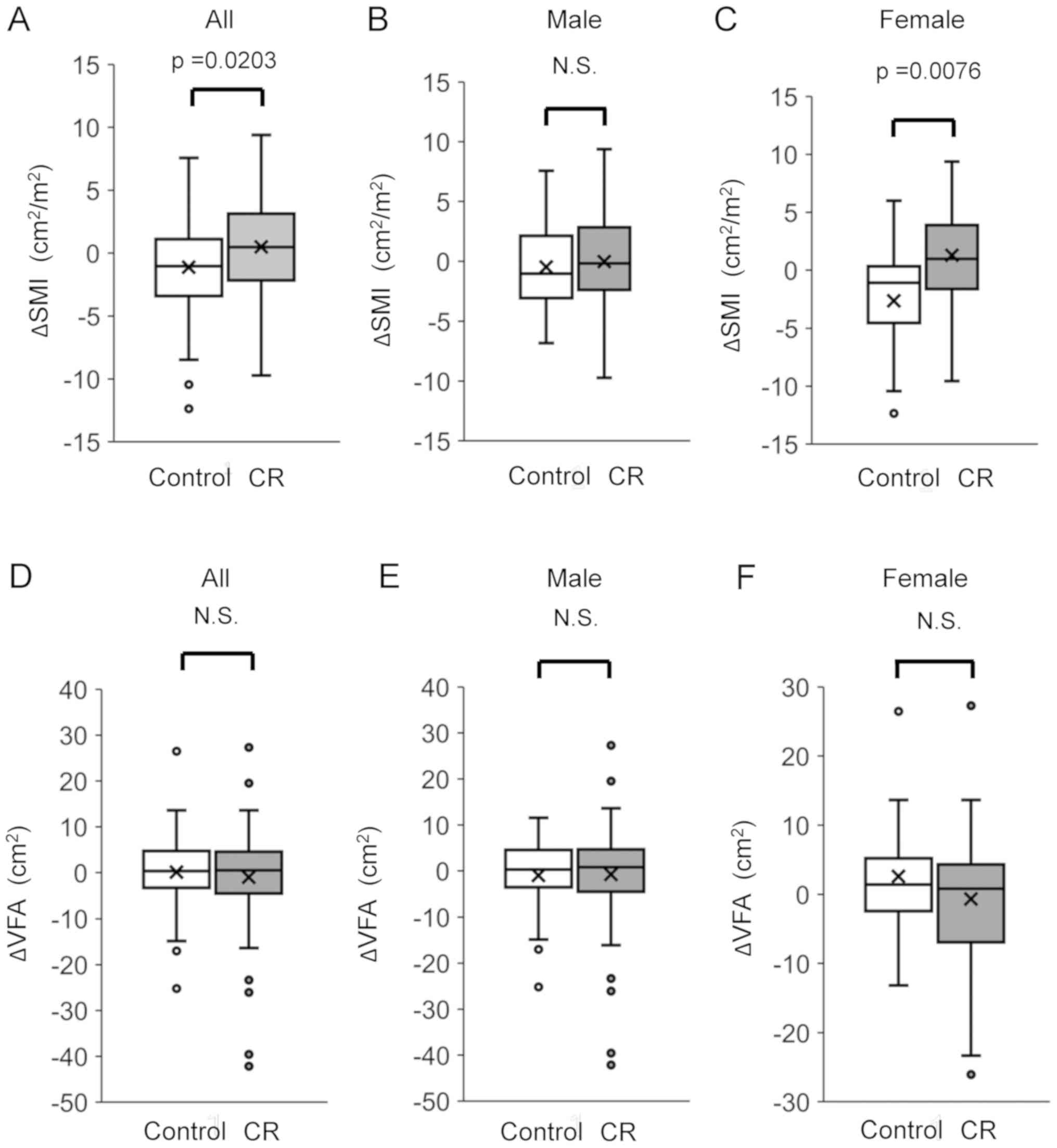
Changes in SMI and VFA
We evaluated changes in SMI by the difference in SMI
between before and after TACE. After treatment with TACE, ΔSMI was
significantly higher in the CR group than in the control group.
There was no significant difference between the CR and control
groups regarding the ΔSMI in male patients. On the other hand,
there was a significant difference between the CR and control
groups regarding ΔSMI in female patients (Fig. 1). The ΔSMI had no significant
association with the etiology of liver disease in the CR group
(Fig. S1). Moreover, in the CR
group, ΔSMI was not significantly different between male and female
patients or between patients classified as Child-Pugh class A and
Child-Pugh class B/C (Fig. S1B and
C). There was no significant difference in the ΔVFA between the
CR and control groups. Moreover, there was no significant
difference in VFA between the CR and control groups in both male
and female patients (Fig. 1).
Correlations between survival period
and Δ each variable
Correlations between survival period and the
alterations in body composition and physical performance were
evaluated using pairwise correlations. There was no significant
correlation between the survival period and ΔSMI or ΔVFA (Table II). Moreover, no significant
correlation was seen between the survival period and Δ grip
strength, Δ10 meter walking speed, or Δ6-min walking test (Table II).
 | Table II.Pairwise correlations between
survival period and Δeach variable. |
Table II.
Pairwise correlations between
survival period and Δeach variable.
| Variables | Correlation
coefficient | P-value |
|---|
| ΔSMI | −0.00029 | 0.9971 |
| ΔVFA | −0.03869 | 0.6338 |
| Δgrip strength | −0.09365 | 0.4767 |
| Δ10 m walking
speed | −0.04024 | 0.9121 |
| Δ6-min walk
test | −0.19864 | 0.1712 |
Associations between survival period
and changes in SMI, and baseline SMI, VFA, and serum levels of LDH
and total protein
We performed stratification analysis according the
changes in SMI. There was no significant difference in survival
rate between the patients with increased and decreased SMI in all
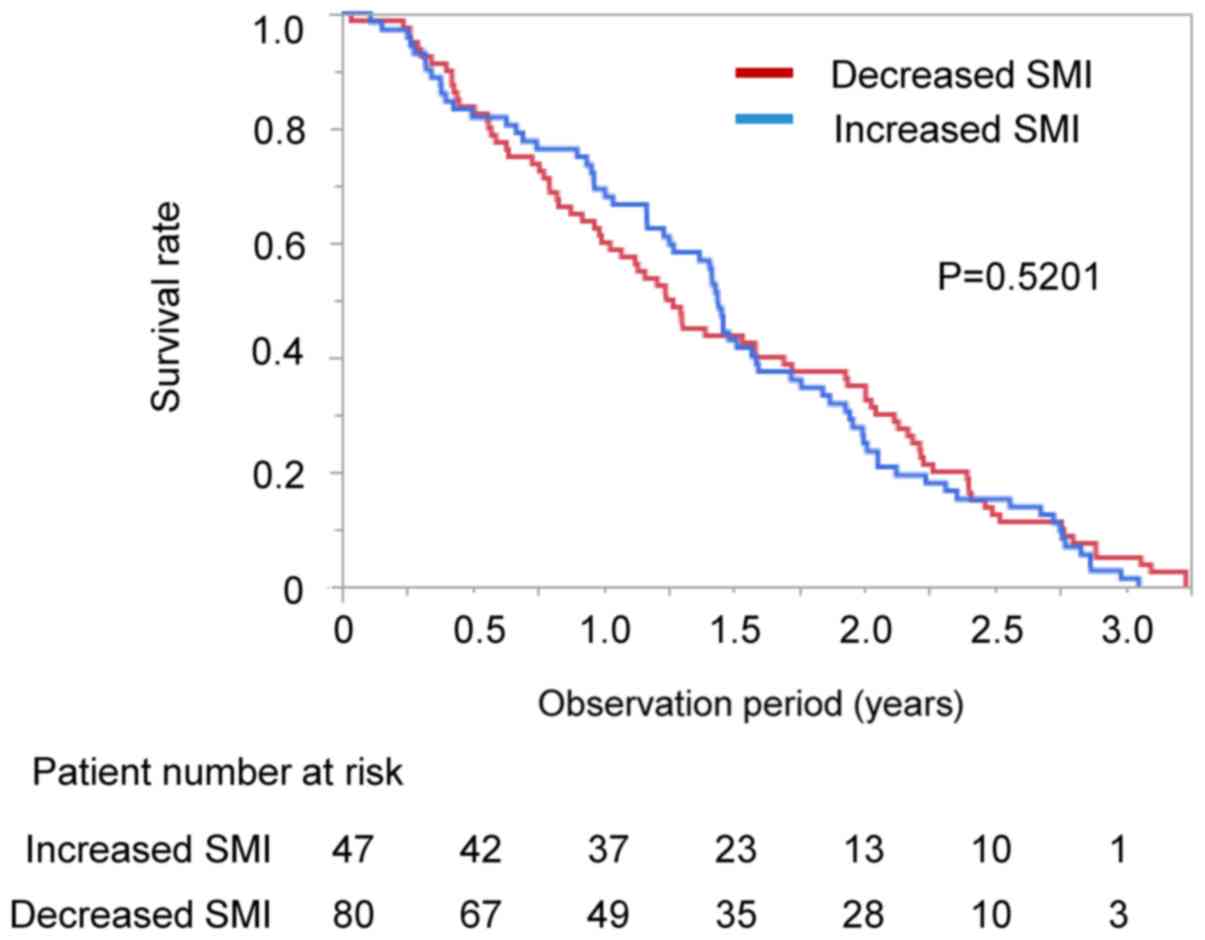
patients (median 526 vs. 459 days, P=0.6206; Fig. 2). The impact of changes in SMI on
survival was also examined in the CR and control groups. No
significant difference was seen in survival between the patients
with increased SMI and decreased SMI in both the CR and control
groups (CR group; increased SMI vs. decreased SMI; median 541 vs.
569 days, P=0.3856; control group: Increased SMI vs. decreased SMI,
median 379 vs. 432 days, P=0.5201; Figs. S2A and B). We also performed
stratified analysis for survival according to the several baseline
clinical parameters. There was no significant difference in
survival rate between the High and Low SMI groups (P=0.7064) and
between the High and Low VFA groups (P=0.7530). There was no
significant difference in the survival rate between the High and
Low LDH groups (P=0.1095). There was no significant difference in
the survival rate between the High and Low total protein groups
(P=1.0330).
Independent factors associated with
survival
We evaluated the independent factors associated with
survival using Cox regression analysis. There was significant
difference in serum level of LDH and total protein between the CR
and control groups in univariate analysis. However, these factors
were not identified as an independent factor for survival in the
Cox regression analysis. The CR group and Child-Pugh class A were
identified as independent positive factors associated with survival
(Table III). Although BCLC stage
was not significantly associated with survival, the number of TACE
sessions at baseline were an independent negative factor for
survival (Table III). The number
of TACE sessions during the observation period and additional HCC
treatment with hepatic arterial infusion of chemotherapy/tyrosine
kinase inhibitors during the observation period were not selected
by a stepwise procedure.
 | Table III.Independent factor for survival. |
Table III.
Independent factor for survival.
| Factors | Estimate | 95% Confidence
interval | P-value |
|---|
| Group (CR) | 1.760 | 0.914–3.226 | 0.0010 |
| Child-Pugh (class
A-B) | 1.602 | 0.426–2.998 | 0.0129 |
| Number of TACE
session at baseline | −0.843 | −1.465–0.337 | 0.003 |
We also evaluated independent factors associated
with survival in the CR and control groups respectively. In the CR
group, the number of TACE sessions during the observation and
Child-Pugh class were identified as independent factors for
survival (P=0.0004 and P=0.0278). In the Control group, BCLC stage
was identified as an independent factor for the survival
(P=0.0034).
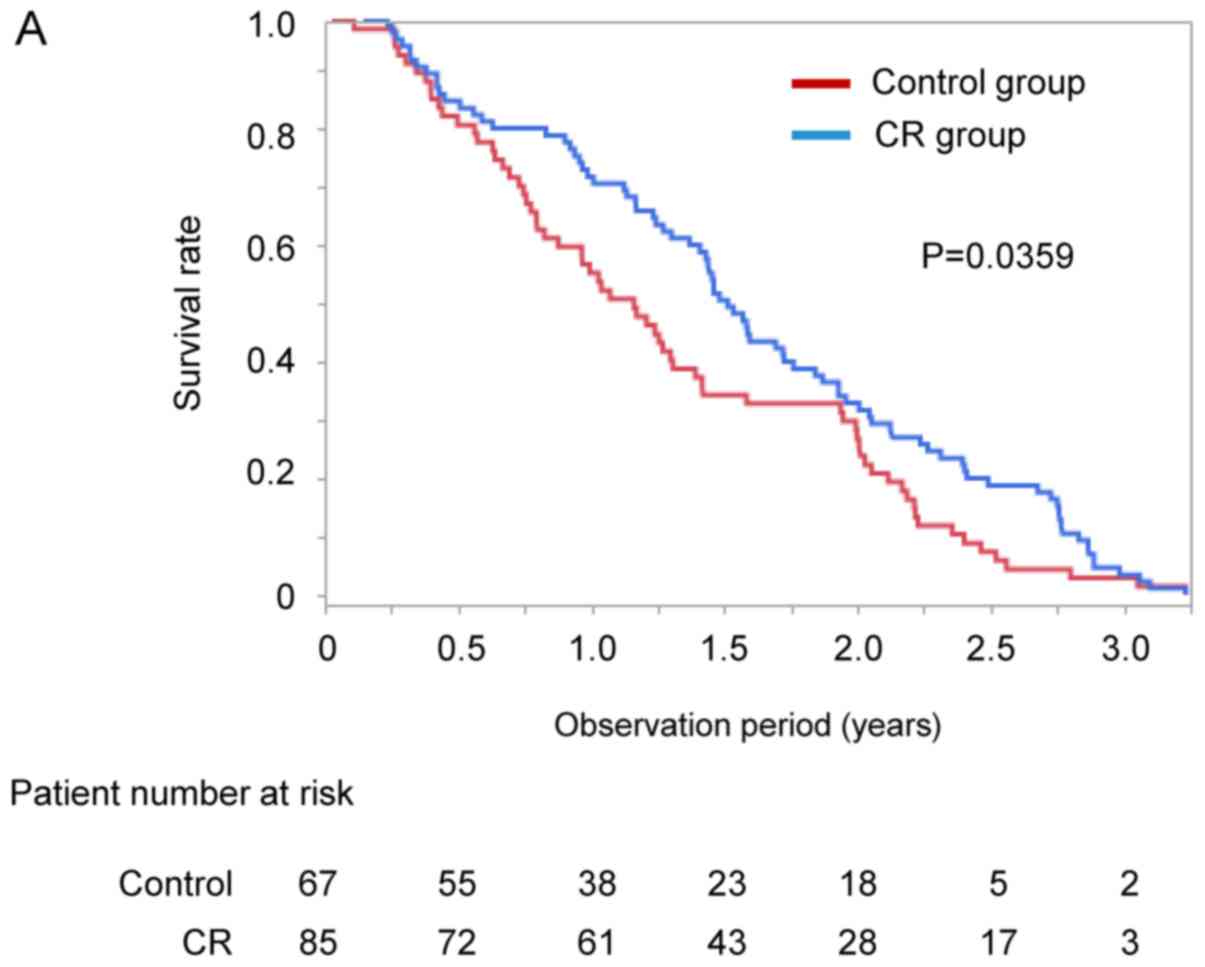
Impact of CR on survival period
Kaplan-Meier analysis was used to compare the
survival rate between the CR and control groups. The median
observation period was 511 days (range, 13–1180 days). The survival
rate of the CR group was significantly higher than that of the
control group on Kaplan-Meier analysis (median 552 vs. 424 days,
P=0.0359; Fig. 3A).
Impact of CR on survival period after
propensity score matching
After propensity score matching, we evaluated
overall survival by Kaplan-Meier analysis. Propensity scores for
all the patients were estimated using the following baseline
characteristics as covariates: Age, sex, BCLC stage, Child-Pugh
score, and BCAA supplementation. There was no significant
difference in observation period between the CR and control groups
(Table IV). No significant
difference was seen in number of TACE session at baseline and SMI
between the two groups (Table IV).
The survival rate of the CR group was significantly higher than
that of the control group (median 529 vs. 369 days, P=0.0332;
Fig. 3B).
 | Table IV.Patient characteristics after
propensity score matching. |
Table IV.
Patient characteristics after
propensity score matching.
|
|
| Control group | Cancer
rehabilitation group |
|
|---|
|
|
|
|
|
|
|---|
| Factors | Reference
value | Median (IQR) or
percentage (n) | Range
(min-max) | Median (IQR) or
percentage (n) | Range
(min-max) | P-value |
|---|
| Observation
period | N/A | 369
(215–659.8) | 13–1114 | 529
(256.5–862) | 56–1131 | 0.0513 |
| Number, n | N/A | 44 |
| 44 |
|
|
| Age, years | N/A | 74.5
(69.25–78.0) | 59.0–84.0 | 73.5
(70.0–77.75) | 63.0–88.0 | 1.0000 |
| Sex,
female/male | N/A | 31.8%/68.2%
(14/30) | N/A | 29.6%/70.5%
(13/31) | N/A | 0.8172 |
| Body mass index,
kg/m2 | 18.5–24.9 | 22.6
(20.1–24.2) | 16.0–32.7 | 22.9
(20.9–25.9) | 17.8–30.5 | 0.3877 |
| Etiology of liver
disease, AIH/ | N/A |
0%/9%/4.6%/77.3%/ | N/A |
2.3%/15.9%/11.4%/56.8%/ | N/A | 0.2052 |
|
Alcohol/HBV/HCV/NASH/others |
| 6.8%/2.3%
(0/4/2/34/3/1) |
| 2.3%/11.3%
(1/7/5/25/1/5) |
|
|
| HCV viremia,
presence/absence | N/A | 30/4 | N/A | 20/5 | N/A | 0.3846 |
| HCC stage,
I/II/III/IV | N/A | 4.6%/25%/50%/20.5%
(2/11/22/9) | N/A |
0%/45.5%/36.4%/18.2% (0/20/16/8) | N/A | 0.1317 |
| BCLC
classification, stage A/B/C | N/A | 4.6%/81.8%/13.6%
(3/36/6) | N/A | 0%/84.1%/15.9%
(0/37/7) | N/A | 0.3516 |
| Number of TACE
session at baseline | N/A | 1(0–2.75) | 0–9 | 1(1–3) | 0–5 | 0.5514 |
| Number of TACE
session during the observation period | N/A | 3 (1–5) | 1–12 | 4 (2–5) | 1–7 | 0.1359 |
| Additional HCC
treatment with HAIC/TKI during the observation period, yes/no | N/A | 15.9%/84.1%
(7/37) | N/A | 25.0%/75.0%
(11/33) | N/A | 0.2905 |
| AFP, ng/ml | ≤10.0 | 58.35
(13.58–391.5) | 1.3–22385.0 | 57.9
(7.01–229.3) | 2.2 −67036.0 | 0.6403 |
| DCP, mAU/ml | ≤40.0 | 153.0
(36.0–1293.0) | 10.0–71031.0 | 159.5
(36.25–2908.25) | 16.0–104513.0 | 0.6589 |
| Child-Pugh class,
A/B/C) | N/A | 52.3%/47.7% /0%
(23/21/0) | N/A | 54.6%/45.5% /0%
(24/20/0) | N/A | 0.8308 |
| AST, IU/l | 13–30 | 49.5 (32–74) | 14–177 | 43 (32.3–57) | 22–113 | 0.1803 |
| ALT, IU/l | 10–30 | 38 (23.3–60) | 7–101 | 29 (23–44.5) | 7–114 | 0.2000 |
| Lactate
dehydrogenase, IU/l | 120–240 | 234.5
(197.5–274) | 118–498 | 212
(180.3–258.5) | 138–624 | 0.1341 |
| ALP, IU/l | 115–359 | 373
(284.8–511.8) | 144–1170 | 325 (291–467) | 214–1467 | 0.8315 |
| GGT, IU/l | 13–64 | 44.5
(29.3–72.8) | 17–395 | 54.5
(27.5–83.5) | 12–551 | 0.5204 |
| Cholinesterase,
U/l | 201–421 | 128
(87.3–175.3) | 47–314 | 157 (103–194) | 57–360 | 0.1829 |
| Total protein,
g/dl | 6.6–8.1 | 7.28
(6.95–7.58) | 6.32–8.2 | 6.9
(6.54–7.49) | 6.04–8.89 | 0.0389 |
| Albumin, g/dl | 4.1–5.1 | 3.36
(2.99–3.63) | 2.43–4.40 | 3.37
(2.99–3.70) | 2.27–4.39 | 0.6856 |
| Total bilirubin,
mg/dl | 0.40–1.20 | 0.99
(0.76–1.28) | 0.39–2.03 | 0.91
(0.68–1.27) | 0.31–2.78 | 0.5017 |
| BUN, mg/dl | 8.6–22.9 | 16.1
(11.7–21.3) | 8.8–31.2 | 17.0
(14.5–19.6) | 5.9–47.6 | 0.7480 |
| Creatinine,
mg/dl | 0.65–1.07 | 0.74
(0.60–0.95) | 0.35–8.50 | 0.74
(0.61–0.94) | 0.43–1.91 | 0.9368 |
| eGFR, ml/min/1.73
m2 | >90.0 | 71.2
(55.5–94.1) | 5.5–129.5 | 72.7
(57.2–90.4) | 27.3–108.4 | 0.9800 |
| Total cholesterol,
mg/dl | 150–199 | 141(124–171.8) | 81–235 | 138
(125.8–150) | 79–233 | 0.6162 |
| Creatine kinase,
U/l | 59–248 | 120.5
(72.3–169.0) | 34.0–386.0 | 89
(58.8–141.0) | 10–297 | 0.2235 |
| HbA1c, % | 4.3–5.8 | 5.9 (5.3–6.5) | 4.7–8.0 | 5.7 (5.3–6.6) | 4.3–13.4 | 0.9609 |
| Prothrombin
activity, % | 80–120 | 78.0
(67.5–94.0) | 14.6–118 | 76.0
(65.3–85.5) | 14.5–117 | 0.5787 |
| Red blood cell
count, ×104/µl | 435–555 | 377
(353–407.8) | 312–466 | 389
(345.3–426.8) | 249–615 | 0.5990 |
| Hemoglobin,
g/dl | 13.7–16.8 | 11.7
(10.53–12.8) | 7.3–15.2 | 11.5
(10.1–12.95) | 7.3–15.6 | 0.8444 |
| White blood cell
count, /µl | 3,300-8,600 | 3,500
(2,700–4175) | 1,700-9,700 | 3,800
(3,025–5,300) | 1,800-21,100 | 0.2104 |
| Platelet count,
×103/mm3 | 15.8–34.8 | 8.6 (6.9–14.4) | 4.7–24.4 | 10.8
(8.5–14.6) | 3.6–46.6 | 0.1873 |
| BCAA
supplementation, yes/no | N/A |
70.4%/29.6%31/13 | N/A |
63.6%/36.4%28/16 | N/A | 0.4963 |
| Hospitalization,
days | N/A | 14.5 (13–21) | 9–35 | 15.5 (11–22.8) | 10–43 | 0.9099 |
| Evaluation period
for CT, days | N/A | 48.5
(31.5–77.5) | 7–309 | 58.5
(41.8–80.8) | 23–312 | 0.1789 |
| Period between TACE
and CT, days | N/A | 10 (9–14) | 6–26 | 10 (8.3–28.8) | 6–125 | 0.4373 |
| Exercise days
during hospitalization, days | N/A | N/A | N/A | 7.0 (5–9.3) | 2–20 | N/A |
| Total times of
exercise, h | N/A | N/A | N/A | 3.3 (2.7–4.4) | 1.3–8.3 | N/A |
| Metabolic
equivalents | N/A | N/A | N/A | 2.5 (2–3) | 2–4 | N/A |
| Grip strength,
kg | N/A | N/A | N/A | 23.8
(19.2–30.4) | 9.1–42.8 | N/A |
| 10-meter walk test,
sec | N/A | N/A | N/A | 7.88
(6.96–12.17) | 6.69–13.55 | N/A |
| 6-min walk test,
meter | N/A | N/A | N/A | 359.9
(302.3–420.0) | 26.2–574.1 | N/A |
| SMI,
cm2/m2 | N/A | 30.5
(22.4–38.7) | 13.8–48.3 | 31.4
(27.0–34.6) | 20.0–46.4 | 0.7417 |
| VFA,
cm2/m2 | N/A | 53.23 (30.85-
85.24) | 5.00–193.06 |
53.02(37.22–95.86) | 4.37–240.75 | 0.6078 |
Discussion
In this study, we investigated effects of CR on the
prognosis of patients with HCC who underwent TACE. The survival
rate was higher in HCC patients with CR than in HCC patients
without CR. Cox regression analysis demonstrated that CR was an
independent factor for survival. These results may indicate that CR
has beneficial effect on prognosis of patients with HCC who
underwent TACE.
In the present study, SMI significantly increased in
the CR group without worsening liver function in patients with CLD
and HCC who underwent TACE. Dawson et al (34) showed that CR improves muscle mass in
patients with prostate cancer during androgen treatment. We also
reported previously that CR increased muscle mass in patients with
HCC during treatment (14). Taken
together, CR may improve the muscle mass of patients with cancer in
spite of treatment for cancer.
In the female, but not in the male, ΔSMI in the CR
group was significantly higher than that in the control group. This
gender difference could be accounted for a marked decrease of ΔSMI
in female of the control group. In our previous study, skeletal
muscle mass was also significantly decreased in female than in male
HCC patients who underwent TACE (35). A possible reason for the gender
difference is sex hormones. Testosterone has potent anabolic
effects on skeletal muscle, leading to muscle protein synthesis and
subsequently increases muscle mass (36). Women have low serum testosterone
levels and serum free testosterone levels are reported to be
positively correlated with skeletal muscle mass in women (37).
The prognosis of solid malignancies is widely known
to be dependent on the cancer stage, such as the TNM system
(38). In this study, BCLC stage was
not identified as an independent factor associated with survival.
It remains unclear why the stage of HCC was not a risk factor for
survival. However, all enrolled patients with HCC were treated with
TACE, and patients with BCLC stage B accounted for approximately
80% of enrolled patients. Therefore, the narrow distribution of HCC
stage may be a possible reason that HCC stage was not a risk factor
for the prognosis of HCC patients treated with TACE.
Loss of muscle mass is an independent prognostic
factor for patients with HCC (11).
Therefore, an increase in skeletal muscle mass is thought to be
related to the improvement of the prognosis of patients with HCC.
We investigated the impact of the change in SMI on survival in
patients with HCC; however, an increase in SMI was not identified
as an independent prognostic factor in patients with HCC. In
addition, there was no significant difference in survival rate
between patients with increased SMI and patients with decreased SMI
in all patients or in the CR group. Although CR is reported to
improve the prognosis of patients with cancer (39), our study indicates that CR may
improve the prognosis regardless of changes in muscle mass in
patients with HCC.
In this study, we first showed that maintaining
physical activity by CR is more important than increasing muscle
mass to improve the prognosis of patients with HCC. The beneficial
effect of CR was also observed after propensity score matching.
Although it remains unclear why CR improved the prognosis, exercise
is reported to exert several beneficial effects against cancer.
First, exercise may suppress the development and
proliferation of HCC through modulation of insulin-like growth
factor 1 signal. Insulin-like growth factor 1 concentration is
related to an increase in tumor growth and development of colon
cancer in in vivo and in vitro studies (40). Exercise improves insulin resistance
and decreases levels of insulin-like growth factor 1 in patients
with breast cancer (39). Moreover,
exercise is also known to enhance the expression of p21,
insulin-like growth factor-binding protein-3, and PTEN, which
suppress insulin-like growth factor 1 signaling in a cancer mouse
model (41).
Second, exercise may suppress HCC growth through
suppression of the Warburg effect. Aerobic exercise is reported to
exert an anti-Warburg effect, leading to an increase in lactate
clearance capacity in animal and human studies (42). Lactate is an important metabolic
compound involved and necessary in all main sequela for
carcinogenesis, specifically: Angiogenesis, immune escape, cell
migration, metastasis, and self-sufficient metabolism (42). Lactate metabolism is directly
correlated with the prognosis of patients with cancer (43). Thus, exercise-induced alteration in
lactate metabolism may suppress HCC growth.
Besides the above mechanisms, myokines are known to
suppress cancer cells directly and indirectly (44). Contracting muscle fibers release
myokines including interleukin, oncostatin, and secreted protein
acidic and rich in cysteine (44).
Interleukin is reported to increase mobilization of natural killer
lymphocytes and to reduce the growth rate of liver cancer in an
in vivo study (45).
Oncostatin M was reported to have an anti-proliferative and
apoptotic effect on breast-cancer cells in an in vivo study
(46). Secreted protein acidic and
rich in cysteine is known to be associated with apoptosis of colon
cancer cells in mice and humans (47). Thus, a possible hypothesis is that CR
may inhibit progression of HCC through up-regulation of myokines,
leading to improvement in the prognosis.
There were limitations in this study. This was not a
randomized controlled trial with constant duration of CR and
irregular time intervals between the first and second CT. Patients
who did not agree to exercise were defined as the control group. In
addition, the exclusion criteria for the CR group would be
associated with death. Thus, patients in the control group might be
more deconditioned than patients in the CR group. Second, we did
not evaluate the physical activity of patients before TACE and
after discharge. Continuous execution of CR should have been
evaluated at outpatient visits. Third, changes in eating habits
were not evaluated in this study. Since exercise is known to affect
eating habits (48) and, changes in
eating habits might be a confounding factor of CR. Fourth, there
were no patients who underwent liver transplantation during the
observation period, suggesting the selection bias. Fifth, we did
not evaluate any immune-related variable, although exercise is
known to activate immunity and may suppress proliferation of cancer
cells through modulation of regulation of the tumor
microenvironment (49,50). Thus, multicenter randomized
controlled trials should be conducted with constant duration of CR
and regular interval of CT examinations, evaluation of physical
activity and eating habits before and after TACE, and various
treatments for HCC after TACE, including liver transplantation. In
addition, effects of CR on immune-related variable should be
evaluated to elucidate the mechanisms for CR-related improvement of
prognosis in patients with HCC.
In conclusion, we showed that CR not only increased
muscle mass but also prolonged survival in patients with HCC who
underwent TACE. Moreover, CR, but not an increase in muscle mass,
was an independent factor for survival. Thus, exercise may exert
beneficial effects on the prognosis independent from muscle
hypertrophy in patients with HCC. Patients with HCC are recommended
to maintain physical activity rather than be on bed rest even after
a diagnosis of HCC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Miwa Sakai
(Division of Gastroenterology, Department of Medicine, Kurume
University School of Medicine) for data acquisition.
Funding
The present study was supported by Program for Basic
and Clinical Research on Hepatitis (AMED; grant no.
JP19fk0210045).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RH, TK, SK and KH participated in the study
conception and design, data acquisition and interpretation, and
manuscript drafting. NG, TY, TO, MB, SI, DN and TN participated in
data acquisition and interpretation. AK participated in analysis
and interpretation of data. HM, NS and TT participated in data
interpretation and revising the manuscript critically for important
intellectual content.
Ethics approval and consent to
participate
The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki, as reflected in the
prior approval given by the Institutional Review Board of Kurume
University (approval no. 15072). An opt-out approach was used to
obtain informed consent from patients, and personal information was
protected during data collection. None of the patients were
institutionalized.
Patient consent for publication
Not applicable.
Competing interests
TK has honoraria (lecture fee) from Mitsubishi
Tanabe Pharma Corporation, MSD K.K, and Otsuka Pharmaceutical Co.,
Ltd. The other authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCAA
|
branched-chain amino acid
|
|
CLD
|
chronic liver disease
|
|
CR
|
cancer rehabilitation
|
|
CT
|
computed tomography
|
|
HCC
|
hepatocellular carcinoma
|
|
SMI
|
skeletal muscle index
|
|
TACE
|
transcatheter arterial
chemoembolization
|
|
VFA
|
visceral fat area
|
References
|
1
|
Aberg MA, Toren K, Nilsson M, Henriksson
M, Kuhn HG, Nyberg J, Rosengren A, Åberg ND and Waern M:
Nonpsychotic mental disorders in teenage males and risk of early
stroke: A population-based study. Stroke. 47:814–821. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimose S, Tanaka M, Iwamoto H, Niizeki T,
Shirono T, Aino H, Noda Y, Kamachi N, Okamura S, Nakano M, et al:
Prognostic impact of transcatheter arterial chemoembolization
(TACE) combined with radiofrequency ablation in patients with
unresectable hepatocellular carcinoma: Comparison with TACE alone
using decision-tree analysis after propensity score matching.
Hepatol Res. 49:919–928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Windsor PM, Nicol KF and Potter J: A
randomized, controlled trial of aerobic exercise for
treatment-related fatigue in men receiving radical external beam
radiotherapy for localized prostate carcinoma. Cancer. 101:550–557.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serra MC, Ryan AS, Ortmeyer HK, Addison O
and Goldberg AP: Resistance training reduces inflammation and
fatigue and improves physical function in older breast cancer
survivors. Menopause. 25:211–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmitz KH, Courneya KS, Matthews C,
Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal
RJ, Lucia A, et al: American College of Sports Medicine roundtable
on exercise guidelines for cancer survivors. Med Sci Sports Exerc.
42:1409–1426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra SI, Scherer RW, Geigle PM,
Berlanstein DR, Topaloglu O, Gotay CC and Snyder C: Exercise
interventions on health-related quality of life for cancer
survivors. Cochrane Database Syst Rev. CD0075662012.PubMed/NCBI
|
|
7
|
Nishikawa H, Shiraki M, Hiramatsu A,
Moriya K, Hino K and Nishiguchi S: Japan Society of Hepatology
guidelines for sarcopenia in liver disease (1st edition):
Recommendation from the working group for creation of sarcopenia
assessment criteria. Hepatol Res. 46:951–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsien C, Davuluri G, Singh D, Allawy A,
Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ,
Engelen MP, et al: Metabolic and molecular responses to
leucine-enriched branched chain amino acid supplementation in the
skeletal muscle of alcoholic cirrhosis. Hepatology. 61:2018–2029.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samoylova ML, Covinsky KE, Haftek M, Kuo
S, Roberts JP and Lai JC: Disability in patients with end-stage
liver disease: Results from the functional assessment in liver
transplantation study. Liver Transpl. 23:292–298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koo BK, Kim D, Joo SK, Kim JH, Chang MS,
Kim BG, Lee KL and Kim W: Sarcopenia is an independent risk factor
for non-alcoholic steatohepatitis and significant fibrosis. J
Hepatol. 66:123–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iritani S, Imai K, Takai K, Hanai T, Ideta
T, Miyazaki T, Suetsugu A, Shiraki M, Shimizu M and Moriwaki H:
Skeletal muscle depletion is an independent prognostic factor for
hepatocellular carcinoma. J Gastroenterol. 50:323–332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Pagàn JC, Santos C, Barberá JA,
Luca A, Roca J, Rodriguez-Roisin R, Bosch J and Rodés J: Physical
exercise increases portal pressure in patients with cirrhosis and
portal hypertension. Gastroenterology. 111:1300–1306. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saló J, Guevara M, Fernández-Esparrach G,
Bataller R, Ginès A, Jimenez W, Ginès P, Rivera F, Arroyo V and
Rodés J: Impairment of renal function during moderate physical
exercise in cirrhotic patients with ascites: Relationship with the
activity of neurohormonal systems. Hepatology. 25:1338–1342. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiraoka A, Michitaka K, Kiguchi D, Izumoto
H, Ueki H, Kaneto M, Kitahata S, Aibiki T, Okudaira T, Tomida H, et
al: Efficacy of branched-chain amino acid supplementation and
walking exercise for preventing sarcopenia in patients with liver
cirrhosis. Eur J Gastroenterol Hepatol. 29:1416–1423. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Locklear CT, Golabi P, Gerber L and
Younossi ZM: Exercise as an intervention for patients with
end-stage liver disease: Systematic review. Medicine (Baltimore).
97:e127742018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koya S, Kawaguchi T, Hashida R, Goto E,
Matsuse H, Saito H, Hirota K, Taira R, Matsushita Y, Imanaga M, et
al: Effects of in-hospital exercise on liver function, physical
ability, and muscle mass during treatment of hepatoma in patients
with chronic liver disease. Hepatol Res. 47:E22–E34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koya S, Kawaguchi T, Hashida R, Hirota K,
Bekki M, Goto E, Yamada M, Sugimoto M, Hayashi S, Goshima N, et al:
Effects of in-hospital exercise on sarcopenia in hepatoma patients
who underwent transcatheter arterial chemoembolization. J
Gastroenterol Hepatol. 34:580–588. 2019.PubMed/NCBI
|
|
18
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Citro V, Milan G, Tripodi FS, Gennari A,
Sorrentino P, Gallotta G, Postiglione A and Tarantino G: Mental
status impairment in patients with West Haven grade zero hepatic
encephalopathy: The role of HCV infection. J Gastroenterol.
42:79–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukui H, Saito H, Ueno Y, Uto H, Obara K,
Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, et al:
Evidence-based clinical practice guidelines for liver cirrhosis
2015. J Gastroenterol. 51:629–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Writing Committee M, ; Yancy CW, Jessup M,
Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci
SA, Horwich T, et al: 2013 ACCF/AHA guideline for the management of
heart failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on practice
guidelines. Circulation. 128:e240–e327. 2013.PubMed/NCBI
|
|
22
|
Heart Failure Society of America, ;
Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA,
Givertz MM, Katz SD, Klapholz M, Moser DK, et al: HFSA 2010
comprehensive heart failure practice guideline. J Card Fail.
16:e1–e194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rochwerg B, Brochard L, Elliott MW, Hess
D, Hill NS, Nava S, Navalesi P; Members Of The Steering Committee,
; Antonelli M, Brozek J, Conti G, et al: Official ERS/ATS clinical
practice guidelines: Noninvasive ventilation for acute respiratory
failure. Eur Respir J. 50:2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wedzicha JA Erc Co-Chair, Miravitlles M,
Hurst JR, Calverley PM, Albert RK, Anzueto A, Criner GJ, Papi A,
Rabe KF, Rigau D, et al: Management of COPD exacerbations: A
European Respiratory Society/American Thoracic Society guideline.
Eur Respir J. 49:2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saeki I, Yamasaki T, Maeda M, Kawano R,
Hisanaga T, Iwamoto T, Matsumoto T, Hidaka I, Ishikawa T, Takami T
and Sakaida I: No muscle depletion with high visceral fat as a
novel beneficial biomarker of sorafenib for hepatocellular
carcinoma. Liver Cancer. 7:359–371. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garber CE, Blissmer B, Deschenes MR,
Franklin BA, Lamonte MJ, Lee IM, Nieman DC and Swain DP; American
College of Sports Medicine, : American College of Sports Medicine
position stand. Quantity and quality of exercise for developing and
maintaining cardiorespiratory, musculoskeletal, and neuromotor
fitness in apparently healthy adults: Guidance for prescribing
exercise. Med Sci Sports Exerc. 43:1334–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: JSH consensus-based clinical practice guidelines for the
management of hepatocellular carcinoma: 2014 update by the liver
cancer study group of Japan. Liver Cancer. 3:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teramoto T, Sasaki J, Ishibashi S, Birou
S, Daida H, Dohi S, Egusa G, Hiro T, Hirobe K, Iida M, et al:
Metabolic syndrome. J Atheroscler Thromb. 21:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hashida R, Matsuse H, Takano Y, Omoto M,
Nago T and Shiba N: Walking exercise combined with neuromuscular
electrical stimulation of antagonist resistance improved muscle
strength and physical function for elderly people: A pilot study. J
Phys Fitness Sports Med. 5:195–203. 2016. View Article : Google Scholar
|
|
32
|
Brooks D, Solway S and Gibbons WJ: ATS
statement on six-min walk test. Am J Respir Crit Care Med.
167:12872003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawaguchi T, Tokushige K, Hyogo H, Aikata
H, Nakajima T, Ono M, Kawanaka M, Sawada K, Imajo K, Honda K, et
al: A data mining-based prognostic algorithm for NAFLD-related
hepatoma patients: A nationwide study by the Japan study group of
NAFLD. Sci Rep. 8:104342018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dawson JK, Dorff TB, Todd Schroeder E,
Lane CJ, Gross ME and Dieli-Conwright CM: Impact of resistance
training on body composition and metabolic syndrome variables
during androgen deprivation therapy for prostate cancer: A pilot
randomized controlled trial. BMC Cancer. 18:3682018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirota K, Kawaguchi T, Hashida R, Koya S,
Bekki M, Goshima N, Yoshiyama T, Otsuka T, Nozoe R, Nakano D, et
al: Profiles associated with Sarcopenia in hepatoma patients
underwent transcatheter arterial chemoembolization: A data-mining
analysis. J Cachexia Sarcopenia Muscle Clin Rep. 3:e000662018.
|
|
36
|
Bhasin S, Woodhouse L and Storer TW: Proof
of the effect of testosterone on skeletal muscle. J Endocrinol.
170:27–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yakabe M, Kojima T, Okumura T, Takiyama S,
Umeda-Kameyama Y, Akishita M and Ogawa S: Serum free testosterone
levels are positively correlated with skeletal muscle mass in older
women aged over 75 years. Geriatr Gerontol Int. 19:460–461. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okamura Y, Sugiura T, Ito T, Yamamoto Y,
Ashida R and Uesaka K: The optimal cut-off value of the
preoperative prognostic nutritional index for the survival differs
according to the TNM stage in hepatocellular carcinoma. Surg Today.
47:986–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meneses-Echávez JF, Jiménez EG, Rio-Valle
JS, Correa-Bautista JE, Izquierdo M and Ramírez-Vélez R: The
insulin-like growth factor system is modulated by exercise in
breast cancer survivors: A systematic review and meta-analysis. BMC
Cancer. 16:6822016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Devin JL, Bolam KA, Jenkins DG and Skinner
TL: The influence of exercise on the insulin-like growth factor
axis in oncology: Physiological basis, current, and future
perspectives. Cancer Epidemiol Biomarkers Prev. 25:239–249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu M, King B, Ewert E, Su X, Mardiyati N,
Zhao Z and Wang W: Exercise activates p53 and negatively regulates
IGF-1 pathway in epidermis within a skin cancer model. PLoS One.
11:e01609392016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
San-Millán I and Brooks GA: Reexamining
cancer metabolism: Lactate production for carcinogenesis could be
the purpose and explanation of the Warburg Effect. Carcinogenesis.
38:119–133. 2017.PubMed/NCBI
|
|
43
|
Hofmann P: Cancer and exercise: Warburg
hypothesis, tumour metabolism and high-intensity anaerobic
exercise. Sports (Basel). 6:2018.PubMed/NCBI
|
|
44
|
Lucia A and Ramírez M: Muscling in on
cancer. N Engl J Med. 375:892–894. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pedersen L, Idorn M, Olofsson GH,
Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC,
Pedersen KS, Dethlefsen C, et al: Voluntary running suppresses
tumor growth through epinephrine- and IL-6-dependent NK cell
mobilization and redistribution. Cell Metab. 23:554–562. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hojman P, Dethlefsen C, Brandt C, Hansen
J, Pedersen L and Pedersen BK: Exercise-induced muscle-derived
cytokines inhibit mammary cancer cell growth. Am J Physiol
Endocrinol Metab. 301:E504–E510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aoi W, Naito Y, Takagi T, Tanimura Y,
Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, et
al: A novel myokine, secreted protein acidic and rich in cysteine
(SPARC), suppresses colon tumorigenesis via regular exercise. Gut.
62:882–889. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Panão I and Carraça EV: Effects of
exercise motivations on body image and eating habits/behaviours: A
systematic review. Nutr Diet. 2019.
|
|
49
|
Kerr J, Anderson C and Lippman SM:
Physical activity, sedentary behaviour, diet, and cancer: An update
and emerging new evidence. Lancet Oncol. 18:e457–e471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koelwyn GJ, Quail DF, Zhang X, White RM
and Jones LW: Exercise-dependent regulation of the tumour
microenvironment. Nat Rev Cancer. 17:620–632. 2017. View Article : Google Scholar : PubMed/NCBI
|