Introduction
In 2018, 22,240 cases of epithelial ovarian cancer
(EOC) were reported in the United States of America, with the
associated death toll being 14,070 individuals (1). EOC is the primary cause of death from
gynecological cancer in the US, with the morbidity and mortality
being 11.5/100,000 and 6.7/100,000, respectively (2). The findings of EOC are usually
advanced, and the 5-year survival rates for EOC diagnosed at stages
III and IV are only 41% and 20%, respectively, in the United States
(2). Histologically, EOC can be
divided into 4 types: Serous carcinoma (68–71%), endometrioid
carcinoma (9–11%), clear cell carcinoma (12–13%) and mucinous
carcinoma (3%) (3,4). Studies have revealed that EOC is a
heterogeneous tumor (5,6). The current standard treatment for EOC
consists of cytoreductive surgery and platinum-based chemotherapy
(7). Despite aggressive treatment,
overall survival rate (OS) has not significantly improved in recent
decades (8,9). Therefore, novel therapeutic targets are
required to improve the current prognosis of patients with EOC.
In recent years, numerous studies have demonstrated
that the tumor microenvironment plays an important role in the
progression and metastasis of cancer (10,11).
Chemokines are important members of the tumor microenvironment and
mediate the recruitment of immune cells to the tumor
microenvironment (12). Thus,
chemokines are directly and indirectly involved in the formation of
the tumor environment and immune milieu (13). C-C motif chemokine 14 (CCL14) is an
important member of the chemokine family and was originally
isolated from blood filters of patients with chronic renal failure.
The protein is composed of 74 amino acids, of which 4 cysteine
residues are linked by disulfide bonds (14). The relative molecular weight of CCL14
is 8,673 kDa and it has 46% sequence homology to macrophage
inflammatory protein (14). CCL14
specifically binds to chemokine receptor 1 (CCR1), CCR3 and
chemokine receptor 5 (CCR5) to exert biological effects (15,16). A
previous study in patients with breast cancer have demonstrated
that CCL14 promotes angiogenesis and metastasis (17). It has also been reported that CCL14
is involved in the occurrence and development of oral cancer
(18). Therefore, the biological
functions of CCL14 are diverse. The involvement of CCL14 in the
formation of the immune microenvironment indicates that the
CCL14/CCR1/CCR5 axis can be used as a potential target for
immunotherapy. The underlying mechanism of action of CCL14 in EOC
is unclear and the significance of CCL14 in the progression and
prognosis of EOC has not been reported. The present study aimed to
elucidate the role of CCL14 in EOC.
Patients and methods
Patient specimens
The Medical Ethics Committees of the Cancer Center
of Sun Yat-Sen University (Guangzhou, China) and Jiangmen Central
Hospital (Jiangmen, China) approved the present study. The
requirement for patient consent was waived by the ethics
committees. A total of 154 patients with EOC were enrolled between
January 2008 and December 2015. The mean age of enrolled patients
was 48.5 years, ranging from 17–86 years. Among these cases, 82
patients had undergone ovariectomy at the Central Cancer Department
of Sun Yat-Sen University and 72 patients had undergone ovariectomy
at Jiangmen Central Hospital. The inclusion criteria for these
patients in the present study were: Tissues could undergo
immunohistochemical examination; no history of chemotherapy,
radiotherapy and surgery prior to ovariectomy; complete immune
function; and no other malignant tumors or secondary primary
tumors. The follow-up period was censored to December 2018. All
cases were classified on the basis of the World Health Organization
Classification of Tumors of Female Reproductive Organs (19), the Tumor Node Metastasis
Classification System of the US Joint Commission (20). The stage of tumors was assessed
according to the International Federation of Gynecology and
Obstetrics (FIGO) (21). The
positive control tissues in the present study were derived from
adjacent renal tissue from the surgical specimens of patients with
renal cancer, which were paraffin-embedded tissue specimens
archived following pathological diagnosis.
Tissue microarray (TMA) and
immunohistochemistry (IHC)
The standard EnVision procedure for tissue
microarrays was used to evaluate the immunohistochemical expression
of the CCL14 protein (22). To avoid
potential bias caused by the heterogeneity of tumors, the TMA
included 154 patient samples and each sample had 3 selected points
with a diameter of 1.5 mm. IHC was performed on all points for each
sample. The results of IHC were calculated by averaging the 3
points. All samples were fixed with 10% neutral buffered formalin
(NBF) at room temperature for 48 h. IHC sections were prepared
using 3-µm TMA paraffin-embedded sections. Xylene was used to
de-wax the sections and then the sections were rehydrated in a
descending alcohol series (100, 95, 85, 75 and 65%) and distilled
water at room temperature. Subsequently, the sections were
pressure-cooked at 100°C with citric acid buffer solution (pH 6.0)
for 3 min to repair antigen, then sections were placed in 3%
hydrogen peroxide for 10 min to block endogenous peroxidase
activity. After the pre-processing in the aforementioned steps, TMA
sections were incubated with CCL14 primary antibody (polyclonal
antibody; cat. no. PA5-28819; Invitrogen; Thermo Fisher Scientific,
Inc.) at a dilution ratio of 1:500 for 50 min at 37°C in the
incubator. Following this, TMA sections were incubated with CCL14
secondary antibody (undiluted; cat. no. K5007; Dako; Agilent
Technologies, Inc.) for 30 min at 37°C in the incubator. The
sections were then stained with 3,3-diaminobenzidine. The last step
involved using hematoxylin to counterstain the sections, which were
finally fixed using dehydration. Human kidney tissue was used as
the positive control (14) and in
the negative control, human kidney tissue were incubated with 0.02
mol/l PBS instead of the primary antibody against CCL14.
IHC evaluation
Expression levels of CCL14 was evaluated by 2
independent pathologists using a light microscope (cat. no. BX51;
Olympus Corp.). Percentages (0–100%) were used to define the number
of positive tumor cells and a scoring system was used to evaluate
dye strength as follows: Negative expression, -; low positive
expression, 1+; moderate positive expression, 2+; and strong
positive expression, 3+. Scoring of each sample was performed using
the following formula: Percentage of positive cells × dye strength
and the total score of each sample ranged from 0–300 (22).
Statistical analysis
SPSS v16.0 (SPSS, Inc.) was used to perform the
statistical analysis of the present study. Data are presented as
mean standard deviation. The χ2 test was used to analyze
the association between CCL14 protein expression levels and
clinicopathological parameters in patients with EOC. Survival
analysis of patients with EOC was performed using the Kaplan-Meier
method with the log-rank test for evaluation. A Cox proportional
hazard model was used to perform multivariate analyses. All
P-values were analyzed bilaterally. P<0.05 was considered to
indicate a statistically significant difference.
Results
Immunohistochemical analysis of CCL14
in tissues from patients with EOC
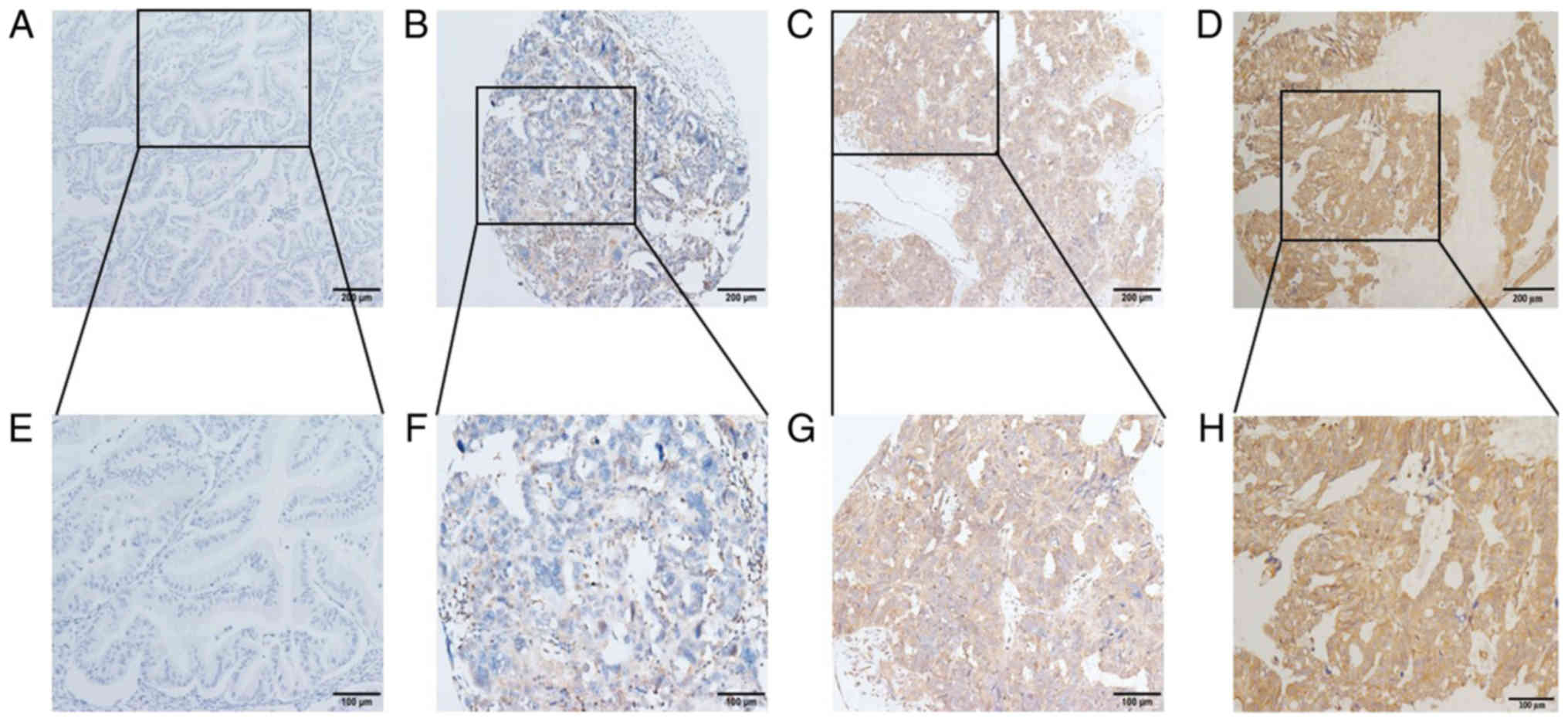
IHC results revealed that CCL14 was expressed in
most cases, mainly in the cytoplasm of cancer cells. Representative
image of expression levels are presented in Fig. 1, from negative to strong positive.
IHC scoring showed that 17 cases were scored between 0–50 (11.04%),
39 cases were scored between ≥50–100 (25.32%); 22 cases were scored
between ≥100–150 (14.29%), 27 cases were scored between ≥150–200
(17.53%), 20 cases were scored between ≥200–250 (12.99%) and 29
cases were scored ≥250 (18.83%) (data not shown).
Cut-off value for CCL14 expression
levels
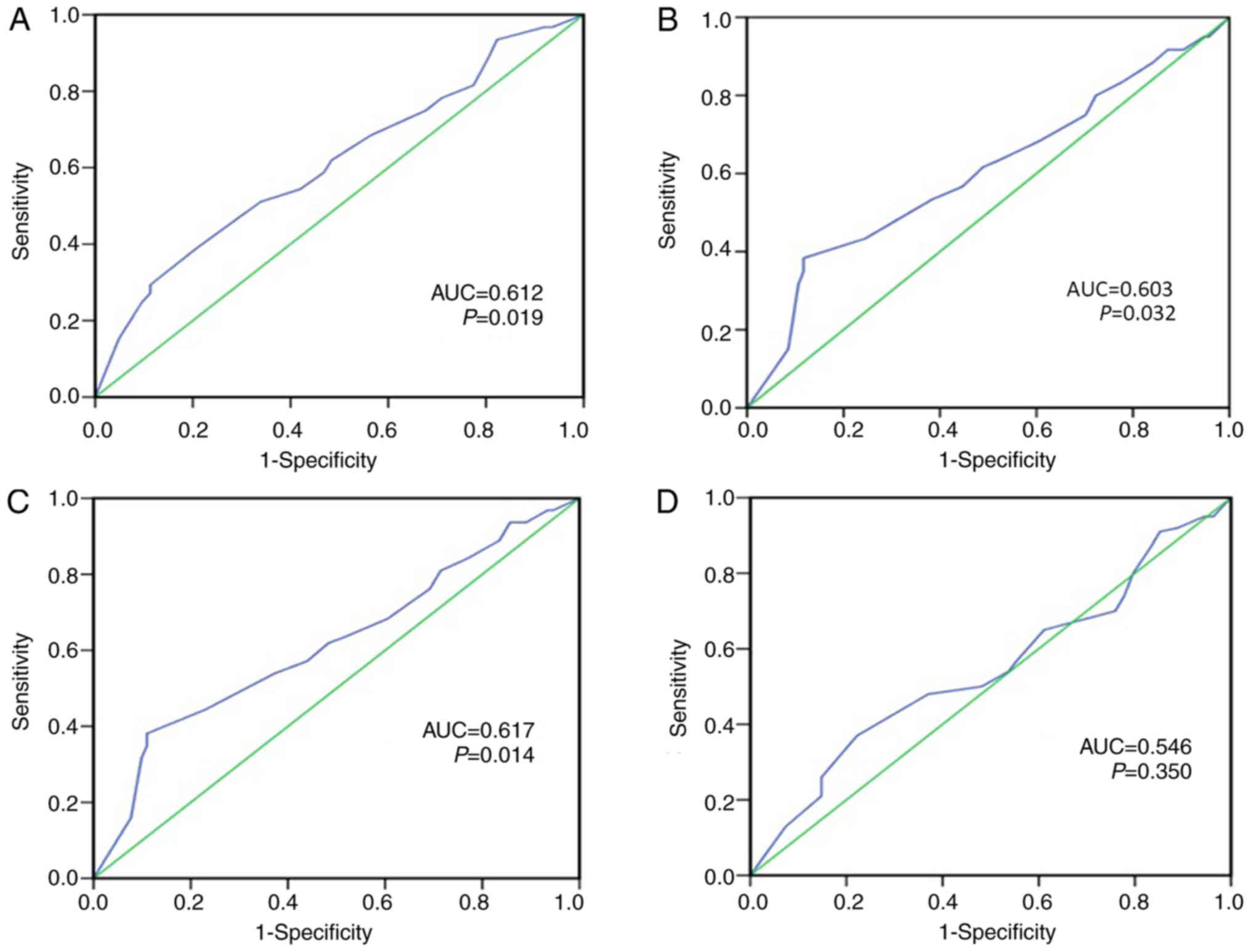
In order to select the appropriate cut-off point of
CCL14 for further analysis, a receiver operating characteristic
(ROC) curve was used to analyze each clinicopathological parameter.
The point of infinite proximity (0.0, 1.0) on the ROC curve for
each clinicopathological parameter had relevant specificity and
sensitivity at the highest points (23), so these areas were defined as the
cut-off points. The ROC curve analysis demonstrated that survival
status, FIGO stage, pN status and relapse were taken as state
variables for ROC analysis (Fig. 2)
and that the classification efficiency of survival status (area
under curve, 0.612; P=0.02) was the best (Fig. 2A). Therefore, survival status was
taken as a state variable and 190 was defined as the cut-off point
for CCL14 protein expression levels. High expression levels of
CCL14 protein were defined as those with higher expression levels
compared with the cut-off value and low expression levels of CCL14
was defined as those with expression levels which were below the
cut-off value.
Association of CCL14 expression levels
with clinicopathological features in patients with EOC
The expression levels of CCL14 is associated with a
number of clinicopathological features. High expression levels of
CCL14 protein were analyzed using the χ2 test which
revealed that it was significantly inversely associated with FIGO
stage (P=0.014; Table I). Among 94
patients with advanced stage EOC (III+IV), 71 (75.5%) had low
expression levels of CCL14 and 23 (24.5%) had high expression
levels. Among the 60 patients with early stage EOC (I+II), 34
(56.7%) had low expression levels and 26 (43.3%) had high
expression levels of CCL14. Patients with pN 1 status had similar
results (P=0.005; Table I), 70
(76.9%) had low expression levels and 21 (23.1%) had high
expression levels of CCL14. In those patients with pN 0 status, 35
(55.6%) had low expression levels of CCL14 and 28 (44.4%) had high
expression levels. However, the other clinical and demographic
parameters of age, histological type, pathological grade, tumor
size, relapse, CA125, CA19-9 and CEA, were not significantly
associated with the expression levels of CCL14.
 | Table I.Association between the
clinicopathological variables and expression of CCL14 in patients
with epithelial ovarian cancer. |
Table I.
Association between the
clinicopathological variables and expression of CCL14 in patients
with epithelial ovarian cancer.
|
|
| CCL14 expression
levels |
|
|---|
|
|
|
|
|
|---|
| Variable | All cases, n | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Age, years |
|
|
| 0.614 |
|
≤49 | 80 | 56 (70.0) | 24 (30.0) |
|
|
>49 | 74 | 49 (66.2) | 25 (33.8) |
|
| FIGO stage |
|
|
| 0.014a |
|
I+II | 60 | 34 (56.7) | 26 (43.3) |
|
|
III+IV | 94 | 71 (75.5) | 23 (24.5) |
|
| Histological
type |
|
|
| 0.132 |
|
Serous | 101 | 73 (72.3) | 28 (27.7) |
|
|
Mucinous | 53 | 32 (60.4) | 21 (39.6) |
|
| Pathological
grade |
|
|
| 0.207 |
| G1 | 27 | 20 (74.1) | 7 (25.9) |
|
| G2 | 48 | 28 (58.3) | 20 (41.7) |
|
| G3 | 79 | 57 (72.2) | 22 (27.8) |
|
| Median tumor size,
cm |
|
|
| 0.271 |
|
≤10.05 | 76 | 55 (72.4) | 21 (27.6) |
|
|
>10.05 | 78 | 50 (64.1) | 28 (35.9) |
|
| Relapse |
|
|
| 0.060 |
| No | 100 | 63 (63.0) | 37 (37.0) |
|
|
Yes | 54 | 42 (77.8) | 12 (22.2) |
|
| pN status |
|
|
| 0.005b |
| 0 | 63 | 35 (55.6) | 28 (44.4) |
|
| 1 | 91 | 70 (76.9) | 21 (23.1) |
|
| CA125, U/ml |
|
|
| 0.821 |
|
≤33 | 17 | 12 (70.6) | 5 (29.4) |
|
|
>33 | 137 | 93 (67.9) | 44 (32.1) |
|
| CA19-9, U/ml |
|
|
| 0.753 |
|
≤35 | 101 | 68 (67.3) | 33 (32.7) |
|
|
>35 | 53 | 37 (69.8) | 16 (30.2) |
|
| CEA, µg/ml |
|
|
| 0.953 |
| ≤5 | 123 | 84 (68.3) | 39 (31.7) |
|
|
>5 | 31 | 21 (67.7) | 10 (32.3) |
|
Association between
clinicopathological characteristics, CCL14 status and patient
survival
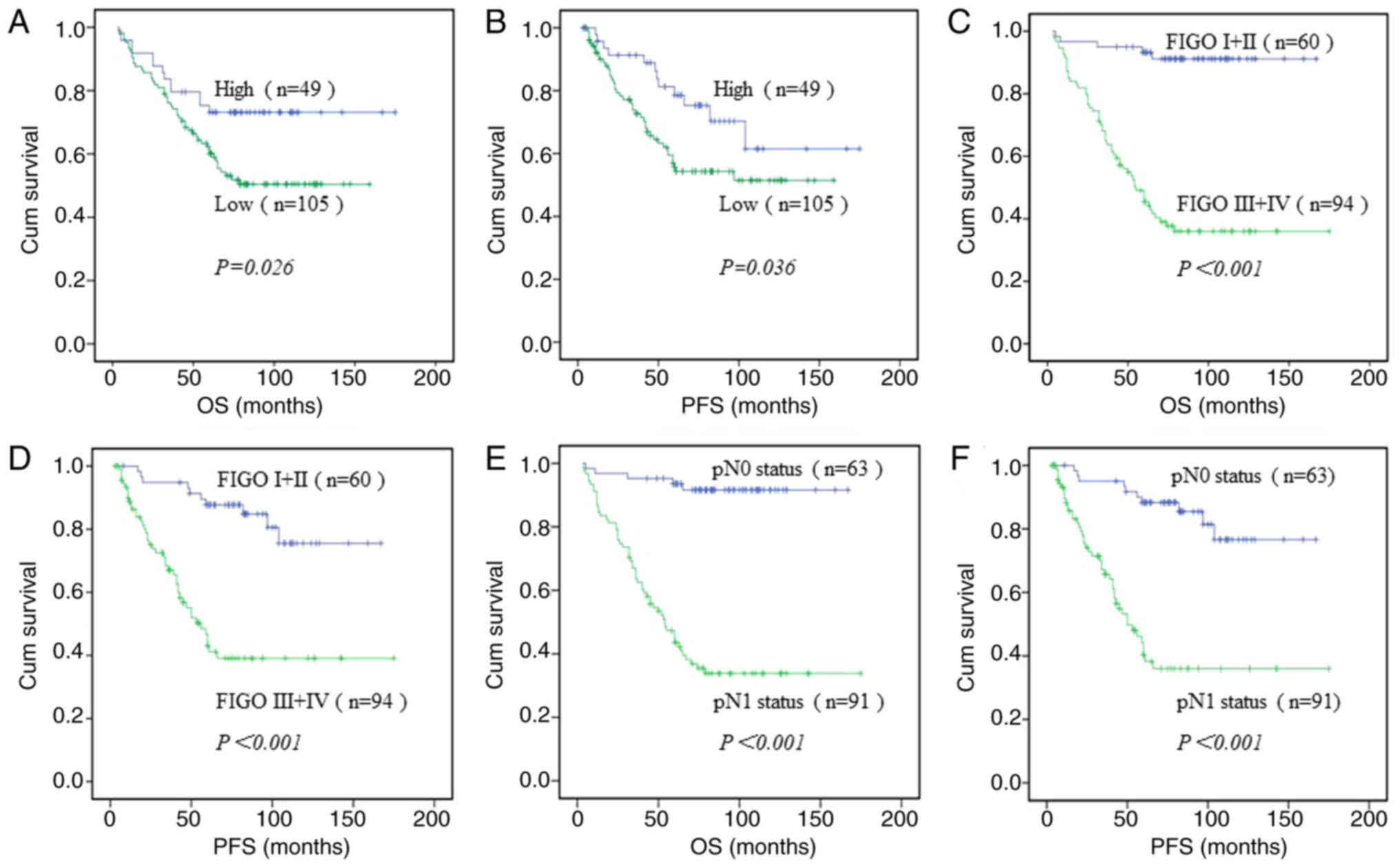
To elucidate the best clinicopathological factors
for the prognosis of EOC, a univariate analysis of each
clinicopathological parameter was performed. The results
demonstrated that patients with high expression levels of CCL14 had
a high OS time (mean, 136.1 months); this was significantly higher
compared with the mean of 98.9 months in patients with low CCL14
expression levels (P=0.026; Table
II; Fig. 3A). A similar trend
was observed for progression-free survival (PFS) (Fig. 3B). For FIGO stage, the mean OS time
of patients with stage I+II cancer was 155.4 months, which was
significantly higher compared with that of patients with stage
III+IV cancer (mean, 86.2 months) (P<0.001; Table II; Fig.
3C). PFS of patients with stage I+II EOC was significantly
higher compared with that of patients with stage III+IV cancer.
(P<0.001; Fig. 3D). For
pathological grade, the mean OS time for patients with a high
differentiation grade was longer compared with that for patients
with a low differentiation grade (G1 mean, 143.3 months; G2 mean,
130.9 months; G3 mean, 90.4 months) (P<0.001; Table II). The mean OS time for relapse was
shorter (mean, 73.8 months) compared with that for no relapse
(mean, 130.9 months) (P=0.001; Table
II). The mean OS time for patients with EOC who had pN 1 status
(mean, 83.1 months) was shorter compared with that for patients
with pN 0 status (mean, 156 months) (P<0.001; Table II; Fig.
3E). A similar trend was observed for PFS (P<0.001; Fig. 3F). The results also revealed that
CA125 is a relevant factor in survival (P=0.006; Table II). However, age, histological type,
tumor size, CA19-9 and CEA did not impact the OS time of patients
with EOC (Table II).
 | Table II.Univariate analysis of
clinicopathological variables in 154 patients with ovarian
cancer. |
Table II.
Univariate analysis of
clinicopathological variables in 154 patients with ovarian
cancer.
| Variable | All cases, n | Mean OS time,
months |
P-valued |
|---|
| Age, years |
|
| 0.057 |
|
≤49 | 80 | 127.19±7.75 |
|
|
>49 | 74 | 90.77±6.76 |
|
| FIGO stage |
|
|
<0.001c |
|
I+II | 60 | 155.37±5.02 |
|
|
III+IV | 94 | 86.20±7.34 |
|
| Histological
type |
|
| 0.413 |
|
Serous | 101 | 112.85±7.08 |
|
|
Mucinous | 53 | 113.01±8.84 |
|
| Pathological
grade |
|
|
<0.001c |
| G1 | 27 | 143.26±8.60 |
|
| G2 | 48 | 130.91±9.96 |
|
| G3 | 79 | 90.38±7.52 |
|
| Tumor size, cm |
|
| 0.897 |
|
≤10.05 | 76 | 116.81±8.07 |
|
|
>10.05 | 78 | 96.35±6.42 |
|
| Relapse |
|
| 0.001b |
| No | 100 | 130.92±6.94 |
|
|
Yes | 54 | 73.77±6.12 |
|
| pN status |
|
|
<0.001c |
| 0 | 63 | 156.00±4.76 |
|
| 1 | 91 | 83.13±7.38 |
|
| CA125, U/ml |
|
| 0.006b |
|
≤33 | 17 | 119.83±6.96 |
|
|
>33 | 137 | 110.06±6.20 |
|
| CA19-9, U/ml |
|
| 0.195 |
|
≤35 | 101 | 106.43±6.78 |
|
|
>35 | 53 | 126.78±9.64 |
|
| CEA, µg/ml |
|
| 0.407 |
| ≤5 | 123 | 109.15±5.67 |
|
|
>5 | 31 | 107.49±13.37 |
|
| CCL14 |
|
| 0.026a |
| High
expression | 49 | 136.06±9.33 |
|
| Low
expression | 105 | 98.86±6.30 |
|
Independent prognostic factors for
patients with EOC
Multivariable analysis using a Cox risk regression
model demonstrated that upregulation of CCL14 protein in patients
with EOC was significantly associated with OS (HR, 0.483; 95% CI,
0.261–0.896; P=0.021; Table III)
and was an independent prognostic factor. Upregulation of CCL14 was
also associated with a favorable PFS (HR, 0.437; 95% CI:
0.228–0.839, P=0.013; Table III).
In addition, two interesting phenomena were observed: Pathological
grade was an independent prognostic factor in OS (HR, 1.865; 95%
CI, 1.179–2.948; P=0.008; Table
III) and PFS (HR, 1.774; 95% CI, 1.128–2.791; P=0.013; Table III) in the patients with EOC.
 | Table III.Multivariate survival analyses of
clinicopathological variables in patients with epithelial ovarian
cancer. |
Table III.
Multivariate survival analyses of
clinicopathological variables in patients with epithelial ovarian
cancer.
|
| OS time | PFS time |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (>49 vs. ≤49
years) | 1.310 | 0.758–2.263 | 0.333 | 1.027 | 0.579–1.821 | 0.927 |
| Histological type
(serous vs. mucinous) | 1.068 | 0.603–1.892 | 0.821 | 1.239 | 0.670–2.289 | 0.494 |
| Pathological grade
(G3 vs. G1+2) | 1.865 | 1.179–2.948 | 0.008 | 1.774 | 1.128–2.791 | 0.013a |
| Tumor size, cm
(>10.05 vs. ≤10.05) | 1.118 | 0.654–1.911 | 0.684 | 0.701 | 0.390–1.258 | 0.233 |
| CA125, U/ml (>33
vs. ≤33) | 6.511 | 0.868–48.846 | 0.068 | 7.126 | 0.946–53.693 | 0.057 |
| CA19-9, U/ml
(>35 vs. ≤35) | 0.709 | 0.389–1.294 | 0.263 | 0.841 | 0.447–1.581 | 0.590 |
| CEA, µg/ml (>5
vs. ≤5) | 1.158 | 0.617–2.172 | 0.648 | 1.309 | 0.659–2.60 | 0.442 |
| CCL14 expression
(high vs. low) | 0.483 | 0.261–0.896 | 0.021 | 0.437 | 0.228–0.839 | 0.013a |
Discussion
Chemokines comprise a group of ~50 small secreted
proteins (8–14 kDa). According to the position of the first 2
cysteine residues, chemokines can be divided into CC-chemokines,
CXC-chemokines, C-chemokines and CX3C-chemokines. The role of these
proteins is to interact with a family of ~20 7-transmembrane
G-protein-coupled receptors (24).
Some studies have demonstrated that chemokines are novel targets
for cancer immunotherapy (25,26).
CCL14 is a member of the chemokine family that has attracted
considerable attention in recent years, as it has a common receptor
(CCR5) against HIV, which may be used as a novel method of treating
HIV (15,27). The gene for the CCL14 protein is
located on human chromosome 17q11.2 and is the product of
transcripts encoding single and double cis-trans and double
cis-trans alignments of tandem genes (28). CCL14 is expressed in certain normal
somatic tissues, including the spleen, liver, skeletal muscle,
myocardium, intestinal tract and bone marrow and is a C-chemokine
with unconventional biological activity (14). CCL14 can be converted into a monomer
at physiological concentrations, which is able to activate the
migration of different leukocytes by inducing Ca2+ flux (29). Studies have shown that the
CCL14/CCR1/CCR5 axis is hypothesized to mediate the chemotaxis of
monocytes, eosinophils and T lymphocytes, which is consistent with
the high expression levels of CCR5/CCR1 in these cells (30,31).
In the present study, IHC was used to detect the
expression levels of CCL14 protein. The results of the current
study revealed that CCL14 protein was expressed in most patients
with EOC. Upregulation of CCL14 accounted for 43.3% of those with
early-stage EOC, which was a significantly higher percentage
compared with that for late-stage patients with EOC (24.5%). The
proportion of patients with high expression levels of CCL14 with pN
0 status was higher compared with that of patients with pN 1
status. The mean OS time of patients with high expression levels of
CCL14 was significantly higher compared with that of the patients
with low expression levels of CCL14. The results revealed that the
upregulation of CCL14 was significantly associated with the OS time
of patients with EOC. Furthermore, multivariate analysis revealed
that the upregulation of CCL14 was significantly associated with OS
and PFS time, which indicated that the upregulation of CCL14 was an
independent prognostic factor for EOC.
Studies suggest that CCL14 is involved in the
incidence and development of breast and oral cancer (17,18);
however, there are no reports that investigate the role of CCL14 in
EOC. Studies have demonstrated that CCL5/CCR5 and chemokine (C-X-C)
motif ligand 12 (CXCL12) β promote tumor immune tolerance and tumor
progression (32,33). CXCL12/CXCR4, CCL18 and CXCL16/CXCR6
can promote the progression and migration of OC (34–36).
However, studies performed on CXCL9 and CXCL10 have revealed that
it can promote an antitumor immune response (37,38).
These studies have demonstrated that chemokines are directly
involved in the formation of the immune microenvironment in OC.
CCL14 appears to be significantly downregulated in 9 types of
cancer, which implicates CCL14 as an important player in the
pathogenesis of cancer (39).
Previous studies have demonstrated that CCL14 is a factor that can
improve the prognosis of patients with hepatocellular carcinoma and
human papilloma virus-related cervical intraepithelial neoplasia
(40,41). Low expression levels of CCL14 is
associated with poor immune function and disease promotion in these
carcinomas (40,41). In multiple myeloma, CCL14 can recruit
polarized macrophages to form an antitumor immune environment and
improve the prognosis of patients with multiple myeloma (42). These studies have also demonstrated
that CCL14 is a tumor suppressor gene.
In intestinal studies, CCL14 can recruit chemotactic
immune cells and prevent pathogenic bacteria from affecting the
intestine (43). This function of
CCL14 plays an important anticancer role in OC, since a study
demonstrated that there are a large number of macrophages and T
lymphocytes in the OC microenvironment (44). Other studies reported that in OC,
macrophages and T lymphocytes upregulate the expression levels of
CCR1 and CCR5 receptors, which creates an antitumor immune
environment leading to the necrosis of OC cells (45,46).
Furthermore, another study investigating multiple myeloma
demonstrated that when CCL14 binds to CCR1 and CCR5, it can lead to
chemoattraction and recruitment of macrophages, which promotes
proliferation by activating the PI3K-AKT, MAPK/ERK, JNK and p38MAPK
pathways (42). These studies
suggest that the CCL14 protein is involved in the generation of
antitumor immunity. It is possible that CCL14 has a similar role
and underlying mechanism in OC, although the mechanism by which it
exerts antitumor activities requires further exploration.
In conclusion, CCL14 is an independent prognostic
factor in EOC. Upregulation of CCL14 is associated with increased
OS and PFS times for patients with EOC. Overall, the present study
revealed that CCL14 may be used for the development of novel
targeted therapies for EOC.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Medical Technology Foundation of Jiangmen (grant no. 2017A4003) and
the Guangdong Medical Science and Technology Research Fund (grant
no. A2016003).
Availability of data and materials
The data that support the findings of the present
study are available from researchdata.org.cn of Sun Yat-sen University Cancer
Center. However, restrictions apply to the availability of these
data, which were used under license for the present study and are
not publicly available. Data are however available from the authors
upon reasonable request and with the permission of Research Data
Deposit public platform of Sun Yat-sen University Cancer
Center.
Authors' contributions
YC and JC designed the present study. HH, XC, YC and
LH acquired and analyzed the data. YC, YL and LH performed all the
experiments and drafted the manuscript. YX and ZZ collected and
assembled the data and performed the experiments. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by The Institute Research
Medical Ethics Committee of Sun Yat-Sen University Cancer Center
(Guangzhou, China) and Jiangmen Central Hospital (Jiangmen, China).
The requirement for informed consent (written or verbal) was waived
for the use of retrospective data and paraffin tissue specimens
from the patients in the present study, the majority of whom were
deceased, since this was not deemed necessary by the Ethics
Committee. All samples were anonymized.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rojas V, Hirshfield KM, Ganesan S and
Rodriguez-Rodriguez L: Molecular characterization of epithelial
ovarian cancer: Implications for diagnosis and treatment. Int J Mol
Sci. 17(pii): E21132016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCluggage WG: Morphological subtypes of
ovarian carcinoma: A review with emphasis on new developments and
pathogenesis. Pathology. 43:420–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kossaï M, Leary A, Scoazec JY and Genestie
C: Ovarian cancer: a heterogeneous disease. Pathobiology. 85:41–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morgan RJ Jr, Armstrong DK, Alvarez RD,
Bakkum-Gamez JN, Behbakht K, Chen LM, Copeland L, Crispens MA,
DeRosa M, Dorigo O, et al: Ovarian cancer, version 1.2016, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
14:1134–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sant M, Allemani C, Santaquilani M, Knijn
A, Marchesi F and Capocaccia R; EUROCARE Working Group, :
EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999.
Results and commentary. Eur J Cancer. 45:931–991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sant M, Chirlaque Lopez MD, Agresti R,
Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, Dimitrova N, Innos
K, Katalinic A, Langseth H, et al: Survival of women with cancers
of breast and genital organs in Europe 1999–2007: Results of the
EUROCARE-5 study. Eur J Cancer. 51:2191–2205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J,
Li J, Li F and Tan HB: Immune cells within the tumor
microenvironment: Biological functions and roles in cancer
immunotherapy. Cancer Lett. Nov 12–2019.(Epub ahead of print).
|
|
11
|
Sonugür FG and Akbulut H: The role of
tumor microenvironment in genomic instability of malignant tumors.
Front Genet. 10:10632019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mukaida N, Sasaki S and Baba T: Chemokines
in cancer development and progression and their potential as
targeting molecules for cancer treatment. Mediators Inflamm.
2014:1703812014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schulz-Knappe P, Mägert HJ, Dewald B,
Meyer M, Cetin Y, Kubbies M, Tomeczkowski J, Kirchhoff K, Raida M,
Adermann K, et al: HCC-1, a novel chemokine from human plasma. J
Exp Med. 183:295–299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Detheux M, Ständker L, Vakili J, Münch J,
Forssmann U, Adermann K, Pöhlmann S, Vassart G, Kirchhoff F,
Parmentier M and Forssmann WG: Natural proteolytic processing of
hemofiltrate CC chemokine 1 generates a potent CC chemokine
receptor (CCR)1 and CCR5 agonist with anti-HIV properties. J Exp
Med. 192:1501–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsou CL, Gladue RP, Carroll LA, Paradis T,
Boyd JG, Nelson RT, Neote K and Charo IF: Identification of C-C
chemokine receptor 1 (CCR1) as the monocyte hemofiltrate C-C
chemokine (HCC)-1 receptor. J Exp Med. 188:603–608. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Shi L, Gui B, Yu W, Wang J, Zhang D,
Han X, Yao Z and Shang Y: Binding of the JmjC demethylase JARID1B
to LSD1/NuRD suppresses angiogenesis and metastasis in breast
cancer cells by repressing chemokine CCL14. Cancer Res.
71:6899–6908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng L, Houck JR, Lohavanichbutr P and
Chen C: Transcriptome analysis reveals differentially expressed
lncRNAs between oral squamous cell carcinoma and healthy oral
mucosa. Oncotarget. 8:31521–31531. 2017.PubMed/NCBI
|
|
19
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumours of female reproductive
organs. 4th edition. World Health Organization; 2014
|
|
20
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. New York:
Springer; 2010
|
|
21
|
Prat J; FIGO Committee on Gynecologic
Oncology, : Staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Abridged republication of
guidelines from the international federation of gynecology and
obstetrics (FIGO). Obstet Gynecol. 126:171–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai MY, Luo RZ, Chen JW, Pei XQ, Lu JB,
Hou JH and Yun JP: Overexpression of ZEB2 in peritumoral liver
tissue correlates with favorable survival after curative resection
of hepatocellular carcinoma. PLoS One. 7:e328382012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai MY, Zhang B, He WP, Yang GF, Rao HL,
Rao ZY, Wu QL, Guan XY, Kung HF, Zeng YX and Xie D: Decreased
expression of PinX1 protein is correlated with tumor development
and is a new independent poor prognostic factor in ovarian
carcinoma. Cancer Sci. 101:1543–1549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Griffith JW, Sokol CL and Luster AD:
Chemokines and chemokine receptors: Positioning cells for host
defense and immunity. Annu Rev Immunol. 32:659–702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mollica Poeta V, Massara M, Capucetti A
and Bonecchi R: Chemokines and chemokine receptors: New targets for
cancer immunotherapy. Front Immunol. 10:3792019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Agostino G, Cecchinato V and Uguccioni
M: Chemokine heterocomplexes and cancer: A novel chapter to be
written in tumor immunity. Front Immunol. 9:21852018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Münch J, Ständker L, Pöhlmann S, Baribaud
F, Papkalla A, Rosorius O, Stauber R, Sass G, Heveker N, Adermann
K, et al: Hemofiltrate CC chemokine 1[9-74] causes effective
internalization of CCR5 and is a potent inhibitor of R5-tropic
human immunodeficiency virus type 1 strains in primary T cells and
macrophages. Antimicrob Agents Chemother. 46:982–990. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Forssmann U, Mägert HJ, Adermann K, Escher
SE and Forssmann WG: Hemofiltrate CC chemokines with unique
biochemical properties: HCC-1/CCL14a and HCC-2/CCL15. J Leukoc
Biol. 70:357–366. 2001.PubMed/NCBI
|
|
29
|
Blain KY, Kwiatkowski W, Zhao Q, La Fleur
D, Naik C, Chun TW, Tsareva T, Kanakaraj P, Laird MW, Shah R, et
al: Structural and functional characterization of CC chemokine
CCL14. Biochemistry. 46:10008–10015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bleul CC, Wu L, Hoxie JA, Springer TA and
Mackay CR: The HIV coreceptors CXCR4 and CCR5 are differentially
expressed and regulated on human T lymphocytes. Proc Natl Acad Sci
USA. 94:1925–1930. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loetscher P, Uguccioni M, Bordoli L,
Baggiolini M, Moser B, Chizzolini C and Dayer JM: CCR5 is
characteristic of Th1 lymphocytes. Nature. 391:344–345. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
You Y, Li Y, Li M, Lei M, Wu M, Qu Y, Yuan
Y, Chen T and Jiang H: Ovarian cancer stem cells promote tumour
immune privilege and invasion via CCL5 and regulatory T cells. Clin
Exp Immunol. 191:60–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Givel AM, Kieffer Y, Scholer-Dahirel A,
Sirven P, Cardon M, Pelon F, Magagna I, Gentric G, Costa A, Bonneau
C, et al: miR200-regulated CXCL12β promotes fibroblast
heterogeneity and immunosuppression in ovarian cancers. Nat Commun.
9:10562018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mao TL, Fan KF and Liu CL: Targeting the
CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther.
24:621–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lane D, Matte I, Laplante C, Garde-Granger
P, Carignan A, Bessette P, Rancourt C and Piché A: CCL18 from
ascites promotes ovarian cancer cell migration through proline-rich
tyrosine kinase 2 signaling. Mol Cancer. 15:582016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong L, Wang S, Li W, Wu D and Chen W:
Tumor-associated macrophages promote the metastasis of ovarian
carcinoma cells by enhancing CXCL16/CXCR6 expression. Pathol Res
Pract. 214:1345–1351. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bronger H, Singer J, Windmüller C, Reuning
U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M
and Avril S: CXCL9 and CXCL10 predict survival and are regulated by
cyclooxygenase inhibition in advanced serous ovarian cancer. Br J
Cancer. 115:553–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
K Au K, Peterson N, Truesdell P,
Reid-Schachter G, Khalaj K, Ren R, Francis JA, Graham CH, Craig AW
and Koti M: CXCL10 alters the tumour immune microenvironment and
disease progression in a syngeneic murine model of high-grade
serous ovarian cancer. Gynecol Oncol. 145:436–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong HS, Chang CM, Liu X, Huang WC and
Chang WC: Characterization of cytokinome landscape for clinical
responses in human cancers. Oncoimmunology. 5:e12147892016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Wan JX, Ke ZP, Wang F, Chai HX
and Liu JQ: TMEM88, CCL14 and CLEC3B as prognostic biomarkers for
prognosis and palindromia of human hepatocellular carcinoma. Tumour
Biol. 39:10104283177089002017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santegoets LA, van Seters M,
Heijmans-Antonissen C, Kleinjan A, van Beurden M, Ewing PC, Kühne
LC, Beckmann I, Burger CW, Helmerhorst TJ and Blok LJ: Reduced
local immunity in HPV-related VIN: Expression of chemokines and
involvement of immunocompetent cells. Int J Cancer. 123:616–622.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Zheng Y, Li T, Wang Q, Qian J, Lu Y,
Zhang M, Bi E, Yang M, Reu F, et al: Chemokines CCL2, 3, 14
stimulate macrophage bone marrow homing, proliferation, and
polarization in multiple myeloma. Oncotarget. 6:24218–24229.
2015.PubMed/NCBI
|
|
43
|
Kotarsky K, Sitnik KM, Stenstad H,
Kotarsky H, Schmidtchen A, Koslowski M, Wehkamp J and Agace WW: A
novel role for constitutively expressed epithelial-derived
chemokines as antibacterial peptides in the intestinal mucosa.
Mucosal Immunol. 3:40–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Negus RP, Stamp GW, Hadley J and Balkwill
FR: Quantitative assessment of the leukocyte infiltrate in ovarian
cancer and its relationship to the expression of C-C chemokines. Am
J Pathol. 150:1723–1734. 1997.PubMed/NCBI
|
|
45
|
Milliken D, Scotton C, Raju S, Balkwill F
and Wilson J: Analysis of chemokines and chemokine receptor
expression in ovarian cancer ascites. Clin Cancer Res. 8:1108–1114.
2002.PubMed/NCBI
|
|
46
|
Ghoneum A, Afify H, Salih Z, Kelly M and
Said N: Role of tumor microenvironment in ovarian cancer
pathobiology. Oncotarget. 9:22832–22849. 2018. View Article : Google Scholar : PubMed/NCBI
|