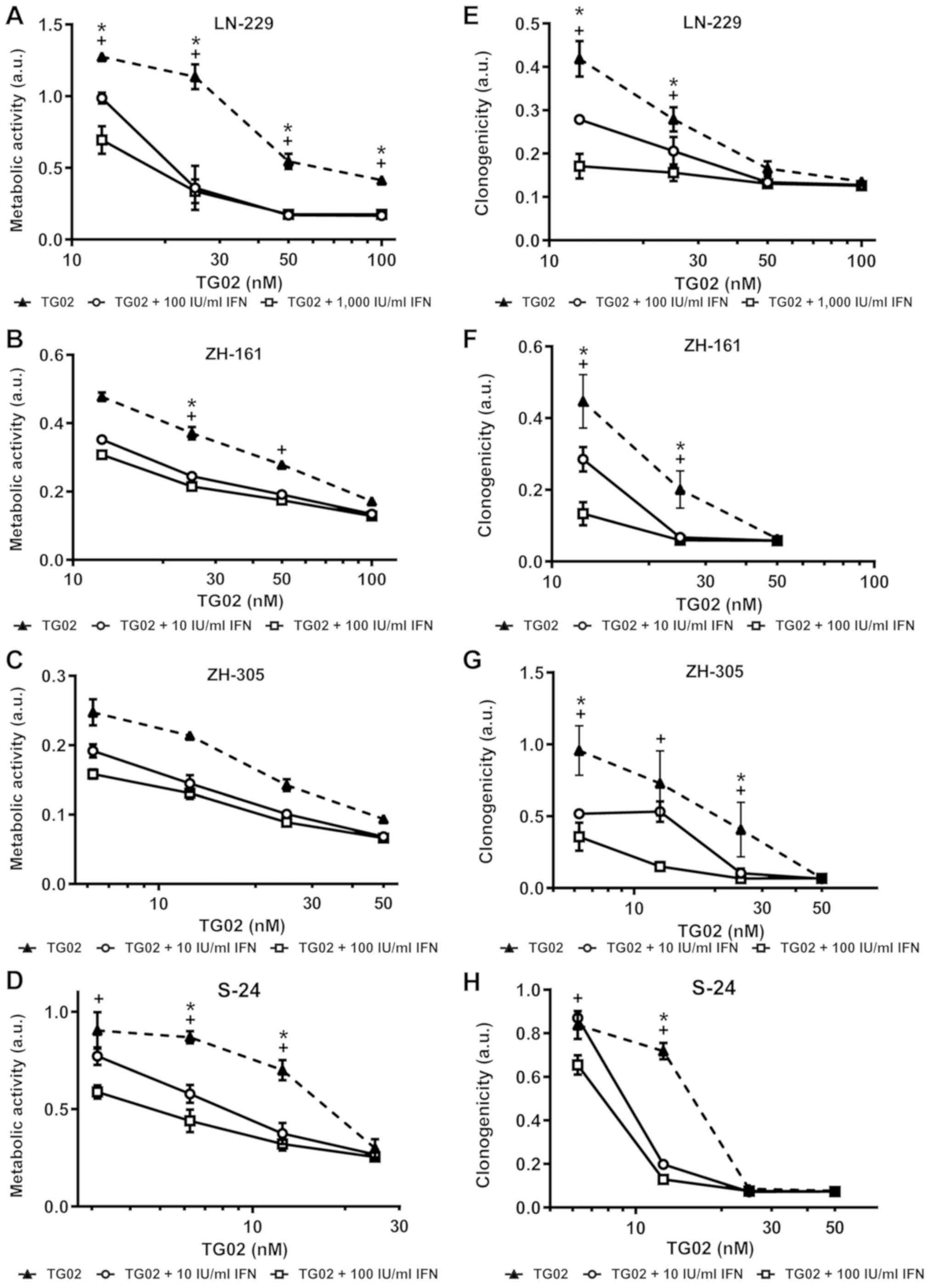
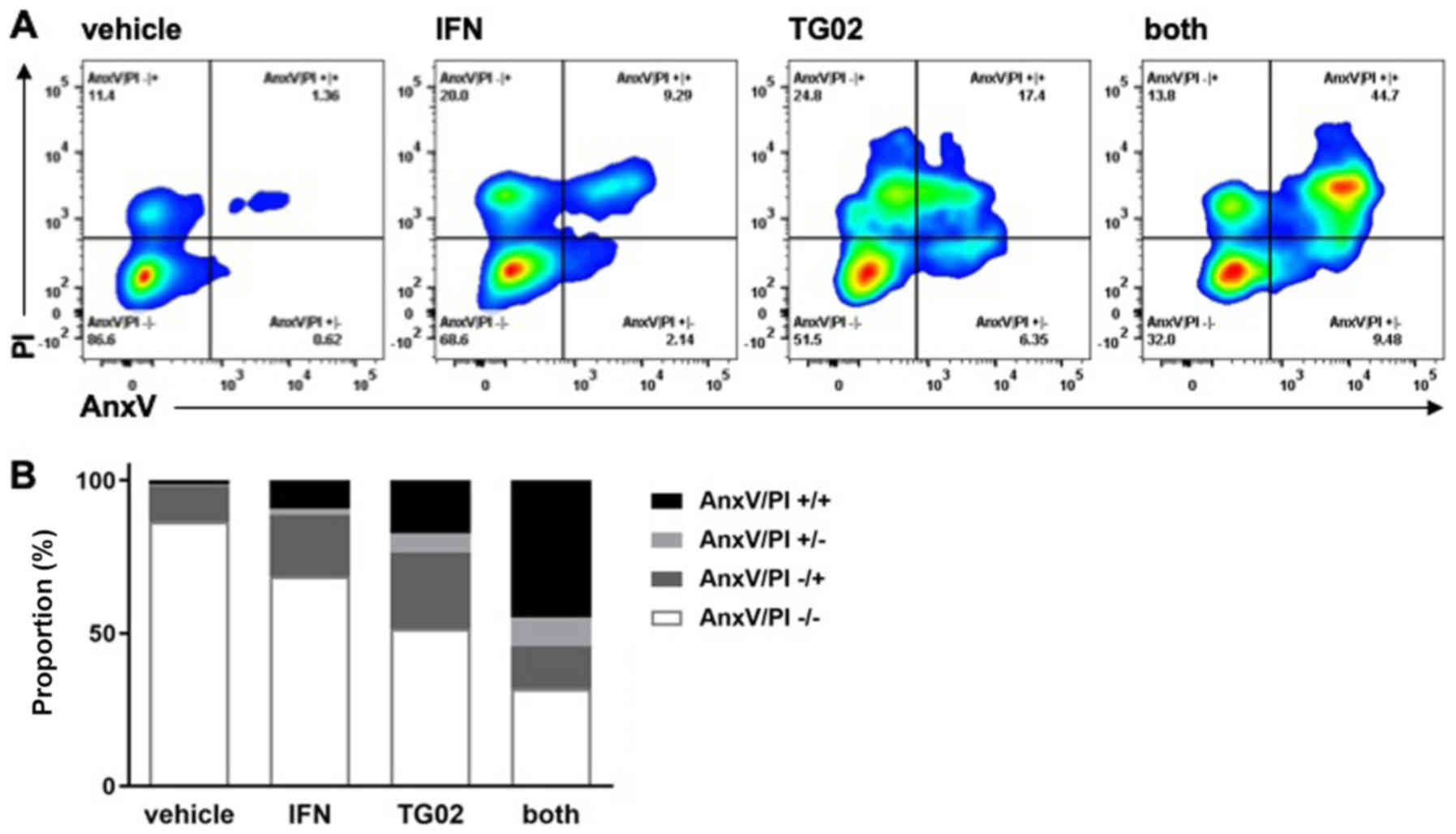
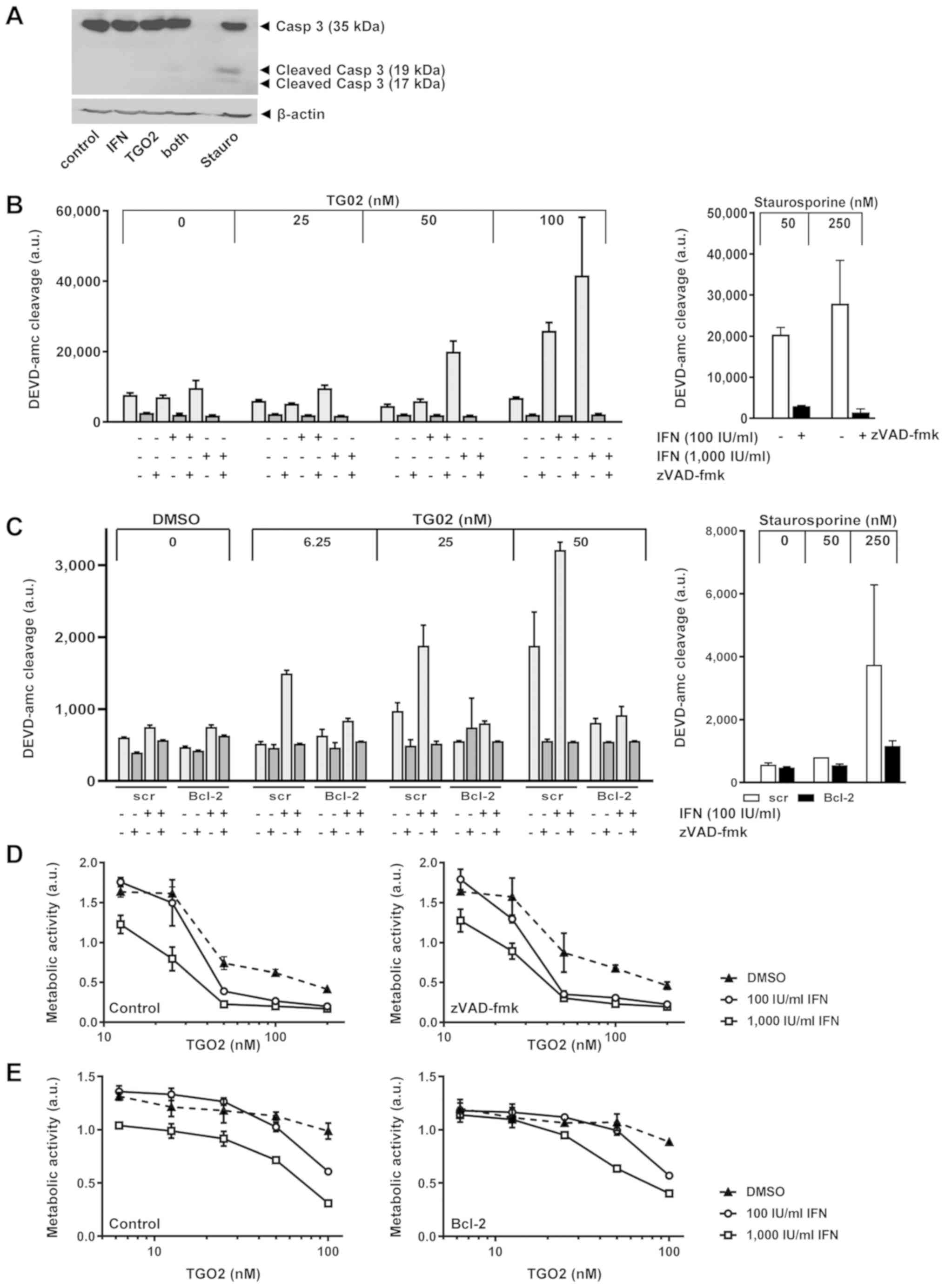
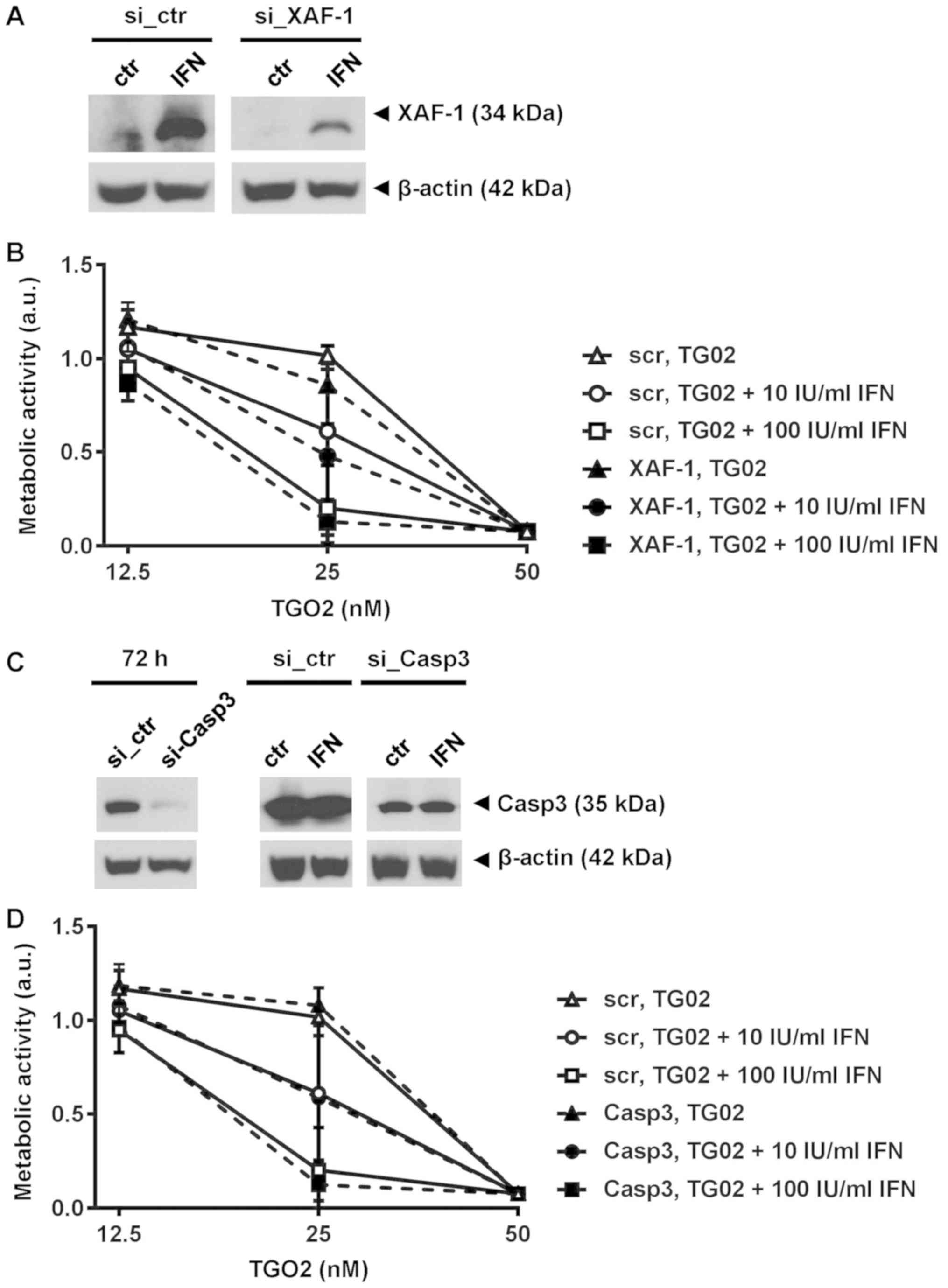
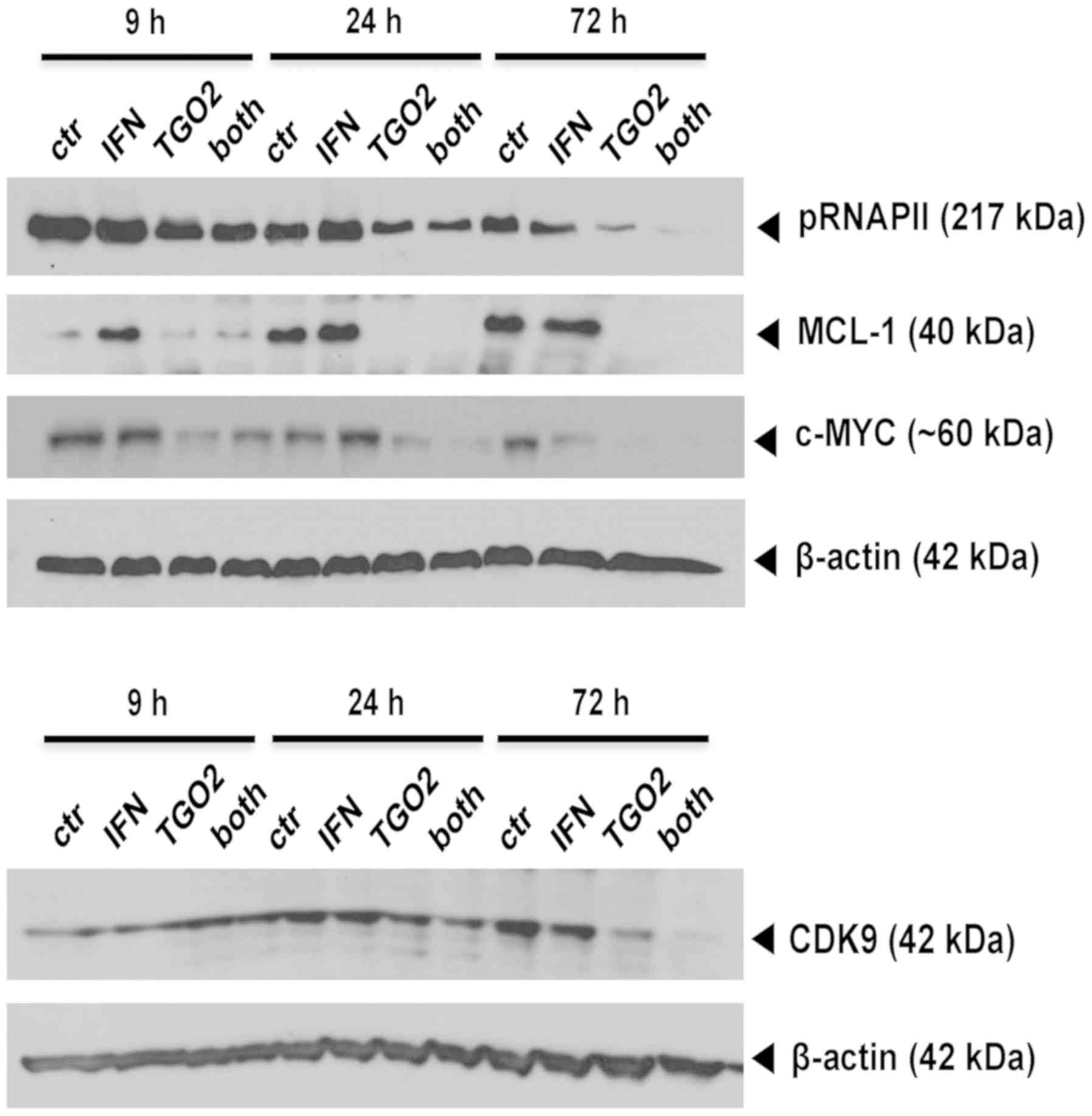
|
1
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the united states in 2011–2015. Neuro Oncol. 20 (Suppl-4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weller M, Wick W, Aldape K, Brada M,
Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and
Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brennan CW, Verhaak RGW, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weller M, van den Bent M, Tonn JC, Stupp
R, Preusser M, Cohen-Jonathan-Moyal E, Henriksson R, Le Rhun E,
Balana C, Chinot O, et al: European association for neuro-oncology
(EANO) guideline on the diagnosis and treatment of adult astrocytic
and oligodendroglial gliomas. Lancet Oncol. 18:e315–e329. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prados MD, Byron SA, Tran NL, Phillips JJ,
Molinaro AM, Ligon KL, Wen PY, Kuhn JG, Mellinghoff IK, de Groot
JF, et al: Toward precision medicine in glioblastoma: The promise
and the challenges. Neuro Oncol. 17:1051–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yue C, Xu J, Tan Estioko MD, Kotredes KP,
Lopez-Otalora Y, Hilliard BA, Baker DP, Gallucci S and Gamero AM:
Host STAT2/type I interferon axis controls tumor growth. Int J
Cancer. 136:117–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du Z, Cai C, Sims M, Boop FA, Davidoff AM
and Pfeffer LM: The effects of type I interferon on glioblastoma
cancer stem cells. Biochem Biophys Res Commun. 491:343–348. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Happold C, Roth P, Silginer M, Florea AM,
Lamszus K, Frei K, Deenen R, Reifenberger G and Weller M:
Interferon-β induces loss of spherogenicity and overcomes therapy
resistance of glioblastoma stem cells. Mol Cancer Ther. 13:948–961.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wakabayashi T, Natsume A, Mizusawa J,
Katayama H, Fukuda H, Sumi M, Nishikawa R, Narita Y, Muragaki Y,
Maruyama T, et al: JCOG0911 INTEGRA study: A randomized screening
phase II trial of interferonβ plus temozolomide in comparison with
temozolomide alone for newly diagnosed glioblastoma. J Neurooncol.
138:627–636. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Rhun E, von Achenbach C, Lohmann B,
Silginer M, Schneider H, Meetze K, Szabo E and Weller M: Profound,
durable and MGMT-independent sensitivity of glioblastoma cells to
cyclin-dependent kinase inhibition. Int J Cancer. 145:242–253.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su YT, Chen R, Wang H, Song H, Zhang Q,
Chen LY, Lappin H, Vasconcelos G, Lita A, Maric D, et al: Novel
targeting of transcription and metabolism in glioblastoma. Clin
Cancer Res. 24:1124–1137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu J, Bryla C, McCoy A, Lisa B, Garren N,
Siegel C, Grajkowska E, Theeler B, Park DM, Parrott T, et al:
ACTR-69. Phase I trial of TG02 plus dose-dense or metronomic
temozolomide for adults with recurrent anaplastic astrocytoma and
glioblastoma. Neuro Oncol. 19 (Suppl-6):vi15. 2017. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greco WR, Bravo G and Parsons JC: The
search for synergy: A critical review from a response surface
perspective. Pharmacol Rev. 47:331–385. 1995.PubMed/NCBI
|
|
17
|
Silginer M, Nagy S, Happold C, Schneider
H, Weller M and Roth P: Autocrine activation of the IFN signaling
pathway may promote immune escape in glioblastoma. Neuro Oncol.
19:1338–1349. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weller M, Trepel M, Grimmel C, Schabet M,
Bremen D, Krajewski S and Reed JC: Hypericin-induced apoptosis of
human malignant glioma cells is light-dependent, independent of
bcl-2 expression, and does not require wild-type p53. Neurol Res.
19:459–470. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fumagalli M, Rossiello F, Mondello C and
d'Adda di Fagagna F: Stable cellular senescence is associated with
persistent DDR activation. PLoS One. 9:e1109692014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim M, Xia Y, Bettegowda C and Weller M:
Current state of immunotherapy for glioblastoma. Nat Rev Clin
Oncol. 15:422–442. 2018. View Article : Google Scholar : PubMed/NCBI
|