Introduction
Hepatocellular carcinoma (HCC) is one of the most
common forms of cancer. The prognosis of patients with HCC is
generally poor, and it is the third leading cause of cancer
mortality worldwide (1). The
recurrence rate within five years of surgical resection or
radiofrequency ablation for HCC is estimated at 70%, which
contributes to the poor prognosis (2). To improve prognosis, it is important to
diagnose recurrence at an early phase and begin appropriate
additional therapies. Although numerous methods for predicting
recurrence have been studied, effective prediction methods have not
yet been established (3,4). At present, alpha-fetoprotein (AFP) and
protein induced by vitamin K absence or antagonist-II (PIVKA-II)
are commonly used as tumor markers and are reported to be
prognostic factors of HCC (3).
However, these markers are sometimes elevated in patients without
HCC who suffer from chronic hepatitis (5). Thus, novel diagnostic markers to detect
HCC with greater sensitivity and specificity are urgently
needed.
In this study, we noted glypican-3 (GPC3), a protein
of about 70 kDa, that attaches to the cell membrane via
glycosylphosphatidylinositol anchors and is expressed in
approximately 70% of HCC cases (6–8). GPC3
has multiple sugar chains and heparan sulfate modification sites
and is known to be related to poor prognosis for HCC (9–12). GPC3
undergoes cleavage at its central region (R358/S359) by an enzyme
called Furin into a N-terminal form of about 40 kDa and a
C-terminal form of about 30 kDa (13). GPC3 is also thought to exist in a
full-length form, the same as the membrane-bound state, with
numerous intermolecular disulfide bonds (14,15).
GPC3 is present in the blood of HCC patients
(16,17). However, the molecular form of GPC3 in
blood is still being debated and there are various opinions about
the soluble form, such as full-length and N-terminal forms
(18,19). Furthermore, levels of GPC3 in blood
differ across reports, with values ranging from several pg/ml to
several µg/ml (12,15,19–22).
Although the roles of GPC3 and its physiological significance have
not yet been elucidated, it is thought to relate to the regulation
of cell differentiation and proliferation and to exert these
functions only after being cleaved and configured into the
full-length form by the disulfide bond between N- and C-terminal
domains (13,23).
There have been several reports about the clinical
usefulness of the N-terminal form of GPC3. Enzyme-linked
immunosorbent assay methods capable of detecting the N-terminal
form of GPC3 have indicated that preoperative N-terminal GPC3 is an
independent biomarker of HCC recurrence (15,19,20).
However, it remains controversial whether full-length GPC3
(FL-GPC3) is a predictive marker of HCC recurrence. Thus, we
developed a new measurement reagent to specifically detect FL-GPC3
and verified its utility for predicting HCC recurrence after radial
surgery, using FL-GPC3 alone or in combination with the
conventional tumor markers AFP and PIVKA-II.
Materials and methods
Samples
Preoperative serum and plasma samples were collected
from 39 patients with HCC who underwent surgical resection at the
National Cancer Research Center East Hospital, Japan. All samples
were frozen and stored at −80°C until measurement. Written informed
consent was obtained from all patients.
Measurement of plasma FL-GPC3, AFP, or
PIVKA-II
AFP and PIVKA-II were measured using an
electrochemiluminescence immunoassay kit (Roche Co.) and
chemiluminescent enzyme immunoassay kit (Eisai Co.), respectively.
The assay system for FL-GPC3 was constructed using a sandwich
immunoassay, in which a monoclonal mouse antibody against its
N-terminus is used to capture the protein and a monoclonal mouse
antibody against the C-terminus is used to detect the protein. The
monoclonal antibody against its N-terminus was labeled with biotin
and the antibody against its C-terminus with alkaline phosphatase.
The immunoassay was performed by first reacting the plasma sample
with the biotinylated antibody, then capturing the immunocomplex
using streptavidin-coated magnetic beads. After washing the beads,
an alkaline phosphatase-labeled antibody was added to form a
sandwich immunocomplex. After a second wash, a luminescent
substrate was added and the luminescence intensity was measured.
All immunoassay steps were performed using a HISCL™ series (Sysmex
Co.), which is an automated immunoassay device. Recombinant GPC3
(R&D Systems Inc.) was used as the assay standard. Standards at
concentrations of 2, 15, 50, 150, 500, and 1,500 pg/ml were
measured and calibration curves were generated by the
four-parameter logistic regression method.
Detecting FL-GPC3 in blood
HepG2 (ATCC HB-8065), a GPC3-positive liver cancer
cell line, was purchased from American Type Culture Collection.
This cell line was authenticated using STR DNA profiling by
PowerPlex® 16 HS system (Promega KK. The Cells were
seeded at 1.6×105 cells/ml and cultured in 250 ml of
Dulbecco's modified Eagle's medium D5796 (Sigma-Aldrich) with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) for 3 days. The
culture supernatant was recovered, filtered through a 0.22 µm
filter, and concentrated 20-fold by centrifugal filters (3 kDa
MWCO). NaCl was added to a final concentration of 400 mM and
Tween-20 was added to a final concentration of 0.1%, after which 50
µg biotinylated N-terminal recognizing antibody and
streptavidin-immobilized beads were added. After reacting at 4°C
for 12 h, beads were collected and washed eight times with 1 ml of
0.1% Tween/PBS. The bound immunocomplex was then eluted with 0.5 ml
of 0.1 M Glycine-HCl pH 2.5/0.1% Tween-20. After concentrating the
eluate with centrifugal filters, 1/5 of the volume was used for
Western blotting and 4/5 was used for analysis by mass
spectrometry. For Western blotting analysis, the sample after
immunoprecipitation and recombinant FL-GPC3 were fractionated by
SDS-PAGE and transferred to a PVDF membrane. Membranes were washed
with 0.1% Tween/TBS three times after incubation with 3% BSA/1%
Tween/TBS for 60 min and incubated with 0.1 µg/ml of primary
antibodies to detect the C-terminus region of GPC3 for 60 min.
Next, membranes were washed three times and incubated with
secondary anti-mouse antibodies conjugated with HRP (dilution,
1:5,000; Cat. No. 330; Medical and Biological Laboratories Co.,
Ltd.) for 60 min. After washing three times, membranes were
incubated with a chemical substrate and measured using the ECL
system LAS-3000 mini (Fuji Firm Co.) according to the
manufacturer's protocols. For mass spectrometry, reverse phase
chromatography was carried out with a L-column Micro (0.1×150 mm,
Chemical Substance Evaluation Research Organization) connected to
an ADVANCE UHPLC SYSTEM (Michrom BioResources, Inc.) using a Thermo
Scientific LTQ Orbitrap XL mass spectrometer. Measurement was
performed under conditions of 0.1% formic acid with acetonitrile as
a solvent at a flow rate of 500 nl/min. The ionization method was
performed under conditions of Nanoflow-LC-ESI with a positive
ionization mode, capillary voltage 1.9 kV, and collision energy
35%.
Statistical analysis
Statistical analyses were performed using Statflex
software (Nankodo, Tokyo, Japan). Patient characteristics were
compared by the Fisher's exact tests for nominal variable and the
Mann-Whitney U test for continuous variables. Survival rates were
analyzed by the Kaplan-Meier method and log rank test. The cutoff
values were determined by receiver operating characteristic (ROC)
curves and area under the curve (AUC) analyses, and Spearman rank
correlation was used for correlation analysis. Statistical
significance was defined as P<0.05.
Results
Patient characteristics
Table I shows the
characteristics of 39 patients with HCC. The median patient age was
67 years (range: 59–71 years) and 31 (79%) were male. 31 patients
(79%) had a hepatic virus infection, 23 of them with HCV and eight
with HBV. Of the total 39 patients, 11 had no recurrence of HCC
within 4 years (non-recurrence group) and 28 had recurrence
(recurrence group). Among the recurrence group, 15 of 28 patients
had early recurrence within 1 year (Table II). Furthermore, six of the 15
patients with early recurrence were stage III or higher, while the
other nine patients were stage II or lower, who were thought to
have a relatively low risk of recurrence (24).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | Number |
|---|
| Age, median
(range) | 67 (59–71) |
| Sex |
|
|
Male | 31 |
|
Female | 8 |
| Liver
condition |
|
|
Cirrhosis | 13 |
| Chronic
hepatitis | 15 |
|
Other | 11 |
| Virus
infection |
|
|
HCV | 23 |
|
HBV | 8 |
|
None | 8 |
| TNM stage |
|
| I | 22 |
| II | 8 |
|
III | 6 |
| IV | 2 |
| Number of
tumors |
|
|
Solitary | 8 |
|
Multiple | 31 |
| Tumor size (mm),
median (range) | 40.0
(25.0–67.5) |
| Vascular
invasion |
|
|
Present | 11 |
|
Absent | 28 |
| Differentiation of
tumor |
|
|
Well | 8 |
|
Moderately | 28 |
|
Poorly | 3 |
| Recurrence |
|
|
Yes | 28 |
| No | 11 |
| AFP (ng/ml), median
(range) | 28.5
(5.7–294.6) |
| PIVKA-II (mAU/ml),
median (range) | 97.0
(26.5–508.5) |
| FL-GPC3 (pg/ml),
median (range) | 21.0
(4.0–55.6) |
 | Table II.Characteristics of patients in the
recurrence and non-recurrence groups. |
Table II.
Characteristics of patients in the
recurrence and non-recurrence groups.
| Variables | Recurrence
(n=28) | Non-recurrence
(n=11) | Fisher's exact test
P-values | Mann-Whitney U test
P-values |
|---|
| Age |
|
| 0.069 |
|
|
≥65 | 8 | 7 |
|
|
|
<65 | 20 | 4 |
|
|
| Sex |
|
| 0.400 |
|
|
Male | 21 | 10 |
|
|
|
Female | 7 | 1 |
|
|
| Liver
condition |
|
| 0.044 |
|
|
Cirrhosis | 23 | 5 |
|
|
| Chronic
hepatitis or other | 5 | 6 |
|
|
| HCV infection |
|
| 0.471 |
|
|
Positive | 18 | 5 |
|
|
|
Negative | 10 | 6 |
|
|
| HBV infection |
|
| 0.400 |
|
|
Positive | 7 | 1 |
|
|
|
Negative | 12 | 10 |
|
|
| Stage |
|
| 0.446 |
|
| I or
II | 18 | 9 |
|
|
| III or
IV | 10 | 2 |
|
|
| Number of
tumors |
|
| 1.000 |
|
|
Solitary | 22 | 9 |
|
|
|
Multiple | 6 | 2 |
|
|
| Tumor size
(mm) |
|
| 0.477 |
|
| ≥5 | 12 | 3 |
|
|
|
<50 | 16 | 8 |
|
|
| Vascular
invasion |
|
| 0.383 |
|
|
Present | 9 | 2 |
|
|
|
Absent | 19 | 9 |
|
|
| Differentiation of
tumor |
|
| 0.259 |
|
| Well or
moderately | 25 | 11 |
|
|
|
Poorly | 3 | 0 |
|
|
| AFP (ng/ml), median
(range) | 35.7
(10.2–420.5) | 5.7 (3.6–59.8) |
| 0.057 |
| PIVKA-II (mAU/ml),
median (range) | 208.0
(25.0–822.0) | 67.0
(30.0–91.0) |
| 0.365 |
| FL-GPC3 (pg/ml),
median (range) | 40.8
(8.5–64.7) | 3.3 (2.9–10.2) |
| 0.002 |
|
≥14.4 | 9 | 10 | 0.001 |
|
|
<14.4 | 19 | 1 |
|
|
| Recurrence within 1
year |
| – | – |
|
|
Yes | 15 |
|
|
|
| No | 13 |
|
|
|
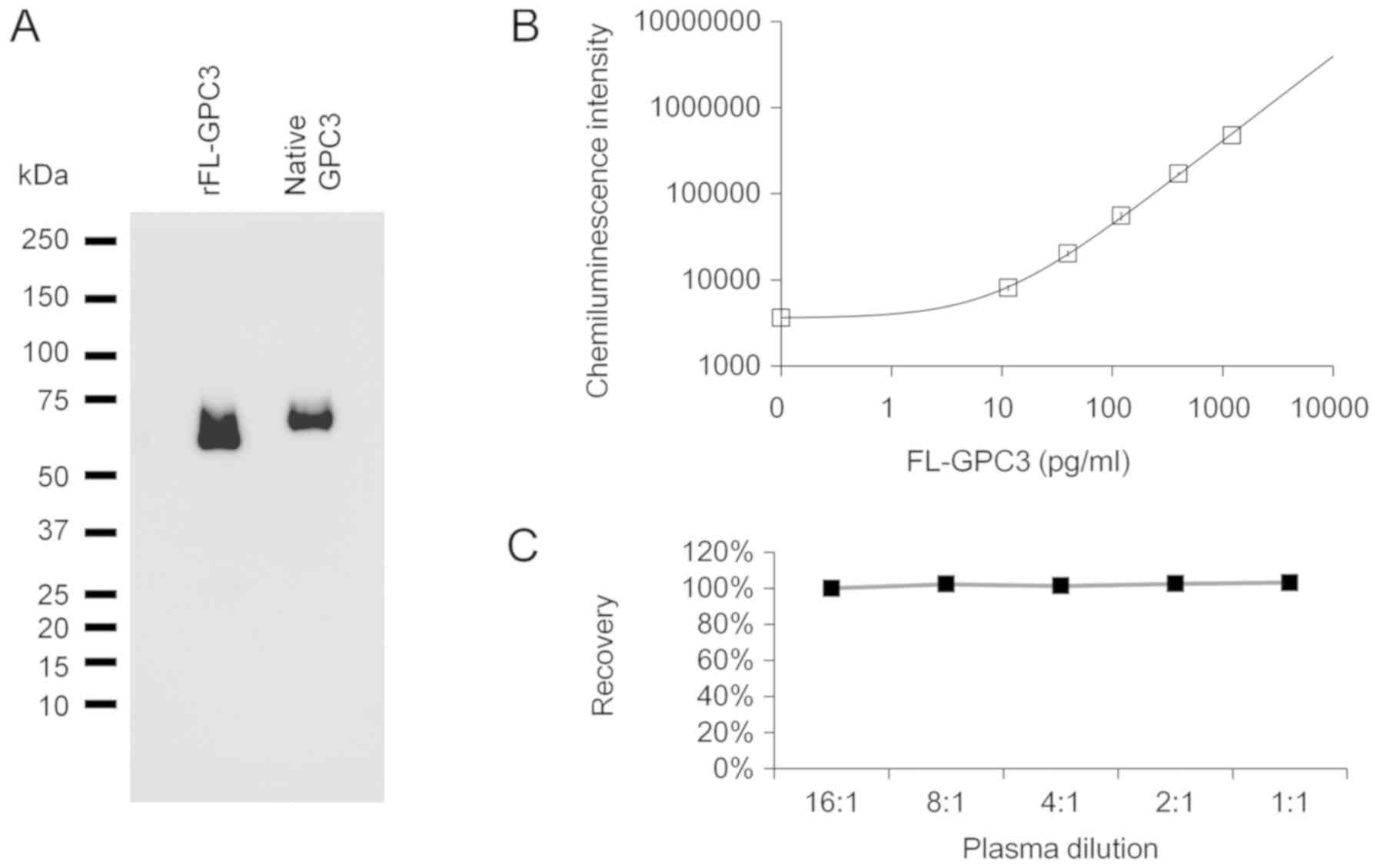
Proof of existence of FL-GPC3 in
cultured cell supernatant
To verify the presence of FL-GPC3 in blood, we
evaluated secreted GPC3 in the supernatant of cultured HepG2 cells,
which has been well characterized as a GPC3-positive liver cancer
cell line (25). We used mass
spectrometry to detect FL-GPC3 containing both the N- and
C-terminal domains. HepG2 cultured supernatant was used based on
the following two conjectures: (i) Proteins secreted from HepG2
cells into supernatant are potentially the same as those secreted
from liver cancer cells into blood in vivo and (ii) using
immunoprecipitation with mass spectrometry to directly detect
FL-GPC3 in blood would require much higher sensitivity (pg/ml
order) than the currently measurable value. GPC3 in the supernatant
of cultured cells was recovered by immunoprecipitation with the
N-terminal recognition antibody and confirmed by Western blotting
with the C-terminal recognizing antibody (Fig. 1A). The molecular weight of the
captured GPC3 was the same as that of the full-length recombinant
GPC3, which was run alongside for comparison. To confirm the
existence of both the N- and C-terminal domains, analysis by mass
spectrometry was performed. Many peptides derived from both the N-
and C-terminals of GPC3 were detected (Table SI). It was thus confirmed that
FL-GPC3 was present in the culture supernatant, implying a high
likelihood of FL-GPC3 being present in blood.
Construction of a measurement method
for FL-GPC3
To measure FL-GPC3 in blood, a sandwich assay was
constructed using an antibody recognizing the N-terminus for
capture and an antibody recognizing the C-terminus for detection.
The determination range of FL-GPC3 by this method was 2–1,500 pg/ml
(Fig. 1B). The coefficient of
variation for the three measurements was less than 5%,
demonstrating that our method measured FL-GPC3 with a very high
precision. In addition, recombinant GPC3 spiked into GPC3 negative
specimens of healthy subjects showed a clear spike recovery ratio
and dilution linearity, indicating that the method was not affected
by the plasma matrix (Fig. 1C).
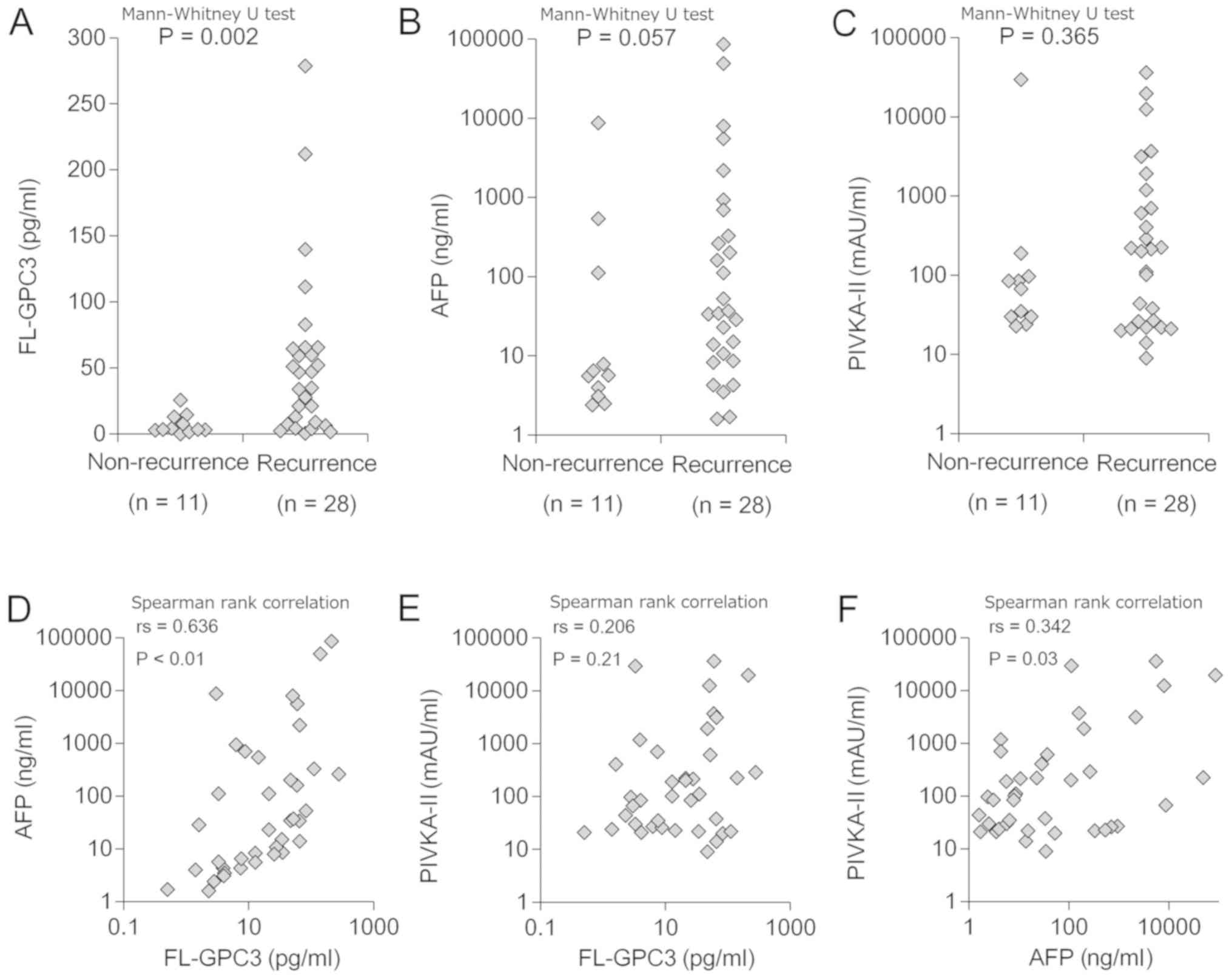
Preoperative plasma FL-GPC3, AFP, and
PIVKA-II levels in HCC patients
Preoperative plasma or serum biomarker levels in HCC
patients were measured using fully-automated immunoassay systems.
The median levels of AFP, PIVKA-II, and FL-GPC3 were 28.5
(5.7–294.6) ng/ml, 97.0 (26.5–294.6) mAU/ml, and 21.0 (4.0–5.6)
pg/ml, respectively (Table I). The
median FL-GPC3 level in the recurrence group was 40.8 pg/ml (range
8.5–64.7), which was significantly higher than that in the
non-recurrence group (3.3 pg/ml, range 2.9–10.2, P<0.01)
(Table II; Fig. 2A). By contrast, there were no
significant differences in median AFP or PIVKA-II levels between
the two groups (recurrence group vs. non-recurrence group: AFP;
35.7 [range, 10.2–420.5] ng/ml vs. 5.7 [range, 3.6–59.8] ng/ml,
P=0.06, PIVKA-II; 208.0 [range, 25.0–822.0] mAU/ml vs. 67.0 [range,
30.0–91.0] mAU/ml, P=0.37) (Table
II; Fig. 2B and C). Weak
correlations between FL-GPC3 and AFP, AFP and PIVKA-II were
observed (Fig. 2D-F). No other
patient background factors, including tumor size, were
significantly associated with FL-GPC3 levels.
FL-GPC3 predicts HCC recurrence within
four years of surgery more precisely than the two existing
biomarkers
ROC analysis was used to verify whether each
biomarker could be used to classify each group. The AUC value of
the ROC curve was highest for FL-GPC3, followed by AFP and PIVKA-II
(0.830, 0.698, and 0.594, respectively; Fig. 3A). When the cutoff value of FL-GPC3
was set to 14.4 pg/ml based on the Youden index, the positive rate
of FL-GPC3 in the recurrence group was 68% (19/28), which was
higher than the 9% (1/11) in the non-recurrence group (Table II). Next, the disease-free survival
(DFS) periods in patients with high (≧14.4 pg/ml, n=20) and low
(<14.4 pg/ml, n=19) FL-GPC3 levels were analyzed and compared
using Kaplan-Meier survival analysis and log rank test, which
revealed significant differences in both groups (60.0% vs. 15.8%
and 95.0% vs. 47.4%, at 1- and 4-year recurrence rates,
respectively; P=0.005, 0.001; Fig.
3B). This clearly supports that FL-GPC3 is a predictive marker
of HCC recurrence that is equal to or better than AFP and PIVKA-II
(Fig. S1A and B).
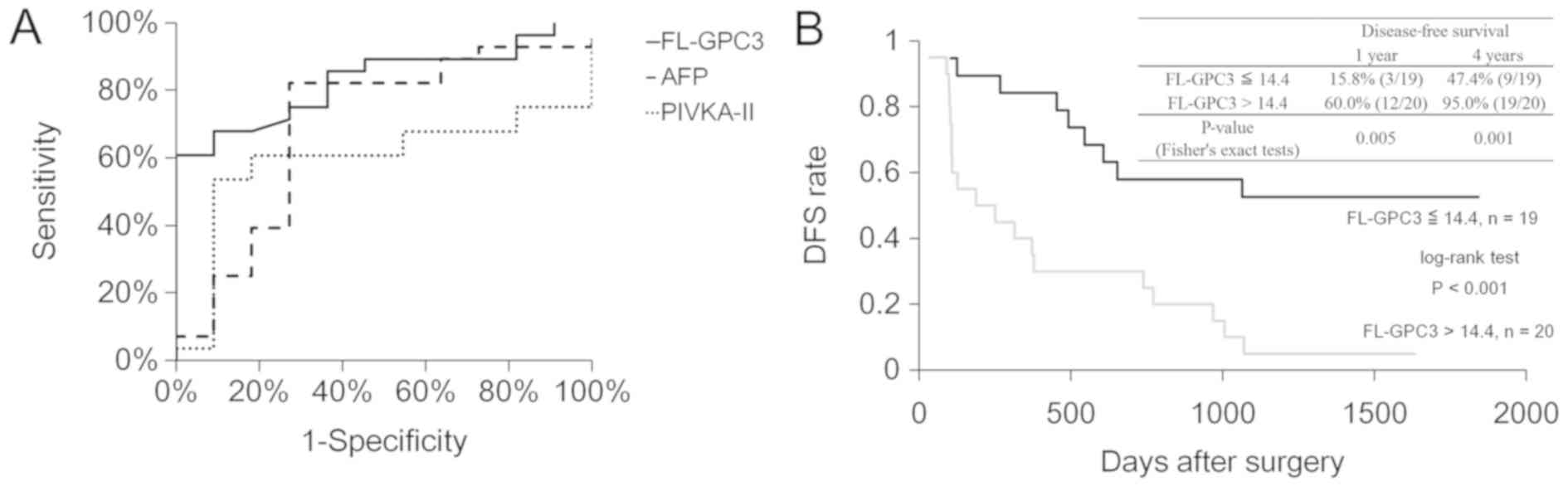 | Figure 3.ROC and Kaplan-Meier survival curves
for DFS after surgery. (A) ROC curves of FL-GPC3, AFP and PIVKA-II
are presented. The area under the ROC curve value for AFP, PIVKA-II
and FL-GPC3 was 0.830 (95% confidence interval, 0.957–0.702), 0.698
(0.893–0.503) and 0.594 (0.782–0.406), respectively. (B)
Kaplan-Meier curves of DFS in patients with low (≤14.4 pg/ml, n=19)
and high (>14.4 pg/ml, n=20) FL-GPC3 are presented. The P-value
of the log rank test was 0.0004. ROC, receiver operating
characteristic; DFS, disease-free survival; FL-GPC3, full-length
glypican-3; AFP, alpha-fetoprotein; PIVKA-II protein induced by
vitamin K absence or antagonist-II. |
Combining three biomarkers enabled
better prediction of HCC recurrence within one year of surgery
Having demonstrated that FL-GPC3 is a predictive
marker of HCC recurrence within four years, we evaluated whether
earlier recurrence (i.e., within one year) could be predicted.
Patients with recurrence were divided into an early recurrence
group (n=15), which showed recurrence within one year, and a second
group with later recurrence or no recurrence (n=24). Biomarker
levels were then compared between groups (Table III). Significant differences were
observed for FL-GPC3 (46.2 pg/ml vs. 6.9 pg/ml, P=0.014), AFP
(160.2 ng/ml vs. 10.9 ng/ml, P=0.026), and PIVKA-II (226.0 mAU/ml
vs. 36.5 mAU/ml, P=0.008). However, the AUC value of the ROC curve
obtained for each biomarker was not high enough to be practical.
The sensitivity of each marker for predicting early recurrence was
high (FL-GPC3 93.3%, AFP 93.3%, PIVKA-II 86.7%), but the
specificity was insufficient (FL-GPC3 45.8%, AFP 58.3%, PIVKA-II
47.8%) when the cutoff values of each marker was set based on the
Youden index (FL-GPC3 >8.0 pg/ml, AFP >7.9 ng/ml, PIVKA-II
>102.0 mAU/ml) (Fig. 4A). Thus,
we examined whether combining the three values was effective for
predicting recurrence. Of the total patients, 14/39 (36%) were
positive for all three markers, and these patients tended to face
recurrence at an earlier stage than other patients (rates of
recurrence within 1 year, 93% (13/14) vs. 8% (2/25), P<0.001)
(Table IV). The sensitivity and
specificity to predict recurrence within 1 year were 86.6 and
95.8%, respectively (Fig. 4B).
Therefore, by using three biomarkers in combination, patients
likely to face recurrence within one year of surgery could be
predicted with a very high sensitivity and specificity.
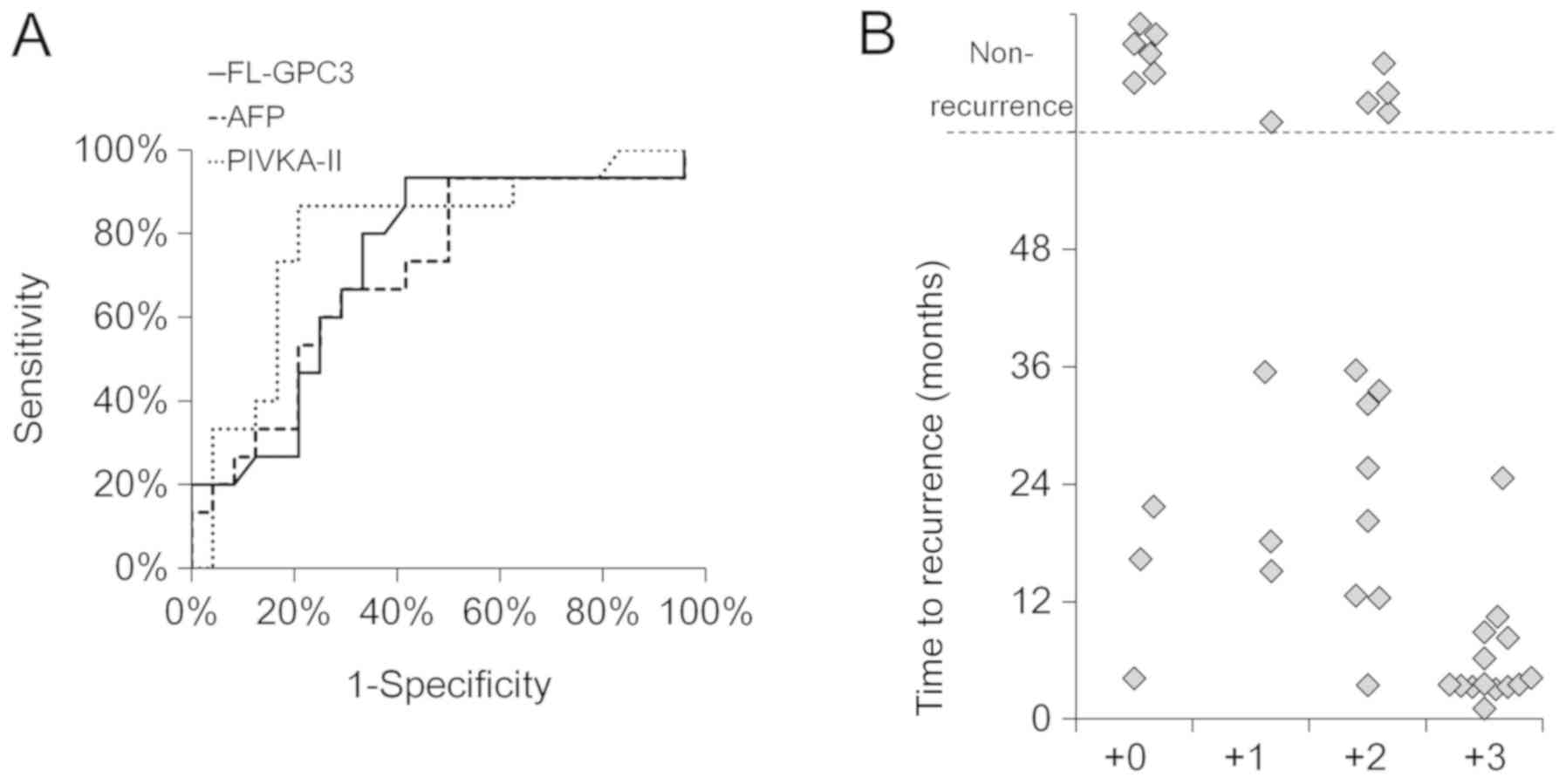 | Figure 4.Early recurrence prediction by
FL-GPC3, AFP, PIVKA-II and their combinations. (A) ROC curves of
FL-GPC3, AFP and PIVKA-II for the diagnosis of HCC recurrence
within one year of surgery are presented. The area under the ROC
curve values for each marker was 0.736 (95% confidence interval,
0.820–0.652), 0.714 (0.809–0.619) and 0.757 (0.837–0.677),
respectively. The cutoff values for each marker were set based on
the Youden index (FL-GPC3 >8.0 pg/ml, AFP >7.9 ng/ml and
PIVKA-II >102.0 mAU/ml). (B) The associations between time to
recurrence and positive numbers of FL-GPC3, AFP and PIVKA-II are
presented. Plus three, two and one indicates the patients that were
positive for all three, two or one marker respectively. Plus zero
indicates the patients that were positive for no markers. The
cutoff value of each marker was FL-GPC3 >8.0 pg/ml, AFP >7.9
ng/ml and PIVKA-II >102.0 mAU/ml. Each patient is indicated by a
dot. FL-GPC3, full-length glypican-3; AFP, alpha-fetoprotein;
PIVKA-II protein induced by vitamin K absence or antagonist-II;
HCC, hepatocellular carcinoma; ROC, receiver operating
characteristic. |
 | Table III.Patient characteristics in the two
groups, early recurrence or others. |
Table III.
Patient characteristics in the two
groups, early recurrence or others.
| Variables | Early recurrence
n=15 | Other n=24 | Fisher's exact test
P-values | Mann-Whitney U test
P-values |
|---|
| Age |
|
| 0.061 |
|
|
≥65 | 12 | 12 |
|
|
|
<65 | 3 | 12 |
|
|
| Sex |
|
| 0.452 |
|
|
Male | 4 | 4 |
|
|
|
Female | 11 | 20 |
|
|
| Liver
condition |
|
| 0.018 |
|
|
Cirrhosis or chronic
hepatitis | 14 | 14 |
|
|
|
Other | 1 | 10 |
|
|
| HCV infection |
|
| 0.150 |
|
|
Positive | 11 | 12 |
|
|
|
Negative | 4 | 12 |
|
|
| HBV infection
(+/−) |
|
| 0.950 |
|
|
Positive | 3 | 5 |
|
|
|
Negative | 12 | 19 |
|
|
| Stage |
|
| 0.323 |
|
| I or
II | 6 | 6 |
|
|
| III or
IV | 9 | 18 |
|
|
| Number of
tumors |
|
| 0.950 |
|
|
Solitary | 3 | 5 |
|
|
|
Multiple | 12 | 19 |
|
|
| Tumor size
(mm) |
|
| 0.131 |
|
|
≥50 | 8 | 7 |
|
|
|
<50 | 7 | 17 |
|
|
| Vascular invasion
(+/−) |
|
| 0.043 |
|
|
Present | 7 | 4 |
|
|
|
Absent | 8 | 20 |
|
|
| Differentiation of
tumor |
|
| 0.849 |
|
| Well or
moderately | 14 | 22 |
|
|
|
Poorly | 1 | 2 |
|
|
| AFP (ng/ml), median
(range) | 160.2
(16.8–1441.4) | 10.9
(4.2–67.4) |
| 0.026 |
| PIVKA-II (mAU/ml),
median (range) | 226.0
(156.0–2532.0) | 36.5
(22.8–120.3) |
| 0.008 |
| FL-GPC3 (pg/ml),
median (range) | 46.7 (21–62.4) | 6.9 (3.2–37.2) |
| 0.014 |
 | Table IV.Patient characteristics in two groups
classified according to the marker positive number. |
Table IV.
Patient characteristics in two groups
classified according to the marker positive number.
| Variables | Three positive
groups n=14 | Other n=25 | Fisher's exact test
P-values | Mann-Whitney U test
P-values |
|---|
| Age |
|
| 0.342 |
|
|
≥65 | 10 | 14 |
|
|
|
<65 | 4 | 11 |
|
|
| Sex
(male/female) |
|
| 0.916 |
|
|
Male | 3 | 5 |
|
|
|
Female | 11 | 20 |
|
|
| Liver
condition |
|
| 0.029 |
|
|
Cirrhosis or chronic
hepatitis | 13 | 15 |
|
|
|
Other | 1 | 10 |
|
|
| HCV infection |
|
| 0.237 |
|
|
Positive | 10 | 13 |
|
|
|
Negative | 4 | 12 |
|
|
| HBV infection |
|
| 0.471 |
|
|
Positive | 2 | 6 |
|
|
|
Negative | 12 | 19 |
|
|
| Stage |
|
| 0.221 |
|
| I or
II | 6 | 6 |
|
|
| III or
IV | 8 | 19 |
|
|
| Number of
tumors |
|
| 0.916 |
|
|
Solitary | 3 | 5 |
|
|
|
Multiple | 11 | 20 |
|
|
| Tumor size
(mm) |
|
| 0.073 |
|
|
≥50 | 8 | 7 |
|
|
|
<50 | 6 | 18 |
|
|
| Vascular
invasion |
|
| 0.024 |
|
|
Present | 7 | 4 |
|
|
|
Absent | 7 | 21 |
|
|
| Differentiation of
tumor |
|
| 0.923 |
|
| Well or
moderately | 13 | 23 |
|
|
|
Poorly | 1 | 2 |
|
|
| AFP (ng/ml), median
(range) | 180.9
(26.4–4701.8) | 7.9 (4.0–52.6) |
| 0.004 |
| PIVKA-II (mAU/ml),
median (range) | 450.5
(216.5–3555.5) | 30.0
(22.0–85.0) |
| 0.000 |
| FL-GPC3 (pg/ml),
median (range) | 51.5 (29.5–64) | 6.3 (3.0–25.6) |
| 0.001 |
| Recurrence within 1
year (+/−) |
|
| <0.001 |
|
|
Yes | 13 | 2 |
|
|
| No | 1 | 23 |
|
|
Discussion
To formulate effective treatment policies, it is
crucial to know the risk of recurrence of HCC before treatment. Our
study has shown that HCC patients with high FL-GPC3 levels have a
higher risk of recurrence within four years, and that there is no
significant correlation between FL-GPC3 level and tumor diameter.
This finding is consistent with previous reports (26,27). It
has been reported that GPC3 is expressed in cancerous cells as well
as non-cancerous surrounding cells, and that the prognosis of
GPC3-positive HCC patients is poor (12,14,28,29).
This may indicate that FL-GPC3 is produced by the tumor itself but
as well as by precancerous lesions located in the surrounding liver
tissue. Thus, it is possible that FL-GPC3 could be used as a
biomarker to qualitatively evaluate the malignancy of a tumor and
its surrounding environment.
In the present study, we revealed that patients
positive for all three markers (FL-GPC3, AFP and PIVKA-II) were
likely to face HCC recurrence within one year. In addition, a weak
correlation was found between FL-GPC3 and AFP and between AFP and
PIVKA-II. It is known that a high value of AFP or PIVKA-II is a
poor prognosis marker of HCC (30,31). Our
study showed that high levels of FL-GPC3 were strongly related to
HCC recurrence, and this might also reflect poor prognosis. Both
AFP and GPC3 are known as fetal cancer antigens. Our study shows a
correlation between the two, but FL-GPC3 did not correlate with
tumor size, whereas AFP is known to correlate well with tumor size
(32). Therefore, even if AFP and
FL-GPC3 are the same fetal cancer antigen, they may reflect some
different factors that contribute to cancer recurrence. In
addition, since there is no correlation between FL-GPC3 and
PIVKA-II, it can be said that it also reflects different risk
factors for recurrence. Our study shows a correlation between AFP
and PIVKA-II, both of which are known to correlate with tumor size
(32,33). However, AFP is elevated in poorly
differentiated hepatocytes, and high levels of PIVKA-II are said to
suggest the presence of vascular invasion (34). This suggests that AFP and PIVKA-II
also reflect different recurrence risk factors. Based on these
facts, FL-GPC3, AFP, and PIVKA-II are all considered to be markers
that reflect different cancer states and risk of recurrence. While
expression of any of these three markers could indicate poor
prognosis, patients with high levels of all three markers are
thought to be at very high risk of early recurrence due to
predisposition to cancer development, even though there is a weak
correlation among these markers. Our results show that patients
with early recurrence have a high prevalence of background liver
disease and a high incidence of vascular invasion, which is
consistent with previous reports (35). Patients with these backgrounds still
need careful follow-up as patients with high recurrence risk. On
the other hand, there was one early recurrence in a patient who had
neither vascular invasion nor background liver disease, and this
case showed high values for FL-GPC3, AFP, and PIVKA-II. As the
sample set used in this study is relatively small, further case
studies will need to be added. However, the measurement of the
three markers may be useful in screening for patients with
high-early recurrence risk that may be overlooked by background
liver disease or vascular invasion alone. In addition, the presence
or absence of mild vascular invasion cannot be known until a
histopathological examination after surgery. Our finding that
patients who are positive for all three markers are more likely to
have early recurrence, so that they know the patient's risk of
early recurrence before treatment. Better knowledge of the risk of
recurrence will allow a more optimal treatment method to be
selected for each patient, promoting the development of
personalized medicine. Future efforts to develop new therapies
combating HCC recurrence would benefit from the selection of
patients with high recurrence risk as well as the possibility of
reducing side effect risk. Diagnosis of HCC recurrence risk by
FL-GPC3 measurement may become a crucial diagnostic technique in
the future.
In the present study, the median concentration of
FL-GPC3 in the blood of HCC patients was 21.0 pg/ml, which is lower
than previously reported levels (400–99,940 pg/ml) (15,19,20).
This difference could be because the previously reported assays
were for N-terminal GPC3, which measures both N-terminal and the
full-length protein, whereas our assay measured only the latter. As
our assay requires the formation of a sandwich by antibodies
simultaneously recognizing the N- and C-terminal sides, it enables
measurement of only FL-GPC3. By contrast, both the N-terminal
domain and full-length protein were measured in a previous study
using a pair of antibodies specific to the N-terminal domain. The
correlation between GPC3 and other markers remains controversial
(15,26,28) but
may be explained by the target form of GPC3 in each report. It is
not clear what form of GPC3-whether the N-terminal domain, the
full-length protein, or the combination of the two-more closely
reflects the prognosis of HCC. A comparative study between
N-terminal GPC3 and our assay in the same group of patients would
be required to confirm this. Verification studies performed for all
GPC3 forms in the same patient group could address this
problem.
Further, while the present study focused on
predicting the risk of post-operative HCC recurrence, FL-GPC3
measurement could be expanded to predict HCC occurrence in patients
with chronic hepatitis (CH) or LC. Past studies have shown that the
N-terminal form of GPC3 does not increase in these patients
(19,21), but FL-GPC3 has not yet been
investigated. Comparing the levels of FL-GPC3 in patients with HCC,
CH, or LC and healthy volunteers could give a clear idea of the
normal level of this protein and help identify the point at which
FL-GPC3 levels increase in blood. We hope that the utility of
FL-GPC3 as a biomarker will be further investigated in larger
scale, multi-center cohort studies.
Supplementary Material
Supporting Data
Acknowledgements
A patent application has been filed relating to this
work. Inventors: Tetsuya Nakatsura, Keigo Saito, Masahiro Miura,
Kozo Suto and Takuya Iino. Title, method for assisting prediction
of recurrence risk in HCC patient, device, computer program
product, and kit. Patent code: JP Patent 6292564. Date filed, March
6, 2017. Date issued, February 23, 2018.
Funding
The present study was supported by the National
Cancer Center Research and Development Fund (grant nos. 25-A-7 and
28-A-8), as well as joint research funding from Sysmex Co.,
Ltd.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM, NF, and KSa and KSu constructed the measurement
system of FL-GPC3 and analyzed the patient data. YS, SM, and TS
analyzed and interpreted the patient data. MK, ST and NG collected
samples and suggested the clinical utility of FL-GPC3. TY and TN
were major contributors in the conception and designing of the
current study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the National Cancer Center of Japan and Sysmex
Corporation. Informed consent was obtained from all patients.
Patient consent for publication
Written consent for publication was obtained from
all patients.
Competing interests
TN was supported by fundamental research funding
obtained from Sysmex Co., Ltd. The remaining authors declare that
they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
AFP
|
alpha-fetoprotein
|
|
PIVKA-II
|
protein induced by vitamin K absence
or antagonist-II
|
|
GPC3
|
glypican-3
|
|
FL-GPC3
|
full-length glypican-3
|
|
ROC
|
receiver operating characteristic
|
|
AUC
|
area under the curve
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawada Y, Yoshikawa T, Ofuji K, Yoshihura
M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato
Y, et al: Phase II study of the GPC3-derived peptide vaccine as an
adjuvant therapy for hepatocellular carcinoma patients.
Oncoimmunology. 5:e11294832016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toyoda H, Kumada T, Osaki Y, Oka H, Urano
F, Kudo M and Matsunaga T: Staging hepatocellular carcinoma by a
novel scoring system (BALAD score) based on serum markers. Clin
Gastroenterol Hepatol. 4:1528–1536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carr BI, Kanke F, Wise M and Satomura S:
Clinical evaluation of lens culinaris agglutinin-reactive
alpha-fetoprotein and des-gamma-carboxy prothrombin in
histologically proven hepatocellular carcinoma in the United
States. Dig Dis Sci. 52:776–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bayati N, Silverman AL and Gordon SC:
Serum alpha-fetoprotein levels and liver histology in patients with
chronic hepatitis C. Am J Gastroenterol. 93:2452–2456. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirakawa H, Kuronuma T, Nishimura Y,
Hasebe T, Nakano M, Gotohda N, Takahashi S, Nakagohri T, Konishi M,
Kobayashi N, et al: Glypican-3 is a useful diagnostic marker for a
component of hepatocellular carcinoma in human liver cancer. Int J
Oncol. 34:649–656. 2009.PubMed/NCBI
|
|
7
|
Shirakawa H, Suzuki H, Shimomura M, Kojima
M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N,
Kinoshita T and Nakatsura T: Glypican-3 expression is correlated
with poor prognosis in hepatocellular carcinoma. Cancer Sci.
100:1403–1407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HL, Anatelli F, Zhai QJ, Adley B,
Chuang ST and Yang XJ: Glypican-3 as a useful diagnostic marker
that distinguishes hepatocellular carcinoma from benign
hepatocellular mass lesions. Arch Pathol Lab Med. 132:1723–1728.
2008.PubMed/NCBI
|
|
9
|
Nakatsura T, Kageshita T, Ito S, Wakamatsu
K, Monji M, Ikuta Y, Senju S, Ono T and Nishimura Y: Identification
of glypican-3 as a novel tumor marker for melanoma. Clin Cancer
Res. 10:6612–6621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toretsky JA, Zitomersky NL, Eskenazi AE,
Voigt RW, Strauch ED, Sun CC, Huber R, Meltzer SJ and Schlessinger
D: Glypican-3 expression in Wilms tumor and hepatoblastoma. J
Pediatr Hematol Oncol. 23:496–499. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saikali Z and Sinnett D: Expression of
glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 89:418–422.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Capurro M, Wanless IR, Sherman M, Deboer
G, Shi W, Miyoshi E and Filmus J: Glypican-3: A novel serum and
histochemical marker for hepatocellular carcinoma.
Gastroenterology. 125:89–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Cat B, Muyldermans SY, Coomans C,
Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B and
David G: Processing by proprotein convertases is required for
glypican-3 modulation of cell survival, Wnt signaling, and
gastrulation movements. J Cell Biol. 163:625–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ho M and Kim H: Glypican-3: A new target
for cancer immunotherapy. Eur J Cancer. 47:333–338. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haruyama Y, Yorita K, Yamaguchi T,
Kitajima S, Amano J, Ohtomo T, Ohno A, Kondo K and Kataoka H: High
preoperative levels of serum glypican-3 containing N-terminal
subunit are associated with poor prognosis in patients with
hepatocellular carcinoma after partial hepatectomy. Int J Cancer.
137:1643–1651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakatsura T, Yoshitake Y, Senju S, Monji
M, Komori H, Motomura Y, Osaka S, Beppu T, Ishiko T, Kamohara H, et
al: Glypican-3, overexpressed specifically in human hepatocellular
carcinoma, is a novel tumor marker. Biochem Biophys Res Commun.
306:16–25. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Filmus J, Capurro M and Rast J: Glypicans.
Genome Biol. 9:2242008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Capurro M and Filmus J: Glypican-3 as a
serum marker for hepatocellular carcinoma. Cancer Res. 65:372–373.
2005.PubMed/NCBI
|
|
19
|
Hippo Y, Watanabe K, Watanabe A,
Midorikawa Y, Yamamoto S, Ihara S, Tokita S, Iwanari H, Ito Y,
Nakano K, et al: Identification of soluble NH2-terminal fragment of
glypican-3 as a serological marker for early-stage hepatocellular
carcinoma. Cancer Res. 64:418–423. 2004. View Article : Google Scholar
|
|
20
|
Chen M, Li G, Yan J, Lu X, Cui J, Ni Z,
Cheng W, Qian G, Zhang J and Tu H: Reevaluation of glypican-3 as a
serological marker for hepatocellular carcinoma. Clin Chim Acta.
423:105–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Beale G, Chattopadhyay D, Gray J, Stewart
S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D and Reeves H:
AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers
for hepatocellular cancer in non-alcoholic and alcoholic fatty
liver disease. BMC Cancer. 8:2002008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozkan H, Erdal H, Kocak E, Tutkak H,
Karaeren Z, Yakut M and Koklu S: Diagnostic and prognostic role of
serum glypican 3 in patients with hepatocellular carcinoma. J Clin
Lab Anal. 25:350–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Capurro M, Shi W, Izumikawa T, Kitagawa H
and Filmus J: Processing by convertases is required for
glypican-3-induced inhibition of Hedgehog signaling. J Biol Chem.
290:7576–7585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu
CL and Wong J: Clinicopathologic features of long-term survivors
and disease-free survivors after resection of hepatocellular
carcinoma: A study of a prospective cohort. J Clin Oncol.
19:3037–3044. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao W, Tang Z, Zhang YF, Feng M, Qian M,
Dimitrov DS and Ho M: Immunotoxin targeting glypican-3 regresses
liver cancer via dual inhibition of Wnt signalling and protein
synthesis. Nat Commun. 6:65362015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Saadany S, El-Demerdash T, Helmy A,
Mayah WW, Hussein BE, Hassanien M, Elmashad N, Fouad MA and Basha
EA: Diagnostic value of Glypican-3 for hepatocellular carcinomas.
Asian Pac J Cancer Prev. 19:811–817. 2018.PubMed/NCBI
|
|
27
|
Chen IP, Ariizumi S, Nakano M and Yamomoto
M: Positive glypican-3 expression in early hepatocellular carcinoma
predicts recurrence after hepatectomy. J Gastroenterol. 49:117–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ofuji K, Saito K, Suzuki S, Shimomura M,
Shirakawa H, Nobuoka D, Sawada Y, Yoshimura M, Tshuchiya N,
Takahashi M, et al: Perioperative plasma glypican-3 level may
enable prediction of the risk of recurrence after surgery in
patients with stage I hepatocellular carcinoma. Oncotarget.
8:37835–37844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaseb AO, Hassan M, Lacin S, Abdel-Wahab
R, Amin HM, Shalaby A, Wolff RA, Yao J, Rashid A, Vennapusa B, et
al: Evaluating clinical and prognostic implications of Glypican-3
in hepatocellular carcinoma. Oncotarget. 7:69916–69926. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Imamura H, Matsuyama Y, Tanaka E, Ohkubo
T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T,
Kawasaki S and Makuuchi M: Risk factors contributing to early and
late phase intrahepatic recurrence of hepatocellular carcinoma
after hepatectomy. J Hepatol. 38:200–207. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Liu Z, Yin X, Qi X, Lu B, Liu Y
and Hou J: Prognostic value of PIVKA-II in hepatocellular carcinoma
patients receiving curative ablation: A systematic review and
meta-analysis. Int J Biol Markers. 33:266–274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abbasi A, Bhutto AR, Butt N and Munir SM:
Corelation of serum alpha fetoprotein and tumor size in
hepatocellular carcinoma. J Pak Med Assoc. 62:33–36.
2012.PubMed/NCBI
|
|
33
|
Poté N, Cauchy F, Albuquerque M, Voitot H,
Belghiti J, Castera L, Puy H, Bedossa P and Paradis V: Performance
of PIVKA-II for early hepatocellular carcinoma diagnosis and
prediction of microvascular invasion. J Hepatol. 62:848–854. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imamura H, Matsuyama Y, Miyagawa Y, Ishida
K, Shimada R, Miyagawa S, Makuuchi M and Kawasaki S: Prognostic
significance of anatomical resection and des-gamma-carboxy
prothrombin in patients with hepatocellular carcinoma. Br J Surg.
86:1032–1038. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamanaka N, Okamoto E, Toyosaka A,
Mitunobu M, Fujihara S, Kato T, Fujimoto J, Oriyama T, Furukawa K
and Kawamura E: Prognostic factors after hepatectomy for
hepatocellular carcinomas. A univariate and multivariate analysis.
Cancer. 65:1104–1110. 1990. View Article : Google Scholar : PubMed/NCBI
|