Introduction
In recent years, high-precision radiotherapy has
played an important role in cancer treatment. However, recurrence
and distant metastasis caused by residual cancer cells remain major
concerns and lead to a poor outcome (1). It has been reported that inflammatory
signaling cascades promote tumor survival, while inducing
detrimental effects in normal tissues (2). Further, inflammatory response has been
reported to play a crucial role in different stages of tumor
development, including, initiation, transformation, invasion, and
metastasis, and is known to promote angiogenesis, proliferation,
and resistance to apoptosis (3,4). These
factors contribute to poor prognosis post radiotherapy. Therefore,
targeting the inflammatory signaling pathway could inhibit
angiogenesis, proliferation, and resistance to apoptosis and help
radio-sensitize the tumor cells. In addition, this targeting could
preferentially radio-sensitize the tumor cells without affecting
the normal cells.
4-Methylumbelliferone (4-MU) is a hyaluronan (HA)
synthesis inhibitor that has been demonstrated to possess
anti-tumor and anti-invasion/metastatic effects by blocking the
interaction between hyaluronan and CD44 and suppressing downstream
signaling (5). In addition, 4-MU has
been reported to inhibit HA synthesis (HAS) (6), and especially HAS2 that is involved in
synthesis of high molecular weight-HA (HMW-HA) (7,8). These
references reported that the interaction between hyaluronan and its
receptor CD44 in bone metastasis and the characteristics of the HAS
including HAS1, HAS2, and HAS3. HA has unique biological effects on
cells, depending on the molecular weight. Generally, it has been
reported that low molecular weight-HA (LMW-HA) has
pro-inflammatory, pro-angiogenic, and pro-tumorigenic effects,
while HMW-HA has anti-inflammatory and anti-tumor effects (9). However, many researchers have
identified that these effects are not entirely accurate. A recent
study demonstrated that HMW-HA correlates with poor survival in
patients with pancreatic cancer (10). In addition, it has been shown that
4-MU suppresses inflammatory cytokines such as IL-6, −8 and
chemokines, and has anti-inflammatory effects (11). The anti-inflammatory effects of 4-MU
may be associated with its ability to block the synthesis of
endogenous HA and inhibit the lipopolysaccharide (LPS)-induced
up-regulation of inflammatory mediators and inflammatory-related
receptors, like the toll-like receptor 4 (TLR4). It has been
reported that nuclear factor kappa-light-chain (NF-κB), a
transcription factor, is activated via the IL-6 signaling network
and promotes the formation of cancer stem cells and mesenchymal
stem cells (12). Moreover, IL-6
activates the phosphorylation of signaling pathways associated with
cancer survival, such as PI3K/Akt and JAK/STAT, upon binding IL-6R.
In addition, overexpression of IL-6 induced higher distant
metastasis by cancer cells (13,14).
In our previous study, administration of a
combination of 100 µM 4-MU and 2 Gy X-ray irradiation caused
downregulation of matrix metalloproteinases-2 and −9 and inhibited
the colony-forming and metastatic potential of HT1080 human
fibrosarcoma cells (15). Although
many studies reveal the relationship cancer mechanism and
hyaluronan, there are few studies that the effect of HA synthesis
inhibition and irradiation. In addition, the relationship between
4-MU mediated inhibition of hyaluronan synthesis and the consequent
inflammatory and radio-sensitizing effects remains unclear.
Therefore, we investigated the sensitization mechanism of 4-MU in
HT1080 cells and sought to clarify these relationships in this
study.
Materials and methods
Reagents
4-MU was purchased from Nacalai Tesque, and diluted
in dimethylsulfoxide (DMSO) (Wako Pure Chemical Industries, Ltd.)
at a working concentration of 500 µM. We used DMSO at a final
concentration of 0.05%. To clearly observe the effects of4-MU, 500
µM 4-MU was used, which also minimized the cytotoxic effects on
normal fibroblast cells (16).
LMW-HA (15–40 kDa, cat. no. GLR001) and HMW-HA (>950 kDa, cat.
no. GLR002) were purchased from R&D Systems and used at working
concentrations of 200 µg/ml and 100 µg/ml, respectively.
Cell culture
HT1080 human fibrosarcoma cells from American Type
Culture Collection were cultured in Roswell Park Memorial Institute
(RPMI) 1640 medium (Thermo Fisher Scientific Inc.) supplemented
with 10% heat-inactivated fetal bovine serum (FBS; Japan Bio Serum)
and 1% penicillin/streptomycin (Life Technologies) at 37°C in a
humidified atmosphere of 5% CO2. HT1080 cell is suitable
for verifying the hyaluronan synthesis inhibition effect of 4-MU
because it has a high self-hyaluronan production and high invasion
abilities.
Clonogenic potency assay
The clonogenic potency of HT1080 cells was evaluated
using the colony formation assay. We seeded the control group with
300 cells, the 500 µM 4-MU group with 20000 cells, the 2 Gy X-ray
irradiation group with 600 cells, and the 4-MU and 2 Gy X-ray
irradiation group with 50000 cells on φ60 culture dishes and
incubated for 2 h, and then subjected to X-ray irradiation at 2 Gy
in the presence of 500 µM 4-MU and 200 µg/ml LMW-HA or 100 µg/ml
HMW-HA, followed by further incubation for 24 h. The 4-MU was then
washed out. To form the appropriate number of colonies, HT1080
cells was seeded different numbers because of cell killing effects
of 4-MU. In addition, we determined the exogenous HA concentration
and use it according to the procedure reported previously (5,17). We
tried to use HMW-HA at the same concentration as LMW-HA, but it did
not dissolve at a concentration of 200 µg/ml. Thus, we used 200
µg/ml LMW-HA or 100 µg/ml HMW-HA. After 7–10 days of incubation,
cells were fixed with methanol (Wako Pure Chemical Industries
Ltd.), stained with Giemsa (Wako Pure Chemical Industries Ltd.).
Colonies consisting of >50 cells were counted. The survival
fraction for HT1080 cells was calculated as the plating efficiency
of the irradiated and/or 4-MU administration samples compared with
that of the control samples.
Irradiation
Ionizing radiation (IR) was delivered using an X-ray
generator (MBR-1520R-3; Hitachi Medical Co.) with 0.5-mm aluminum
and 0.3-mm copper filters at a distance of 45 cm between the focus
and target (150 kV, 20 mA, 1.0 Gy/min). During X-ray exposure, the
total dose and dose rate were monitored with a thimble ionization
chamber (MZ-BD-3; Hitachi Medical Co.) placed next to the sample.
We use the dose rate in air, where air kerma is converted to dose
rate.
HA density quantitation
HA concentration was measured by using the
Hyaluronan Quantikine® ELISA kit (R&D Systems, Inc.)
using the procedure reported previously (15). HA concentration was calculated from a
standard curve of the measured at 450 nm absorbance.
RNA extraction and analysis
Total RNA was extracted from HT1080 cells, and RNA
quality was confirmed according to the procedure reported
previously (15).
Cyanine (Cy)3-labeled cRNA samples were synthesized
from total RNA, and hybridized to 8×60K format SurePrint G3 Human
GE v2 microarray slides (eArray Design ID=039494) according to the
manufacturer's instructions (Agilent Technologies Inc.). Cy3
fluorescence was detected with a DNA microarray scanner (G2600A
SureScan), and processed using Feature Extraction software (both
from Agilent Technologies Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
cDNA was synthesized using a procedure reported
previously with reaction mixture containing forward and reverse
primers (Table I) (15). The reaction was carried out in a
real-time PCR system (StepOne Plus; Life Technologies) under the
following conditions: 95°C for 30 sec, followed by 40 cycles of
95°C for 5 sec, and 54°C for 30 sec. Target gene expression levels
were calculated relative to that of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH, internal control) mRNA using the comparative
ΔΔCq method (18).
 | Table I.Primer sequence of the target
gene. |
Table I.
Primer sequence of the target
gene.
| Primers | Sequence (5–3) |
|---|
| HAS1 forward |
TGTGTATCCTGCATCAGCGGT |
| HAS1 reverse |
CTGGAGGTGTACTTGGTAGCATAACC |
| HAS2 forward |
CTCCGGGACCACACAGAC |
| HAS2 reverse |
TCAGGATACATAGAAACCTCTCA |
| HAS3 forward |
ACCATCGAGATGATTCGAGT |
| HAS3 reverse |
CCATGAGTCGTACTTGTTGAGG |
| IL-1α forward |
GGTTGAGTTTAAGCCAATCCA |
| IL-1α reverse |
TGCTGACCTAGGCTTGATGA |
| IL-1β forward |
TACCTGTCCTGCGTGTTGAA |
| IL-1β reverse |
TCTTTGGGTAATTTTTGGGATCT |
| IL-6 forward |
CACTGGGCACAGAACTTATGTTG |
| IL-6 reverse |
AAAATAATTAAAATAGTGTCCTAACGCCAT |
| IL-36α forward |
CATCTACCTGGGCCTGAATG |
| Il-36α reverse |
GGGTTGGTTTACAAATCCATTA |
| IL-36γ forward |
AAGTGACAGTGTGACCCCAGT |
| IL-36γ reverse |
GGATTCTGGATTCCCAAATAAA |
| IL-37 forward |
CCCCAGTGCTGCTTAGAAGA |
| IL-37 reverse |
TCACCTTTGGACTTGTGTGAA |
| GAPDH forward |
GTGAGGTCGGAGTCAACG |
| GAPDH reverse |
TGAGGTCAATGAAGGGGTC |
Invasion assay
The invasive potential of HT1080 cells was assessed
using a BioCoat Matrigel invasion chamber (BD Biosciences). The
suspension containing 2.5×104 HT1080 cells was plated in
24-well chambers dishes, and incubated for 22 h at 37°C. Invasive
cells were fixed and stained using a Diff-Quik staining kit (Dade
Behring, Inc., Siemens Healthcare GmbH). Invasive cells were
counted, and invasion rate was calculated using the procedure
reported previously (15).
Statistical analysis
Statistical analysis of microarray data was
performed using GeneSpring (Agilent Technologies Inc.). Up- and
downregulated mRNAs transcripts were selected based on fold change
(>2-fold) in irradiated and/or 4-MU-administered samples
relative to control samples. Ingenuity Pathway Analysis (IPA;
Qiagen Silicon Valley) was used for functional analysis of each
transcript. The significance of differences between control and
experimental cultures was evaluated using one-way analysis of
variance and the Tukey-Kramer test. Statistical analyses were
performed using Microsoft Excel 2010 (Microsoft Corporation) with
the add-on software Statcel v3 (OMS Publishing). P<0.05 was
considered to indicate a statistically significant difference.
Results
4-MU inhibits HA synthesis and
invasion/metastasis
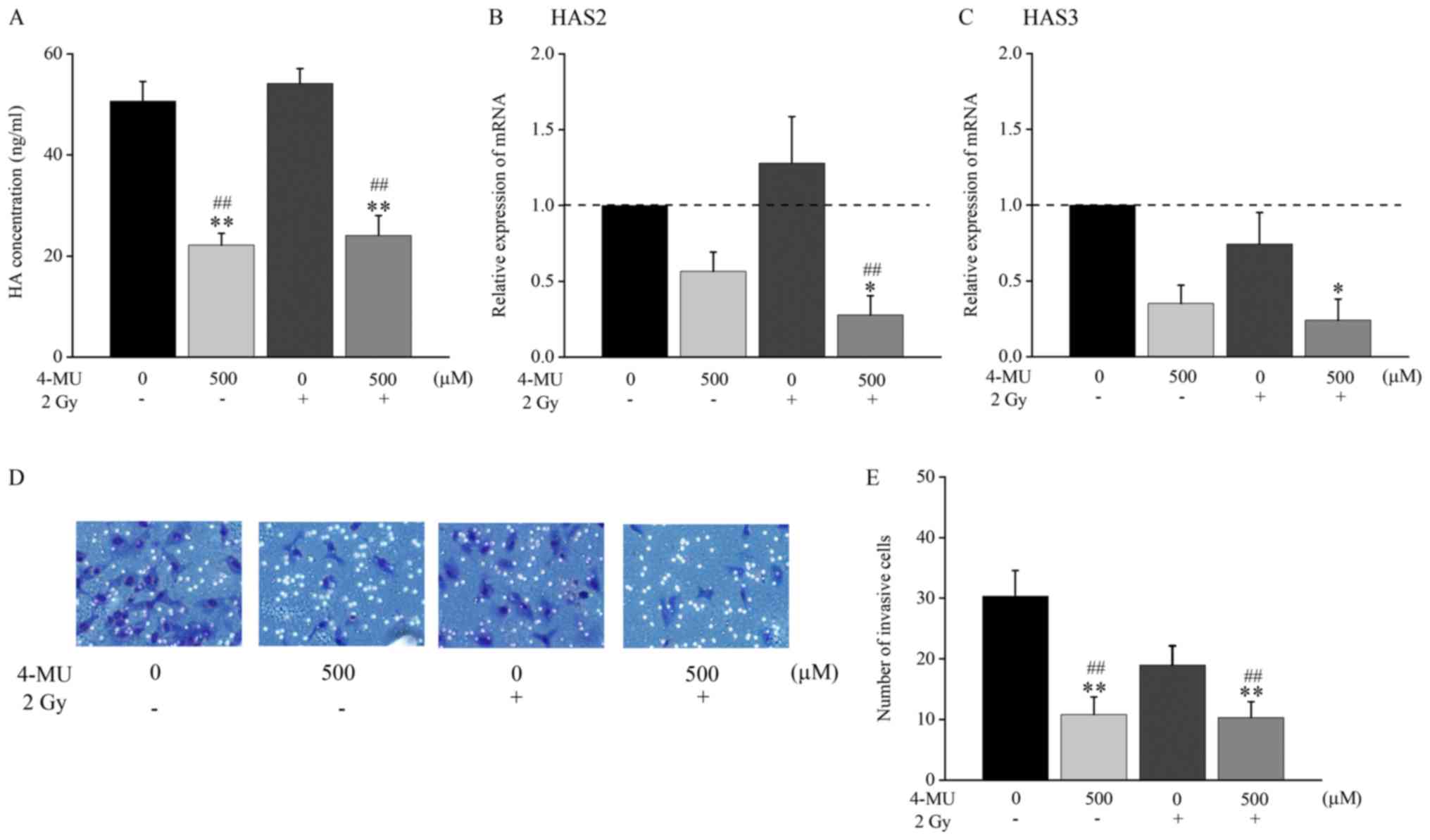
The HA concentration in the cell culture supernatant
following administration of 2 Gy X-ray irradiation and/or 4-MU was
analyzed by ELISA. The concentration of HA was significantly
decreased in the presence of 4-MU as compared to the control (22.17
± 2.36 vs. 50.65 ± 3.90 ng/ml). The concentration of HA in the
combination of 500 µM 4-MU and 2 Gy X-ray irradiation treatment was
also significantly decreased as compared to 2 Gy X-ray irradiation
alone (24.06 ± 3.94 vs. 54.15 ± 2.95 ng/ml; Fig. 1A).
To confirm the effects of 4-MU on HAS, the mRNA
expression of HAS1-3 was evaluated by RT-qPCR. The mRNA expression
of HAS1 was undetectable (data not shown). The mRNA expression of
HAS2 and HAS3 was decreased 0.57 and 0.35 folds, respectively,
against the baseline of 4-MU treatment alone (Fig. 1B and C). Furthermore, the expression
of HAS2 following treatment with 4-MU and 2 Gy X-ray irradiation
was significantly decreased as compared to 2 Gy irradiation alone
(0.27 ± 0.13 vs. 1.28 ± 0.31; Fig.
1B). These results suggested that HAS2 and HAS3 expression were
significantly decreased by 4-MU.
To verify invasion at the cellular level, the
invasion rate potential was measured using a BioCoat Matrigel
invasion chamber (Fig. 1D and E).
The invasion rate of cells treated with 4-MU was significantly
lower than that of the control (10.8 ± 2.91 vs. 30.3 ± 4.23 cells
in the field of view) (Fig. 1E). In
addition, the invasion rate of cells treated with the combination
of 500 µM 4-MU and 2 Gy X-ray irradiation was significantly lower
than that of the cells treated with 2 Gy X-ray irradiation alone
(10.3 ± 2.62 vs. 19.0 ± 3.16 cells in the field of view) (Fig. 1E). This result suggested that
invasion potential was significantly inhibited by 500 µM 4-MU.
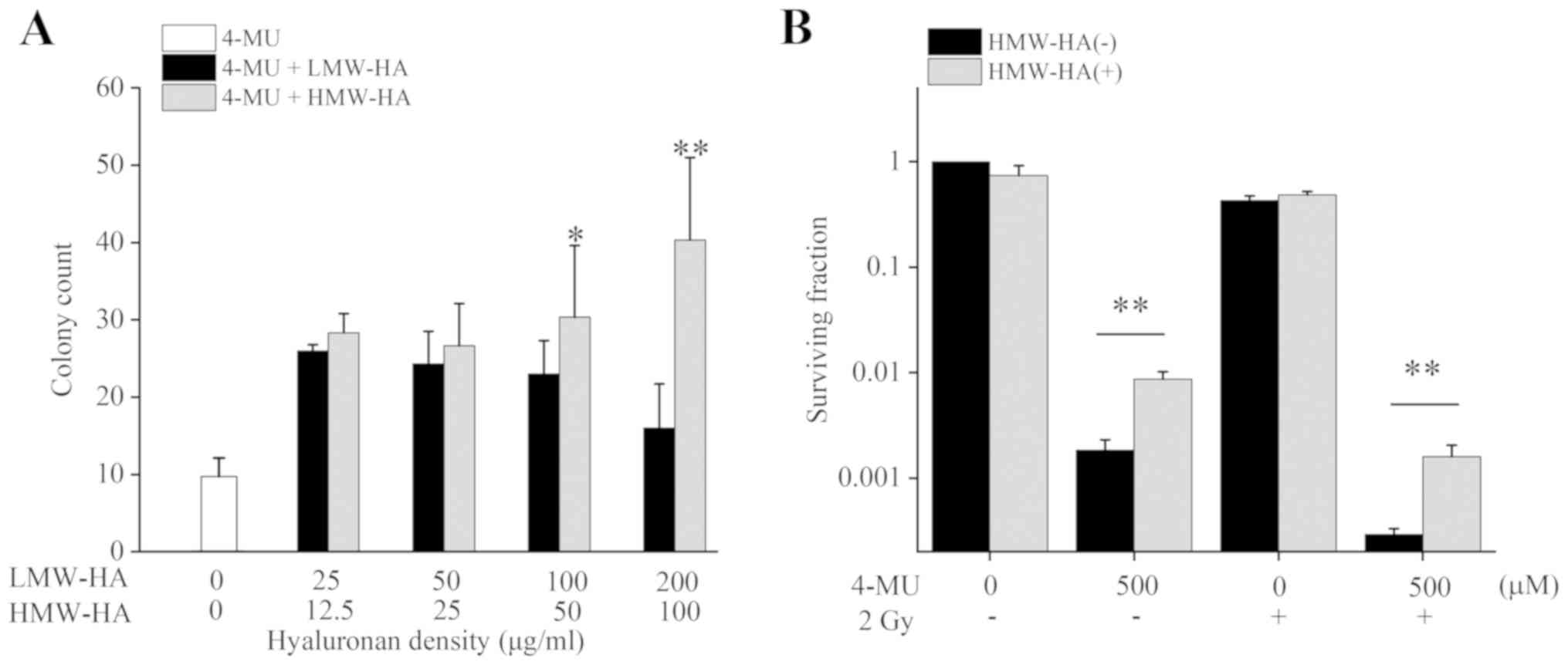
The effects of molecular weight of HA
on surviving fraction
We evaluated the clonogenic potency of HT1080 cells
exposed to 4-MU with LMW-HA or HMW-HA using the colony formation
assay. The number of colony counts following administration of 500
µM 4-MU and exogenous HA significantly increased for both LMW-HA
and HMW-HA (Fig. 2A). The number of
colony counts following 25 µg/ml LMW-HA and 100 µg/ml HMW-HA
treatments 3.1 and 4.8 folds higher than 4-MU administration alone,
respectively, compared to non-HA controls. In addition, cell
survival increased significantly when 100 µg/ml HMW-HA was
administered along with 4-MU (Fig.
2B). These results suggested that exogenous HA administration
suppresses the 4-MU mediated cell-killing effect, with HMW-HA being
more effective than LMW-HA.
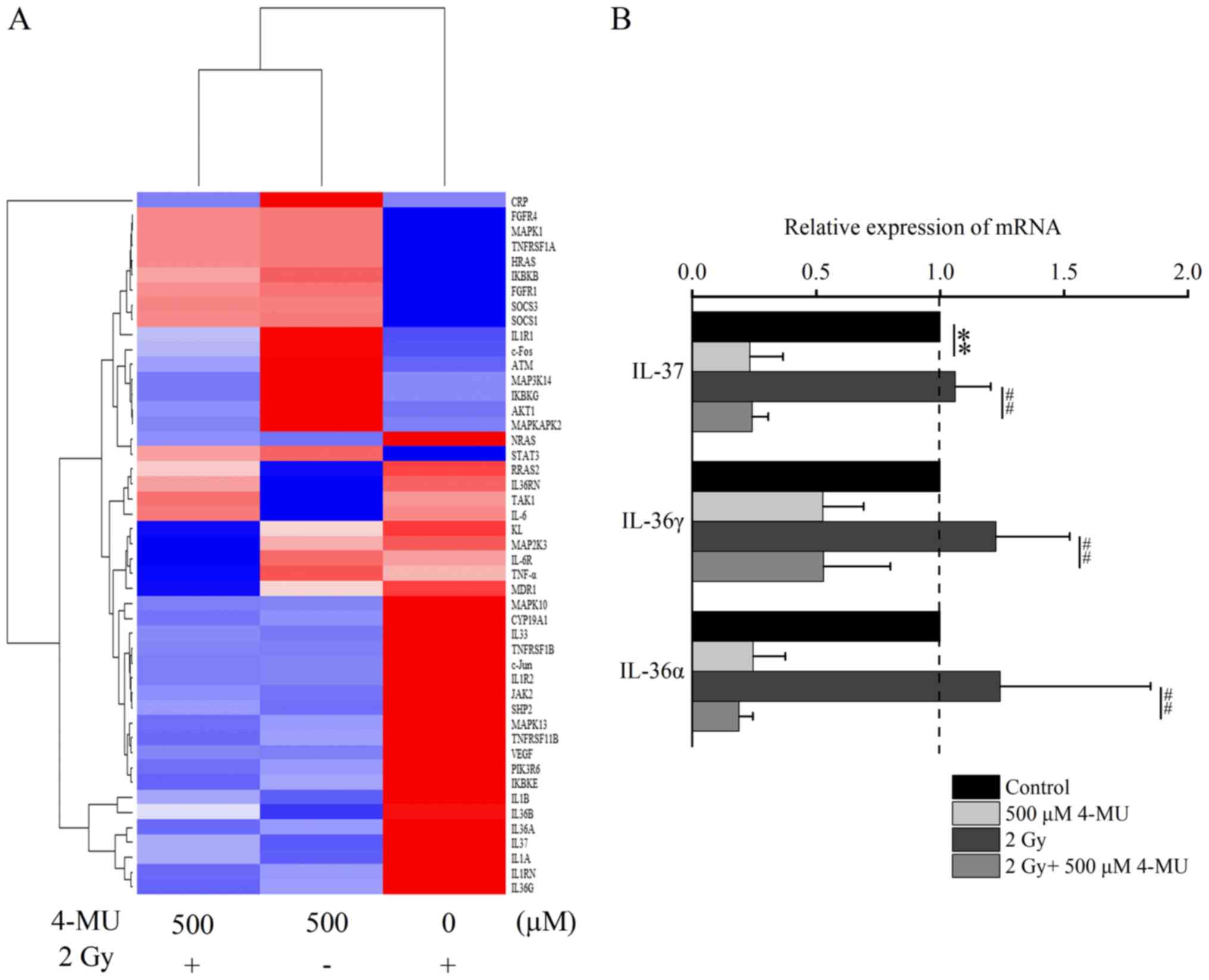
The analysis of IL-1 family mRNA
following exogenous HMW-HA administration
Our previous studies showed that IL-6 mRNA
expression was elevated by 2 Gy X-ray irradiation, and inactivated
by 4-MU (15). Clustering analysis
of genes contained in the IL-6 signaling pathway revealed a total
of 85 genes, with 47 genes showing differential expression pattern
in each treatment. The IL-1 family genes were specifically
identified in 4-MU treatment following clustering analysis of these
47 genes (Fig. 3A). Therefore, we
focused on the IL-1α, −1β, −36α, −36γ, and −37. Our previous study
identified IL-1α, −1β and −6, and this study newly identified
IL-36α, −36γ, and −37 with expression pattern like that of IL-1α
and −1β. To validate the expression of the newly identified genes
IL-36α, −36γ, and −37, we performed RT-qPCR (Fig. 3B). The mRNA expression of IL-36α,
−36γ, −37 was decreased in the presence of 4-MU.
We speculated that the genes suppressed by 4-MU were
associated with HMW-HA since co-administration of exogenous HMW-HA
increased the cell surviving fraction compared to
non-administration HMW-HA. Therefore, we evaluated the mRNA
expression level of IL-1α, −1β, −6, −36α, −36γ, and −37 by RT-qPCR
following exogenous HMW-HA administration. The mRNA expression of
IL-1β, −6, and −36α treated with 4-MU alone was decreased by
exogenous HMW-HA administration (Fig.
4). On the other hand, the mRNA expression level of IL-1α,
−36γ, and −37 was significantly increased. The mRNA expression
following HMW-HA co-administration in 4-MU treatment alone was
increased 8-, 5- and 4-fold for IL-1α, IL-36γ, and IL-37,
respectively, as compared to HMW-HA non-administration. The mRNA
expression following HMW-HA co-administration in the combined
treatment with 4-MU and 2 Gy X-ray irradiation was also increased
4-, 3- and 2.5-fold for IL-1α, IL-36γ, and IL-37, respectively,
compared to HMW-HA non-administration. These results suggested that
HMW-HA up-regulated IL-1α, −36γ, and −37expression, while 4-MU
downregulated them.
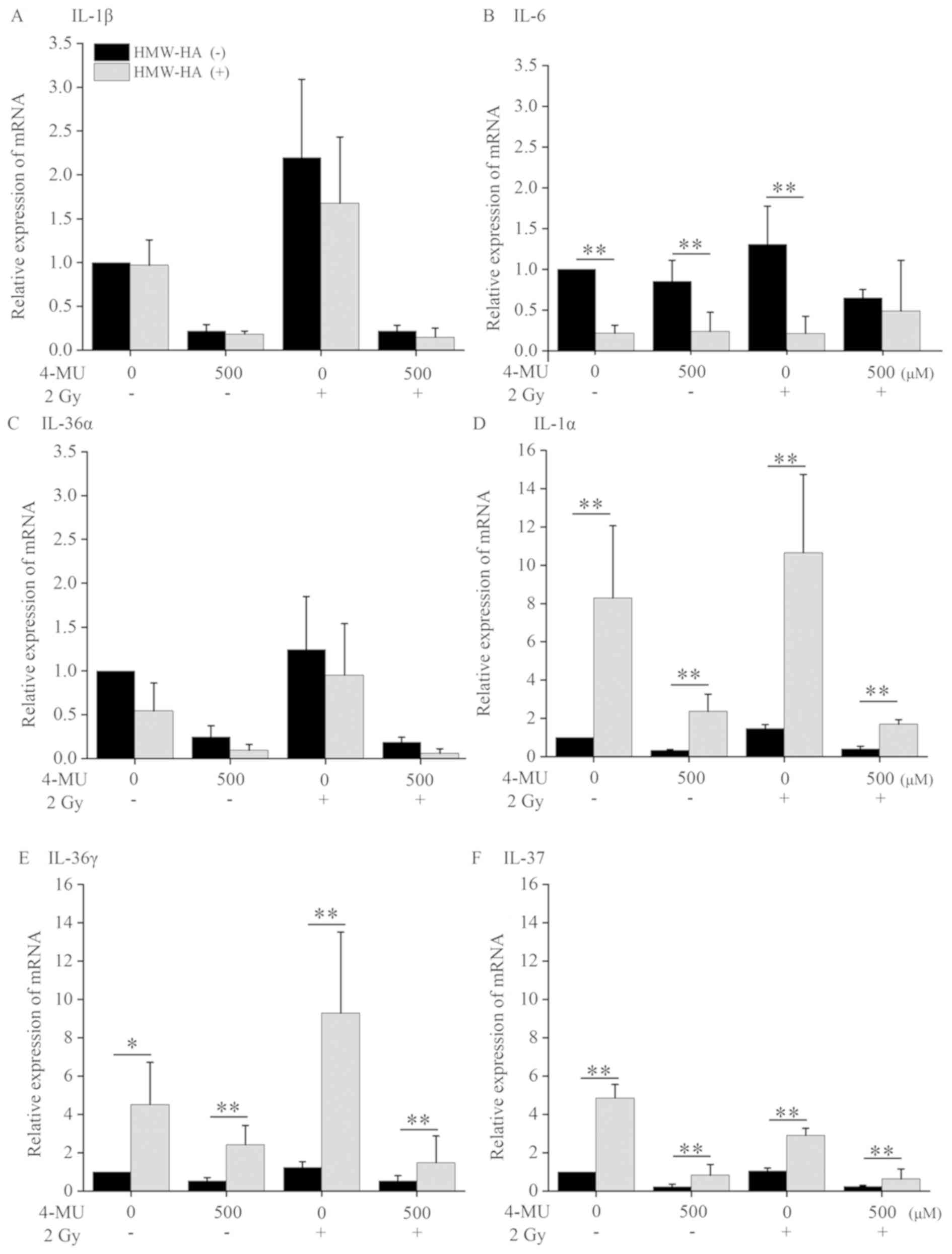 | Figure 4.mRNA expression of the IL-1 family.
HT1080 cells treated with 500 μM 4-MU and/or 2 Gy X-ray
irradiation, with or without HMW-HA, were cultured for 24 h, after
which the mRNA levels of (A) IL-1β, (B) IL-6, (C) IL-36α, (D)
IL-1α, (E) IL-36γ and (F) IL-37 were evaluated by reverse
transcription-quantitative PCR. Data are presented as the mean ±
standard deviation. *P<0.05 and **P<0.01 vs. the HMW-HA (−)
group. IL, interleukin; 4-MU, 4-methylumbelliferone; HMW, high
molecular weight; LMW, low molecular weight; HA, hyaluronan. |
Discussion
We found that 4-MU inhibits HA production and mRNA
expression of HAS2 and −3 in HT1080 cells (Fig. 1A-C). HAS2 is involved in the
synthesis and secretion of HMW-HA (7,8), and it
has been suggested that 4-MU could inhibit the HMW-HA production.
Our results confirmed that cell-killing effect of 4-MU was
suppressed by exogenously administered HMW-HA (Fig. 2A and B). In addition, we found that
mRNA expression of IL-1α, −36γ, and −37, that are associated with
IL-6 signaling, were increased by exogenous administration of
HMW-HA (Fig. 4D, E and F), and that
it could rescue cells from cell-killing effect of 4-MU. On the
other hand, the surviving fraction following 2 Gy X-ray irradiation
alone did not change with or without HMW-HA, which suggests that
HMW-HA is not involved in the radio-sensitization effect of
4-MU.
The mRNA expression levels of IL-1α, −36γ, and −37
were increased by HMW-HA. IL-1α has been reported to consolidate
cellular scaffolds in HT1080 cells (19). It has been reported that IL-36 has a
pro-inflammatory effect in endothelial cells; while it has also
been shown to have anti-inflammatory effect, its exact role remains
to be determined (20). In a recent
study, it was reported that IL-36γ activated pro-inflammatory
signaling pathway on binding on the IL-36 receptor (IL-1R6), and
compensated IL-1RAcp protein that is shared by IL-1α and −1β
(21,22). As a result, IL-36γ activated the
signaling pathway like IL-1α and IL-1β. Given that IL-37 suppresses
the expression of IL-1β (23,24), we
speculated that the expression of IL-1β would be increased by 4-MU
because 4-MU suppresses IL-37 expression. However, we found that
IL-1β was also suppressed by 4-MU. Therefore, this suggested that
IL-37 is not involved in the 4-MU-mediated killing effect. However,
since IL-37 has not yet been fully characterized, further
investigations are necessary to determine its exact role. Although
anoikis, a type of programmed cell death that kills floating cells,
was not measured in this study, it is possible that it was
suppressed by the increased mRNA expression of IL-1α and −36γ by
exogenous HMW-HA administration. It was considered that the
percentage of cells that can survive increased in spite of anoikis
suppression. The results of this study suggested that HMW-HA
rescues cells from the cytotoxicity of 4-MU possibly due to the
enhancement of cellular scaffolds that control anoikis by IL-1α and
−36γ. However, there are still many unclear points regarding the
effects of the IL-36 family on the cancer cells. The IL-36 family
is a factor mainly involved in skin inflammation, and our knowledge
there has been no report describing its function in cancer cells.
Therefore, the results of this study might provide a new facet of
IL-36 cytokines. IL-6 has been shown to inhibit the oxidative
stress response and DNA repair in non-small cell lung cancer cells,
and promote cancer cell resistance to radio- and chemotherapy
(25–27). We evaluated the concentration of the
reactive oxygen species (ROS) as a final messenger in bystander
effects. The concentration of the intracellular ROS increased by
4-MU administration, and attenuated the effect of 4-MU by ROS
scavenger dimethyl sulfoxide and c-PTIO (unpublished data).
However, since IL-6 mRNA expression was suppressed by HMW-HA in
this study, it was inferred that that IL-6 is not the 4-MU
sensitization mechanism in HT1080 cells.
In conclusion, it was suggested that the
cell-killing effect of 4-MU was inhibited by HMW-HA, while the
surviving fraction with 2 Gy X-ray irradiation alone did not
increase with exogenous HA. This result suggested that the
radio-sensitizing effect of 4-MU and its inhibitory effect on
hyaluronan synthesis are not related. Therefore, an alternate
sensitivity mechanism, other than hyaluronan synthesis inhibition,
possibly exists. On the other hand, this study was revealed that
IL-1α, −36γ and −37 are involved in the cell-killing effect of 4-MU
on HT1080.
Acknowledgements
Not applicable.
Funding
The present study was supported by the JSPS
Grant-In-Aid for Young Scientists (grant no. 17K16413).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KH, RS, KO, ET and YH conceived and designed the
present study, performed the experiments and drafted the
manuscript. KH, RS, RT, RF and MC performed the experiments, and
analyzed and interpreted the data. KO, ET, and YH critically
reviewed the article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hara T, Iwadate M, Tachibana K, Waguri S,
Takenoshita S and Hamada N: Metastasis of breast cancer cells to
the bone, lung, and lymph nodes promotes resistance to ionizing
radiation. Strahlenther Onkol. 193:848–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deorukhkar A and Krishnan S: Targeting
inflammatory pathways for tumor radiosensitization. Biochem
Pharmacol. 80:1904–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michael C. Haffner, Chiara Berlato and
Wolfgang Doppler: Exploiting our knowledge of NF-κB signaling for
the treatment of mammary cancer. J Mammary Gland Biol Neoplasia.
11:63–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yates TJ, Lopez LE, Lokeshwar SD, Ortiz N,
Kallifatidis G, Jordan A, Hoye K, Altman N and Lokeshwar VB:
Dietary supplement 4-methylumbelliferone: An effective
chemopreventive and therapeutic agent for prostate cancer. J Natl
Cancer Inst. 107:djv0852015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiraga T, Ito S and Nakamura H: Cancer
stem-like cell marker CD44 promotes bone metastases by enhancing
tumorigenicity, cell motility, and hyaluronan production. Cancer
Res. 73:4112–4122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Itano N, Sawai T, Yoshida M, Lenas P,
Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y,
et al: Three isoforms of mammalian hyaluronan synthases have
distinct enzymatic properties. J Biol Chem. 274:25085–25092. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itano N and Kimata K: Mammalian hyaluronan
synthases. IUBMB Life. 54:195–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Passi A, Vigetti D, Buraschi S and Iozzo
RV: Dissecting the role of hyaluronan synthases in the tumor
microenvironment. FEBS J. 286:2937–2949. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Franklin O, Billing O, Öhlund D, Berglund
A, Herdenberg C, Wang W, Hellman U and Sund M: Novel prognostic
markers within the CD44-stromal ligand network in pancreatic
cancer. J Pathol Clin Res. 5:130–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Hao P, Liu G, Wang W, Han R, Jiang Z
and Li X: Effects of 4-methylumbelliferone and high molecular
weight hyaluronic acid on the inflammation of corneal stromal cells
induced by LPS. Graefes Arch Clin Exp Ophthalmol. 255:559–566.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bharti R, Dey G and Mandal M: Cancer
development, chemoresistance, epithelial to mesenchymal transition
and stem cells: A snapshot of IL-6 mediated involvement. Cancer
Lett. 375:51–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeda K, Fujii N, Nitta Y, Sakihara H,
Nakayama K, Rikiishi H and Kumagai K: Murine tumor cells
metastasizing selectively in the liver: Ability to produce
hepatocyte-activating cytokines interleukin-1 and/or −6. Jpn J
Cancer Res. 82:1299–1308. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reichner JS, Mulligan JA, Palla ME, Hixson
DC, Albina JE and Bland KI: Interleukin-6 production by rat
hepatocellular carcinoma cells is associated with metastatic
potential but not with tumorigenicity. Arch Surg. 131:360–365.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saga R, Monzen S, Chiba M, Yoshino H,
Nakamura T and Hosokawa Y: Anti-tumor and anti-invasion effects of
a combination of 4-methylumbelliferone and ionizing radiation in
human fibrosarcoma cells. Oncol Lett. 13:410–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saga R, Hasegawa K, Murata K, Chiba M and
Nakamura T: Okumura K, Tsuruga E and Hosokawa Y: Regulation of
radiosensitivity by 4-methylumbelliferone via the suppression of
interleukin-1 in fibrosarcoma cell. Oncol Lett. 17:3555–3561.
2019.PubMed/NCBI
|
|
17
|
Lokeshwar VB, Lopez LE, Munoz D, Chi A,
Shirodkar SP, Lokeshwar SD, Escudero DO, Dhir N and Altman N:
Antitumor activity of hyaluronic acid synthesis inhibitor
4-methylumbelliferone in prostate cancer cells. Cancer Res.
70:2613–2623. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H,
Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, et al:
High-molecular-weight hyaluronan--a possible new treatment for
sepsis-induced lung injury: A preclinical study in mechanically
ventilated rats. Crit Care. 12:R1022008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nazarenko I, Marhaba R, Reich E, Voronov
E, Vitacolonna M, Hildebrand D, Elter E, Rajasagi M, Apte RN and
Zöller M: Tumorigenicity of IL-1alpha- and IL-1beta-deficient
fibrosarcoma cells. Neoplasia. 10:549–562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bridgewood C, Stacey M, Alase A, Lagos D,
Graham A and Wittmann M: IL-36γ has proinflammatory effects on
human endothelial cells. Exp Dermatol. 26:402–408. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carrier Y, Ma HL, Ramon HE, Napierata L,
Small C, O'Toole M, Young DA, Fouser LA, Nickerson-Nutter C,
Collins M, et al: Inter-regulation of Th17 cytokines and the IL-36
cytokines in vitro and in vivo: Implications in psoriasis
pathogenesis. J Invest Dermatol. 131:2428–2437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Buhl AL and Wenzel J: Interleukin-36 in
Infectious and Inflammatory Skin Diseases. Front Immunol.
10:11622019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tete S, Tripodi D, Rosati M, Conti F,
Maccauro G, Saggini A, Cianchetti E, Caraffa A, Antinolfi P,
Toniato E, et al: IL-37 (IL-1F7) the newest anti-inflammatory
cytokine which suppresses immune responses and inflammation. Int J
Immunopathol Pharmacol. 25:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamari Y, Kashino G and Mori H:
Acquisition of radioresistance by IL-6 treatment is caused by
suppression of oxidative stress derived from mitochondria after
γ-irradiation. J Radiat Res (Tokyo). 58:412–420. 2017. View Article : Google Scholar
|
|
26
|
Chen Y, Zhang F, Tsai Y, Yang X, Yang L,
Duan S, Wang X, Keng P and Lee SO: IL-6 signaling promotes DNA
repair and prevents apoptosis in CD133+ stem-like cells of lung
cancer after radiation. Radiat Oncol. 10:2272015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan S, Tsai Y, Keng P and Chen Y, Lee SO
and Chen Y: IL-6 signaling contributes to cisplatin resistance in
non-small cell lung cancer via the up-regulation of anti-apoptotic
and DNA repair associated molecules. Oncotarget. 6:27651–27660.
2015. View Article : Google Scholar : PubMed/NCBI
|