Introduction
Intrahepatic cholangiocarcinoma (iCCA) is a highly
malignant neoplasm arising from the intrahepatic bile ducts, which
accounts for 5–15% of primary hepatic malignancies, 3% of
gastrointestinal malignancies, and 10% of biliary tract
malignancies (1). Although iCCA is
relatively rare, there is an increasing morbidity and mortality
rate worldwide, at least in part, due to late diagnosis at advanced
stages when surgery is no longer possible. Furthermore, despite the
use of systemic chemotherapy and radiotherapy, the median survival
time of patients with advanced stages still remains short (7–12
months) (2). Surgical resection is
considered to be the optimal therapeutic intervention to increase
patient survival time; however, only one third of patients with
iCCA are eligible for surgery following diagnosis (3). Furthermore, the recurrence rate is
reported to be greater than 60% with the 5-year survival rate
ranging from 20 to 40% following curative surgery (4). Considering the overall poor prognosis
of patients with iCCA, there is a requirement for the
identification of novel diagnostic tumor biomarkers and the
development of molecular targeted therapies.
As important marker proteins of lipid microdomains,
flotillin-1 and flotillin-2 are widely distributed in mammals,
plants, bacteria and fungi (5). The
interactions between flotillins and various proteins, which lead to
extensive effects on signaling molecules, enable flotillins to
participate in various cellular processes, including endocytosis,
proliferation and adhesion (6–8). In
mammals, flotillin-1 is highly expressed in the brain, heart,
placenta and hematopoietic cells, while flotillin-2 shows
ubiquitous expression in numerous different tissues (9–11).
However, in addition to participating in physiological functions,
flotillins serve important roles in cancer progression. Flotillin-2
has been used as a candidate marker for predicting poor prognosis
and as a useful therapeutic target for cancer (12). Furthermore, flotillin-2 is an
important regulator of lung metastasis in breast cancer (13). However, the expression level and the
clinical significance of flotillin-2 in iCCA have not yet been
reported.
To the best of our knowledge, the present study was
the first to investigate the expression of flotillin-2 in human
iCCA tissues and cell lines. The expression of flotillin-2 in
frozen resected samples was detected using western blotting, which
demonstrated that flotillin-2 was upregulated in iCCA tissues
compared with matched adjacent non-tumor tissues. Furthermore,
immunohistochemistry was used to detect flotillin-2 expression in
92 iCCA samples. Moreover, flotillin-2 knockdown decreased the
invasion of iCCA cell lines in vitro, further supporting the
notion that flotillin-2 serves important roles in iCCA invasion and
malignant progression. The present study may provide the basis for
the development of targeted therapy to reduce iCCA progression.
Materials and methods
Patients and surgical specimens
For the preliminary conduction of western blotting,
12 paired fresh iCCA tissues and adjacent non-tumor tissues were
collected from patients who underwent radical surgery between
September 2016 and July 2018 at the Affiliated Yantai Yuhuangding
Hospital of Qingdao University. In addition, a cohort of 92
patients with iCCA with complete clinical information who had
received surgery were recruited between March 2008 and February
2017 according to the following criteria: Radical resection alone,
no preoperative adjuvant chemotherapy or radiation therapy, and no
severe perioperative complications that may influence the survival
time. The intact clinical information of the patients is summarized
in Table I. The follow-up period
ranged between 5 and 116 months, with a median follow-up period of
30 months. Clinicopathological classification and pathologic
tumor-node-metastasis (pTNM) staging was determined according to
the 8th edition AJCC/UICC staging system (2017). The experimental
protocols were approved by the Ethics Committee of the Affiliated
Yantai Yuhuangding Hospital of Qingdao University, and written
informed consent was obtained from all patients.
 | Table I.Associations between the expression of
flotillin-2 and the clinicopathological features of patients with
intrahepatic cholangiocarcinoma. |
Table I.
Associations between the expression of
flotillin-2 and the clinicopathological features of patients with
intrahepatic cholangiocarcinoma.
|
|
| Flotillin-2 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | n | Low | High | P-value |
|---|
| Sex |
|
|
| 0.930 |
| Male | 58 | 21 | 37 |
|
|
Female | 34 | 12 | 22 |
|
| Age, years |
|
|
| 0.438 |
|
<60 | 48 | 19 | 29 |
|
|
≥60 | 44 | 14 | 30 |
|
| Liver cirrhosis
status |
|
|
| 0.912 |
| No | 62 | 22 | 40 |
|
|
Yes | 30 | 11 | 19 |
|
|
Differentiation |
|
|
| 0.335 |
|
Well | 19 | 9 | 10 |
|
|
Moderately | 45 | 13 | 32 |
|
|
Poorly | 28 | 11 | 17 |
|
| Tumor size, cm |
|
|
| 0.827 |
|
<5 | 32 | 11 | 21 |
|
| ≥5 | 60 | 22 | 38 |
|
| Tumor nodule |
|
|
| 0.920 |
|
Solitary | 84 | 30 | 54 |
|
|
Multiple | 8 | 3 | 5 |
|
| Hepatolith |
|
|
| 0.653 |
| No | 80 | 28 | 52 |
|
|
Yes | 12 | 5 | 7 |
|
| Tumor stage |
|
|
| 0.335 |
|
T1+T2 | 70 | 27 | 43 |
|
|
T3+T4 | 22 | 6 | 16 |
|
| Lymph node
metastasis |
|
|
| 0.029a |
| No | 56 | 25 | 31 |
|
|
Yes | 36 | 8 | 28 |
|
| M stage |
|
|
| 0.651 |
| M0 | 87 | 32 | 55 |
|
| M1 | 5 | 1 | 4 |
|
| TNM stage |
|
|
| 0.016a |
| I | 32 | 18 | 14 |
|
| II | 14 | 5 | 9 |
|
|
III | 15 | 4 | 11 |
|
| IV | 31 | 6 | 25 |
|
Tissue microarray and
immunohistochemistry
Representative areas of tumor cores in the paraffin
embedded tissue blocks were selected according to hematoxylin-eosin
(H&E) staining, and then transferred into a recipient master.
Antigen retrieval was performed by immersing the sections in
citrate buffer (pH 6.0; cat. no. AR0024; Boster Biological
Technology) and heating in a microwave for 5 min. The sections were
subsequently treated with 3% hydrogen peroxide for 30 min at room
temperature to quench endogenous peroxidase activity, followed by
10% normal goat serum for 30 min at 37°C. Sections were incubated
with mouse monoclonal antibody against human flotillin-2 (cat. no.
sc-28320; 1:200; Santa Cruz Biotechnology, Inc.) overnight at 4°C
followed by incubation with anti-mouse secondary antibody for 30
min at 37°C. The sections were subsequently stained with DAB
(Beyotime), and the staining was evaluated by two independent
observers.
Cell culture and small interfering RNA
(siRNA) transfection
The human iCCA cell lines RBE and HuCCT1 were both
purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and tested for mycoplasma. Cells were cultured in
RPMI-1640 containing 10% fetal bovine serum (FBS). Both RBE and
HuCCT1 cells were transfected with siRNA targeting flotillin-2
using Lipofectamine 3000 (cat. no. L3000015; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The sequence of flotillin-2 siRNA was
5′-GACCTTGAAATCCATGACG-3′, with the AllStars siRNA (cat. no.
1027281; Qiagen) as the negative control.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from tumor cells using an
RNeasy Plus Mini Kit (cat. no. 74104; Qiagen) and
reverse-transcribed into cDNA using the SuperScript First Strand
cDNA system (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. qPCR was performed using the
SYBR-Green and a LightCycler 480 PCR system (Roche Applied
Science). The following primer pairs were used for qPCR:
Flotillin-2 forward, 5′-TTGCTGACTCTAAGCGAGCC-3′ and reverse,
5′-TCCACGGCAATCTGTTTCTTG-3′; and β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The following thermocycling conditions
were used for qPCR: 95°C for 10 min followed by 40 cycles of
two-step PCR (95°C for 15 sec and 60°C for 30 sec). mRNA levels
were quantified using the 2−ΔΔCq method (14) and normalized to the internal
reference gene β-actin. Every experiment was carried out in
triplicate and repeated three times.
Assessment of flotillin-2
immunostaining
Flotillin-2 immunostaining was determined according
to the intensity and proportion of immunohistochemical staining by
a semi-quantitative method as previously described (15). Briefly, the intensity was graded as 0
(no staining), 1 (weak staining), 2 (moderate staining) or 3
(strong staining), and the proportion was scored as 1 (0–10%), 2
(11–50%), 3 (51–75%) or 4 (76–100%). The product of the intensity
and proportion was used as the final immunohistochemistry score.
The optimal cutoff point of flotillin-2 immunostaining was
determined according to the log-rank test with respect to overall
survival (OS), with the ultimate immunostaining score of 5 to
define flotillin-2 expression as low or high.
Western blotting
Cells and tissues were lysed in RIPA buffer
containing protease inhibitor cocktail. After determination of
protein concentration by a Bio-Rad BCA protein assay, equal amounts
of proteins were separated on SDS-PAGE and were transferred to PVDF
membranes, which were blocked in 5% non-fat milk in TBST buffer.
The membranes were subsequently incubated with specific mouse
monoclonal antibodies against human flotillin-2 (cat. no. sc-28320;
1:100; Santa Cruz Biotechnology, Inc.) and human β-actin (cat. no.
sc-8432; 1:1,000; Santa Cruz Biotechnology, Inc.). Following
washing with TBST at room temperature, the membranes were incubated
with horseradish peroxidase-conjugated anti-mouse secondary
antibodies for 1 h and subsequently developed through ECL detection
reagent (Thermo Fisher Scientific).
Cell invasion assay
The invasion ability of tumor cells was assessed
using Boyden chambers pre-coated with Matrigel (8-µm pore size;
Thermo Fisher Scientific, Inc.). After transfection of flotillin-2
siRNA, 1×105 transfected RBE or HuCCT1 cells were seeded
on the upper chamber of the insert in serum-free medium. Medium
supplemented with 10% FBS was added to the lower chamber. After 24
h, the cells on the upper side of the membrane were removed by
cotton swab. The invading cells on the lower surface were fixed and
stained. Stained cells were counted in four-randomly selected
fields (magnification, ×400).
Statistical analyses
Statistical analyses were performed with SPSS 18.0
software. The χ2 or Fisher's exact tests were used to
compare qualitative variables and to determine the association
between flotillin-2 expression and clinicopathological variables.
Quantitative variables were compared through two-tailed Student's
t-test. The OS was analyzed by the Kaplan-Meier method and the
survival curves were compared by the log-rank test. The significant
variables in the univariate analyses were further evaluated through
multivariate analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression pattern of flotillin-2 in
iCCA tissues
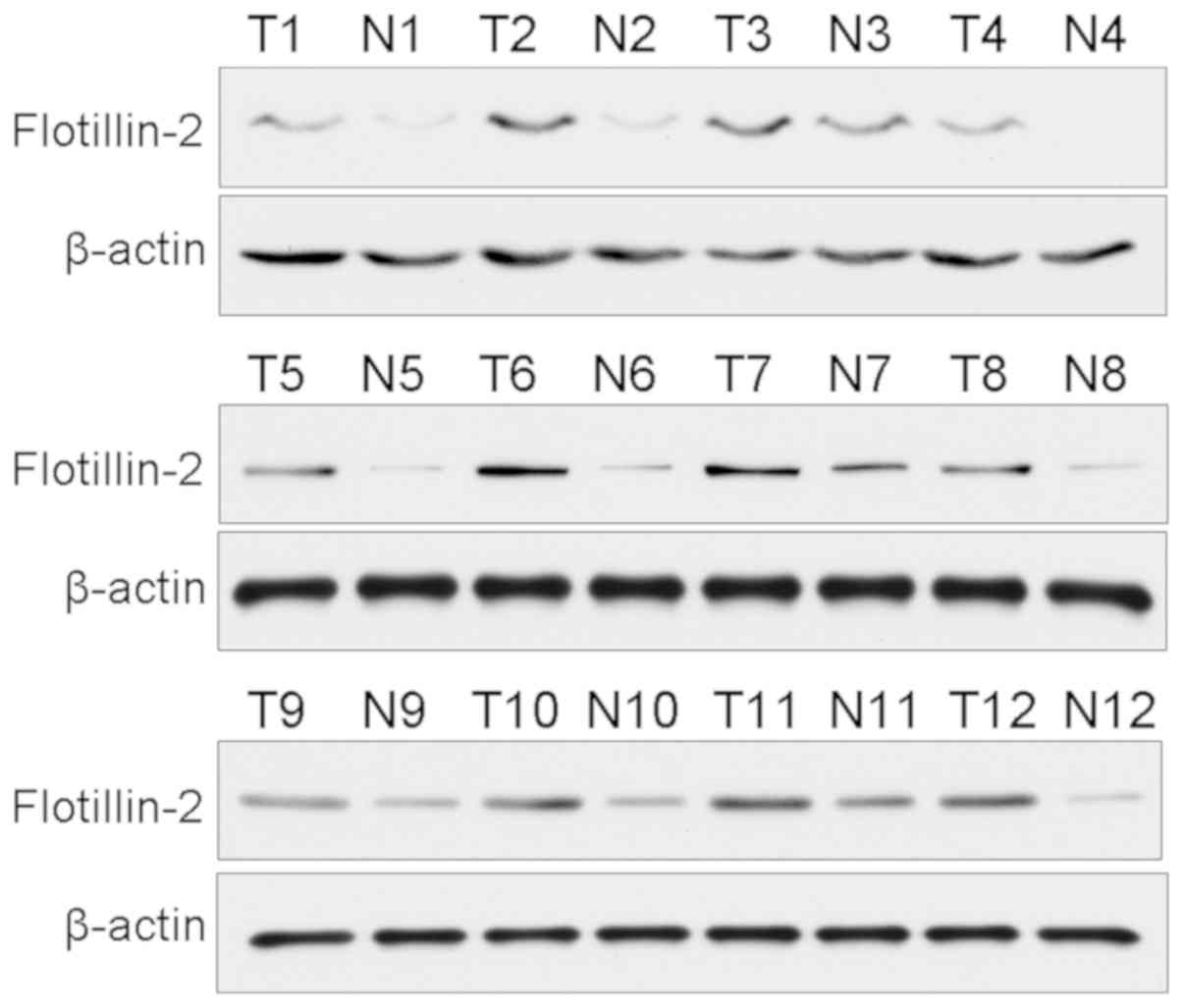
Western blotting was used to investigate the protein
levels of flotillin-2 in paired frozen iCCA and matched adjacent
tissues. As shown in Fig. 1,
flotillin-2 protein levels were significantly upregulated in iCCA
tissues compared with matched non-tumor bile duct tissues. The
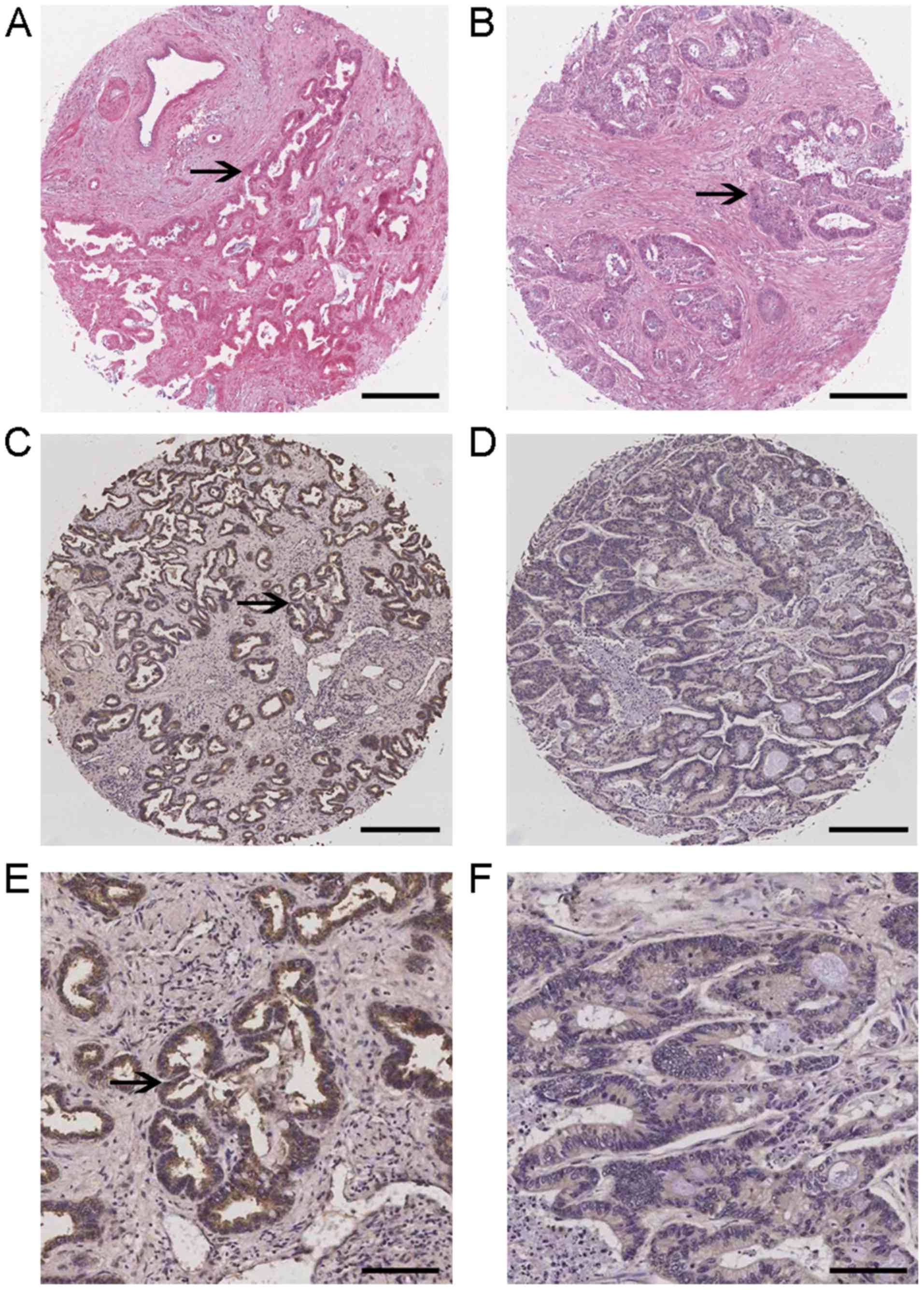
expression levels of flotillin-2 in a tissue microarray consisting
of human iCCA samples were subsequently investigated using
immunohistochemistry (Figs. 2 and
S1). Flotillin-2 expression was
observed predominantly in the cytoplasm and cell membranes of tumor
cells, which were confirmed by H&E staining showing the tumor
islands. Out of the 92 carcinoma specimens, 59 samples (64.1%) and
33 samples (35.9%) exhibited high and low flotillin-2 expression,
respectively.
Associations between flotillin-2
expression and clinicopathological characteristics in patients with
iCCA
The role of flotillin-2 in iCCA was further
investigated by evaluating its potential association with
clinicopathological parameters. The association between flotillin-2
upregulation and clinicopathological characteristics are presented
in Table I. The upregulation of
flotillin-2 was associated with lymph node metastasis (P=0.029) and
TNM stage (P=0.016). However, there was no association between
flotillin-2 expression and factors such as age, gender, tumor
differentiation, tumor size or tumor nodule.
Prognostic indicator of flotillin-2
upregulation in iCCA
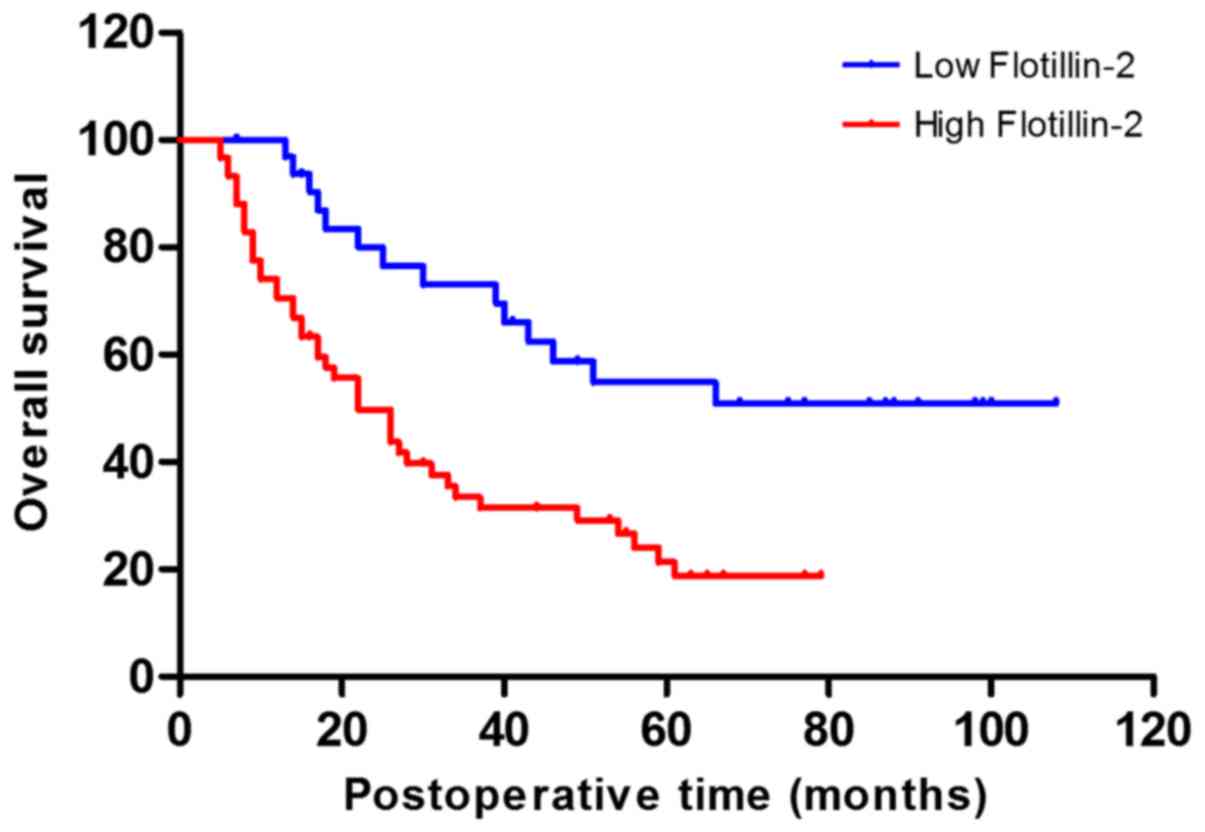
Kaplan-Meier analysis was performed to investigate
whether there was an association between flotillin-2 upregulation
and patient OS. As demonstrated in Fig.
3, patients with high flotillin-2 expression showed a worse OS
outcome compared with those with low expression (P=0.003). In
addition, univariate analysis revealed that tumor differentiation,
lymph node metastasis, M stage, TNM stage as well as flotillin-2
upregulation were significantly associated with decreased OS but
not with the other parameters (Table
II). Furthermore, multivariate analyses revealed that high
flotillin-2 expression (P=0.005), lymph node metastasis (P=0.003)
and TNM stage (P=0.012) were independent markers for poor
prognosis, which further demonstrated high flotillin-2 expression
as an indicator for poor OS in patients with iCCA after curative
resection (Table III).
 | Table II.Univariate analysis of the
clinicopathological features for overall survival of the 92
patients with intrahepatic cholangiocarcinoma. |
Table II.
Univariate analysis of the
clinicopathological features for overall survival of the 92
patients with intrahepatic cholangiocarcinoma.
|
Characteristics | n | Survival rate,
% | P-value |
|---|
| Sex |
|
| 0.422 |
|
Male | 58 | 37.9 |
|
|
Female | 34 | 41.2 |
|
| Age, years |
|
| 0.583 |
|
<60 | 48 | 41.7 |
|
|
≥60 | 44 | 36.4 |
|
| Liver cirrhosis
status |
|
| 0.330 |
| No | 62 | 41.9 |
|
|
Yes | 30 | 33.3 |
|
|
Differentiation |
|
| 0.029a |
|
Well | 19 | 57.9 |
|
|
Moderately | 45 | 42.8 |
|
|
Poorly | 28 | 21.4 |
|
| Tumor size, cm |
|
| 0.695 |
|
<5 | 32 | 43.8 |
|
| ≥5 | 60 | 36.7 |
|
| Tumor nodule |
|
| 0.228 |
|
Solitary | 84 | 39.2 |
|
|
Multiple | 8 | 37.5 |
|
| Hepatolith |
|
| 0.736 |
| No | 80 | 38.8 |
|
|
Yes | 12 | 41.6 |
|
| Tumor stage |
|
| 0.115 |
|
T1+T2 | 70 | 41.4 |
|
|
T3+T4 | 22 | 31.8 |
|
| Lymph node
metastasis |
|
| 0.016a |
| No | 56 | 51.8 |
|
|
Yes | 36 | 19.4 |
|
| M stage |
|
| 0.021a |
| M0 | 87 | 41.4 |
|
| M1 | 5 | 0 |
|
| TNM stage |
|
| 0.001a |
| I | 32 | 65.6 |
|
| II | 14 | 35.7 |
|
|
III | 15 | 33.3 |
|
| IV | 31 | 16.1 |
|
| Flotillin-2
expression |
|
| 0.003b |
|
Low | 33 | 57.6 |
|
|
High | 59 | 28.8 |
|
 | Table III.Multivariate analysis of the
clinicopathological features for overall survival of the 92
patients with intrahepatic cholangiocarcinoma. |
Table III.
Multivariate analysis of the
clinicopathological features for overall survival of the 92
patients with intrahepatic cholangiocarcinoma.
| Factors | P-value | HR | 95% CI |
|---|
| Flotillin-2
expression, low, high | 0.005b | 2.974 | 1.112–4.386 |
| Tumor
differentiation, well, moderately, poorly | 0.461 | 0.852 | 0.510–2.839 |
| Lymph node
metastasis, no, yes | 0.003b | 1.105 | 0.882–3.456 |
| M stage, M0,
M1 | 0.114 | 0.806 | 0.410–3.447 |
| TNM stage, I, II,
III, IV | 0.012a | 0.584 | 0.213–1.795 |
Role of flotillin-2 in cell
invasion
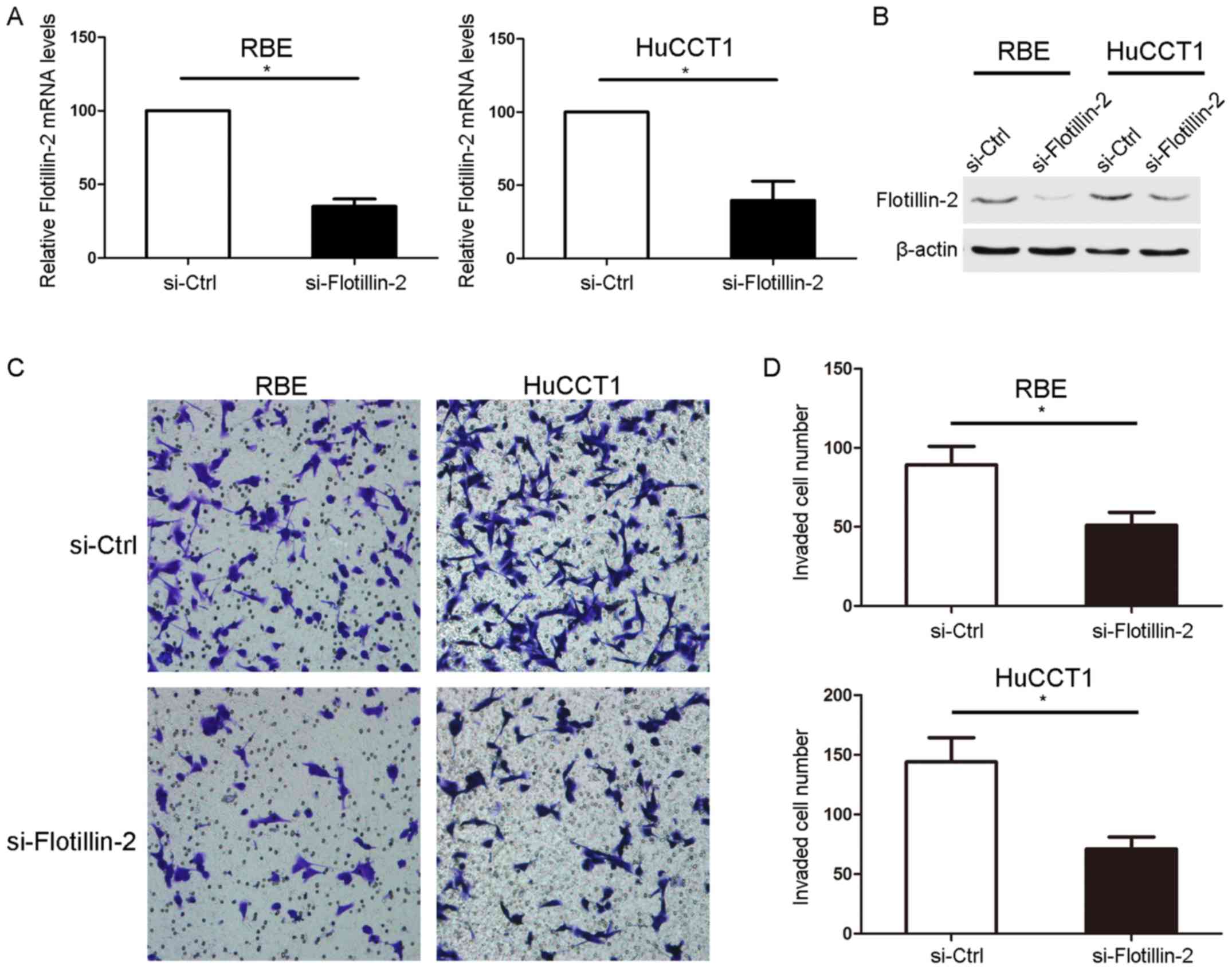
Flotillin-2 siRNA was used to knockdown the mRNA as
well as protein expression levels of flotillin-2 in RBE and HuCCT1
cells (Fig. 4A and B). The effect of
flotillin-2 knockdown on the invasion ability of the cells was
subsequently determined using an invasion assay, which demonstrated
that flotillin-2 knockdown significantly inhibited the invasion
ability of RBE and HuCCT1 cells (Fig. 4C
and D).
Discussion
As highly conserved membrane proteins that assemble
cholesterol- and sphingolipid-rich membrane microdomains,
flotillins are not only considered to be scaffold proteins of lipid
rafts (16), but are also associated
with cellular trafficking, signal transduction, cytoskeleton
remodeling and adhesion (17). In
addition, flotillins serve important roles in tumor development.
The dysregulation of flotillin-2 has been reported in a variety of
tumors. The present study revealed that there was significantly
increased flotillin-2 expression in iCCA tissues compared with the
matched adjacent non-tumor tissues. Flotillin-2 expression was
subsequently detected in an iCCA tissue microarray to investigate
its potential association with clinicopathological parameters and
patient OS. Immumohistochemistry revealed that high flotillin-2
expression was seen in 59 of the totally 92 iCCA samples (64.1%).
Even though the mechanism underlying the upregaultion of
flotillin-2 in iCCA remains still unclear, microRNAs have been
shown to regulate the expression of flotillin-2. Previous studies
found that miR-485 and miR-133 could target flotillin-2, resulting
in inhibition of metastasis or epithelial-mesenchymal transition
(EMT) in lung adenocarcinoma (18,19).
Furthermore, flotillin-2 was targeted by miR-449 in glioma
(20). In addition, miR-802 and
miR-34a have also been reported to target flotillin-2 in prostate
cancer and melanoma respectively (21,22).
Therefore, the dysregulation of microRNAs may account for the
upregulation of flotillin-2, leading to the progression of
iCCA.
The present study revealed that high flotillin-2
expression was closely associated with lymph node metastasis and
TNM stage. Further in vitro study demonstrated that
knockdown of flotillin-2 inhibited the invasion ability of iCCA
cell lines, suggesting that flotillin-2 may serve a role in tumor
invasion and metastasis. This is consistent with several other
reports, which revealed that flotillin-2 is upregulated and
significantly associated with advanced TNM stage and metastasis in
a variety of tumors (23).
Flotillin-2 could act as a biomarker for lymphatic and distant
metastasis, and promote cell metastasis in nasopharyngeal carcinoma
(24). Moreover, flotillin-2 is
associated with lymphovascular invasion in gastric cancer (25), as well as depth of invasion in
colorectal cancer (15), supporting
the participation of flotillin-2 in tumor invasion. Additionally,
flotillin-2 is associated with differentiation in breast, gastric
and cervical cancer (25–27), but not in colorectal cancer (15). In the present study, flotillin-2 had
no association with differentiation, which suggested that this
might be tumor type specific since flotillin-2 was also associated
with tumor size in gastric cancer (25), but not in others. The consensus among
the majority of studies is that flotillin-2 is related to invasion
and metastasis in several types of cancer.
Metastasis marks tumor progression from local
tumorigenesis to an incurable status as well as an increase in
tumor aggressiveness, and significantly affects OS. The present
study revealed a significantly poorer OS outcome in patients with
high flotillin-2 expression compared to those with low flotillin-2
expression. More importantly, the multivariate Cox proportional
hazards model revealed that high flotillin-2 expression was an
independent indicator for poor prognosis. Flotillin-2 has been
shown to be promising as new biomarkers to predict poor prognosis
of patients with a few different kinds of tumors (28), such as non-small-cell lung cancer,
esophageal squamous cell carcinoma and renal cell carcinoma
(29). A link between flotillin-2
and tumor progression has been established, however, the mechanisms
underlying the roles of flotillin-2 in cancer malignancy have not
been completely elucidated. Flotillin-2 is involved in
drug-resistance of colorectal cancer cells, potentially by
mediating the PI3K/Akt signaling pathway (30). Moreover, flotillin-2 plays a
pro-neoplastic role in nasopharyngeal carcinoma and promotes
metastasis through both PI3K/AKT3 and NF-κB signaling pathways
(31). In breast cancer, flotillin-2
induces tumor proliferation through modulation of AKT/FOXO
signaling pathway (32).
Furthermore, flotillin-2 modulated the cell cycle and induced EMT
via the upregulation of twist as a result of ERK1/2 pathway
activation in hepatocellular carcinoma (33).
In the multivariate analysis of our study, lymph
node metastasis and advanced TNM stage, act as independent
indicators for poor prognosis while some known clinicopathological
parameters like tumor differentiation and M stage do not. There are
several potential reasons for this. The sample size as well as the
criteria utilized to enroll the sample, could affect independent
prognostic factors associated with OS. Meanwhile, this may have
been attributed to the interaction of TNM stage with T, N and M
stage (34). In addition, the study
design, for example, whether a study is prospective or
multi-center, may also affect the results. Huang et al
(35) showed that lymphatic
metastasis, rather than TNM stage and tumor differentiation, was an
independent risk factor for OS. By contrast, Yamaoka et al
(36) revealed that tumor
differentiation and lymph node metastasis were not independent
pronostic factors. A large-scale study with multicenter analysis
showed that lymph node metastasis was a significant factor
affecting OS, while tumor differentiation was not (37). Therefore, additional multi-center
studies with large sample numbers are required to validate the
results obtained in the present study.
The present study had a number of limitations.
Firstly, only patients that received radical surgery, without any
pre-operative treatment, were enrolled. It should be noted that the
majority of patients with iCCA had received chemotherapy instead of
radical surgery due to late diagnosis, and were therefore not
enrolled. Meanwhile, 9 patients diagnosed with TNM stage IV in the
present study received chemotherapy following radical surgery.
Therefore, the influence of adjuvant chemotherapy on clinical
outcome is not clear, and whether there is an association between
flotillin-2 expression and chemoresistance in iCCA requires further
investigation. Furthermore, the limited sample size of this study
requires future studies with a larger sample size.
In summary, the present study suggested that
upregulation of flotillin-2 was associated with unfavorable
clinicopathological factors, and more importantly, with decreased
OS in patients with iCCA. Furthermore, flotillin-2 expression
served as an independent marker of poor prognosis in patients with
iCCA and promoted invasion of iCCA cells in vitro.
Therefore, targeting flotillin-2 may provide a potential direction
for the development of novel therapeutic agents for iCCA.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Natural
Science Foundation of Shandong Province of China (grant no.
ZR2017BH095).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZX and TW were involved in the study concept and
design. ZX acquired the samples, and conducted the western blotting
and immunohistochemistry analysis. TW conducted the in vitro
study. ZX and TW performed the analysis and interpretation of data.
HS and XJ were responsible for the design of the study, and the
writing, review and revision of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of The Affiliated Yantai
Yuhuangding Hospital of Qingdao University approved the study. All
patients received an explanation of the aims of the study and
provided written informed consent.
Patient consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maithel SK, Gamblin TC, Kamel I,
Corona-Villalobos CP, Thomas M and Pawlik TM: Multidisciplinary
approaches to intrahepatic cholangiocarcinoma. Cancer.
119:3929–3942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao R, Krishnan S, Bhosale PR, Javle MM,
Aloia TA, Shroff RT, Kaseb AO, Bishop AJ, Swanick CW, Koay EJ, et
al: Ablative radiotherapy doses lead to a substantial prolongation
of survival in patients with inoperable intrahepatic
cholangiocarcinoma: A retrospective dose response analysis. J Clin
Oncol. 34:219–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan KM, Tsai CY, Yeh CN, Yeh TS, Lee WC,
Jan YY and Chen MF: Characterization of intrahepatic
cholangiocarcinoma after curative resection: Outcome, prognostic
factor, and recurrence. BMC Gastroenterol. 18:1802018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moeini A, Sia D, Bardeesy N, Mazzaferro V
and Llovet JM: Molecular Pathogenesis and Targeted Therapies for
Intrahepatic Cholangiocarcinoma. Clin Cancer Res. 22:291–300. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivera-Milla E, Stuermer CA and
Malaga-Trillo E: Ancient origin of reggie (flotillin), reggie-like,
and other lipid-raft proteins: Convergent evolution of the SPFH
domain. Cell Mol Life Sci. 63:343–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Planchon D, Rios Morris E, Genest M,
Comunale F, Vacher S, Bièche I, Denisov EV, Tashireva LA,
Perelmuter VM, Linder S, et al: MT1-MMP targeting to endolysosomes
is mediated by upregulation of flotillins. J Cell Sci.
131:jcs2189252018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banning A, Babuke T, Kurrle N, Meister M,
Ruonala MO and Tikkanen R: Flotillins regulate focal adhesions by
interacting with α-actinin and by influencing the activation of
focal adhesion kinase. Cells. 7:E282018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Z, Cheng F, Yang Y, Zhang F, Chen G
and Liu D: Expression and functional analysis of flotillins in
Dugesia japonica. Exp Cell Res. 374:76–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Head BP, Patel HH and Insel PA:
Interaction of membrane/lipid rafts with the cytoskeleton: Impact
on signaling and function: Membrane/lipid rafts, mediators of
cytoskeletal arrangement and cell signaling. Biochim Biophys Acta.
1838:532–545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stuermer CA: How reggies regulate
regeneration and axon growth. Cell Tissue Res. 349:71–77. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao F, Zhang J, Liu YS, Li L and He YL:
Research advances on flotillins. Virol J. 8:4792011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao K, Xie D, Cao P, Zou Q, Lu C, Xiao S,
Zhou J and Peng X: SiRNA-mediated flotillin-2 (Flot2)
downregulation inhibits cell proliferation, migration, and invasion
in gastric carcinoma cells. Oncol Res. 21:271–279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berger T, Ueda T, Arpaia E, Chio II,
Shirdel EA, Jurisica I, Hamada K, You-Ten A, Haight J, Wakeham A,
et al: Flotillin-2 deficiency leads to reduced lung metastases in a
mouse breast cancer model. Oncogene. 32:4989–4994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Cao C, Xiong Q and Liu D: FLOT2
overexpression is associated with the progression and prognosis of
human colorectal cancer. Oncol Lett. 17:2802–2808. 2019.PubMed/NCBI
|
|
16
|
Vega-Cabrera LA and Pardo-Lopez L:
Membrane remodeling and organization: Elements common to
prokaryotes and eukaryotes. IUBMB life. 69:55–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bodin S, Planchon D, Rios Morris E,
Comunale F and Gauthier-Rouviere C: Flotillins in intercellular
adhesion-from cellular physiology to human diseases. J Cell Sci.
127:5139–5147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mou X and Liu S: MiR-485 inhibits
metastasis and EMT of lung adenocarcinoma by targeting Flot2.
Biochem Biophys Res Commun. 477:521–526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei G, Xu Y, Peng T and Yan J: miR-133
involves in lung adenocarcinoma cell metastasis by targeting FLOT2.
Artif Cells Nanomed Biotechnol. 46:224–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang S, Zheng S, Huang S, Cheng H, Lin Y,
Wen Y and Lin W: Flot2 targeted by miR-449 acts as a prognostic
biomarker in glioma. Artif Cells Nanomed Biotechnol. 47:250–255.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang D, Lu G, Shao Y and Xu D:
microRNA-802 inhibits epithelial-mesenchymal transition through
targeting flotillin-2 in human prostate cancer. Biosci Rep.
37:BSR201605212017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu R, Xie H, Luo C, Chen Z, Zhou X, Xia
K, Chen X, Zhou M, Cao P, Cao K and Zhou J: Identification of FLOT2
as a novel target for microRNA-34a in melanoma. J Cancer Res Clin
Oncol. 141:993–1006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu FT, Qu QG and Zhu ZM: Up-regulation of
Flot-2 protein is related to lymph node metastasis and poor
prognosis in human solid tumors. Minerva Chir. 72:146–156.
2017.PubMed/NCBI
|
|
24
|
Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J
and Liao W: Flotillin-2 promotes nasopharyngeal carcinoma
metastasis and is necessary for the epithelial-mesenchymal
transition induced by transforming growth factor-β. Oncotarget.
6:9781–9793. 2015.PubMed/NCBI
|
|
25
|
Zhu Z, Wang J, Sun Z, Sun X, Wang Z and Xu
H: Flotillin2 expression correlates with HER2 levels and poor
prognosis in gastric cancer. PLoS One. 8:e623652013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Yang Q, Guo L, Li XH, Zhao XH,
Song LB and Lin HX: Flotillin-2 is associated with breast cancer
progression and poor survival outcomes. J Transl Med. 11:1902013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Lin L, Huang Z, Ji B, Mei S, Lin Y
and Shen Z: High expression of flotillin-2 is associated with poor
clinical survival in cervical carcinoma. Int J Clin Exp Pathol.
8:622–628. 2015.PubMed/NCBI
|
|
28
|
Deng Y, Ge P, Tian T, Dai C, Wang M, Lin
S, Liu K, Zheng Y, Xu P, Zhou L, et al: Prognostic value of
flotillins (flotillin-1 and flotillin-2) in human cancers: A
meta-analysis. Clin Chim Acta. 481:90–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu XX, Liu WD, Wang L, Zhu B, Shi X, Peng
ZX, Zhu HC, Liu XD, Zhong MZ, Xie D, et al: Roles of flotillins in
tumors. J Zhejiang Univ Sci B. 19:171–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye DM, Ye SC, Yu SQ, Shu FF, Xu SS, Chen
QQ, Wang YL, Tang ZT and Pan C: Drug-resistance reversal in
colorectal cancer cells by destruction of flotillins, the key lipid
rafts proteins. Neoplasma. 66:576–583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Huang W, Ren C, Wen Q, Liu W, Yang
X, Wang L, Zhu B, Zeng L, Feng X, et al: Flotillin-2 promotes
metastasis of nasopharyngeal carcinoma by activating NF-κB and
PI3K/Akt3 signaling pathways. Sci Rep. 5:116142015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie G, Li J, Chen J, Tang X, Wu S and Liao
C: Knockdown of flotillin-2 impairs the proliferation of breast
cancer cells through modulation of Akt/FOXO signaling. Oncol Rep.
33:2285–2290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM,
Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al: Flot2 promotes
tumor growth and metastasis through modulating cell cycle and
inducing epithelial-mesenchymal transition of hepatocellular
carcinoma. Am J Cancer Res. 7:1068–1083. 2017.PubMed/NCBI
|
|
34
|
Xu YF, Liu HD, Liu ZL, Pan C, Yang XQ,
Ning SL, Zhang ZL, Guo S and Yu JM: Sprouty2 suppresses progression
and correlates to favourable prognosis of intrahepatic
cholangiocarcinoma via antagonizing FGFR2 signalling. J Cell Mol
Med. 22:5596–5606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang C, Tian Y, Peng R, Zhang C, Wang D,
Han S, Jiao C, Wang X, Zhang H, Wang Y and Li X: Association of
downregulation of WWOX with poor prognosis in patients with
intrahepatic cholangiocarcinoma after curative resection. J
Gastroenterol Hepatol. 30:421–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaoka R, Ishii T, Kawai T, Yasuchika K,
Miyauchi Y, Kojima H, Katayama H, Ogiso S, Fukumitsu K and Uemoto
S: CD90 expression in human intrahepatic cholangiocarcinoma is
associated with lymph node metastasis and poor prognosis. J Surg
Oncol. 118:664–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchiyama K, Yamamoto M, Yamaue H, Ariizumi
S, Aoki T, Kokudo N, Ebata T, Nagino M, Ohtsuka M, Miyazaki M, et
al: Impact of nodal involvement on surgical outcomes of
intrahepatic cholangiocarcinoma: A multicenter analysis by the
Study Group for Hepatic Surgery of the Japanese Society of
Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci.
18:443–452. 2011. View Article : Google Scholar : PubMed/NCBI
|