Introduction
Osteosarcoma is a type of primary bone tumor that
affects approximately four out of one million people in some
countries, such as Argentina (1).
With the efforts made in treatment of osteosarcoma, survival rate
and time of patients with this disease has been markedly improved
(2). However, no further significant
improvement has been achieved since the 1990s and the overall
survival is still poor (3,4). Patients with early stage osteosarcoma
usually exhibit satisfactory treatment outcomes, following radical
resection. Once distant tumor metastasis occurs surgical resection
is not applicable and survival is extremely low (5). At present, active treatment after early
diagnosis is still the key to treatment of patients with
osteosarcoma.
Transforming growth factor β (TGF-β) is a
multifunctional cytokine that regulates the activation of different
regulatory proteins and downstream substrates that are involved in
cell differentiation, proliferation, chemotaxis and activation of
immune cells (6,7). TGF-β is generally considered as a
double-edged sword in the development of most types of human
malignancies (8); activation of
TGF-β signaling inhibits cancer cell proliferation at the
initiation of tumorigenesis but promotes tumor cell migration and
invasion by mediating epithelial-mesenchymal transition (9). A growing body of literature has
revealed that TGF-β signaling achieves signal transduction in
cancer biology not only by interacting with regulatory proteins,
but also through crosstalk with non-coding RNAs, such as long
non-coding RNAs (lncRNAs) (10,11).
Long intergenic non-coding RNA for kinase activation (LINK-A)
lncRNA is a recently characterized oncogenic lncRNA in
triple-negative breast cancer and ovarian carcinoma (12,13). The
present study demonstrated that LINK-A lncRNA was upregulated in
osteosarcoma and may regulate migration, invasion and stemness of
osteosarcoma cells through TGF-β1.
Materials and methods
Human tissues and cell lines
Plasma samples were collected from 66 patients with
osteosarcoma and 54 healthy volunteers who were admitted by Jining
First People's Hospital (Shizhong, China) between January 2014 and
April 2018. The 66 patients with osteosarcoma included 30 males and
26 females, aged between 16 and 32 years (mean age 23.1±2.1 SD,
years). There were 12 cases in stage I, 14 cases in stage II, 16
cases in stage III and 24 cases in stage IV. The following
inclusion criteria were used: i) Patients diagnosed by biopsies;
ii) patients who were diagnosed for the first time; iii) patients
who fully understood the experimental protocol and signed informed
consent. The exclusion criteria were: i) Patients who were
diagnosed with multiple diseases; ii) patients who were treated
within 6 months before sample collection. The 54 healthy volunteers
included 29 males and 25 females, aged between 17 and 30 years
(mean age, 22.9±2.4 years). The two groups had similar age and sex
distributions.
MG-63 and U2OS osteosarcoma cell lines were
purchased from the American Type Tissue Collection (ATCC; Manassas,
VA, USA). Eagle's minimum essential medium (MEM; cat. no. 30-2003;
ATCC) supplemented with 10% heat-inactivated fetal bovine serum
(FBS, Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to
cultivate cancer cells under normal conditions (37°C; 5%
CO2). In cases of TGF-β1 treatment, cells were treated
in medium containing exogenous TGF-β1 (Sigma-Aldrich; Merck KGaA)
at doses of 10, 20, 30, 40 and 50 ng/ml for 24 h prior to further
experiments.
Cell transfection
Vectors (pcDNA3.1) containing LINK-A lncRNA short
hairpin RNA (shRNA
5′-AAAAGCTTCTCTCACCCTTCAAATTGGATCCAATTTGAAGGGTGAGAGAAGC-3′) were
designed and synthesized by Shanghai GeneChem Co., Ltd. (Shanghai,
China). MISSION shRNA Control Vector was purchased from
Sigma-Aldrich (cat. no. SHC001; Merck KGaA). LINK-A (NCBI ID,
NR_015407.1) lncRNA overexpression vectors (pcDNA3.1) and empty
vectors (pcDNA3.1) were provided by Shanghai Sangong Pharmaceutical
Co., Ltd. (Shanghai, China). Lipofectamine® 2000 reagent
(cat. no. 11668-019; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to transfect 15 nM vectors into
5×105 MG-63 and U2OS cells. Transfections were performed
at 37°C for 6 h. Non-transfected cells were used as control cells.
Cells transfected with empty vectors were used as negative
transfection control cells. Transfection efficiency was determined
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). Cells were harvested 24 h post-transfection prior to
subsequent experiments.
RT-qPCR
Total RNA extraction from 0.3 ml plasma and in
vitro cultivated cells (1×105) was performed using
Monarch® Total RNA Miniprep kit (New England Biolabs,
Inc., Ipswich, MA, USA). Reverse transcription was performed using
High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher
Scientific, Inc.) to synthesize cDNA. PCR reaction systems were
prepared using Luna® Universal One-Step RT-qPCR kit
(SYBR; New England Biolabs, Inc.). CFX96 Touch™ Real-Time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to perform all qPCR reactions. The qPCR thermocycling
conditions were as follows: Initial denaturation at 95°C for 52
sec, followed by 40 cycles of 95°C for 14 sec and 58.5°C for 26
sec. Sequences of primers used were as follows: Human LINK-A,
forward 5′-TTCCCCCATTTTTCCTTTTC-3′, reverse
5′-CTCTGGTTGGGTGACTGGTT-3′; β-actin, forward
5′-GACCTCTATGCCAACACAGT-3′, reverse 5′-AGTACTTGCGCTCAGGAGGA3′. This
experiment was performed in triplicate, and all quantitation cycle
values were normalized to β-actin and relative expression was
quantified by the 2−ΔΔCq method (14).
ELISA
TGF-β1 in 0.3 ml plasma was detected using Human
TGF-β1 Quantikine ELISA Kit (cat. no. DB100B; R&D Systems,
Inc.). All operations were performed following manufacturer's
protocol. Optical density values were detected at 540 nm.
Transwell migration and invasion
assays
QCM Chemotaxis Cell Migration assay, 24-well (8 µm),
colorimetric (cat. no. ECM508; Sigma-Aldrich; Merck KGaA), and QCM
ECMatrix Cell Invasion assay, 24-well (8 µm), colorimetric (cat.
no. ECM550; Sigma-Aldrich; Merck KGaA), were used. In cases of
TGF-β1 treatment, 10 ng/ml TGF-β1 (Sigma-Aldrich; Merck KGaA) was
added to the MEM according to manufacturer's instructions. Briefly,
serum-free cell suspensions (3×104 cells/ml) were made
and 0.1 ml of a cell suspension was transferred to the upper
chamber of the Transwell plates. Culture medium containing 20% FBS
was added into the lower chamber. Cells were cultivated for 24 h at
37°C. Membranes were cleaned using a cotton swab, followed by
staining with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for
20 min at room temperature. This protocol was used for both
invasion and migration assays; prior to the invasion assay the
upper chamber was coated with Matrigel (cat. no. 356234; EMD
Millipore, Billerica, MA, USA). The experiments were performed in
triplicate. Cells were observed and counted under Olympus CX43
light microscope (×40 magnification).
Flow cytometry
MG-63 and U2OS cells (3×105) were
trypsinized, harvested, and incubated with phycoerythrin
(PE)-conjugated CD133 (1:1,500; cat. no. 566593; BD Biosciences,
San Jose, CA, USA) or immunoglobulin (Ig) G1-PE antibody (1:1,500;
cat. no. 130-093-193; Miltenyi Biotec, Bergisch Gladbach, Germany)
in buffer (1X PBS + 0.5% BSA, Sigma-Aldrich; Merck KGaA) for 20 min
at 4°C. Signals were detected using a FACS Aria flow cytometry
system (BD Immunocytometry Systems, San Jose, CA, USA) and
processed by Cell Quest software v5.1 (BD Biosciences). This
experiment was performed in triplicate.
Western blotting
Total protein was extracted from in vitro
cultivated cells (1×105) using the Total Protein
Extraction kit provided by Merck KGaA (cat. no. 2140). Protein
concentrations were measured using BCA assay (Sangon Biotech Co.,
Ltd., Shanghai, China). Proteins (30 µg per lane) were separated by
12% SDS-PAGE and gel transferred to PVDF membranes. Blocking was
performed using PBS containing 5% skimmed milk for 1 h at room
temperature. Primary antibodies against TGF-β1 (cat. no. ab9758;
rabbit anti-human; 1:1,200; Abcam, Shanghai, China) and GAPDH
(ab9485; rabbit anti-human; 1:1,400; Abcam) were used at 4°C for 18
h. The secondary antibody was horseradish peroxidase-conjugated
goat anti-rabbit IgG (cat. no. MBS435036; 1:1,000; MyBioSource,
Inc., San Diego, CA, USA). ECL™ western blotting reagents
(Sigma-Aldrich; Merck KGaA) were added to develop signals. ImageJ
software v.1.46 (National Institutes of Health, Bethesda, MD, USA)
was used for densitometry analysis. TGF-β1 expression was
normalized to GAPDH. This experiment was performed in
triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation;
values were calculated and processed using GraphPad Prism 6
software (GraphPad Software, Inc., La Jolla, CA, USA). Pearson
correlation coefficient was used for correlation analysis.
Student's t-test was used for comparisons between two groups.
One-way analysis of variance followed by Tukey test was used for
comparisons among multiple groups. Diagnostic analysis was
performed by receiver operating characteristics (ROC) curve.
P<0.05 was considered to indicate a statistically significant
difference.
Results
LINK-A lncRNA and TGF-β1 are
upregulated in patients with osteosarcoma compared with healthy
controls
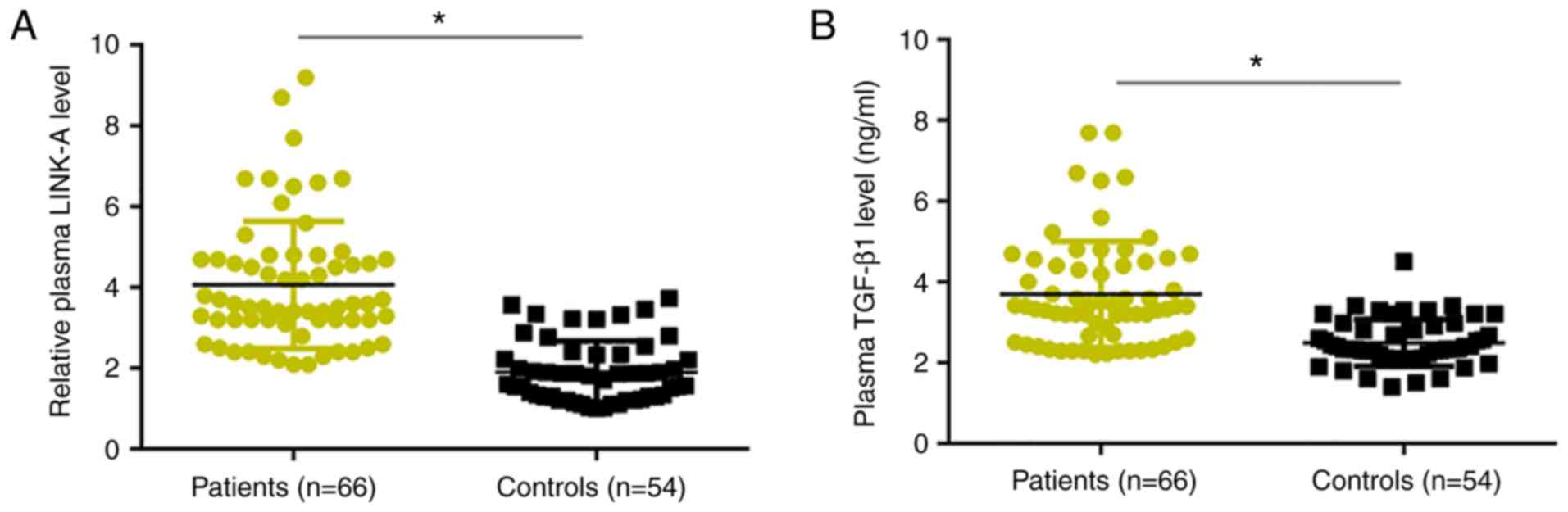
RT-qPCR results revealed that, compared with healthy
controls, plasma levels of LINK-A lncRNA were significantly
increased in patients with osteosarcoma (Fig. 1A). Similarly, ELISA results
demonstrated that plasma levels of TGF-β1 were significantly higher
in patients with osteosarcoma compared with levels in healthy
controls (Fig. 1B).
Plasma LINK-A lncRNA and TGF-β1 are
positively correlated in patients with osteosarcoma and in healthy
controls
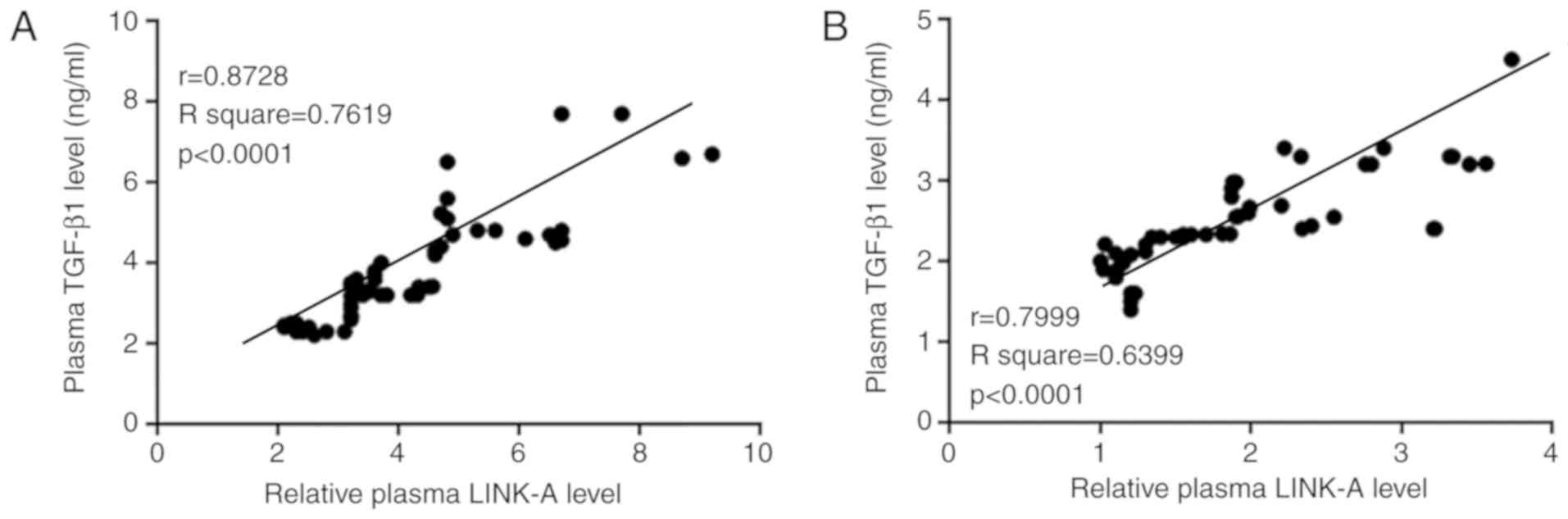
Pearson correlation coefficient analysis
demonstrated that plasma levels of LINK-A lncRNA and TGF-β1 were
positively correlated in both osteosarcoma patients (Fig. 2A) and in healthy controls (Fig. 2B).
Plasma LINK-A lncRNA has diagnostic
potentials for early stage osteosarcoma
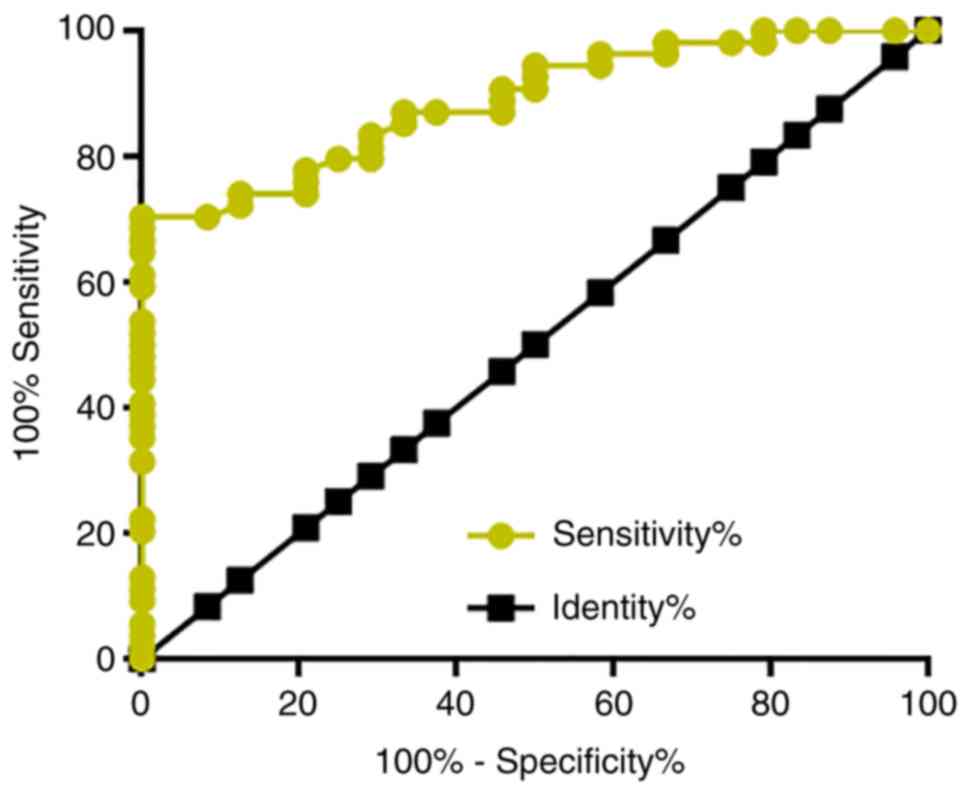
Among the 66 patients with osteosarcoma, there were
26 cases in stage I and II, which are considered as the early
stages of cancer development. ROC curve analysis was performed to
evaluate the diagnostic value of plasma LINK-A lncRNA for early
stage osteosarcoma. The area under the curve was 0.8877, with
standard error of 0.03549 and 95% confidence interval of
0.8182–0.9673 (P<0.0001; Fig.
3).
LINK-A lncRNA positively regulates
TGF-β1 in MG-63 and U2OS osteosarcoma cell lines
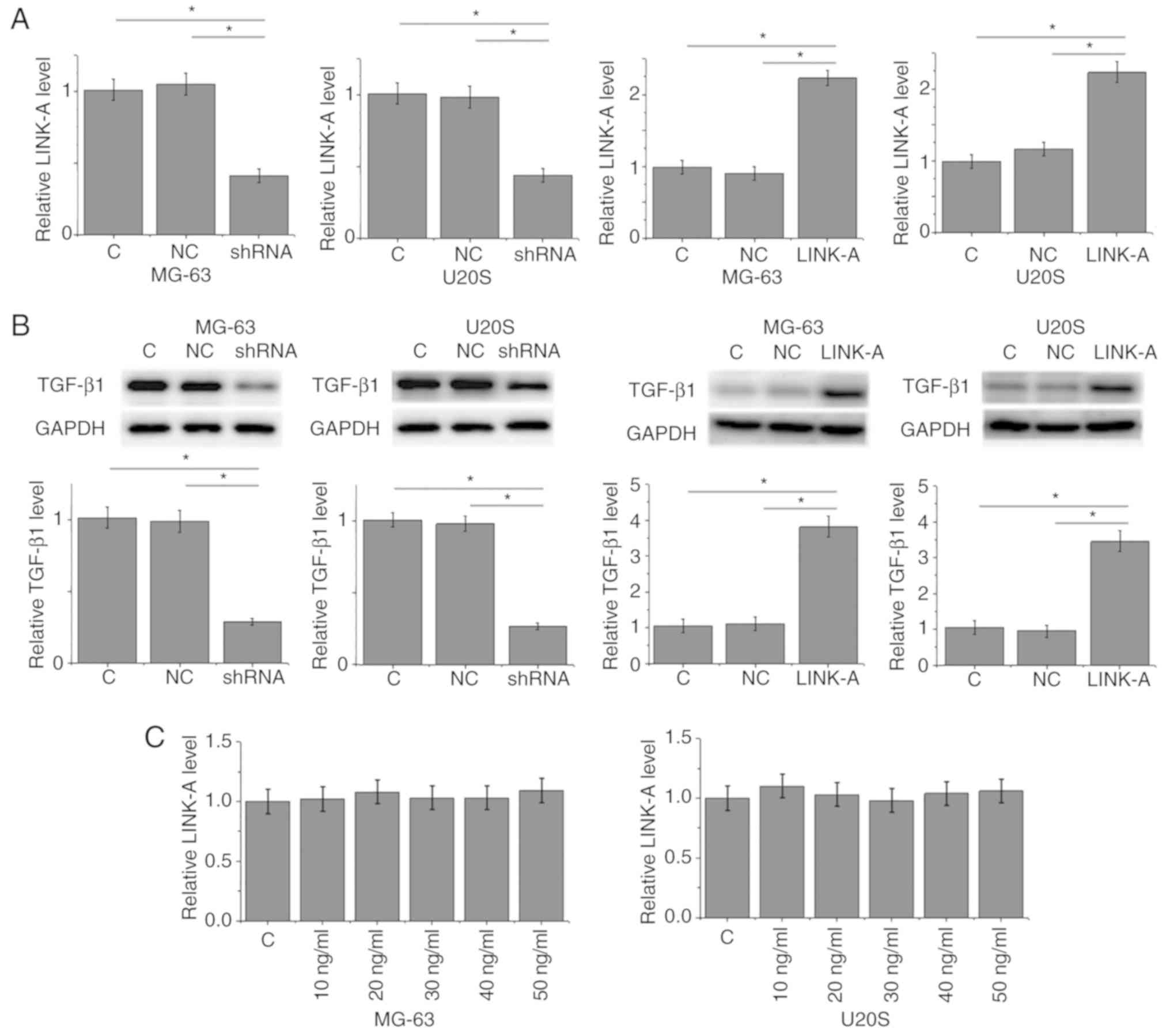
RT-qPCR analysis was used to confirm that LINK-A
lncRNA expression was decreased or increased following shRNA or
overexpression vector transfection, respectively, in MG-63 and U2OS
osteosarcoma cell lines compared with the control groups (Fig. 4A). LINK-A lncRNA shRNA mediated the
downregulation, whereas LINK-A lncRNA overexpression mediated the
upregulation of TGF-β1 expression in the two osteosarcoma cell
lines (Fig. 4B). By contrast,
exogenous TGF-β1 treatment at doses of 10, 20, 30, 40 and 50 ng/ml
failed to significantly alter the expression of LINK-A lncRNA in
these cells (Fig. 4C).
LINK-A lncRNA knockdown inhibits
migration, invasion and stemness of MG-63 and U2OS cells
Compared with the control groups, LINK-A lncRNA
knockdown significantly inhibited, whereas treatment with TGF-β1 at
a dose of 10 ng/ml significantly promoted the migration (Fig. 5A), invasion (Fig. 5B) and stemness (reflected by
percentage of CD133+ cells; Fig. 5C) of cells of MG-63 and U2OS
osteosarcoma cell lines. In addition, exogenous TGF-β1
significantly attenuated the effects of LINK-A lncRNA knockdown
(Fig. 5).
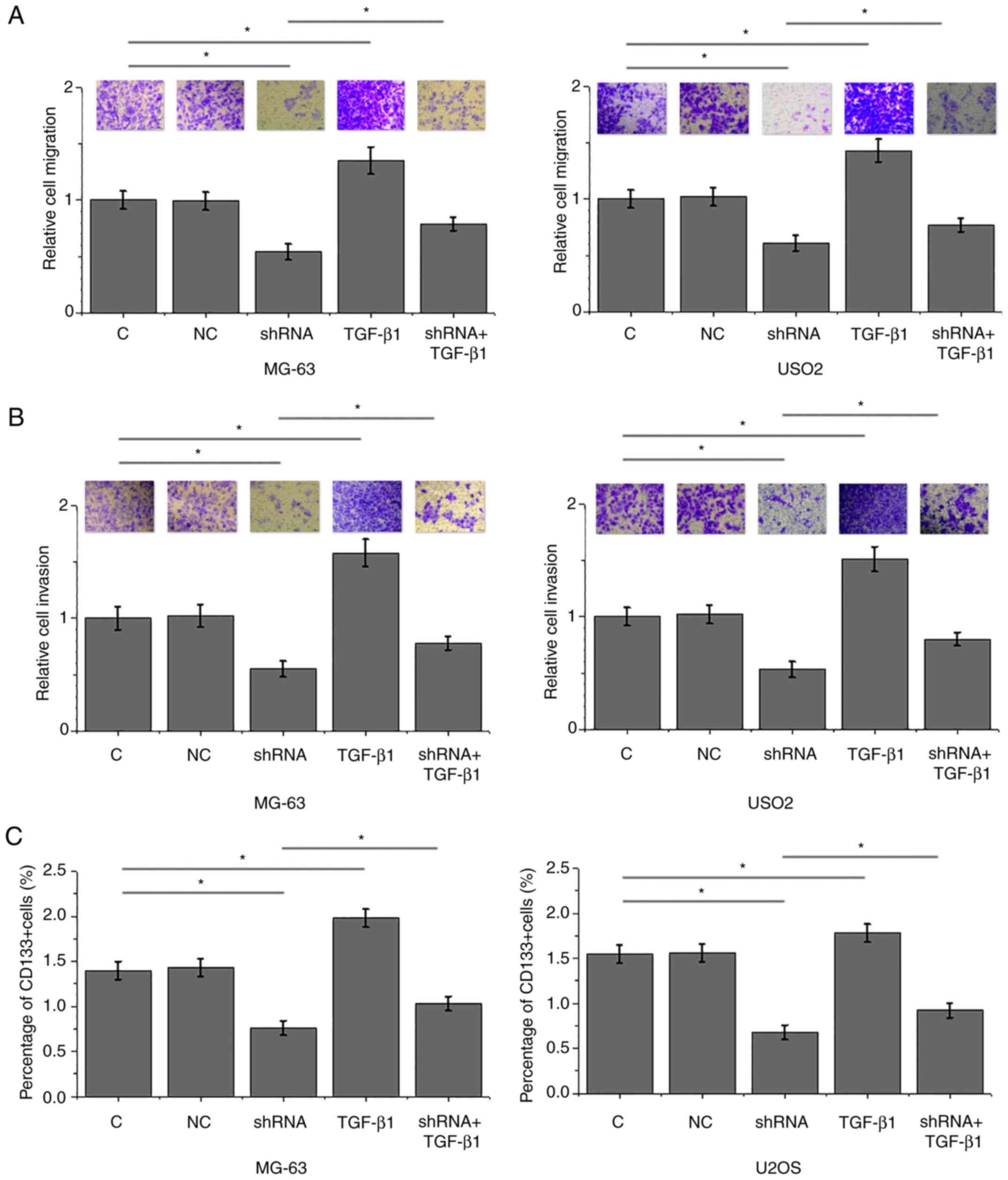 | Figure 5.LINK-A lncRNA knockdown mediates the
inhibition of migration, invasion and stemness of MG-63 and U2OS
osteosarcoma cell lines through TGF-β1. (A-C) LINK-A lncRNA
knockdown significantly inhibited, whereas treatment with TGF-β1
(10 ng/ml) significantly promoted the (A) migration, (B) invasion
and (C) stemness of cells of MG-63 and U2OS osteosarcoma cell
lines. In addition, exogenous TGF-β1 co-treatment significantly
attenuated these effects of LINK-A lncRNA knockdown. For invasion
and migration data, C group was set to ‘100’ and all other groups
were normalized to C. *P<0.05. C, control; LINK-A, long
intergenic non-coding RNA for kinase activation; lncRNA, long
non-coding RNA; NC, negative control; TGF-β1, transforming growth
factor β1. |
Discussion
LINK-A lncRNA is a recently characterized lncRNA
with oncogenic functionality in triple-negative breast cancer and
ovarian carcinoma (12,13). The key finding of the present study
was that LINK-A lncRNA may also be an oncogenic lncRNA in
osteosarcoma and may participate in cell migration, invasion and
stemness. The actions of LINK-A lncRNA were at least partially
mediated by TGF-β1.
It has been reported that the development of
osteosarcoma affects the expression of a large set of genes in the
human body, including lncRNAs (15,16). In
the present study LINK-A lncRNA was significantly upregulated in
plasma from patients with osteosarcoma compared with healthy
controls. A recent study has demonstrated that LINK-A lncRNA is
involved in the regulation of migration and invasion of ovarian
carcinoma cells (13). The present
study revealed that inhibition of LINK-A lncRNA inhibited migration
and invasion and reduced stemness of cancer cells in osteosarcoma
in vitro. Therefore, inhibition of LINK-A lncRNA may serve
as a potential therapeutic target for osteosarcoma. It is also
worth mentioning that inhibition of LINK-A lncRNA exhibited no
significant effects on osteosarcoma cell proliferation (data not
shown), which indicated that LINK-A lncRNA is unlikely to be
involved in the regulation of proliferation of osteosarcoma, which
is consistent with the previous study (13).
Early diagnosis is crucial for the survival of
patients with osteosarcoma. The present study enrolled 26 patients
with osteosarcoma at stages I and II. ROC curve analysis revealed
that the upregulation of LINK-A lncRNA distinguished patients with
early stage osteosarcoma from healthy controls. Therefore,
circulating LINK-A lncRNA may be a potential marker for the early
diagnosis of osteosarcoma. However, clinical trials are needed to
further test the applicability.
Activation of TGF-β is frequently observed in cancer
development (17,18). Inhibition of TGF-β signaling is
considered a promising therapeutic target for cancers (19). The present study also demonstrated
significantly higher plasma levels of TGF-β1 in patients with
osteosarcoma compared with healthy controls. It is known that TGF-β
signals in cancer by interacting with lncRNAs (10,11). The
present study indicated that LINK-A lncRNA may be upstream of
TGF-β1 in the regulation of migration, invasion and stemness of
osteosarcoma. As the plasma levels of LINK-A lncRNA and TGF-β1 were
revealed to be positively correlated in both osteosarcoma and
healthy controls, the interaction between LINK-A lncRNA and TGF-β1
is unlikely to be disease-specific. However, whether this
interaction is direct or indirect is still unknown. LINK-A lncRNA
overexpression and knockdown failed to affect TGF-β1 expression at
the mRNA level (data not shown); therefore, LINK-A lncRNA may
affect TGF-β1 accumulation or degradation, but not gene
transcription.
In conclusion, LINK-A lncRNA and TGF-β1 were both
upregulated in osteosarcoma. LINK-A lncRNA may regulate TGF-β1 to
participate in the regulation of migration, invasion and stemness
of cancer cells in osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YK did the experiments, analyzed the data and was a
major contributor in writing the manuscript. ZN, CM and HG
performed the experiments and literature reviews, and analyzed
data. All authors read and approved the final manuscript and all
authors should confirm its accuracy.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of Jining First People's Hospital, Shizhong, Jining,
China. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and national research committees, the 1964
Declaration of Helsinki and its later amendments. Informed written
consent was obtained from all patients and controls following an
explanation of the nature and possible consequences of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moreno F, Cacciavillano W, Cipolla M,
Coirini M, Streitenberger P, López Martí J, Palladino M, Morici M,
Onoratelli M, Drago G, et al: Childhood osteosarcoma: Incidence and
survival in Argentina. Report from the national pediatric cancer
registry, ROHA network 2000–2013. Pediatr Blood Cancer. 64:2017.
View Article : Google Scholar
|
|
2
|
Durfee RA, Mohammed M and Luu HH: Review
of osteosarcoma and current management. Rheumatol Ther. 3:221–243.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berner K, Johannesen TB, Berner A,
Haugland HK, Bjerkehagen B, Bøhler PJ and Bruland ØS: Time-trends
on incidence and survival in a nationwide and unselected cohort of
patients with skeletal osteosarcoma. Acta Oncol. 54:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slade AD, Warneke CL, Hughes DP, Lally PA,
Lally KP, Hayes-Jordan AA and Austin MT: Effect of concurrent
metastatic disease on survival in children and adolescents
undergoing lung resection for metastatic osteosarcoma. J Pediatr
Surg. 50:157–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakao A, Afrakhte M, Morén A, Nakayama T,
Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH
and ten Dijke P: Identification of Smad7, a TGFbeta-inducible
antagonist of TGF-beta signalling. Nature. 389:631–635. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soderberg SS, Karlsson G and Karlsson S:
Complex and context dependent regulation of hematopoiesis by
TGF-beta superfamily signaling. Ann N Y Acad Sci. 1176:55–69. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akhurst RJ and Derynck R: TGF-beta
signaling in cancer-a double-edged sword. Trends Cell Biol.
11:S44–S51. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Willis BC and Borok Z: TGF-beta-induced
EMT: Mechanisms and implications for fibrotic lung disease. Am J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mondal T, Subhash S, Vaid R, Enroth S,
Uday S, Reinius B, Mitra S, Mohammed A, James AR, Hoberg E, et al:
MEG3 long noncoding RNA regulates the TGF-β pathway genes through
formation of RNA-DNA triplex structures. Nat Commun. 6:77432015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Dong M, Fan D, Hou P, Li H, Liu L,
Lin C, Liu J, Su L, Wu L, et al: lncRNA ANCR down-regulation
promotes TGF-β-induced EMT and metastasis in breast cancer.
Oncotarget. 8:67329–67343. 2017.PubMed/NCBI
|
|
12
|
Lin A, Li C, Xing Z, Hu Q, Liang K, Han L,
Wang C, Hawke DH, Wang S, Zhang Y, et al: The LINK-A lncRNA
activates normoxic HIF1α signalling in triple-negative breast
cancer. Nat Cell Biol. 18:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma J and Xue M: LINK-A lncRNA promotes
migration and invasion of ovarian carcinoma cells by activating
TGF-β pathway. Biosci Rep. 38(pii): BSR201809362018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atiye J, Wolf M, Kaur S, Monni O, Böhling
T, Kivioja A, Tas E, Serra M, Tarkkanen M and Knuutila S: Gene
amplifications in osteosarcoma-CGH microarray analysis. Genes
Chromosomes Cancer. 42:158–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li JP, Liu LH, Li J, Chen Y, Jiang XW,
Ouyang YR, Liu YQ, Zhong H, Li H and Xiao T: Microarray expression
profile of long noncoding RNAs in human osteosarcoma. Biochem
Biophys Res Commun. 433:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Solar M and Paul W: Chain relaxation in
thin polymer films: Turning a dielectric type-B polymer into a
type-A' one. Soft Matter. 13:1646–1653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hawinkels LJ, Paauwe M, Verspaget HW,
Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman
JH, Mesker W, ten Dijke P and Sier CF: Interaction with colon
cancer cells hyperactivates TGF-β signaling in cancer-associated
fibroblasts. Oncogene. 33:97–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colak S and Ten Dijke P: Targeting TGF-β
signaling in cancer. Trends Cancer. 3:56–71. 2017. View Article : Google Scholar : PubMed/NCBI
|