Introduction
Lung cancer is the leading cause of cancer death in
the world, accounting for >1/4 of all cancer-related deaths
(1). Almost 85% of patients with
lung carcinoma exhibit non-small cell lung cancer (NSCLC), of which
lung squamous cell carcinoma (LUSC) accounts for ~30% and results
in ~400,000 deaths annually (2). The
primary strategy for LUSC treatment at present remains surgical
resection. However, this treatment is generally not effective once
the disease progresses to a metastatic stage. Individuals with
advanced disease have a poor prognosis. Indeed, chemotherapy
generally fails to treat patients with metastatic LUSC, and this
disease has a <20% 5-year survival rate, with no optimal
targeted therapeutic having yet been identified to treat this
disease (1). The low survival rate
of patients with LUSC is at least partially attributable to the
disease often not being diagnosed until it is relatively advanced,
thus precluding surgical treatment (2). The present study aims to provide a
useful reference in the future diagnosis of LUSC.
Cancer cells and normal cells exhibit distinct
patterns of protein production and secretion, with numerous tumors
exhibiting marked shifts in proteolytic activity as their signaling
alters during the progression towards malignant disease (3). As such, it is possible to detect
specific cancer-associated proteins in the biofluids of patients,
and these proteins as biomarkers can offer an insight into disease
type and stage. Such biomarkers have been sought as a means of
facilitating LUSC diagnosis and monitoring, since their detection
is easier than a more invasive biopsy procedure and allow rapid
screening. Minimally invasive tumor biomarkers that are readily
accessible in biofluids such as plasma, urine and bronchoalveolar
lavage fluid (BALF) would thus offer a means of easily and
effectively differentiating between patients with cancer and those
with benign disease (4–6). Urine markers can be detected without
exposing individuals to any risk, and urine is highly amenable to
large-scale screening efforts. Therefore, urine is a particularly
promising biospecimen for biomarker screening. In theory, such an
approach would allow population-level screening of individuals,
thereby facilitating the early detection of LUSC and other types of
cancer. However, the current biomarkers for the diagnosis of LUSC
are mainly blood tumor markers such as squamous cell carcinoma
(SCC) antigen and cytokeratin 19 fragment 21-1, but their
sensitivity and specificity are low. There arw few studies on
biomarkers of LUSC in easily accessible specimens, such as urine
and BALF (4,5).
Kininogen 1 (KNG1) is a cysteine proteinase
inhibitor known to inhibit endothelial cell proliferation and
angiogenesis (7). Certain groups
have reported the detection of KNG1 using mass spectrometry, and
recently KNG1 has been identified as a serum biomarker for advanced
colorectal adenoma and colorectal cancer, in addition to being a
salivary biomarker useful for oral SCC detection and for the
monitoring of high-risk individuals (8,9).
However, the disease relevance of KNG1 levels in patients with LUSC
remains to be assessed. Osteopontin (OPN) is expressed in a variety
of tissues, and is abundant in body fluids such as blood, milk and
urine (10). It is a multifunctional
phosphorylated glycoprotein involved in cell migration and
adhesion, and mediates the invasion and metastasis of tumor cells
(6). OPN is associated with
tumorigenesis, progression, metastasis and cancer prognosis
(11). α-1-Antitrypsin (AAT) is also
known as serine proteinase inhibitor A1. It was reported that the
level of AAT was elevated in the tissues and serum of patients with
lung cancer, wherein it was thought to promote tumor invasion and
metastasis (12–14). At present, the levels of OPN and AAT
in the urine and BALF of patients with lung cancer remain unclear.
Moreover, the combined assessment of the diagnostic relevance of
KNG1, OPN and AAT levels in urine, BALF and plasma have not been
studied in LUSC thus far.
The present study aimed to assess whether KNG1, OPN,
and AAT levels in specific biofluids, as measured by ELISA, may be
a viable diagnostic biomarker for LUSC. In addition, the levels of
these three proteins in LUSC tissues were assessed via
immunohistochemistry (IHC).
Materials and methods
Study subjects
Patients with LUSC patients and controls were
recruited from Beijing Shijitan Hospital between October 2014 and
March 2017. The Ethics Committee of Beijing Shijitan Hospital,
Capital Medical University approved the present study (approval no.
10, 2014). All study participants provided written informed
consent, and the study was performed according to the Declaration
of Helsinki. For patients with LUSC, two senior pathologists
confirmed the diagnosis based on pathology findings. The control
group included various benign lung disorders such as chronic cough,
benign pulmonary nodules, hemoptysis, bronchitis, sarcoidosis,
asthma, bronchiectasis and tuberculosis. The characteristics of the
patients with LUSC and the control subjects are presented in
Table I. All plasma, BALF and urine
samples were collected prior to radiological, surgical or
chemotherapeutic treatment. There was no evidence of hematuresis in
any urine samples, with all albumin/creatinine ratios in urine
samples being <30 mg/g. From 20 of the total number of patients
in this study, pairs of LUSC tumor tissue and adjacent normal
tissue located ≥5 cm from the tumor site were also used. Patients
who underwent preoperative radio- or chemo-therapeutic treatment
were excluded from the study. Patients with LUSC were classified
based on the 2009 TNM classification system for malignant tumor
staging produced by the International Union Against Cancer and the
American Joint Committee on Cancer.
 | Table I.Demographics of patients with cancer
and control subjects. |
Table I.
Demographics of patients with cancer
and control subjects.
|
| Biofluids set | Tissue set |
|---|
|
|
|
|
|---|
|
Characteristics | Patients with
cancer (n=31) | Normal controls
(n=20) | P-value | Tumor/adjacent
normal pairs (n=20) |
|---|
| Age, years | 65.7±9.7 | 67.4±9.4 | 0.55 | 63.0±8.2 |
| Sex |
|
Female | 10 (32%) | 8 (40%) | 0.57 | 6 (30%) |
|
Male | 21 (68%) | 12 (60%) |
| 14 (70%) |
| Smoking habit |
|
Nonsmoker | 11 (35%) | 10 (50%) | 0.30 | 8
(40%) |
| Ever
smoker | 20 (65%) | 10 (50%) |
| 12 (60%) |
| Clinical stage |
|
I–II | 13 |
|
| 9 |
|
III–IV | 18 |
|
| 11 |
| Pleural
invasion |
|
Absent | 27 |
|
| 20 |
|
Present | 4 |
|
| 0 |
| Lymphatic
invasion |
|
Positive | 25 |
|
| 15 |
|
Negative | 6 |
|
| 5 |
Plasma collection
From each subject, 6 ml of venous blood was
collected using closed syringes containing a coagulation activator,
and samples were then immediately centrifuged at 3,600 × g for 10
min. The plasma fraction was transferred to a separate Eppendorf
tube and stored at −80°C. Of note, subjects were fasting at the
time of sample collection.
Urine collection
A total of 50 ml of mid-stream urine from each
subject was collected into a sterile polypropylene tube. Samples
were immediately centrifuged at 400 × g for 15 min, and the
supernatant was then aliquoted and frozen at −80°C.
BALF collection
Patients were first administered 2% lidocaine for
local anesthesia. Subsequently, a fiber-optic bronchoscope (Olympus
EXERA BF 240; Olympus Corporation) was used to perform a
bronchoscopy. Lavage was conducted before biopsy or brushing-based
specimen collection to prevent any possibility of blood
contamination. Lavage was performed by washing the bronchus of the
side affected by the disease twice with 50 ml sterile saline
solution, and then slowly withdrawing this solution into a
siliconized tube and placing it in ice water. A recovered BALF
volume of 40 ml was considered acceptable. After isolation, debris
and cells were immediately removed via centrifugation at 1,500 × g
for 10 min, and the supernatants were subsequently frozen at
−80°C.
ELISA
The KNG1, OPN and AAT levels in plasma, urine and
BALF were measured using a commercially available ELISA kit Abnova,
Abcam and Abcam, respectively, according to the manufacturer's
instructions. Plasma and BALF samples were diluted 200- and
100-fold, respectively. Next, plasma, BALF and urine samples were
incubated in KNG1 ELISA plates. Urine samples were diluted
1,500-fold and then plasma, BALF and urine samples were incubated
in OPN ELISA plates. The AAT ELISA plates were incubated with
plasma, urine and BALF samples at dilutions of 1:400,000, 1:400 and
1:500, respectively. The optical density was measured at 450 nm
with a Model 680 microplate reader (Bio-Rad Laboratories, Inc.). A
standard curve was drawn for each plate using the concentration of
the standard sample and the corresponding optical density value of
each well. Both positive and negative controls were used for
validation.
IHC
Following surgical collection, LUSC and adjacent
healthy tissue samples were formalin-fixed, paraffin-embedded and
cut into 4-mm sections. Next, xylene was used to deparaffinize the
samples for 20 min, and an ethanol gradient (100, 100, 95 and 75%,
2 min each) was then used to dehydrate samples. PBS was next used
to wash the samples (5 times, 10 min each), and the samples were
then heated under pressure with an antigen unmasking reagent to
facilitate antigen retrieval. After an additional 10-min wash with
PBS, 3% H2O2 was used to treat the samples
for 15 min, followed by an additional wash step. Tissues were then
probed overnight at 4°C with appropriate primary antibodies against
OPN (1:300, Abcam), AAT (1:300, Abcam) and KNG1 (1:200, Abnova).
Samples were again washed and then probed for 20 min with a
secondary antibody conjugated to HRP (Beijing Zhongshan Jinqiao
Biotechnology, Co., Ltd.) at 37°C the following day. An additional
15-min wash was then performed, and the chromogenic
3,3′-diaminobenzidine mixture(Beijing Zhongshan Jinqiao
Biotechnology, Co. Ltd.) was next used to stain the samples for 5
min. Hematoxylin was used for counterstaining for 2 min, and then
the samples were dehydrated with ethanol (75, 95, 100 and 100%) and
washed with xylene, and natural gum was used to seal the
samples.
Two independent pathologists blinded to the
patients' information independently assessed the IHC slides via
light microscopy. A Nikon Ci-S (Nikon Corporation) microscope with
NIS-Elements F software (Nikon Corporation) was used to capture
images of the samples. Sample scoring was conducted as in previous
studies based on 10 different fields of view (15). Both staining intensity (intensity)
and area (extent) were scored for each sample. With respect to
intensity, samples were scored as either 0, 1, 2, or 3, which
corresponded to no, mild, moderate, or intense staining,
respectively. With respect to area, samples were scored as either
0, 1, 2, 3, or 4, which corresponded to 0, 1–10, 11–50, 51–80, and
81–100% of positive cells, respectively. These two scores were
multiplied together to yield an overall score, with overall scores
of 4–12 being considered positive, and scores of 0–3 being
considered negative.
Statistical analysis
All statistical analyses were conducted using SPSS
v22.0 (IBM Corp.). Normally distributed data were compared via
Student's t-tests, while Mann-Whitney U tests was used for
comparisons of non-normally distributed data. The sensitivity and
specificity of these biomarkers were assessed based on the area
under the curve (AUC) of the receiver operating characteristic
(ROC) curve. χ2 test was used to assess the baseline
characteristic differences between the LUSC and control groups, and
to compare the proteins levels in LUSC and adjacent normal lung
tissues. All tests were two-sided, and P<0.05 was considered to
indicate a statistically significant difference.
Results
ELISA
The KNG1 levels in the plasma, BALF and urine of
patients with LUSC were significantly higher than those in benign
controls (P<0.001) (Table II).
The KNG1 level in BALF was significantly lower than that in plasma
(P<0.0001), but significantly higher than that in urine
(P<0.0001).
 | Table II.Levels of OPN, AAT and KNG1 in the
plasma, BAFL and urine of patients with LUSC and benign disease
controls. |
Table II.
Levels of OPN, AAT and KNG1 in the
plasma, BAFL and urine of patients with LUSC and benign disease
controls.
| Marker | Unit | LUSC | Benign | P-value |
|---|
| KNG1 |
|
|
|
|
|
Plasma | µg/ml | 1,664.1±292.7 | 1,310.6±265.4 | <0.0001 |
|
BALF | µg/ml | 67.3±35.9 | 20.9±17.8 | <0.0001 |
|
Urine | µg/ml | 3.4±1.8 | 1.3±1.5 | 0.0010 |
| OPN |
|
|
|
|
|
Plasma | ng/ml |
4,8108.2±37,757.3 |
21,316.5±11,255.8 | 0.0107 |
|
BALF | ng/ml | 160.3±223.0 | 32.7±47.1 | 0.0004 |
|
Urine | ng/ml | 86.1±43.2 | 132.5±58.4 | 0.0088 |
| AAT |
|
|
|
|
|
Plasma | µg/ml |
25,082.7±9,145.2 |
16,589.1±9,138.7 | 0.0022 |
|
BALF | ng/ml |
30,577.0±13,047.6 |
16,768.7±13,427.0 | 0.0014 |
|
Urine | ng/ml |
2,176.9±1,536.9 | 788.2±690.0 | 0.0005 |
The OPN levels in the plasma and BALF of patients
with LUSC were significantly higher than those in the controls
(P<0.05 for both); however, the OPN level in urine tended to be
lower in patients with LUSC than that in controls (P<0.05)
(Table II). The OPN level in plasma
was significantly higher than that in BALF and urine (P<0.001
for both), and there was also a significant difference in OPN level
between BALF and urine (P<0.05). Notably, the OPN level in BALF
was higher than that in urine in patients with LUSC (P<0.05),
but lower than that in urine in the controls (P<0.05).
The AAT levels in the plasma, BALF and urine of
patients with LUSC were significantly higher than those in the
controls (P<0.01) (Table II).
The AAT level in BALF was significantly lower than that in plasma
(P<0.0001) but significantly higher than that in urine
(P<0.0001).
ROC analysis
The AUC of the ROC curve, sensitivity and
specificity values for KNG1, OPN and AAT in plasma, BALF and urine
are shown in Table III. The
results indicated that the combination of KNG1, OPN and AAT could
improve the respective AUC values in plasma, BALF and urine
(Table III).
 | Table III.Area under curve, sensitivity and
specificity for KNG1, OPN and AAT in plasma, BALF and urine. |
Table III.
Area under curve, sensitivity and
specificity for KNG1, OPN and AAT in plasma, BALF and urine.
| Marker | Cut-off level | AUC | Sensitivity, % | Specificity, % | P-value |
|---|
| KNG1 |
|
Plasma | 1,445.2 µg/ml | 0.81 | 74 | 75 | 0.0002 |
|
BALF | 30.0 µg/ml | 0.91 | 92 | 73 | <0.0001 |
|
Urine | 1.3 µg/ml | 0.81 | 90 | 59 | 0.0015 |
| OPN |
|
Plasma | 46,469.5 ng/ml | 0.71 | 45 | 100 | 0.0115 |
|
BALF | 40.9 ng/ml | 0.83 | 81 | 93 | 0.0008 |
|
Urine | 111.4 ng/ml | 0.75 | 85 | 65 | 0.0105 |
| AAT |
|
Plasma | 20,393.7 µg/ml | 0.74 | 65 | 75 | 0.0046 |
|
BALF | 37,621.5 ng/ml | 0.74 | 52 | 100 | 0.0020 |
|
Urine | 628.4 ng/ml | 0.86 | 100 | 53 | 0.0008 |
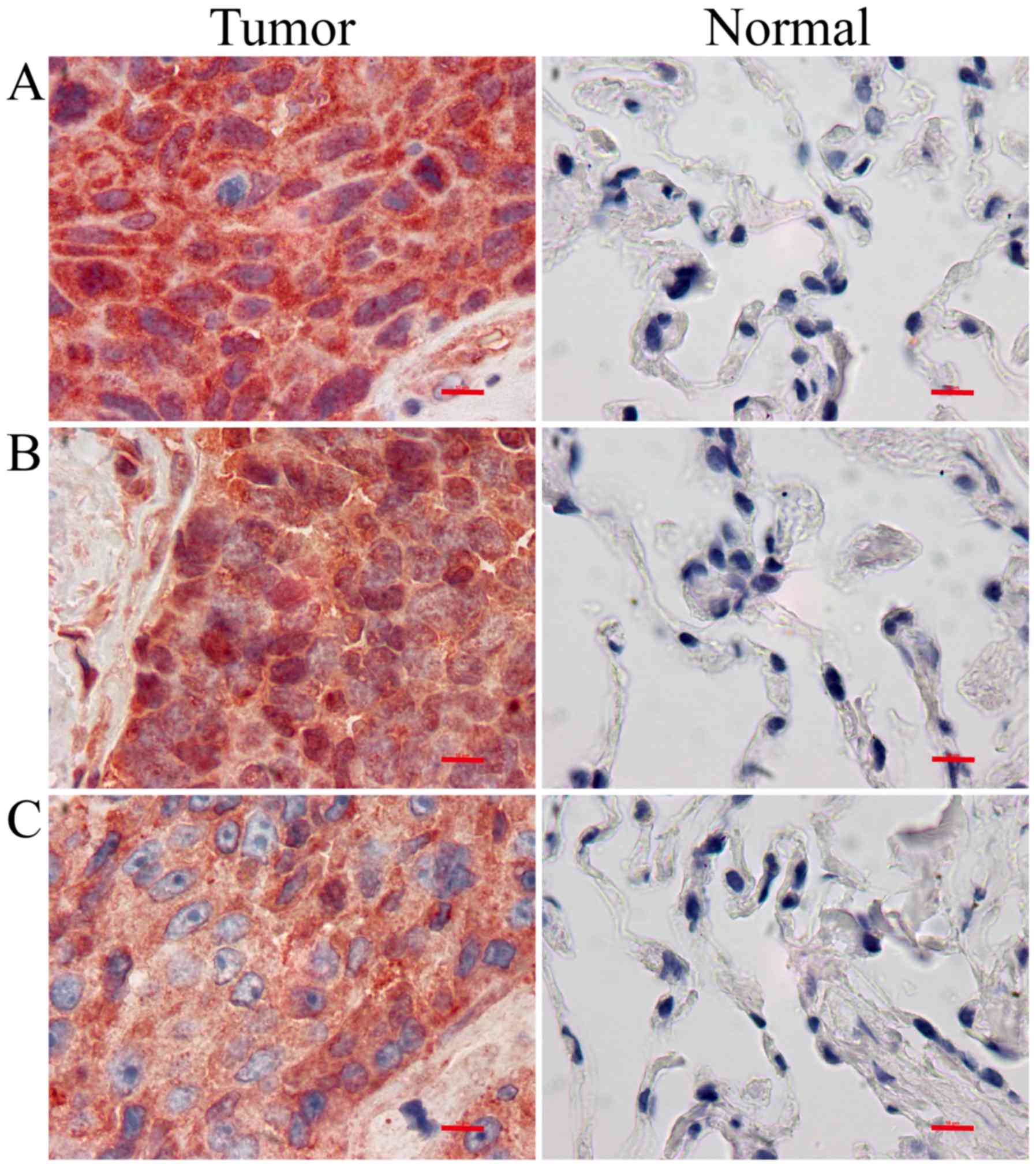
KNG1, OPN and AAT protein expression
in LUSC tissues
The results from IHC showed that KNG1, OPN, and AAT
proteins were primarily expressed in the cytoplasm. The protein
expression levels of KNG1, OPN and AAT were significantly increased
in LUSC tissues compared with those in the controls (P<0.05)
(Fig. 1, Table IV).
 | Table IV.Expression levels of KNG1, OPN and
AAT in LUSC and adjacent normal lung tissues. |
Table IV.
Expression levels of KNG1, OPN and
AAT in LUSC and adjacent normal lung tissues.
|
| LUSC tissue, n
(n=20) | Normal lung tissue,
n (n=20) |
|
|---|
|
|
|
|
|
|---|
| Proteins | 4–12 | 0–3 | 4–12 | 0–3 | P-value |
|---|
| KNG1 | 9 | 11 | 2 | 18 | 0.031 |
| OPN | 8 | 12 | 1 | 19 | 0.020 |
| AAT | 7 | 13 | 1 | 19 | 0.044 |
Discussion
Identification of biomarkers in various biological
fluids is a promising strategy for lung cancer detection. Blood is
the most studied biofluid with respect to biomarker discovery;
thus, there is a wealth of information available regarding the
blood proteome in multiple diseases. Considering its close
association with lung tissue, BALF offers a means of assessing
lung-related biomarkers, and previous BALF analysis results have
shown some equivalence with biopsy findings (16). Urine is an ideal biofluid for
biomarkers assessment owing to its ease of repeated noninvasive
collection in large quantities. In addition, protein levels in
urine tend to remain fairly stable due to low levels of proteolytic
degradation.
The present study demonstrated the combined use of
three biofluids (plasma, BALF, and urine) and tissues for lung
cancer diagnosis purpose. To the best of our knowledge, this study
is the first to describe the potential for KNG1, OPN and AAT to be
used as diagnostic biomarkers of LUSC in plasma, BALF and urine
specimens. The areas under the ROC curve of KNG1 in BALF was 0.91,
and the AUC of KNG1 in urine was 0.81, which as good as in the
plasma. The AUC of OPN in BALF was 0.83, while the AUC of OPN in
urine was 0.75, which was better than that in plasma. The AUC of
AAT in urine was 0.86, which was better than that in plasma and
BALF. These results emphasized the potential of the above markers
in urine and BALF to be used as diagnostic tools.
KNG1 protein is encoded by the KNG1 gene, and is a
cysteine proteinase inhibitor that plays key roles in the process
of blood coagulation. In addition, KNG1 has been found to play
roles in cancer development, with different expression in different
tumors. For example, KNG1 levels were decreased in the serum of
patients with breast cancer (17),
cervical cancer (18), and
endometrial cancer (18), as well as
in the urine of ovarian carcinoma (19) and renal cell carcinoma (20). Moreover, KNG1 was expressed at low
levels in glioma cells (21) and
renal cell carcinoma tissue (22).
By contrast, increased KNG1 levels have been reported in the serum
of patients with hepatocellular carcinoma (23), gastric carcinoma (24) and colorectal cancer (5). IHC staining revealed KNG1 expression to
be significantly higher in colorectal cancer and advanced
colorectal adenoma tissues than that in normal mucosa (25). Bioinformatics analyses suggested that
KNG1 might play critical roles in colorectal cancer liver
metastasis (26). KNG1 levels were
also upregulated in other biofluids, such as the saliva of patients
with oral squamous cell carcinoma (8) and the bile of patients with
cholangiocarcinoma and pancreatic cancer (27). In our study, plasma, BALF and urine
KNG1 levels were significantly higher in patients with LUSC
compared with those in the controls, and this was also consistent
with IHC data from 20 patients, who displayed higher KNG1 protein
levels in tumor tissues than in normal tissues. We hypothesized
that the observed increase in KNG1 levels in the assessed biofluids
of patients with LUSC was a result of cancer cell-mediated
production of this protein. While its specific mechanistic function
in LUSC remains uncertain, KNG1 was thought to exert
anti-angiogenic and anti-endothelial cell proliferative activities
in certain cancers types (18). A
previous study found that aberrant ceRNA-mediated regulation of
KNG1 contributed to glioblastoma-induced angiogenesis, which
provided potential targets for the development of novel therapeutic
strategies for glioblastoma (21).
Importantly, KNG1 levels in plasma, BALF, and urine might serve as
a potential LUSC biomarker, although the mechanism of KNG1 in LUSC
needs further study.
OPN is a protein that plays crucial roles in
immunity, remodeling of tissues and malignant transformation of
tumor cells. The present study observed elevated OPN expression in
the tumors of patients with LUSC, which was consistent with
previous findings. Indeed, one study observed a significant link
between OPN levels and gender, TNM stage, tumor differentiation,
and poor outcomes in patients with NSCLC (28,29). OPN
could play a variety of functions, binding with cluster of
differentiation 44 or certain integrins to trigger the activation
of the PI3K/AKT, Janus kinase 2, and focal adhesion kinase
signaling pathways, thereby serving as a vital regulator of the
epithelial-mesenchymal transition (30–32).
Thus, OPN plays an essential role in cancer progression. OPN is a
secreted protein that can be detected in different biofluids. High
OPN levels in plasma were associated with higher levels of hypoxia
in tumors and a higher risk of recurrence in patients with early
stage NSCLC (33). In individuals
with advanced lung cancer, higher circulating OPN levels were
associated with a poorer prognosis and worse therapeutic responses
(34–36). Prior to this study, BALF and urine
OPN levels had not been assessed. Our findings revealed that OPN
was upregulated in the plasma and BALF of patients with LUSC
compared with those of healthy controls, but it was downregulated
in the urine of patients with LUSC compared with that of healthy
controls. In addition to tissue and blood, the levels of OPN in
BALF and urine might also serve as a potential marker of lung
squamous cell carcinoma.
AAT is a serine protease inhibitor that, while
mainly produced by the liver, can also be found in other tissues
and can be produced by cancer cells. Elevated AAT levels have been
reported in patients with lung cancer (12,37),
with higher plasma AAT levels being detected in these patients
(14,38,39). AAT
plays essential roles in the migration and invasion of cancer
cells, regulating the assembly of fibronectin in the area
surrounding a cell (13,40). The C-terminal portions of AAT were
able to induce cell proliferation and invasion in human pancreatic
adenocarcinoma (41), melanoma
(42) and breast carcinoma (43). When AAT expression was reduced in a
human or murine model, this led to a reduction in the observed
proliferative, metastatic, and adhesive behavior of these tumor
cells (37). AAT deficiency has been
reported to increase the risk of lung cancer (44), and tumors positive for AAT had a
poorer prognosis than those that were negative for this protein
(45). In the present study, AAT
protein levels were increased in LUSC tumor samples, suggesting
that this protein might play a role in oncogenesis. Our results
demonstrated that AAT levels in the plasma, BALF and urines of
patients with LUSC differed from those in the normal controls. The
AUC of AAT in urine was better than that in plasma and BALF, which
suggested that detection of AAT in urine might be a non-invasive
tool for LUSC screening.
Our findings suggest that the measurement of KNG1,
OPN and AAT in body fluids, particularly in urine, represents a
simple, non-invasive strategy that might be of value for LUSC
diagnosis. To the best of our knowledge, this is the first study to
simultaneously evaluate these three proteins in the plasma, urine
and BALF of patients with LUSC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Beijing
Natural Science Foundation (grant no. 7172106), Beijing Municipal
Administration of Hospitals' Ascent Plan (grant no. DFL20150701)
and Enhancement Funding of Beijing Key Laboratory of Urinary
Cellular Molecular Diagnostics (grant no. 2019-JS02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ and WW designed the study. WW and SW performed
the experiments. WW wrote the article. SW and MZ reviewed and
edited the manuscript. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Beijing Shijitan Hospital,
Capital Medical University approved the present study. All study
participants provided written informed consent, and the study was
performed following the Declaration of Helsinki.
Patient consent for publication
Patients provided written informed consent for the
publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kikuchi T, Hassanein M, Amann JM, Liu Q,
Slebos RJ, Rahman SM, Kaufman JM, Zhang X, Hoeksema MD, Harris BK,
et al: In-depth proteomic analysis of nonsmall cell lung cancer to
discover molecular targets and candidate biomarkers. Mol Cell
Proteomics. 11:916–932. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uribarri M, Hormaeche I, Zalacain R,
Lopez-Vivanco G, Martinez A, Nagore D and Ruiz-Argüello MB: A new
biomarker panel in bronchoalveolar lavage for an improved lung
cancer diagnosis. J Thorac Oncol. 9:1504–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimura T, Iwasaki H, Kitagawa M, Ebi M,
Yamada T, Yamada T, Katano T, Nisie H, Okamoto Y, Ozeki K, et al:
Urinary cysteine-rich protein 61 and trefoil factor 3 as diagnostic
biomarkers for colorectal cancer. Transl Oncol. 12:539–544. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn JM, Sung HJ, Yoon YH, Kim BG, Yang WS,
Lee C, Park HM, Kim BJ, Kim BG, Lee SY, et al: Integrated
glycoproteomics demonstrates fucosylated serum paraoxonase 1
alterations in small cell lung cancer. Mol Cell Proteomics.
13:30–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdullah-Soheimi SS, Lim BK, Hashim OH and
Shuib AS: Patients with ovarian carcinoma excrete different altered
levels of urine CD59, kininogen-1 and fragments of
inter-alpha-trypsin inhibitor heavy chain H4 and albumin. Proteome
Sci. 8:582010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu JS, Chen YT, Chiang WF, Hsiao YC, Chu
LJ, See LC, Wu CS, Tu HT, Chen HW, Chen CC, et al: Saliva protein
biomarkers to detect oral squamous cell carcinoma in a high-risk
population in Taiwan. Proc Natl Acad Sci USA. 113:11549–11554.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Wang X, Lin S, Chen C, Wang C, Ma
Q and Jiang B: Identification of kininogen-1 as a serum biomarker
for the early detection of advanced colorectal adenoma and
colorectal cancer. PLoS One. 8:e705192013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Briones-Orta MA, Avendaño-Vázquez SE,
Aparicio-Bautista DI, Coombes JD, Weber GF and Syn WK: Osteopontin
splice variants and polymorphisms in cancer progression and
prognosis. Biochim Biophys Acta Rev Cancer. 1868:93–108A. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Chen Q, Alam A, Cui J, Suen KC,
Soo AP, Eguchi S, Gu J and Ma D: The role of osteopontin in the
progression of solid organ tumour. Cell Death Dis. 9:3562018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Wang S and Zhang M: Identification
of urine biomarkers associated with lung adenocarcinoma.
Oncotarget. 8:38517–38529. 2017.PubMed/NCBI
|
|
13
|
Li Y, Miao L, Yu M, Shi M, Wang Y, Yang J,
Xiao Y and Cai H: α1-antitrypsin promotes lung adenocarcinoma
metastasis through upregulating fibronectin expression. Int J
Oncol. 50:1955–1964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang Y, Ma T, Thakur A, Yu H, Gao L, Shi
P, Li X, Ren H, Jia L, Zhang S, et al: Differentially expressed
glycosylated patterns of alpha-1-antitrypsin as serum biomarkers
for the diagnosis of lung cancer. Glycobiology. 25:331–340. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu S, Meng Q, Hu H and Zhang M:
Correlation of ANXA1 expression with drug resistance and relapse in
bladder cancer. Int J Clin Exp Pathol. 7:5538–5548. 2014.PubMed/NCBI
|
|
16
|
Pastor MD, Nogal A, Molina-Pinelo S,
Quintanal-Villalonga Á, Meléndez R, Ferrer I, Romero-Romero B, De
Miguel MJ, López-Campos JL, Corral J, et al: IL-11 and CCL-1: Novel
protein diagnostic biomarkers of lung adenocarcinoma in
bronchoalveolar lavage fluid (BALF). J Thorac Oncol. 11:2183–2192.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doustjalali SR, Yusof R, Yip CH, Looi LM,
Pillay B and Hashim OH: Aberrant expression of acute-phase reactant
proteins in sera and breast lesions of patients with malignant and
benign breast tumors. Electrophoresis. 25:2392–2401. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdul-Rahman PS, Lim BK and Hashim OH:
Expression of high-abundance proteins in sera of patients with
endometrial and cervical cancers: Analysis using 2-DE with silver
staining and lectin detection methods. Electrophoresis.
28:1989–1996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu AK, Lim BK, Hashim OH and Shuib AS:
Identification of O-glycosylated proteins that are aberrantly
excreted in the urine of patients with early stage ovarian cancer.
International journal of molecular sciences. 14:7923–7931. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sandim V, Pereira Dde A, Kalume DE,
Oliveira-Carvalho AL, Ornellas AA, Soares MR, Alves G and Zingali
RB: Proteomic analysis reveals differentially secreted proteins in
the urine from patients with clear cell renal cell carcinoma. Urol
Oncol. 34:5.e11–25. 2016. View Article : Google Scholar
|
|
21
|
Xu J, Fang J, Cheng Z, Fan L, Hu W, Zhou F
and Shen H: Overexpression of the Kininogen-1 inhibits
proliferation and induces apoptosis of glioma cells. J Exp Clin
Cancer Res. 37:1802018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schrödter S, Braun M, Syring I, Klümper N,
Deng M, Schmidt D, Perner S, Müller SC and Ellinger J:
Identification of the dopamine transporter SLC6A3 as a biomarker
for patients with renal cell carcinoma. Mol Cancer. 15:102016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang W, Zhang L, Guo Q, Wang H, Ma M, Sun
J and Chen C: Identification of the pathogenic biomarkers for
hepatocellular carcinoma based on RNA-seq analyses. Pathol Oncol
Res. 25:1207–1213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu W, Liu B, Cai Q, Li J, Chen X and Zhu
Z: Proteomic identification of serum biomarkers for gastric cancer
using multi-dimensional liquid chromatography and 2D differential
gel electrophoresis. Clinica Chimica Acta. 413:1098–1106. 2012.
View Article : Google Scholar
|
|
25
|
Quesada-Calvo F, Massot C, Bertrand V,
Longuespée R, Blétard N, Somja J, Mazzucchelli G, Smargiasso N,
Baiwir D, De Pauw-Gillet MC, et al: OLFM4, KNG1 and Sec24C
identified by proteomics and immunohistochemistry as potential
markers of early colorectal cancer stages. Clin Proteomics.
14:92017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao B, Yu T, Xue D, Sun B, Shao Q,
Choudhry H, Marcus V, Ragoussis J, Zhang Y, Zhang W and Gao ZH: A
multidimensional integration analysis reveals potential bridging
targets in the process of colorectal cancer liver metastasis. PLoS
One. 12:e01787602017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navaneethan U, Lourdusamy V, Gk Venkatesh
P, Willard B, Sanaka MR and Parsi MA: Bile proteomics for
differentiation of malignant from benign biliary strictures: A
pilot study. Gastroenterol Rep. 3:136–143. 2015. View Article : Google Scholar
|
|
28
|
Li S, Yang R, Sun X, Miao S, Lu T, Wang Y,
Wo Y and Jiao W: Identification of SPP1 as a promising biomarker to
predict clinical outcome of lung adenocarcinoma individuals. Gene.
679:398–404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Q, Guo L, Lin G, Chen Z, Chen T, Lin
J, Zhang B and Gu X: Clinical and prognostic significance of OPN
and VEGF expression in patients with non-small-cell lung cancer.
Cancer Epidemiol. 39:539–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kothari AN, Arffa ML, Chang V, Blackwell
RH, Syn WK, Zhang J, Mi Z and Kuo PC: Osteopontin-a master
regulator of epithelial-mesenchymal transition. J Clin Med.
5:E392016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B
and Song G: Osteopontin promotes mesenchymal stem cell migration
and lessens cell stiffness via integrin β1, FAK, and ERK pathways.
Cell Biochem Biophys. 65:455–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24− stem
cell-like breast cancer cells in human tumors. J Clin Invest.
121:2723–2735. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rouanne M, Adam J, Goubar A, Robin A,
Ohana C, Louvet E, Cormier J, Mercier O, Dorfmüller P, Fattal S, et
al: Osteopontin and thrombospondin-1 play opposite roles in
promoting tumor aggressiveness of primary resected non-small cell
lung cancer. BMC Cancer. 16:4832016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ostheimer C, Schweyer F, Reese T, Bache M
and Vordermark D: The relationship between tumor volume changes and
serial plasma osteopontin detection during radical radiotherapy of
non-small-cell lung cancer. Oncol Lett. 12:3449–3456. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Y, Yang J, Liu H, Bi JR, Liu Y, Chen
YY, Cao JY and Lu YJ: The association between osteopontin and
survival in non-small-cell lung cancer patients: A meta-analysis of
13 cohorts. Onco Targets Ther. 8:3513–3521. 2015.PubMed/NCBI
|
|
36
|
Mack PC, Redman MW, Chansky K, Williamson
SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ and
Gandara DR; SWOG, : Lower osteopontin plasma levels are associated
with superior outcomes in advanced non-small-cell lung cancer
patients receiving platinum-based chemotherapy: SWOG Study S0003. J
Clin Oncol. 26:4771–4776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang SH, Cho KC, Yu KN, Hong SH, Park S,
Lee AY, Kim S, Lee S, Kang JW, Chae C, et al: Alpha 1-antitrypsin
activates lung cancer cell survival by acting on cap-dependent
protein translation, vesicle-mediated transport, and metastasis.
Oncotarget. 2016.
|
|
38
|
Rodríguez-Piñeiro AM, Blanco-Prieto S,
Sánchez-Otero N, Rodríguez-Berrocal FJ and de la Cadena MP: On the
identification of biomarkers for non-small cell lung cancer in
serum and pleural effusion. J Proteomics. 73:1511–1522. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
El-Akawi ZJ, Abu-Awad AM, Sharara AM and
Khader Y: The importance of alpha-1 antitrypsin (alpha1-AT) and
neopterin serum levels in the evaluation of non-small cell lung and
prostate cancer patients. Neuro Endocrinol Lett. 31:113–116.
2010.PubMed/NCBI
|
|
40
|
Chang YH, Lee SH, Liao IC, Huang SH, Cheng
HC and Liao PC: Secretomic analysis identifies alpha-1 antitrypsin
(A1AT) as a required protein in cancer cell migration, invasion,
and pericellular fibronectin assembly for facilitating lung
colonization of lung adenocarcinoma cells. Mol Cell Proteomics.
11:1320–1339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kataoka H, Uchino H, Iwamura T, Seiki M,
Nabeshima K and Koono M: Enhanced tumor growth and invasiveness in
vivo by a carboxyl-terminal fragment of alpha1-proteinase inhibitor
generated by matrix metalloproteinases: A possible modulatory role
in natural killer cytotoxicity. Am J Pathol. 154:457–468. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zelvyte I, Sjögren HO and Janciauskiene S:
Effects of native and cleaved forms of alpha1-antitrypsin on ME
1477 tumor cell functional activity. Cancer Detect Prev.
26:256–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zelvyte I, Lindgren S and Janciauskiene S:
Multiple effects of alpha1-antitrypsin on breast carcinoma MDA-MB
468 cell growth and invasiveness. Eur J Cancer Prev. 12:117–124.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang P, Sun Z, Krowka MJ, Aubry MC, Bamlet
WR, Wampfler JA, Thibodeau SN, Katzmann JA, Allen MS, Midthun DE,
et al: Alpha1-antitrypsin deficiency carriers, tobacco smoke,
chronic obstructive pulmonary disease, and lung cancer risk. Arch
Intern Med. 168:1097–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Higashiyama M, Doi O, Kodama K, Yokouchi H
and Tateishi R: An evaluation of the prognostic significance of
alpha-1-antitrypsin expression in adenocarcinomas of the lung: An
immunohistochemical analysis. Br J Cancer. 65:300–302. 1992.
View Article : Google Scholar : PubMed/NCBI
|