Introduction
Thyroid cancer is a common malignant tumor of the
endocrine system, its incidence keeps increasing year by year
driven by the change of social environment (1). However, due to the latent onset of
thyroid cancer, many patients are diagnosed with metastasis,
hazarding the prognosis of patients (2,3). The
fact that the current diagnosis and treatment of thyroid cancer
offer few options results in unsatisfactory therapeutic effect for
many patients (4). Therefore,
exploring the pathological mechanism of thyroid cancer is of great
clinical significance for the diagnosis and treatment of patients
with thyroid cancer.
miRNAs are non-coding microRNAs, which mainly
influence the biological function of cells through mRNA matching
with downstream target genes (5).
Studies have shown that miRNAs play a vital part in the occurrence
and development of thyroid cancer. For example, miR-26a-5p has been
reported to inhibit proliferation, invasion and migration of
thyroid papillary cancer cells by inhibiting expression of Wnt5a
(6). According to some other studies
(7), miR-15a can affect the
proliferation and apoptosis of thyroid cancer cells by regulating
AKT. Among those miRNAs, miR-298, located on human chromosome
20q13.32, is related to the proliferation and invasion of tumor
cells according to recent studies (8). For example, a study found that miR-298
could affect the proliferation and invasion of ovarian cancer cells
by regulating the expression of EZH2 (9). However, the role and mechanism of
miR-298 in thyroid cancer remains a subject of investigation. While
CDK6 is a kinase-catalyzed group of a protein kinase complex that
primarily affects the cell cycle, which can increase cell
proliferation by accelerating cell cycle (10). However, similarly to miR-298 little
research has been conducted on the effect of CDK6 on thyroid cancer
cells.
Both Targetscan and miRDB databases predict that
CDK6 is a target gene of miR-298, so it was speculated that miR-298
could affect thyroid cancer cells by regulating CDK6. Therefore,
thyroid cancer cells were selected as the research subjects in the
present study to evaluate the effect and mechanism of miR-298 on
thyroid cancer cells, in an attempt to provide a new target
direction for the research on thyroid cancer.
Patients and methods
Clinical specimens
Seventy-five patients who underwent thyroidectomy in
Cangzhou Medical College (Cangzhou, China) from January 2016 to
January 2018 were enrolled. Paired thyroid cancer tissues and
adjacent cancer tissues were obtained from each patients during the
operation, and stored in a liquid nitrogen tank. The patient
information is detailed in Table I.
Inclusion criteria: Patients pathologically diagnosed as thyroid
cancer for the first time were included. In contrast, the exclusion
criteria were as follows: Patients who had received
chemoradiotherapy, associated with other malignant tumors, severe
liver or kidney dysfunction, severe infectious diseases, or those
refused to provide experimental specimens were excluded.
 | Table I.General information of patients. |
Table I.
General information of patients.
| Categories | Thyroid cancer
patients (n=75) |
|---|
| Sex |
| Male | 39 (52.00) |
|
Female | 36 (48.00) |
| Age (years) | 58.25±8.92 |
| BMI
(kg/m2) | 22.35±1.12 |
| Pathological
types |
| Papillary
carcinoma | 31 (41.33) |
|
Follicular carcinoma | 15 (20.00) |
|
Undifferentiated
carcinoma | 17 (22.67) |
| Medullary
carcinoma | 12 (16.00) |
| Pathological
stage |
| I | 32 (42.67) |
| II | 27 (36.00) |
| III | 16 (21.33) |
| Differentiation
degree |
| High | 33 (44.00) |
|
Medium | 26 (34.67) |
| Low | 16 (21.33) |
The study was approved by the Ethics Committee of
Cangzhou Medical College (Cangzhou, China). Patients who
participated in this research had complete clinical data. Patients
and their families agreed to participate in the experiment and
signed informed consents were obtained from the patients or the
guardians.
Experimental reagents and
materials
Human thyroid cancer lines SW579, KHM-2, B-CPAP and
human normal thyroid cell line Nthy-ori3-1 (Conservation Genetics
CAS Shanghai Cell Bank, China). QRT-PCR and reverse transcription
kit (AQ201-01, AQ202-01; TransGen Biotech Co., Ltd.), PBS, fetal
bovine serum (FBS) (10010049 and 10437028; Gibco; Thermo Fisher
Scientific, Inc.), TRIzol reagent (15596018; Gibco; Thermo Fisher
Scientific, Inc.), dual luciferase reporter detection kit
(KFS303-TFX; Beijing Biolab Technology Co., Ltd.), CCK-8 kit
(Promega Corporation), transwell kit (Beijing Yaanda Biotechnology
Co., Ltd.), RIPA, BCA protein kit (Gibco; Thermo Fisher Scientific,
Inc.), Annexin V-FITC/PI cell apoptosis kit (Zp327-1; Beijing Zoman
Biotechnology Co., Ltd.), CDK6, caspase-3, Bax, Bcl-2 and β-actin
antibodies (Cell Signaling Technology Co.), goat anti-rabbit IgG
secondary antibody (Wuhan Boster Biological Technology Co., Ltd.),
ECL developer (Gibco; Thermo Fisher Scientific, Inc.), PCR
instrument (7500 real-time PCR system; Applied Biosystems; Thermo
Fisher Scientific, Inc.). All primers were designed and synthesized
by Shanghai Sangon Biotechnology Co., Ltd.
RT-PCR detection for miR-298 and CDK6
expression
Thyroid tissues and adjacent tissues were removed
from the liquid nitrogen tank for grinding. The total RNA in the
tissue was extracted with TRIzol reagent, whose concentration and
purity of total RNA were then detected by ultraviolet
spectrophotometer, and those within OD260/OD280 >1.8 were
selected for further experiments. Then, 5 µg of total RNA was taken
for cDNA reverse transcription according to the kit instructions.
The reaction parameters were: 37°C for 15 min, 42°C for 35 min, and
70°C for 5 min. miR-298 amplification conditions: PCR reaction
conditions: Pre-denaturation at 94°C for 45 sec, denaturation at
94°C for 10 sec, annealing at 60°C for 45 sec, totaling 40 cycles.
CDK6 amplification conditions: Pre-denaturation at 95°C for 30 sec,
denaturation at 95°C for 10 sec, annealing at 60°C for 35 sec, and
a total of 40 cycles were performed. Three replicate wells were set
per sample, and the experiment was carried out 3 times. Finally,
with U6 as the internal reference of miR-298, and β-actin for CDK6,
2−∆∆ct was applied to analyze the data.
Cell culture and transfection
Thyroid cancer cell lines were cultured in a medium
containing 10% PBS DMEM at 37°C and 5% CO2. When the
cell adherent growth and fusion reached 85%, 25% trypsin was added
for digestion before further culture and passage in the culture
medium. After passage, the cells were taken for detection of the
expression levels of miR-298 and CDK6 mRNA by the method described.
Expression of miR-298 in SW579 and KHM-2 cells was lower than that
in B-CPAP cells, thus, SW579 and KHM-2 cells were selected for
transfection and subsequent experiments. miR-298-mimics
(overexpression sequence), miR-298-inhibitor (inhibition sequence),
miR negative control (miR-NC), targeted inhibition of CDK6 RNA
(si-CDK6), negative control RNA (Si-NC), Targeting overexpressing
CDK6 RNA (sh-CDK6), negative control (Sh-NC) were transfected into
cells using Lipofectamine™ 2000 kit, strictly following the kit
instructions.
Western blot analysis
RIPA lysis was applied to lyse the cells and extract
total protein, then the protein concentration was measured by BCA
assay. The protein concentration was adjusted to 4 µg/µl,
electrophoretically separated by 12% SDS-PAGE before transferring
to PVDF membrane, then sealed for 2 h with 5% skim milk powder.
CDK6 (1:500), caspase-3 (1:500), Bax (1:500), Bcl-2 (1:500) and
β-actin (1:1,000) were then added and sealed overnight at 4°C.
After that, the first antibody was removed by washing the membrane,
followed by the addition of horseradish peroxidase-labeled goat
anti-rabbit secondary antibody (1:1,000), incubated at 37°C for 1
h, and rinsed with PBS 3 times, 5 min each. Then, developed in a
dark room, excess liquid was dried on the membrane with filter
paper, then developed with ECL luminescence.
Cell proliferation test
The proliferation ability of SW579 and KHM-2 cells
was evaluated by CCK-8 kit. Cells were collected 48 h after
transfection, adjusted to 3×104 cells and inoculated on
96-well plates. Then, 100 µl cells were implanted in each well and
cultured in an environment of 37°C and 5% CO2. Next,
each well was added with 10 µl CCK8 solution at 0, 24, 48, and 72 h
after cell adherent growth. After adding the reagent, the culture
was continued for 2 h in an incubator at 37°C and 5% CO2
for 2 h. Then the OD value was measured at 450 nm with a microplate
reader to detect cell proliferation and draw a growth curve. The
experiment was repeated 3 times.
Apoptosis
The transfected cells were digested with 0.25%
trypsin, washed twice with PBS, and added with 100 µl binding
buffer to prepare a suspension of 1×106 cells/ml.
Followed by successive addition of Annexin V-FITC and PI, incubated
at room temperature for 5 min in the dark, and then detected by
FACSVerse flow cytometry system. The experiment was repeated 3
times for average value.
Dual luciferase assay
Lipofectamine 2000 kit was employed to transfect
CDK6-3′UTR wild-type (Wt), CDK6-3′UTR mutant (Mut) as well as
miR-298-mimics and miR-NC into SW579 and KHM-2 cells. Luciferase
activity was measured 48 h after transfection using a dual
luciferase reporter assay kit (Promega Corporation).
Statistical analysis
In the present study, the collected data was
analysed using SPSS20.0 (IBM Corp.), and the picture rendering was
performed by GraphPad 7. Independent t-test was employed for
inter-group comparison, while the inter-group comparison was
conducted by one-way ANOVA. LSD-t was adopted for post pairwise
comparison, and repeated measurement ANOVA was applied for
multi-time expression. Bonferroni was used for post hoc test, and
Pearson test was utilized to analyze the correlation between
micR-298 and CDK6 in tissues. P<0.05 indicates a statistically
significant difference.
Results
Expression levels of miR-298 and CDK6
in thyroid carcinoma tissues
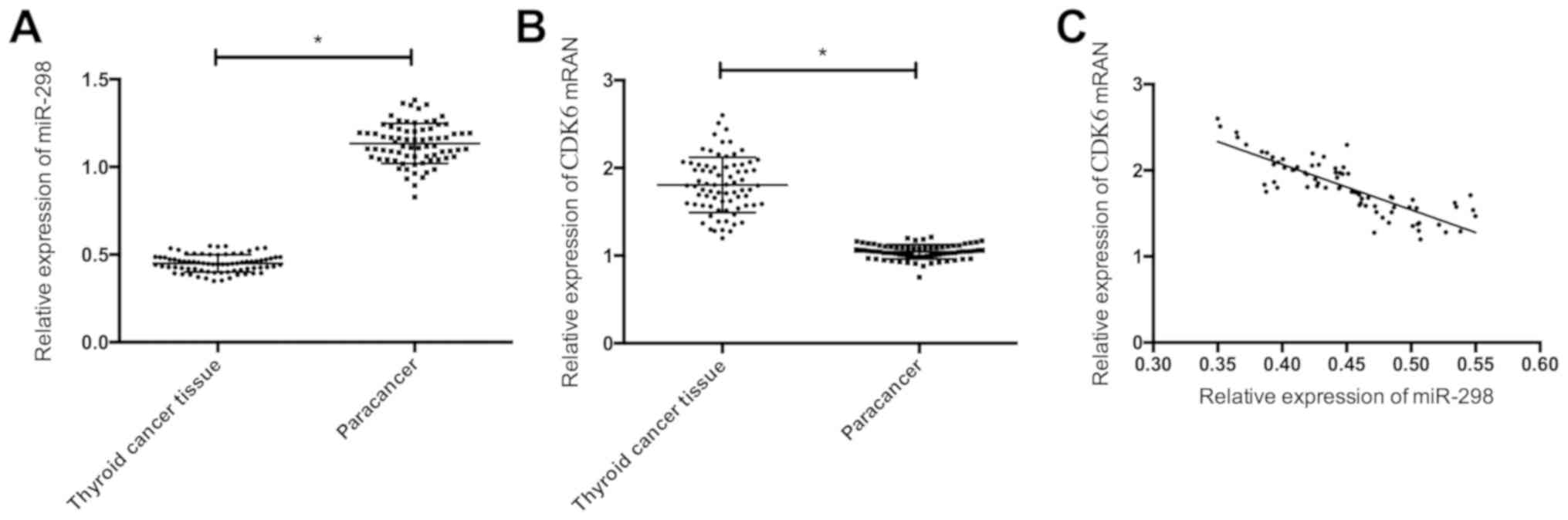
RT-PCR showed that compared with the adjacent
tissues, miR-298 decreased notably (P<0.05), and CDK6 increased
significantly in thyroid cancer tissues (P<0.05). There was a
negative correlation between the expression levels of miR-298 and
CDK6 (r=−0.845, P<0.05) (Fig.
1).
Effects of miR-298 on proliferation
and apoptosis of thyroid cancer cells
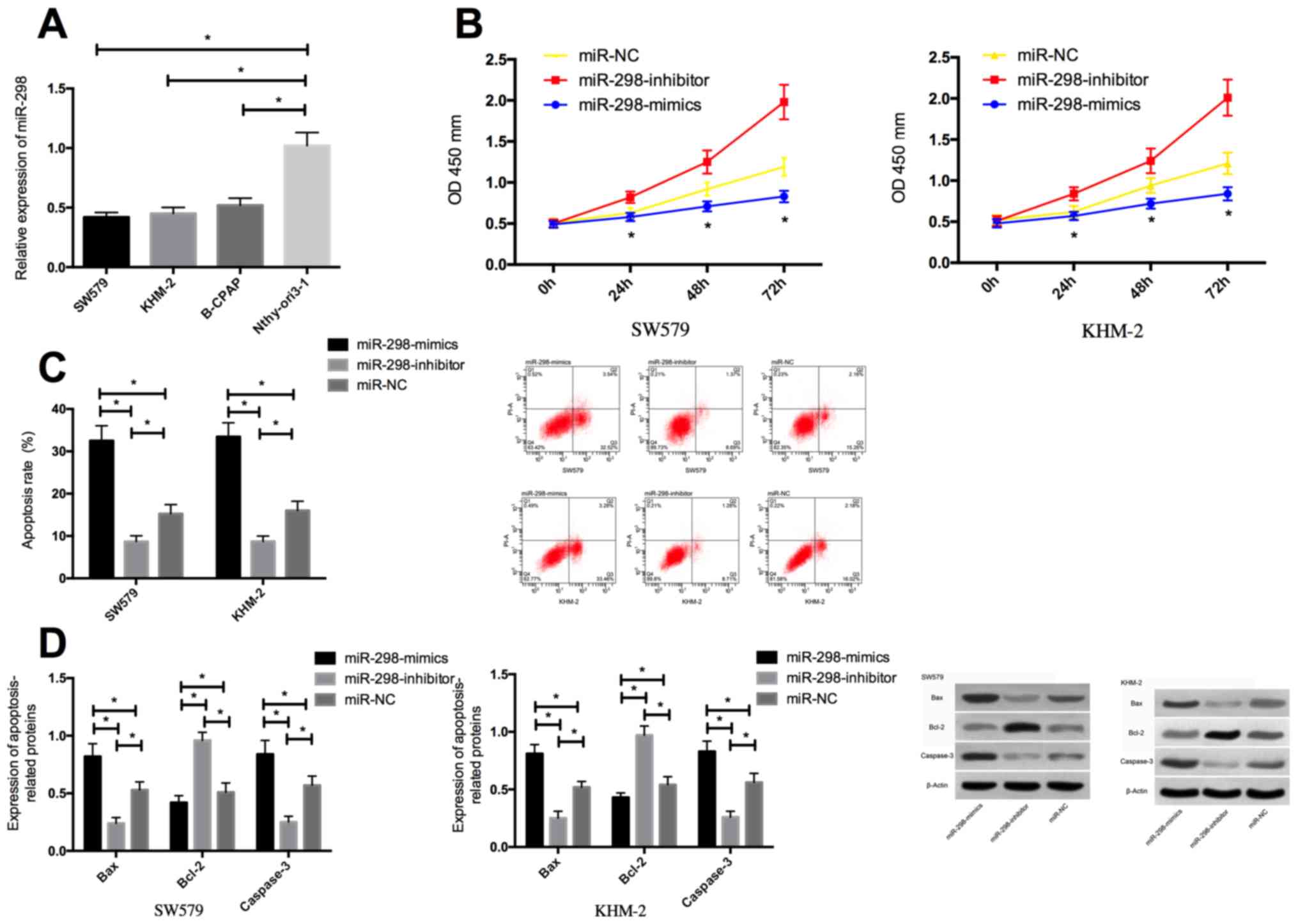
The detection of expression levels of miR-298 in
SW579, KHM-2, B-CPAP and human normal thyroid cells Nthy-ori3-1
revealed that the miR-298 expression in thyroid cancer cells SW579,
KHM-2, B-CPAP were significantly lower than that in Nthy-ori3-1
cells. miR-298 expression in SW579 and KHM-2 cells transfected with
miR-298-mimics was significantly higher than that in cells
transfected with miR-NC, while the miR-298 expression in
miR-298-inhibitor cells was significantly decreased. The biological
functions of the cells in the two groups showed that, compared with
the miR-NC group, the proliferation of transfected miR-298-mimics
was remarkably decreased, the apoptosis rate was significantly
increased, while the proliferation of transfected miR-298-inhibitor
cells was significantly enhanced, and the apoptosis rate was
significantly decreased. The transfected miR-298-mimics cells
presented markedly reduced Bcl-2 expression and significantly
increased expression levels of caspase-3 and Bax protein in
contrast with the miR-NC group, while the opposite effect was
observed between transfected miR-298-inhibitor cells and the miR-NC
group (Fig. 2).
Effects of CDK6 on the biological
function of thyroid cancer cells
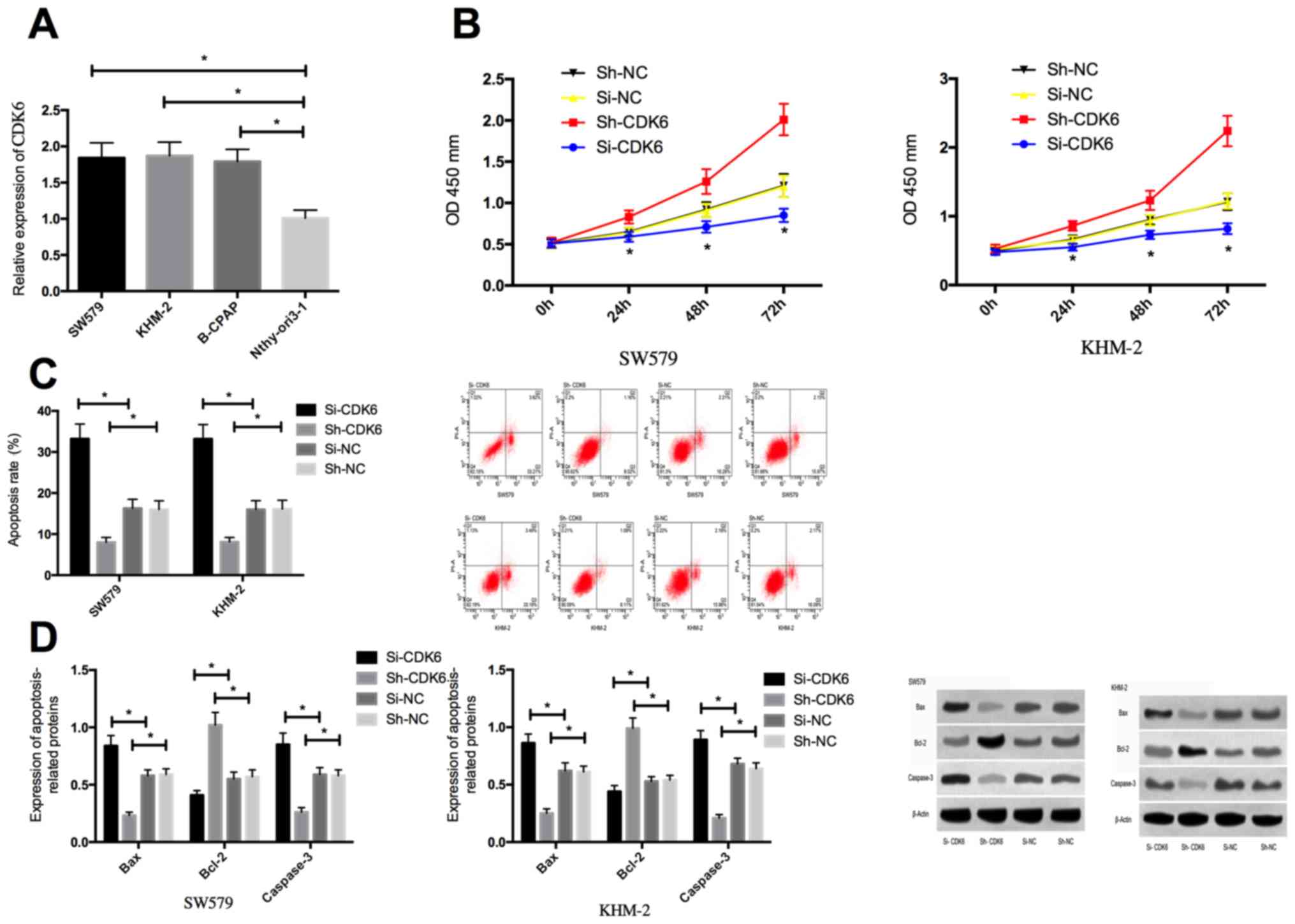
Detection of expression levels of CDK6 in SW579,
KHM-2, B-CPAP and the human normal thyroid cells Nthy-ori3-1
revealed that the CDK6 expression in thyroid cancer cells SW579,
KHM-2 and B-CPAP were significantly higher than that in Nthy-ori3-1
cells. Compared with si-nc transfected cells, the expression of
CDK6 in SW579 and KHM-2 cells transfected with Si-CDK6 was
significantly downregulated, while the expression of CDK6 in
Sh-CDK6 transfected cells was markedly declined. The biological
functions of the cells in the two groups indicated that, compared
with the Si-NC group, the proliferation of transfected Si-CDK6
cells was remarkably decreased, apoptosis was significantly
increased, the expression of Bcl-2 was markedly reduced, and the
expression levels of caspase-3 and Bax protein were significantly
enhanced. While the proliferation of transfected Sh-CDK6 cells was
significantly enhanced, the apoptosis rate was notably decreased,
the expression of Bcl-2 protein was significantly increased, and
the expression of caspase-3 and Bax protein was significantly
decreased in contrast to cells in the Si-NC group (Fig. 3).
Identification of miR-298 target
genes
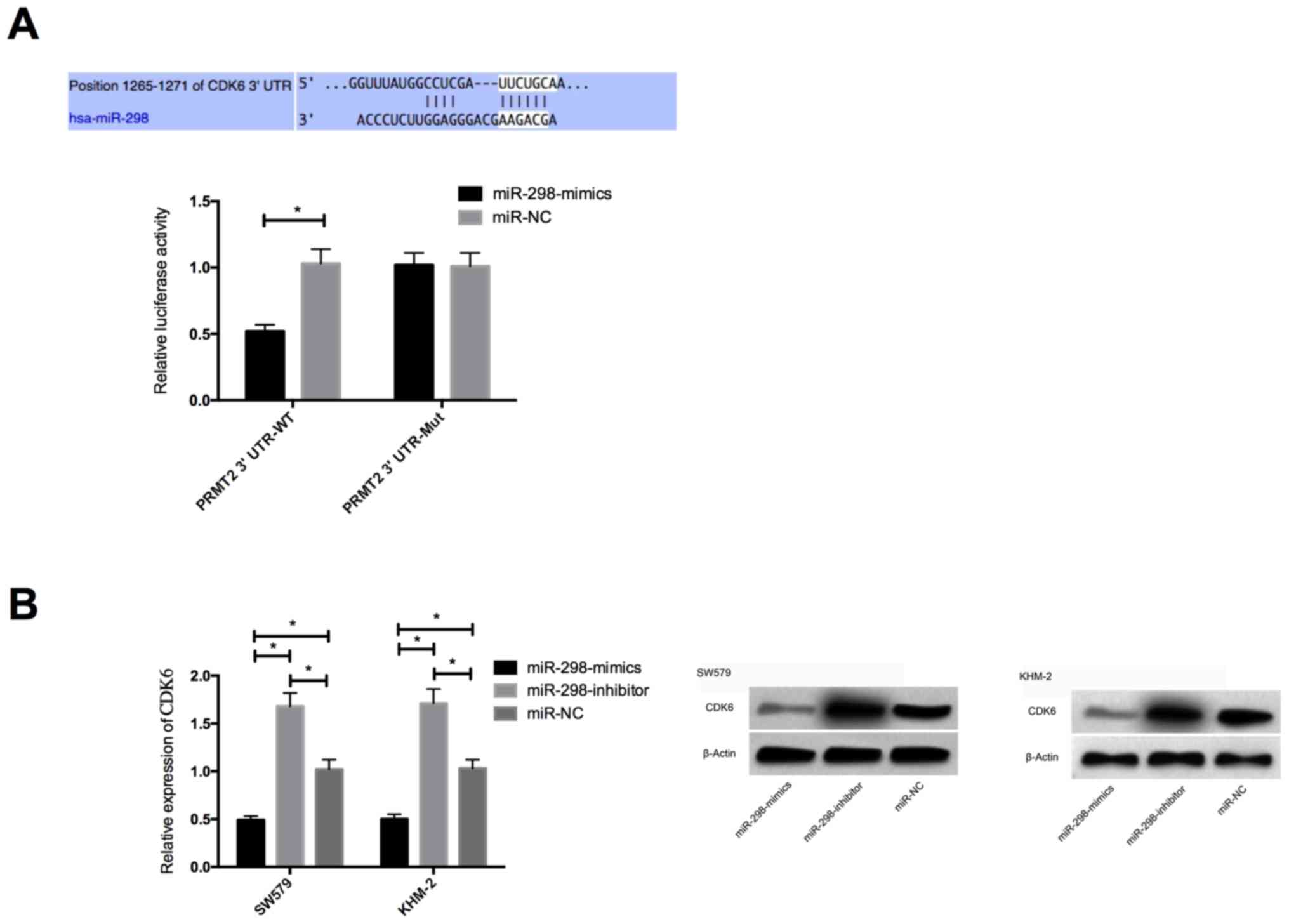
To further validate the relationship between miR-298
and CDK6, we first predicted the presence of targeted binding sites
between CDK6 and miR-298 by predicting the target gene downstream
of miR-298 by Targetscan 7.2. Then dual luciferase activity was
performed to verify that prediction. The results showed that the
luciferase activity of CDK6-3′UT Wt was markedly decreased after
miR-298 overexpression (P<0.05), but had no effect on that of
CDK6-3′UTR Mut (P>0.05). WB detection indicated that CDK6
protein expression of SW579 and KHM-2 cells was significantly
decreased after transfection with miR-298-mimics, while it was
significantly increased after transfection with miR-298-inhibitor
(P<0.05) (Fig. 4).
Discussion
As a common malignant tumor of the endocrine system,
thyroid cancer presents a relatively intricate pathogenesis
(11). In recent years, the role of
miRNA in thyroid cancer has been gradually recognized, and many
studies have reported that miRNAs can regulate the biological
function of thyroid cancer cells (12,13).
In our study, miR-298 was found to be significantly
downregulated in thyroid cancer tissues, suggesting that miR-298
may be associated with the development of thyroid cancer. The
expression of miR-298 in thyroid cancer cells was further tested
and a consistent conclusionwas reached. Studies in the past
demonstrated that miR-298 exerted oncogene function in some
malignant tumors, for example, it could inhibit the progress of
hepatocellular carcinoma by inhibiting expression of CTNND1
(14). In order to confirm our
hypothesis that miR-298 also played a role in tumor suppressor
genes in thyroid cancer, we subsequently overexpressed and
underexpressed miR-298 in thyroid cancer cells SW579 and KHM-2
respectively. The results showed that after the overexpression of
miR-298, the proliferation of SW579 and KHM-2 cells was
significantly inhibited, the apoptosis rate was significantly
increased, and the apoptosis-related protein was also consistent
with the apoptosis rate. Vice versa, the underexpressed miR-298
brought about significantly enhanced proliferation ability, and
notably reduced apoptosis rate of SW579 and KHM-2 cells, suggesting
that miR-298 also plays a role of oncogene in thyroid cancer, which
was consistent with the role of miR-298 in other tumors. In turn,
it also suggested that the low expression of miR-298 might be one
of the causes of thyroid cancer.
However, the mechanism of miR-298 in thyroid cancer
remains poorly understood. Generally speaking, miRNAs regulate
tumor cells by acting on their target genes (15), and we found a targeted relationship
between miR-298 and CDK6 through Targetscan and miRDB database
analysis. CDK6, as a kinase-catalyzed subunit, has been suggested
by previous studies that the overexpression or activation of CDK6
is closely related to the occurrence of many malignant tumors, such
as glioblastoma and lung adenocarcinoma (16,17). In
addition, some studies suggested that overexpression of CDK6 could
lead to the acceleration of G1/S checkpoint in the cell cycle,
which directly led to the enhancement of cell proliferation
(18). This study found that CDK6
was highly expressed in thyroid cancer tissues and thyroid cancer
cells, suggesting that CDK6 may also play an oncogenic part in
thyroid cancer. Accordingly, CDK6 in SW579 and KHM-2 cells was
regulated and observed. When CDK6 was inhibited, the proliferation
of SW579 and KHM-2 cells was markedly suppressed, the apoptosis
rate was significantly increased, and the detection of
apoptosis-related proteins was consistent with the apoptotic rate.
However, the phenotype observed in the further upregulated CDK6 was
contrary, which confirmed our hypothesis. As stated in previous
studies (19,20), CDK6 activation first occurred in the
middle of G1 phase, and could regulate the activity of Rb by
phosphorylation. Some revealed that CDK6 regulated the cell growth
and cell cycle progression mainly through transcriptional
regulation, and that (21) CDK6 is a
cofactor of NF-κB, which could regulate cell cycle by interacting
with NF-κB subunit p65. Furthermore, it was shown that CDK6 was
overexpressed in non-small cell lung cancer, and that
phosphorylation of CDK6 could lead to E2F-dependent transcription
of essential cyclase and regulatory factors, as well as the
assembly of prereplication complexes (22).
In conclusion, miR-298 can inhibit the proliferation
of thyroid cancer cells and promote their apoptosis by inhibiting
the expression of CDK6, which may be a new target for thyroid
cancer therapy.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science Project
of CangzhouMedical College (18Z017).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL wrote the manuscript. CuL and XZ conceived and
designed the study. RW and NG were responsible for the collection
and analysis of the experimental data. HS and XL interpreted the
data and drafted the manuscript. LW and ChL performed the
experiments and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Cangzhou Medical College (Cangzhou, China). Patients who
participated in this research had complete clinical data. Patients
and their families agreed to participate in the experiment and
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang T, He L, Sun W, Qin Y, Zhang P and
Zhang H: 1,25-Dihydroxyvitamin D3 enhances the susceptibility of
anaplastic thyroid cancer cells to adriamycin-induced apoptosis by
increasing the generation of reactive oxygen species. Mol Med Rep.
20:2641–2648. 2019.PubMed/NCBI
|
|
2
|
Luo Y, Hao T, Zhang J, Zhang M, Sun P and
Wu L: MicroRNA-592 suppresses the malignant phenotypes of thyroid
cancer by regulating lncRNA NEAT1 and downregulating NOVA1. Int J
Mol Med. 44:1172–1182. 2019.PubMed/NCBI
|
|
3
|
Bai J, Gao Y, Du Y, Yang X and Zhang X:
MicroRNA-300 inhibits the growth of hepatocellular carcinoma cells
by downregulating CREPT/Wnt/β-catenin signaling. Oncol Lett.
18:3743–3753. 2019.PubMed/NCBI
|
|
4
|
Zou L, Gao Z, Zeng F, Xiao J, Chen J, Feng
X, Chen D, Fang Y, Cui J, Liu Y, et al: Sulfasalazine suppresses
thyroid cancer cell proliferation and metastasis through T-cell
originated protein kinase. Oncol Lett. 18:3517–3526.
2019.PubMed/NCBI
|
|
5
|
Fuziwara CS, Saito KC and Kimura ET:
Interplay of TGFβ signaling and microRNA in thyroid cell loss of
differentiation and cancer progression. Arch Endocrinol Metab.
63:536–544. 2019.PubMed/NCBI
|
|
6
|
Shi D, Wang H, Ding M, Yang M, Li C, Yang
W and Chen L: MicroRNA-26a-5p inhibits proliferation, invasion and
metastasis by repressing the expression of Wnt5a in papillary
thyroid carcinoma. OncoTargets Ther. 12:6605–6616. 2019. View Article : Google Scholar
|
|
7
|
Jin J, Zhang J, Xue Y, Luo L, Wang S and
Tian H: miRNA-15a regulates the proliferation and apoptosis of
papillary thyroid carcinoma via regulating AKT pathway. OncoTargets
Ther. 12:6217–6226. 2019. View Article : Google Scholar
|
|
8
|
Mo Y, He L, Lai Z, Wan Z, Chen Q, Pan S,
Li L, Li D, Huang J, Xue F, et al: LINC01287/miR-298/STAT3 feedback
loop regulates growth and the epithelial-to-mesenchymal transition
phenotype in hepatocellular carcinoma cells. J Exp Clin Cancer Res.
37:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou F, Chen J and Wang H: MicroRNA-298
inhibits malignant phenotypes of epithelial ovarian cancer by
regulating the expression of EZH2. Oncol Lett. 12:3926–3932. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meyerson M and Harlow E: Identification of
G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell
Biol. 14:2077–2086. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrari SM, Fallahi P, Galdiero MR,
Ruffilli I, Elia G, Ragusa F, Paparo SR, Patrizio A, Mazzi V,
Varricchi G, et al: Immune and inflammatory cells in thyroid cancer
microenvironment. Int J Mol Sci. 20:202019. View Article : Google Scholar
|
|
12
|
Liu W: lncRNA LINC-PINT inhibits cancer
cell proliferation, invasion, and migration in osteosarcoma by
downregulating miRNA-21. Cancer Biother Radiopharm. 34:258–263.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu S, Liao Y, Zheng J, Gou L, Regmi A,
Zafar MI and Chen L: In silico integration approach reveals key
microRNAs and their target genes in follicular thyroid carcinoma.
BioMed Res Int. 2019:27251922019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao N, Mu L, Yang W, Liu L, Liang L and
Zhang H: MicroRNA-298 represses hepatocellular carcinoma
progression by inhibiting CTNND1-mediated Wnt/β-catenin signaling.
Biomed Pharmacother. 106:483–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin Y-P, Hu Y-P, Wu X-S, Wu YS, Ye YY, Li
HF, Liu YC, Jiang L, Liu FT, Zhang YJ, et al: miR-143-3p targeting
of ITGA6 suppresses tumour growth and angiogenesis by
downregulating PLGF expression via the PI3K/AKT pathway in
gallbladder carcinoma. Cell Death Dis. 9:1822018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lam PY, Di Tomaso E, Ng HK, Pang JC,
Roussel MF and Hjelm NM: Expression of p19INK4d, CDK4, CDK6 in
glioblastoma multiforme. Br J Neurosurg. 14:28–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendrzyk F, Radlwimmer B, Joos S,
Kokocinski F, Benner A, Stange DE, Neben K, Fiegler H, Carter NP,
Reifenberger G, et al: Genomic and protein expression profiling
identifies CDK6 as novel independent prognostic marker in
medulloblastoma. J Clin Oncol. 23:8853–8862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz EI, Smilenov LB, Price MA,
Osredkar T, Baker RA, Ghosh S, Shi FD, Vollmer TL, Lencinas A,
Stearns DM, et al: Cell cycle activation in postmitotic neurons is
essential for DNA repair. Cell cycle. 6:318–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grossel MJ and Hinds PW: From cell cycle
to differentiation: The role of cdk6 continues to expand. Cell
Cycle. 5:266–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Handschick K, Beuerlein K, Jurida L,
Bartkuhn M, Müller H, Soelch J, Weber A, Dittrich-Breiholz O,
Schneider H, Scharfe M, et al: Cyclin-dependent kinase 6 is a
chromatin-bound cofactor for NF-κB-dependent gene expression. Mol
Cell. 53:193–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li B, He H, Tao BB, Zhao ZY, Hu GH, Luo C,
Chen JX, Ding XH, Sheng P, Dong Y, et al: Knockdown of CDK6
enhances glioma sensitivity to chemotherapy. Oncol Rep. 28:909–914.
2012.PubMed/NCBI
|
|
22
|
Ma H, Chen J, Pan S, Dai J, Jin G, Hu Z,
Shen H and Shu Y: Potentially functional polymorphisms in cell
cycle genes and the survival of non-small cell lung cancer in a
Chinese population. Lung Cancer. 73:32–37. 2011. View Article : Google Scholar : PubMed/NCBI
|