Introduction
Hepatocellular carcinoma (HCC) is a common cause of
cancer-related death worldwide (1).
Only 30% of patients with HCC receive potentially curative
therapies worldwide (2–5). Recently, there has been an increase in
the number of patients with intermediate HCC, mainly due to
frequent recurrence/progression after treatment of HCC and an
increase in the prevalence of non-viral HCC, including nonalcoholic
steatohepatitis-related HCC (6–8). The
majority of patients with intermediate or advanced-stage HCC
generally undergo palliative treatments such as transcatheter
arterial chemoembolization (TACE) (9–11) and
systemic chemotherapy including multi-kinase inhibitors (MKIs)
therapy (12–14).
TACE is a standard therapy for unresectable
intermediate HCC, especially for patients with Barcelona Clinic
Liver Cancer (BCLC) stage B (15).
Several studies have shown that TACE significantly improves patient
survival compared to the best supportive care and prolongs survival
in patients with multiple HCC tumors and no macrovascular invasion
(11,16,17).
However, further TACE could be associated with a high rate of
treatment failure, worsening liver function, and poor prognosis in
patients with HCC recurrence after TACE (18). Since tumor factors vary in the
intermediate stage of HCC, it is important to identify the
indications for suitable TACE in patients with HCC.
Recurrence/progression of HCC is frequently seen
after initial TACE. For recurrent HCC, further TACE is a
therapeutic option that can result in complete response (CR) even
in patients with advanced HCC and portal vein tumor thrombosis
(19). On the other hand, sorafenib,
an MKI, is a standard first-line systemic treatment for advanced
HCC (20,21). Lenvatinib is a newly developed MKI
that has been shown to be non-inferior to sorafenib in overall
survival (OS) (22) and has been
approved as a first-line systemic treatment for advanced HCC
(22). Sorafenib is reported to
improve OS and time to progression in patients with intermediate or
advanced HCC that is refractory to TACE (23,24).
Moreover, the Transcatheter Arterial Chemoembolization Therapy in
Combination with Sorafenib (TACTICS) trial showed that the
combination of TACE with sorafenib significantly improved the time
to progression compared to TACE alone in patients with HCC
(25,26). However, it remains unclear if further
TACE or switching to MKIs is more beneficial for patients with HCC
recurrence after TACE.
The aim of this study was to identify the
indications for suitable TACE in patients with intermediate stage
HCC. We also investigated whether further TACE or switching to MKIs
was more beneficial for patients with HCC recurrence after initial
TACE.
Patients and methods
Study design
This retrospective study was carried out in a single
institution. The study protocol conformed to the ethical guidelines
of the 1975 Declaration of Helsinki, as reflected by the prior
approval of the ethical committee of Kurume University School of
Medicine (approved no: 17205). An opt-out approach was used to
obtain informed consent from the patients, and personal information
was protected during data collection.
Patients
A total of 385 consecutive patients with HCC
underwent TACE between 2009 and 2016 and were registered at the
Kurume University School of Medicine. Patients meeting any of the
exclusion criteria below were excluded from the analysis (n=147). A
total of 238 patients were included in this study. Among the
included patients, 204 patients had been previously treated with
radiofrequency ablation or hepatic rejection, and no patients had
previously been treated with TACE.
Inclusion and exclusion criteria
The following patient inclusion criteria were used:
i) Intermediate stage HCC (BCLC stage B) according to the American
Association for the Study of Liver Diseases guidelines (15,27); ii)
age >18 years; iii) no previous treatment with TACE for HCC; iv)
World Health Organization performance status (PS) 0, and v)
complete follow-up from the initial treatment for HCC until death
or the study censor date (November 30, 2018). The exclusion
criteria were as follows: i) history of a malignant tumor other
than HCC in the 5 years preceding this study; ii) participation in
any drug trial; iii) BCLC stage 0, A, C, or D; iv) PS >1; v)
creatinine >1.5 mg/dl; vi) infiltrative HCC; vii) presence of
portal vein tumor thrombosis or extrahepatic metastasis, viii)
uncontrollable ascites; ix) active esophageal varices, and x)
received liver transplantation.
Evaluation of liver function
Liver function was evaluated by Child-Pugh score,
was scored with five clinical measures of liver disease, such as
total bilirubin level, serum albumin level, prothrombin activity,
ascites (none/mild/moderate-severe), hepatic encephalopathy
(none/grade I, II/grade III, IV). For example, Child-Pugh class A
is 5–6 points and least severe liver disease (28).
Diagnosis and distribution of HCC
HCC was diagnosed by a tumor biopsy or a combination
of tests for serum tumor makers such as alpha-fetoprotein (AFP) and
des-γ-carboxy prothrombin (DCP) and imaging procedures such as
ultrasonography, computed tomography, magnetic resonance imaging,
and/or angiography.
For evaluation of distribution of HCC, we used
arteriobiliary segmentation of liver (Healey and Schroy
classification), which is consisted of following 5 segments:
Anterior, posterior, medial, lateral segments, and caudate lobe
(29). Regardless of the number of
nodules, it defined the number of the liver segments occupied with
nodules.
Treatment for HCC
TACE was selected based on the evidence-based
clinical practice guidelines for HCC of-BCLC staging and treatment
strategy (15).
TACE procedure
The hepatologist who performed the TACE procedures
had more than 10 years of experience in interventional therapy at
the start of this study. TACE was performed for the celiac artery
and the common hepatic artery, which were catheterized with a 3 or
4 Fr catheter, and digital subtraction angiography was performed
with a nonionic iodine contrast agent. After evaluation of the
tumor-containing segment using imaging techniques including
cone-beam computed tomography, a 1.7 or 1.9 Fr microcatheter
(Piolax Inc.) was inserted into the sub- or sub-sub-hepatic segment
to locate the tumor using the adapted microwire (Piolax Inc.). The
catheter was advanced toward the tumor-feeding artery. Then, in
conventional TACE, epirubicin was manually emulsified with lipiodol
(Guerbet Co., Ltd.) depending on the size and number of tumors, and
was administered followed by embolization with absorbable gelatin
sponge particles (Nippon Kayaku Co., Ltd.) (30). A total of 20–50 mg of epirubicin or
cisplatin was used. In the drug-eluting (DC) beads-TACE procedure,
30 mg of epirubicin was dissolved in 2 ml saline and loaded into
the DC Beads (Eisai Co., Ltd.). After epirubicin loading, the DC
Beads containing epirubicin were diluted with 18 ml of a dilution
solution (1:1 saline/contrast agent). The particle size of the DC
Beads was 100–300 µm. All loading procedures were performed
according to the manufacturer's recommended protocol (31). After catheterization into the artery
that flowed into the area where the tumor was located, the diluted
DC beads were administered slowly into the artery. DC beads were
administered until the disappearance of blood flow.
Follow-up schedule after treatment of
HCC
The first follow-up visit was performed
approximately 1 month after TACE to assess the therapeutic
efficacy, and the patients were followed up every 3 months until
death or the study censor time (November 30, 2018). Each follow-up
consisted of a physical examination, serum AFP and DCP
examinations, and at least one imaging examination (abdominal
ultrasound, enhanced computed tomography, or magnetic resonance
imaging). Modified response evaluation criteria in solid tumors
(RECIST) was used as the standard response criterion (32); CR is the disappearance of any
intratumoral arterial enhancement in all target lesions, partial
response (PR) is at least a 30% decrease in the sum of diameters of
viable (contrast enhancement in the arterial phase) target lesions,
taking as reference the baseline sum of the diameters of target
lesions, progression disease (PD) is an increase of at least 20% in
the sum of the diameters of viable (enhancing) target lesions,
taking as reference the smallest sum of the diameters of viable
(enhancing) target lesions recorded since the treatment started,
stable disease (SD) is any cases that do not qualify for either PR
or progressive disease. When HCC recurred, additional treatment for
HCC was selected based on the evidence-based clinical practice
guidelines for HCC of the BCLC staging and treatment strategy
(15).
Additional treatment for recurrence of
HCC after initial TACE
For the recurrence of HCC after initial TACE,
further TACE was generally employed according to the evidence-based
clinical practice guidelines for HCC of the BCLC staging and
treatment strategy (15). However,
for the 14 patients with intermediate stage HCC and Child-Pugh
class A who refused further TACE, MKIs were selected for the
treatment [sorafenib alone (n=11), sorafenib and regorafenib (n=1),
and lenvatinib (n=2)].
Safety evaluation
Adverse events (AEs) and serious adverse events
(SAEs) were monitored and recorded. AEs were assessed during both
the treatment and the follow-up periods. AEs were assessed
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events (CTCAE) version 4.0. For this study,
adverse events were defined as those classified as greater than
grade 3 according to CTCAE.
Clinical outcomes
The primary endpoint of this study was the OS of the
patients.
Decision-tree algorithm
A decision-tree algorithm was constructed to reveal
profiles associated with the prognosis of treatment with TACE-HCC
and CR after initial TACE according to the instructions provided
with the R software package as previously described (6). Following variables were used for the
decision-tree analysis for OS and CR: gender, age, cause of HCC,
Child-Pugh score, mRECIST, maximum nodule diameter, number of
nodules, up-to-seven criteria, number of liver segments with
nodule, gross classification, AFP level, and DCP level.
Statistical analysis
All data are expressed as the number or median
(range). All statistical analyses were carried out using a
statistical analysis software (JMP Pro v.13; SAS Institute Inc.).
OS was calculated by the Kaplan-Meier method and analyzed by the
log-rank test and Bonferroni methods. Factors associated with OS
and CR were evaluated using multivariate stepwise analysis and
decision-tree analysis. A two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The patients' median age was 74 years, and 33.6% of
the patients (80/238) were female (Table
I). The etiology of HCC was hepatitis C virus in 77% of
patients (185/238), and 68% of patients (163/238) showed Child-Pugh
class A. The median tumor size was 31 mm, multiple nodules were
seen in 76% of patients (183/238), and 56% of patients (135/238)
were within the up-to-seven criteria which means within 7 being the
sum of the maximum size and number of tumors for any given HCC
(33) (Table I). Fifty-one percent (120/238) of
patients had <3 liver segments with nodule. In gross
classification, in 59% of patients (142/238) had simple nodular
type (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Median (range) or n
(%) |
|---|
| Number | 238 |
| Therapy (C-TACE/DC
Beads-TACE) | 218/20 |
| Age, years
(range) | 74 (48–88) |
| Sex
(female/male) | 80 (33.6)/158
(66.4) |
| Cause of HCC
(HBV/HCV/Others) | 12 (6)/185 (77)/41
(17) |
| Child-Pugh score
(A/B) | 163 (68)/75
(32) |
| Maximum nodule
diameter, mm | 31 (10–127) |
| Number of
nodules |
| 1 | 55 (23) |
| 2 | 29 (13) |
| 3 | 21 (9) |
| 4 | 46 (19) |
| 5 | 36 (15) |
|
>6 | 51 (21) |
| Up-to-seven
criteria (within/out) | 135 (56)/103
(42) |
| Number of liver
segments with tumor |
| (<3/≥3) | 120 (50.5)/118
(49.5) |
| Gross
classification (simple nodular/other than simple nodular) | 142 (59)/96
(41) |
| AFP, ng/ml | 36.2
(1.8–62,546) |
| DCP, mAU/ml | 83 (9–75,000) |
Evaluation with mRECIST after initial
TACE
An overall CR was observed in 27% of patients
(65/238), PR in 32% of patients (77/238), stable disease (SD) in
11% of patients (28/238), and progressive disease (PD) in 30% of
patients (68/238). The objective response was 59%, and the disease
control rate was 70%.
OS rate and decision-tree analysis for
OS in patients with intermediate stage HCC treated with initial
TACE
The OS rates were 86, 28, and 8% at 1, 3, and 5
years, respectively. The median survival time (MST) was 26.6
months. At the study censor time, 10% (24/238) of included subjects
were alive. Decision-tree analysis for OS demonstrated that mRECIST
was selected as the variable for the initial split, and 21% of
patients with CR were alive (Fig.
1A). In patients with PR, SD, and PD, gross classification was
selected as the second split, and 10% of patients with simple
nodular type were alive (Fig.
1A).
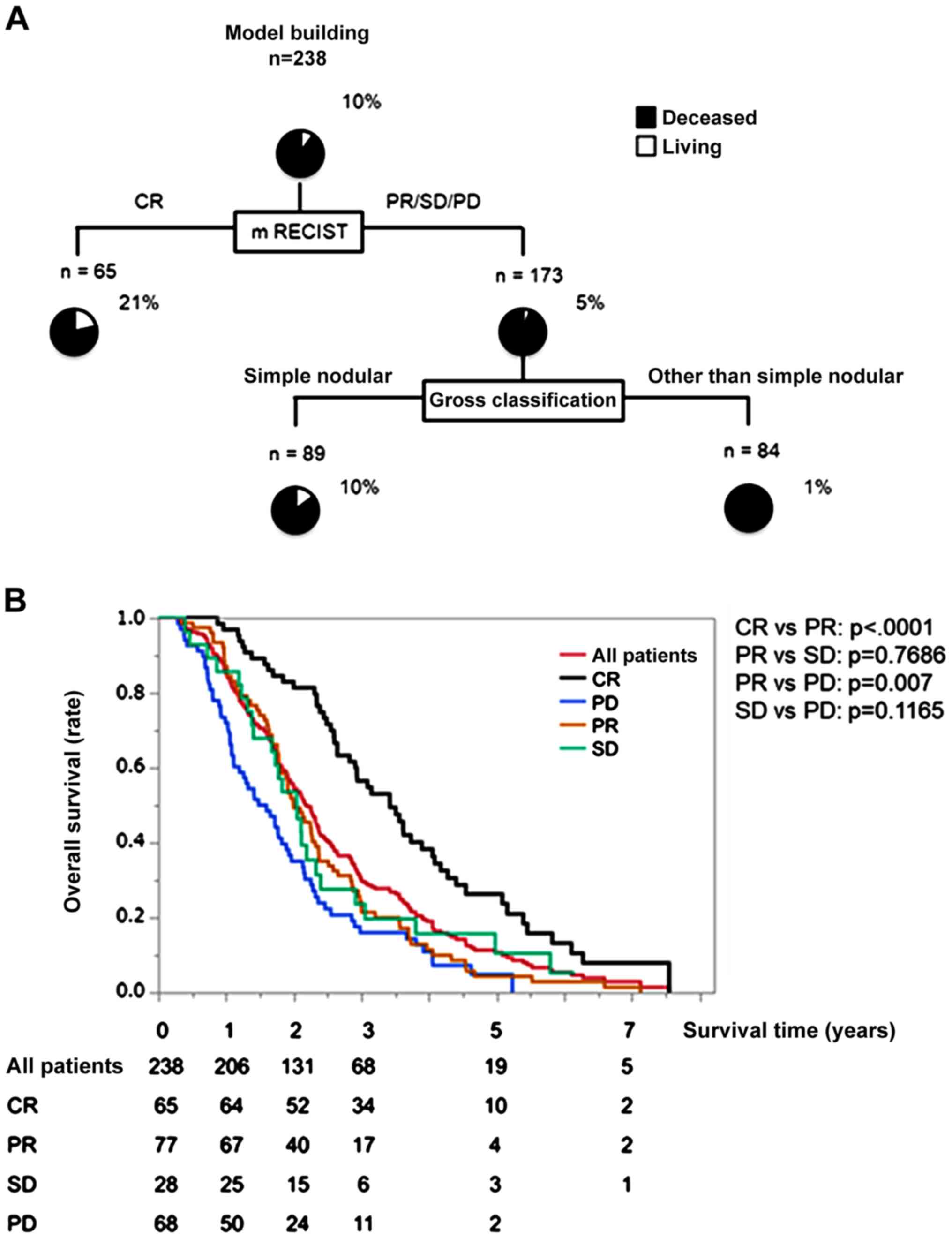 | Figure 1.Decision-tree algorithm for survival
and OS time in patients with intermediate stage HCC. (A) Subjects
were classified according to the indicated cut-off values of the
variables. The pie graphs indicate the percentage of living
(white)/deceased (black) patients in each group. (B) Kaplan-Meier
curves for OS in all patients, and according to mRECIST for initial
TACE treatment. The MST was 26.6 months in all patients. In the
present study, MST was defined as the length of time after which
50% of the patients had died from the first TACE for HCC. OS,
overall survival; HCC, hepatocellular carcinoma; mRECIST, modified
response evaluation criteria in solid tumors; CR, complete
response; PR, partial response; SD, stable disease; PD, progressive
disease; MST, median survival time; TACE, transcatheter arterial
chemoembolization. |
OS rate according to mRECIST
In the CR group, the OS rates were 98, 52, and 15%
at 1, 3, and 5 years, respectively (Fig.
1B). Meanwhile, the rates were 87, 22, and 5% in the PR group,
89, 24, and 10% in the SD group, and 73, 16, and 2% in the PD group
(Fig. 1B). According to bonferroni
methods, the OS rate in the CR group was significantly higher than
that in the PR, SD, and PD groups (all P<.0001). Also, the OS
rate in the PR group was significantly higher than that in the PD
group (P=0.007). On the other hands, there was no significant
difference in the OS rate between the PR group and the SD groups
(P=0.7686), and between the SD group and the PD group (P=0.1165;
Fig. 1B).
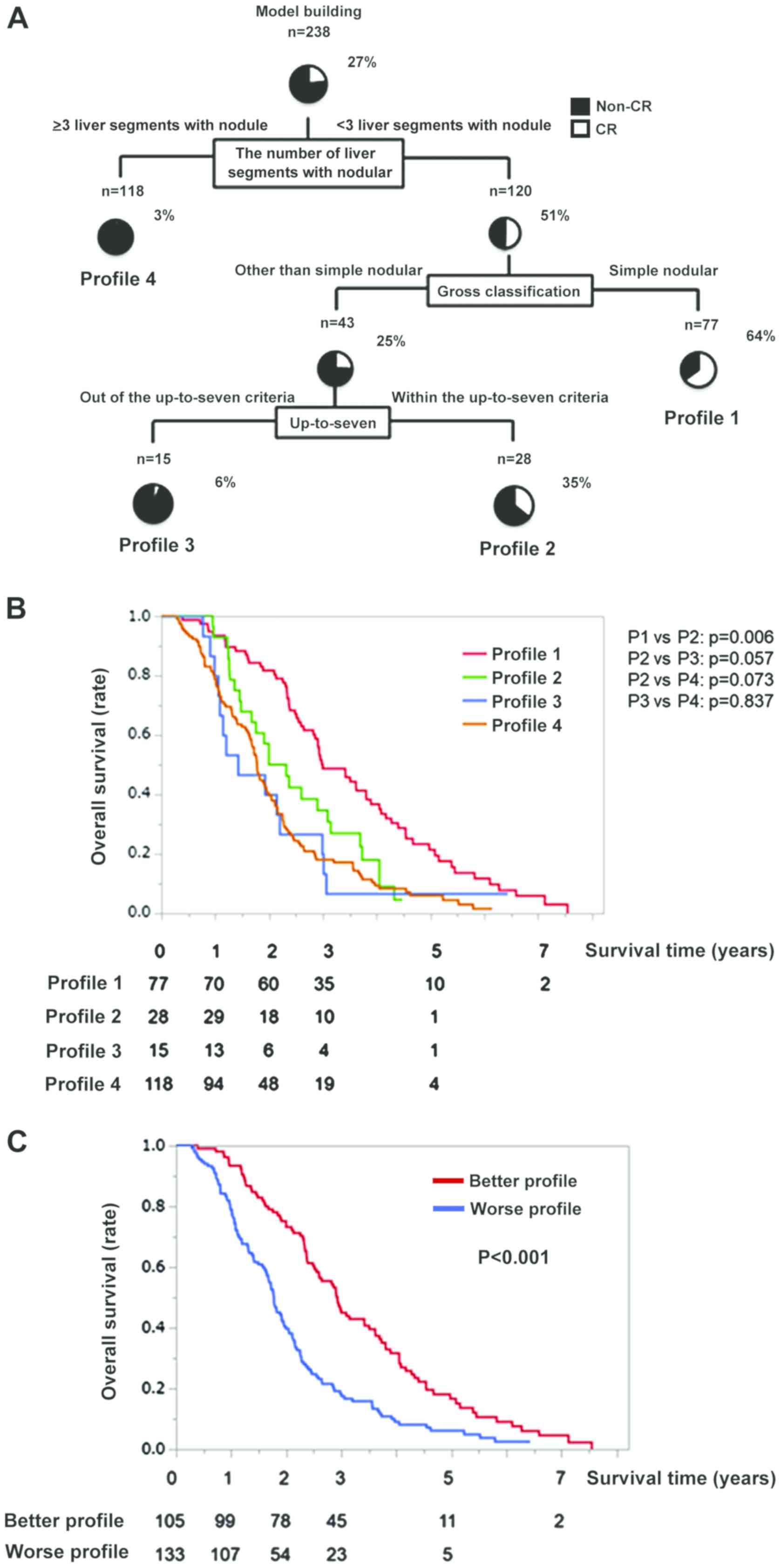
Decision-tree analysis for CR
In this study, CR rate was 27% (65/238) at the study
censor time. To determine the profile for CR, a decision-tree
analysis was performed. A decision-tree analysis identified the
optimal number of liver segments to distinguish between Non-CR and
CR, which nodule localized was selected as the variable for the
initial split, and the CR rate was 51% in patients with <3 liver
segments with nodule (Fig. 2A). In
patients with <3 liver segments with nodule, gross
classification was selected as the second split, and the CR rate
was 64% in patients with simple nodular type (Profile 1; Fig. 2A). In patients with disease other
than simple nodular type, the up-to-seven criteria were selected as
the third split, and the CR rate was 35% for patients within the
up-to-seven criteria (Profile 2; Fig.
2A). Meanwhile, the CR rate was 6 and 3% in Profiles 3 and 4,
respectively (Fig. 2A).
Logistic regression analysis for
CR
Fewer than 3 liver segments with nodule, simple
nodular type, and within the up-to-seven criteria were selected as
the variables in a logistic regression analysis by a stepwise
procedure. In the logistic regression analysis, all 3 variables
were identified as independent factors for CR (Table II).
 | Table II.Multivariate analysis for complete
response. |
Table II.
Multivariate analysis for complete
response.
| Factors | Odds ratio | 95% CI | P-value |
|---|
| <3 liver
segments with nodule | 21.73 | 8.06–76.92 | <0.0001 |
| Simple nodular
type | 4.18 | 1.89–9.79 | 0.0003 |
| Within the
up-to-seven criteria | 3.52 | 1.39–9.76 | 0.0072 |
Difference in the OS among each
profile based on decision-tree analysis for CR
In Profile 1, the OS rates were 93, 45, and 13% at
1, 3, and 5 years, respectively (Fig.
2B). In Profile 2, the OS rates were 96, 35, and 3% at 1, 3,
and 5 years, respectively. Meanwhile, the OS rates were 86, 26, and
6% in Profile 3 and was 79, 16, and 3% in Profile 4 at 1, 3, and 5
years, respectively (Fig. 2B). The
OS rate in the Profile 1 was significantly higher than that in the
Profile 2, Profile 3, and Profile 4 groups (P=0.006, <.0001, and
<.0001, respectively). On the other hands, there was no
significant difference in the OS rate between the Profile 2 and the
Profile 3 or Profile 4 (P=0.057 and P=0.073, respectively), and
between the Profile 3 and the Profile 4 (P=0.837; Fig. 2B). MST was 36.3 months in Profile 1,
28.2 months in Profile 2, 17.2 months in Profile 3, and 21.6 months
in Profile 4 (Fig. 2B). The MST of
Profile 1 and 2 was longer than that of all patients (26.6 months),
and these 2 profiles were categorized as the Better Profile.
Meanwhile, the MST of Profiles 3 and 4 was shorter than that of all
patients, and these 2 profiles were categorized as the Worse
Profile. The MST was 35.7 months in the Better Profile and 21.6
months in the Worse Profile. There was a significant difference in
OS between the Better Profile and the Worse Profile (Fig. 2C).
Patients' characteristics for the
worse profile with child-pugh class A
To investigate the impact of MKIs for patients with
HCC recurrence after TACE, we analyzed patients with Child-Pugh
class A in the Worse Profile. The median age was 73 years, and 27%
of patients (24/87) were female (Table
III). The etiology for HCC was hepatitis C virus in 79% of
these patients (69/87). The median tumor size was 24 mm, 81% of
patients (71/87) had multiple nodules, and 31% of patients (27/87)
were within the up-to-seven criteria. In gross classification,
simple nodular type was seen in 59% of patients (51/87). For HCC
recurrence after TACE, MKIs were selected for 16.1% of patients
(14/87). Meanwhile, further TACE was selected for 83.9% of patients
(73/87) (Table III).
 | Table III.Patient characteristics for the Worse
Profile with Child-Pugh A. |
Table III.
Patient characteristics for the Worse
Profile with Child-Pugh A.
|
Characteristics | All patients, mean
(range) or n (%) |
|---|
| Number | 87 |
| Age, years | 73 (52–88) |
| Sex
(female/male) | 24 (27)/63
(73) |
| Cause of HCC
(HBV/HCV/Others) | 6 (7)/69 (79)/12
(14) |
| Maximum nodule
diameter, mm | 24 (10–127) |
| Number of
nodules |
| 1 | 4 (5) |
| 2 | 4 (5) |
| 3 | 8 (10) |
| 4 | 17 (19) |
| 5 | 22 (25) |
|
>6 | 32 (36) |
| Up-to-seven
criteria (within/out) | 27 (31)/60
(69) |
| Gross
classification (simple nodular/other than simple nodular) | 45 (59)/42
(51) |
| AFP, ng/ml | 36.2
(1.8–62,546) |
| DCP, mAU/ml | 83 (9–75,000) |
| Therapy following
TACE (MKIs/further TACE) | 14 (16)/73
(84) |
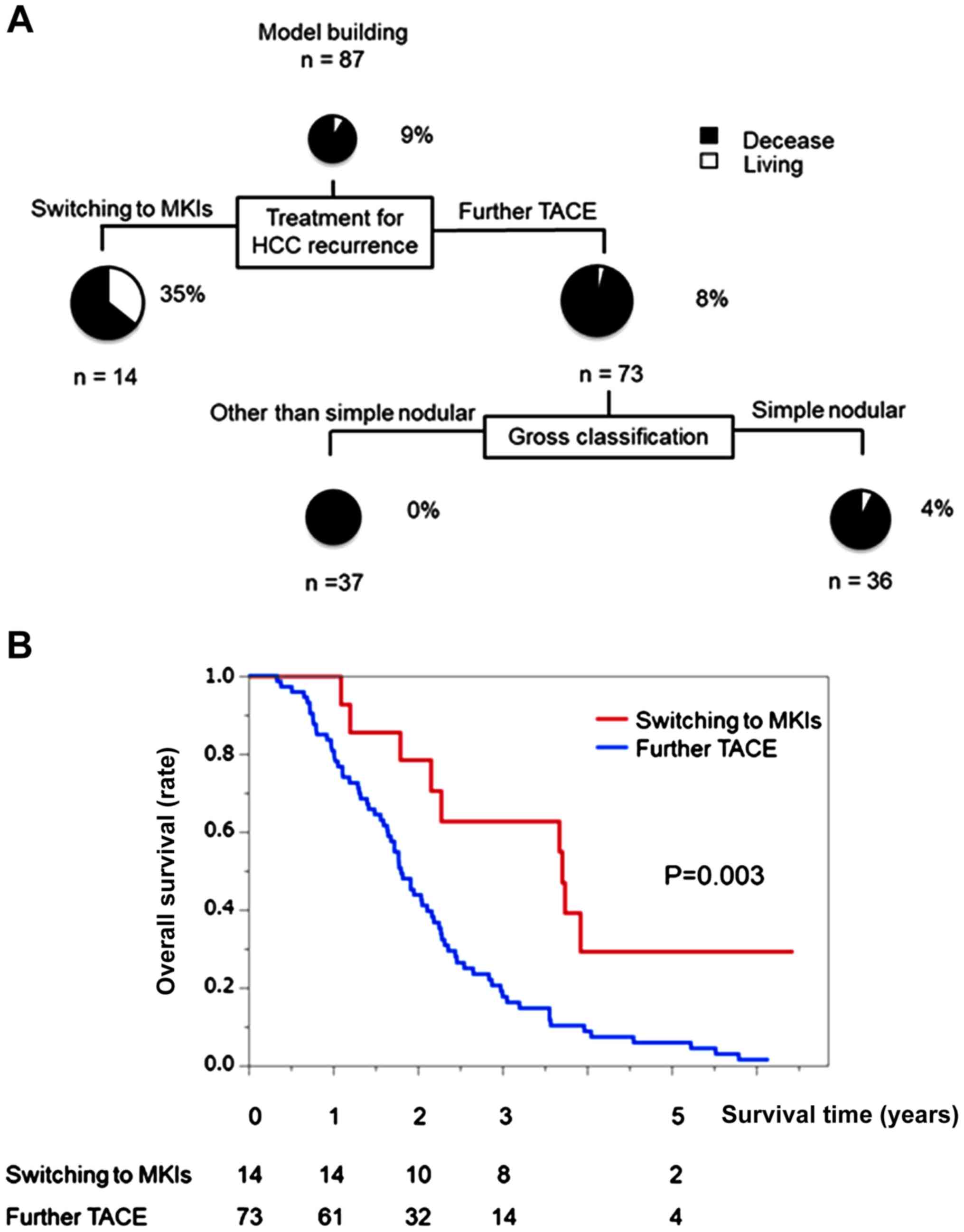
Decision-tree analysis for OS in the
worse profile with child-pugh class A
The OS rate was 9% at the study censor time. MKIs
were selected as the variable for the initial split. Although the
OS rate was 4% in patients with further TACE, the OS rate was 35%
in patients treated with MKIs (Fig.
3A).
Difference in OS between patients
treated with MKIs and further TACE in the worse profile with
child-pugh class A
In patients who were switched to MKIs, the OS was
100, 57, and 14% at 1, 3, and 5 years, respectively (Fig. 3B). Meanwhile, the OS was 83, 19, and
5%, respectively, in patients treated with further TACE. The OS
rate in patients switched to MKIs was significantly higher than
that in patients who underwent further TACE (Fig. 3B). The MST was 44.9 months in
patients switched to MKIs and 21.9 months in patients who underwent
further TACE.
Logistic regression analysis for the
prognosis of patients with the worse profile and child-pugh class
A
Switching to MKIs and gross classification were
selected as the variables in a logistic regression analysis by a
stepwise procedure. In the logistic regression analysis, both
switching to MKIs and simple nodular type were identified as
independent factors for prognosis (Table IV).
 | Table IV.Multivariate analysis for prognosis
in patients with the Worse Profile with Child-Pugh class A. |
Table IV.
Multivariate analysis for prognosis
in patients with the Worse Profile with Child-Pugh class A.
| Factors | Odds ratio | 95% CI | P-value |
|---|
| Switching to
MKIs | 0.26 | 0.11–0.56 | <0.001 |
| Gross
classification (simple nodular) | 0.42 | 0.23–0.74 | 0.002 |
AE and SAE
Among all patients, 2 patients (0.4%) experienced
SAEs that were assessed as greater than grade 3 AEs according to
CTCAE. The complication was hepatic failure in 2 (0.4%) patients
after the initial TACE. However, these patients recovered
completely with no aftereffects.
Discussion
In the present study, we demonstrated that CR by
initial TACE was the most important variable for OS in patients
with intermediate stage HCC. We found that profiles associated with
a high CR rate were ‘<3 liver segments with nodule,’ ‘simple
nodular type,’ and ‘within the up-to-seven criteria.’, which are
considered as suitable TACE criteria. Moreover, we showed that, in
patients with ineligible for suitable TACE criteria, switching to
MKIs may improve the prognosis to a greater degree than further
TACE in cases of HCC recurrence after initial TACE.
TACE is a recommended therapy for intermediate stage
HCC in the clinical guidelines for HCC worldwide (15,34). In
this study, we demonstrated that the MST was 26.6 months in
patients with intermediate stage HCC who underwent initial TACE.
Since the MST of intermediate stage HCC has been reported to be
about 20 months (15), the prognosis
of the patients in our study seems to be better than that in
previous reports (15,27,35). HCC
treatment is reported to affect the prognosis of patients with HCC
(11). It is important to identify
the best candidates for TACE to prolong the OS of patients with
intermediate stage HCC.
A combination of Child-Pugh class and the
up-to-seven criteria has been used as the indication for TACE
(36). TACE is recommended for HCC
within the up-to-seven criteria (BCLC-B1) with an MST of 41.0
months (36). However, TACE was also
recommended for HCC above the up-to-seven criteria (BCLC-B2 and
BCLC-B3), and the MST of BCLC-B2 and BCLC-B3 has been reported to
be 22.1 and 14.1 months, respectively (36). In our study, the CR for initial TACE
was selected as the first split factor for OS. The CR was
associated with the following two profiles: Profile 1) ‘<3 liver
segments with nodule’ and ‘simple nodular type’ and Profile 2)
‘<3 liver segments with nodule,’ ‘other than simple nodule,’ and
‘within the up-to-seven criteria.’ The MST was 35.7 months in
patients with Profiles 1 and 2 (Better Profile). Thus, besides
being within the up-to-seven criteria, patients with these 2
profiles are thought to be suitable for treatment with TACE.
Nevertheless, the up-to-seven criteria has been reported as a
prognostic factor for TACE therapy (36,37),
patients with the Profile 1 were divided into ‘within the
up-to-seven criteria’ (Profile 1a; n=67) or ‘out of the up-to-seven
criteria’ (Profile 1b; n=10). The CR rate was 67 and 50% in the
Profile 1a and 1b groups, respectively (Fig. S1). There was no significant
difference in the survival time between Profile 1a and 1b (P=0.691;
Fig. S2). These findings further
support our hypothesis that gross classification was more important
than up-to-seven criteria for OS in intermediate stage HCC patients
treated with TACE Based on these findings, we demonstrated tumor
development and tumor properties such as < 3 liver segments with
nodule and simple nodular type to be more important prognostic
factors than the up-to-seven criteria for TACE in this study.
Previous studies demonstrated that sorafenib
improved OS in TACE-refractory patients with intermediate stage HCC
(23,24,38), and
the MST was prolonged by about 12 months with conversion to
sorafenib compared to the MST with further TACE (23,38).
However, the effects of MKIs on OS remain unclear for patients with
intermediate stage HCC who undergo treatments other than refractory
TACE. In our study, we demonstrated that switching to MKIs improved
the OS rates compared to further TACE in patients with HCC
recurrence who did not meet our TACE criteria, in other words,
those with 1) ‘≥3 liver segments with noduler,’ 2) ‘other than
simple nodule,’ and 3) ‘out of the up-to-seven criteria’.
In this study, it was difficult to achieve CR with
further TACE in such patients. Moreover, further TACE is known to
worsen liver function (18,39). Furthermore, TACE could induce a
significant neoangiogenetic reaction by an increase of vascular
endothelial growth factor level, which is a prognostic parameter of
patients with HCC (40). Meanwhile,
MKIs block vascular endothelial growth factor receptors, leading to
suppression of tumor angiogenesis (41). Moreover, tumor growth related
molecules such as Raf-1, c-kit, and platelet-derived growth factors
are known prognostic factors for patients with HCC (42–44).
Since MKIs inhibit Raf-1, c-kit, and platelet-derived growth factor
signaling cascade (45), MKIs may
improve the prognosis of patients with HCC through suppression of
tumor angiogenesis and tumor growth. Taken together, switching to
MKIs may improve the survival rate compared to further TACE in
patients with HCC recurrence who do not meet the suitable TACE
criteria.
The present study has several limitations. First,
the study design was retrospective. Second, selection bias exists
for the classification of the MKI and further TACE groups. Third,
we did not evaluate the impact of progression-free survival on OS.
Fourth, we do not have a weighted test program. However, a weighted
test should be used for survival plots where crossover between the
groups is observed. Thus, a randomized controlled prospective
validation study, which incorporates progression-free survival, is
required in large number of patients with intermediate stage HCC to
determine the indications for suitable TACE and the optimal timing
to switch to MKIs.
In conclusion, the indications for suitable TACE in
patients with intermediate stage HCC may be ‘<3 liver segments
with nodule,’ ‘simple nodular type,’ and ‘within the up-to-seven
criteria.’ Moreover, in patients with HCC who do not meet the
criteria for suitable TACE, switching to MKIs may be associated
with a better prognosis than further TACE in patients with HCC
recurrence after TACE.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Drs. Ryoko
Kuromatsu, Masahito Nakano, Shusuke Okamura and Yu Noda (all
affilited with Division of Gastroenterology, Department of
Medicine, Kurume University School of Medicine) for clinical
management of patients.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS and MT participated in the study conception and
design, acquisition of data, interpretation of data and drafting of
the manuscript. HI, TN and TS participated in the acquisition of
data and drafting of the manuscript. TK participated in the
analysis and interpretation of data and drafting of the manuscript.
HK and TT participated in the study conception and design and the
critical revision. All authors agree to be accountable for all
aspects of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
committee of Kurume University School of Medicine (approval no.
17205), and an opt-out approach was used to obtain informed consent
from the patients.
Patient consent for publication
Not applicable.
Competing interests
TK received honoraria (lecture fees) from Mitsubishi
Tanabe Pharma Corporation, MSD K.K., and Otsuka Pharmaceutical Co.,
Ltd. The other authors disclose no conflicts of interest.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
TACE
|
transcatheter arterial
chemoembolization
|
|
MKIs
|
multi-kinase inhibitors
|
|
BCLC
|
Barcelona Clinic Liver Cancer
|
|
OS
|
overall survival
|
|
PS
|
performance status
|
|
AFP
|
alpha-fetoprotein
|
|
DCP
|
des-γ-carboxy prothrombin
|
|
DC
|
drug-eluting
|
|
mRECIST
|
modified response evaluation criteria
in solid tumors
|
|
AEs
|
adverse events
|
|
SAEs
|
serious adverse events
|
|
CTCAE
|
common terminology criteria for
adverse events
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
MST
|
median survival time
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Llovet JM: Prognostic
prediction and treatment strategy in hepatocellular carcinoma.
Hepatology. 35:519–524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belghiti J and Fuks D: Liver resection and
transplantation in hepatocellular carcinoma. Liver Cancer. 1:71–82.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee Cheah Y and P KHC: Liver
transplantation for hepatocellular carcinoma: An appraisal of
current controversies. Liver Cancer. 1:183–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin SM: Local ablation for hepatocellular
carcinoma in Taiwan. Liver Cancer. 2:73–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamada S, Kawaguchi A, Kawaguchi T,
Fukushima N, Kuromatsu R, Sumie S, Takata A, Nakano M, Satani M,
Tonan T, et al: Serum albumin level is a notable profiling factor
for non-B, non-C hepatitis virus-related hepatocellular carcinoma:
A data-mining analysis. Hepatol Res. 44:837–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tateishi R, Uchino K, Fujiwara N, Takehara
T, Okanoue T, Seike M, Yoshiji H, Yatsuhashi H, Shimizu M, Torimura
T, et al: A nationwide survey on non-B, non-C hepatocellular
carcinoma in Japan: 2011–2015 update. J Gastroenterol. 54:367–376.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marasco G, Colecchia A, Colli A, Ravaioli
F, Casazza G, Bacchi Reggiani ML, Cucchetti A, Cescon M and Festi
D: Role of liver and spleen stiffness in predicting the recurrence
of hepatocellular carcinoma after resection. J Hepatol. 70:440–448.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa H, Kita R, Kimura T and Osaki Y:
Transcatheter arterial embolic therapies for hepatocellular
carcinoma: A literature review. Anticancer Res. 34:6877–6886.
2014.PubMed/NCBI
|
|
10
|
Bruix J, Sala M and Llovet JM:
Chemoembolization for hepatocellular carcinoma. Gastroenterology.
127 (5 Suppl 1):S179–S188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takayasu K, Arii S, Ikai I, Omata M, Okita
K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ando E, Tanaka M, Yamashita F, Kuromatsu
R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K and Sata M:
Hepatic arterial infusion chemotherapy for advanced hepatocellular
carcinoma with portal vein tumor thrombosis: Analysis of 48 cases.
Cancer. 95:588–595. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeda H, Nishikawa H, Osaki Y, Tsuchiya
K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y and Izumi N;
Japanese Red Cross Liver Study Group, : Clinical features
associated with radiological response to sorafenib in unresectable
hepatocellular carcinoma: A large multicenter study in Japan. Liver
Int. 35:1581–1589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Sakata K, Matsugaki S, Kajiwara M, Fukuizumi K, Tajiri N,
Matsukuma N, et al: Efficacy, safety, and survival factors for
sorafenib treatment in Japanese patients with advanced
hepatocellular carcinoma. Oncology. 84:108–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uraki J, Yamakado K, Nakatsuka A and
Takeda K: Transcatheter hepatic arterial chemoembolization for
hepatocellular carcinoma invading the portal veins: Therapeutic
effects and prognostic factors. Eur J Radiol. 51:12–18. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piscaglia F and Ogasawara S: Patient
selection for transarterial chemoembolization in hepatocellular
carcinoma: Importance of benefit/risk assessment. Liver Cancer.
7:104–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, et al:
Hepatic function during repeated TACE procedures and prognosis
after introducing sorafenib in patients with unresectable
hepatocellular carcinoma: Multicenter analysis. Dig Dis.
35:602–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Zhao Y, Bai W and Han G: Response
assessment for HCC patients treated with repeated TACE: The optimal
time-point is still an open issue. J Hepatol. 63:1530–1531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikeda M, Mitsunaga S, Shimizu S, Ohno I,
Takahashi H, Okuyama H, Kuwahara A, Kondo S, Morizane C, Ueno H, et
al: Efficacy of sorafenib in patients with hepatocellular carcinoma
refractory to transcatheter arterial chemoembolization. J
Gastroenterol. 49:932–940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogasawara S, Chiba T, Ooka Y, Kanogawa N,
Motoyama T, Suzuki E, Tawada A, Kanai F, Yoshikawa M and Yokosuka
O: Efficacy of sorafenib in intermediate-stage hepatocellular
carcinoma patients refractory to transarterial chemoembolization.
Oncology. 87:330–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Jesus VHF and Dettino ALA: Update on
hepatocellular carcinoma from the 2018 gastrointestinal cancer
symposium (ASCO GI). J Hepatocell Carcinoma. 5:87–90. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo M: Proposal of primary endpoints for
TACE combination trials with systemic therapy: Lessons learned from
5 negative trials and the positive TACTICS trial. Liver Cancer.
7:225–234. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heimbach JK, Kulik LM, Finn RS, Sirlin CB,
Abecassis MM, Roberts LR, Zhu AX, Murad MH and Marrero JA: AASLD
guidelines for the treatment of hepatocellular carcinoma.
Hepatology. 67:358–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease-should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Healey JE Jr, Schroy PC and Sorensen RJ:
The intrahepatic distribution of the hepatic artery in man. J Int
Coll Surg. 20:133–148. 1953.PubMed/NCBI
|
|
30
|
Horikawa M, Miyayama S, Irie T, Kaji T and
Arai Y: Development of conventional transarterial chemoembolization
for hepatocellular carcinomas in Japan: Historical, strategic, and
technical review. AJR Am J Roentgenol. 205:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lammer J, Malagari K, Vogl T, Pilleul F,
Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S,
et al: Prospective randomized study of doxorubicin-eluting-bead
embolization in the treatment of hepatocellular carcinoma: Results
of the PRECISION V study. Cardiovasc Intervent Radiol. 33:41–52.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
D'Amico F, Schwartz M, Vitale A, Tabrizian
P, Roayaie S, Thung S, Guido M, del Rio Martin J, Schiano T and
Cillo U: Predicting recurrence after liver transplantation in
patients with hepatocellular carcinoma exceeding the up-to-seven
criteria. Liver Transpl. 15:1278–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan society of hepatology 2013 update (3rd JSH-HCC
Guidelines). Hepatol Res. 45:2015. View Article : Google Scholar
|
|
35
|
Han K and Kim JH: Transarterial
chemoembolization in hepatocellular carcinoma treatment: Barcelona
clinic liver cancer staging system. World J Gastroenterol.
21:10327–10335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bolondi L, Burroughs A, Dufour JF, Galle
PR, Mazzaferro V, Piscaglia F, Raoul JL and Sangro B: Heterogeneity
of patients with intermediate (BCLC B) hepatocellular carcinoma:
Proposal for a subclassification to facilitate treatment decisions.
Semin Liver Dis. 32:348–359. 2012.PubMed/NCBI
|
|
37
|
Yamakado K and Hirota S:
Sub-classification of intermediate-stage (Barcelona Clinic Liver
Cancer stage-B) hepatocellular carcinomas. World J Gastroenterol.
21:10604–10608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arizumi T, Ueshima K, Minami T, Kono M,
Chishina H, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, et al:
Effectiveness of sorafenib in patients with transcatheter arterial
chemoembolization (TACE) refractory and intermediate-stage
hepatocellular carcinoma. Liver Cancer. 4:253–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ikeda M, Arai Y, Park SJ, Takeuchi Y, Anai
H, Kim JK, Inaba Y, Aramaki T, Kwon SH, Yamamoto S, et al:
Prospective study of transcatheter arterial chemoembolization for
unresectable hepatocellular carcinoma: An Asian cooperative study
between Japan and Korea. J Vasc Interv Radiol. 24:490–500. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sergio A, Cristofori C, Cardin R, Pivetta
G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A and
Farinati F: Transcatheter arterial chemoembolization (TACE) in
hepatocellular carcinoma (HCC): The role of angiogenesis and
invasiveness. Am J Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rezaĭkina AV and Komarova VD: An
immunoenzyme method of detecting anti-DNA antibodies in lupus
erythematosus. Lab Delo. 40–43. 1988.(In Russian).
|
|
42
|
Chen B, Liu J, Wang X, Shen Q, Li C and
Dai C: Co-expression of PDGF-B and VEGFR-3 strongly correlates with
poor prognosis in hepatocellular carcinoma patients after
hepatectomy. Clin Res Hepatol Gastroenterol. 42:126–133. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen L, Shi Y, Jiang CY, Wei LX, Wang YL
and Dai GH: Expression and prognostic role of pan-Ras, Raf-1, pMEK1
and pERK1/2 in patients with hepatocellular carcinoma. Eur J Surg
Oncol. 37:513–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan W, Zhu Z, Pan F, Huang A and Dai GH:
Overexpression of c-kit(CD117), relevant with microvessel density,
is an independent survival prognostic factor for patients with
HBV-related hepatocellular carcinoma. Onco Targets Ther.
11:1285–1292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weintraub JL and Salem R: Treatment of
hepatocellular carcinoma combining sorafenib and transarterial
locoregional therapy: State of the science. J Vasc Interv Radiol.
24:1123–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|