Introduction
Breast cancer (BC) is common in women and is one of
the main causes of cancer-associated mortality in the female
population worldwide, with an incidence rate of 1 in 8 women (13%)
(1–3). Environmental contamination serves a
vital role in cancer development, as well as other diseases,
including endometrial cancer and endometriosis (4,5). Reports
show that >20% of global disease burden and >30% of disease
burden in children is due to contaminated environments (2,3,5). Therefore, it has been speculated that a
healthy environment would prevent or decrease the incidence of many
diseases/disorders and ultimately reduce morbidity (4). A comprehensive understanding of the
mechanisms involved in the etiology of BC and the identification of
new biomarkers of its risk are key components for the improvement
of BC prevention (6). Even though
genetic modifications for BC have been widely investigated, further
advancements are required to uncover the metabolic changes
associated with this disease (7,8).
Metabolomics (one of the newest ‘omics’) is a
rapidly developing branch of science and medicine aimed at
identifying biomarkers for a number of human diseases or disorders.
It has assisted in further understanding the underlying mechanisms
of cancers and therefore treatment strategies (9). The pathophysiological status of
biological systems can be reflected by changes in the metabolome,
which may be owing to genetic alterations in metabolic pathways or
changes in catabolism and enzymes activities (9,10). The
metabolome is an amplified culmination of biological systems, as
small alterations in enzyme activities may result in major changes
in metabolite levels (10). Lipids
are reported to be risk factors for BC reoccurrence and development
(11,12). Amino acids have also been reported to
be associated with BC development (13,14).
Nuclear magnetic resonance (NMR) spectroscopy has been extensively
used in metabolome studies, due to its ability to detect
metabolites in intact tissues and even in in vivo (15). There are many advantages for NMR
spectroscopy, such as the following: No need for sample
purification; both hydrophilic and hydrophobic metabolites can be
detected; quantitative analysis can be performed; a fast method
(1-dimensional techniques, excluding solid-state NMR); small
amounts of sample are required; a non-invasive and non-destructive
method; and high reproducibility (9). Due to the abundance of hydrogen in
nature (>99.98%), low relaxation time and an appreciable nuclear
spin, proton nuclear (1H) NMR is the most popular NMR
technique applied in metabolome investigation (16); it can be used to detect metabolomic
changes in cells, tissues or biofluids (17,18) and
to provide novel insights into disease etiology or underlying
mechanisms (7,19–21).
The metals chromium (Cr), cobalt (Co), copper (Cu)
and nickel (Ni) are important trace elements for humans, since they
are components of enzymes. However, at high concentrations the
metals can cause serious issues, such as disease or toxicity, owing
to their inhibition of enzyme activity (22–25).
Some other non-essential heavy metals, such as cadmium (Cd), lead
(Pb), mercury (Hg) and tin (Sn), are toxic at high levels, as they
can block the functions of other essential metals (26). In addition, in natural conditions
these elements cannot be decomposed, or may even be bioaccumulated
and biomagnified in food chains (27,28). It
has been reported that Cd, Cr, Ni, Cu, Pb and Hg are carcinogens
(29,30). Furthermore, it has been recorded that
Cd, Cr, Ni, Cu, Co, Pb and Hg can cause lung cancer; Cr can
increase the probability of liver, larynx, esophageal, and
gastrointestinal cancer; Cd and Ni can result in renal and prostate
cancer; Cu can cause non-Hodgkin's lymphoma or skin cancer; Pb and
Hg may increase the risk of glioma and stomach, prostate or bladder
cancer; and Ni, Cb, Hg, Pb and Cr (VI) may cause breast sarcoma and
carcinoma (29,30). Environmental exposure to heavy metals
may be mainly through the food chain, smoking and even drinking
water (31,32). Heavy metal anthropogenic
contamination comes mainly from power industries, waste deposits
and even fertilizers (33).
Some heavy metals, such as Cd, have been found at
high levels (20–30 µg/g tissue) in breast tissue (34). Furthermore, heavy metals can
accumulate in breast tissue, cause DNA damage and even increase
tumor development (35). However, to
the best of our knowledge, the association between plasma heavy
metals and the metabolome in patients with BC is unknown, as is the
association between plasma heavy metals and the metabolome in BC
development. Therefore, the present investigation aimed to examine
the metabolome and heavy metals present in the plasma of patients
with BC at first diagnosis.
Materials and methods
Patients and plasma sample
collection
Plasma samples from female patients with malignant
BC (n=105; 50.22±9.83 years) and age-matched healthy female
controls (n=35; 49.76±10.07 years) were collected from the
Affiliated Tengzhou Central People's Hospital of Jining Medical
University (Jining, China) between November 2017 and May 2018.
Written informed consent was obtained from the patients and
controls in the present study. The patients with BC and the control
population were from the same local area of Tengzhou. Heavy metal
contamination is relatively high in this area owing to the number
of mining operations. The study was performed in accordance with
the standards of the Institutional Ethical Committee and the
Helsinki Declaration of 1975, as revised in 1983, and was approved
by the Institutional Review Board of the Affiliated Tengzhou
Central People's Hospital of Jining Medical University. The
patients were chosen based on the following criteria: i) All
patients were female; ii) all patients received positive pathology
for BC; iii) all patients were in the early stages of BC (stages
I–II), according the clinical Tumor-Node-Metastasis staging method
(6,7); iv) no patients received pre-operative
treatment, including adjuvant chemotherapy or radiotherapy; and v)
patients did not have diabetes or any other diseases. The selected
healthy controls included age- and sex-matched healthy subjects
with no metabolic diseases and who were confirmed to have no breast
lesions following a physical examination followed by mammography
and breast ultrasonography. Prior to surgery in the patients and
following overnight fasting for all subjects, 10 ml of venous blood
was collected from each subject in a vessel tube, containing
heparin as the anticoagulant, and was subsequently centrifuged
(1,500 × g for 15 min at 4°C) to collect clear plasma. The plasma
was then transferred into sterile vials and immediately stored at
−80°C until further analysis.
NMR spectroscopy
Nuclear magnetic resonance (NMR) analyses were
performed as described previously (36,37).
Briefly, prior to the NMR spectroscopy, 200 µl of plasma sample was
mixed with 80 µl D2O solution containing sodium
phosphate buffer (0.1 M, pH 7.4) and sodium 3-trimethylsilyl-
2,2,3,3-d4-propionate as an internal standard (δ=0 ppm). The
1H NMR spectra was acquired using a 600.13 MHz Bruker
AV600 spectrometer (Bruker Corporation) with a 5-mm CryoProbe at
300 K. Nuclear Overhauser effect spectroscopy and a zg pulse
sequence of 1H NMR spectra and zggpr pulse sequence of
J-resolved NMR spectra were used to acquire the NMR information
(38). The low molecular weight
metabolite (LMWM) model and the lipid molecules in lipoprotein
particles (LIPO) model were used in this study, as previously
described (37). The LIPO model
provides information on lipoprotein lipids, and subclasses, which
are acquired through the water-suppressed 1H NMR
spectrum. Alternately, the LMWM model suppresses most of the broad
macromolecules and lipoprotein lipid signals, therefore improving
the sensitivity of low molecular weight metabolites (37–41).
NMR spectral processing and
analysis
The 1H NMR spectra were processed by
MestRe-C software (version 3.0; Mestrelab Research) as described
previously (36,38). Briefly, the spectra were binned with
a unit of 0.005 ppm between 0.2 and 10.0 ppm, and then integrated
spectral intensity for each bin. The binned data were adjusted by
generalized log transformation and mean-centered prior to
multivariate analysis.
Multivariate analyses
The processed NMR datasets were examined by
principal component analysis (PCA) and partial least squares
discriminant analysis using the SIMCA-P10.0 10.0 software package
(MKS Umetrics AB), as previously described (36,38).
Mineral element quantification
Determination of tissue mineral elements was
performed as previously described (42,43).
Briefly, 0.2 ml aliquots of plasma were transferred to 120-ml
Teflon digestion vessels, followed by the addition of 5 ml of
nitric acid. Analysis of metals in plasma was preceded by microwave
digestion with concentrated nitric acid to destroy organic matter
and mineralize the sample. The multi-element calibration standard
was provided as 10 mg/l in 5% nitric acid and was not in the plasma
matrix. An Agilent 7500 (Agilent Technologies, Inc.) inductively
coupled plasma mass spectrometry system was used for simultaneous
determination of Cd, Cr, Arsenic (As), Pb and Hg. Positive
ionisation mode was used (38). The
voltage for the ion lens was set at 6 V; the argon gas flow rate in
the spray chamber was 0.88 l/min; the power output for the RF
generator was 1,100 W; the auxiliary gas flow rate was 1.2 l/min;
and the nebulizer gas flow rate of the plasma was 16 l/min at room
temperature. All the certified reference materials (in solution)
were purchased from the National Institute of Metrology. Blank
controls (n=3) underwent the same procedures (38).
Statistical analysis
Data were statistically analyzed with SPSS
statistics software (version 22; IBM Corp.) and Student's t-test.
Differences were compared for every parameter and data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference
(38).
Results
Baseline characteristics of the study
population
Table I shows the
baseline characteristics of the patients with BC and the control
population. There was no significant difference between the mean
age of the patients with BC (50.22±9.83 years) and the control
population (49.76±10.07 years). Similarly, there was no significant
difference for average body mass index. Following diagnosis with BC
at stages I or II, blood samples were drawn from the patients for
analysis.
 | Table I.Baseline characteristics of the study
population. |
Table I.
Baseline characteristics of the study
population.
|
Characteristics | Patients with BC
(n=105) | Control group
(n=35) |
|---|
| Mean age ± SD,
years | 50.22±9.83 | 49.76±10.07 |
| Mean BMI ± SD,
kg/m2 | 24.32±3.39 | 24.11±3.89 |
| Normal
(18.5–24.9), n (%) | 67 (63.81) | 25 (71.43) |
|
Preobese (25–29.9), n (%) | 37 (35.24) | 10 (28.57) |
| Obesity
(≥30), n (%) | 1 (0.95) | 0 (0.00) |
| Stage, n (%) |
|
|
| I | 65 (61.90) |
|
| II | 40 (38.10) |
|
Plasma metabolome changes
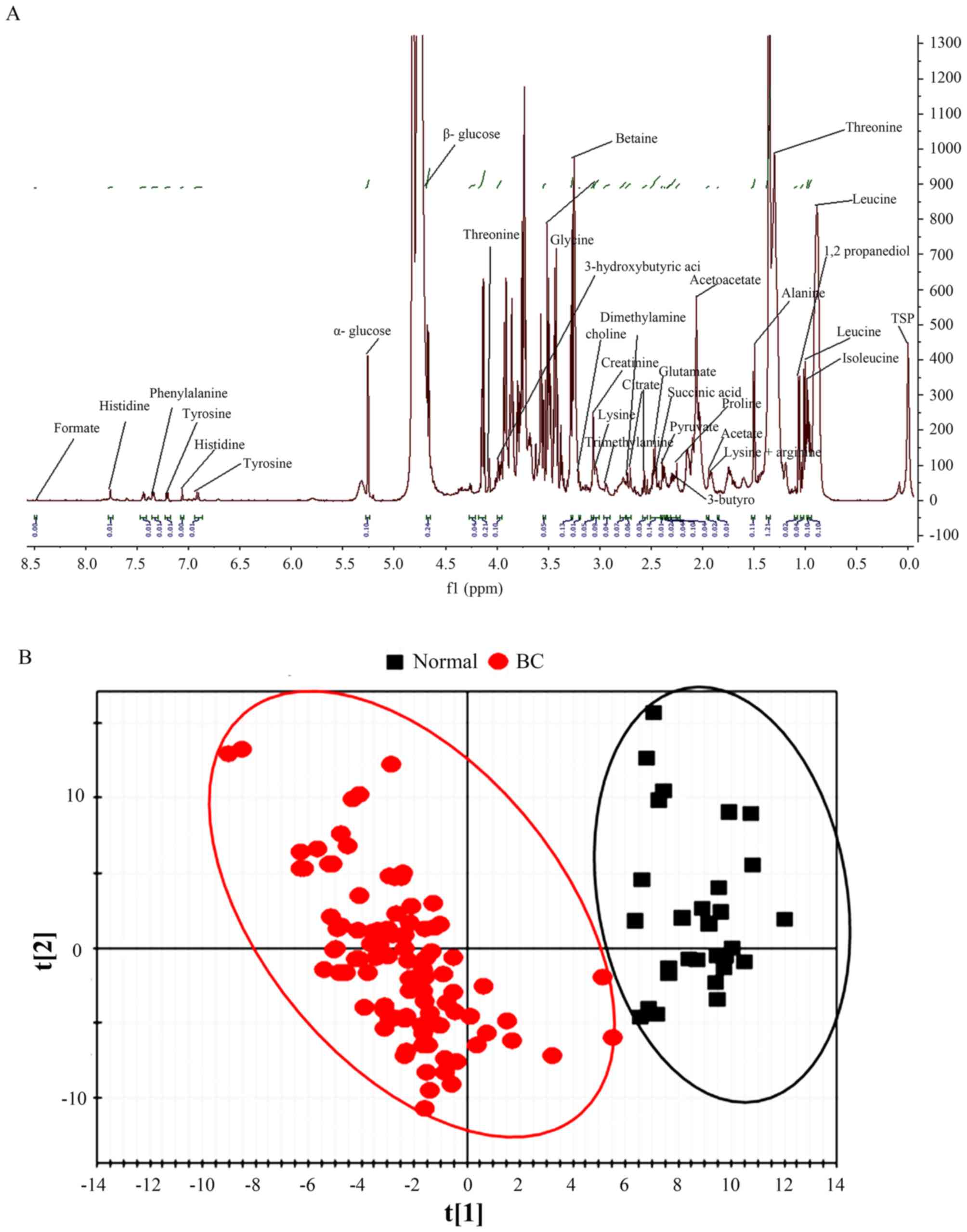
The LMWM 1H NMR spectra, the metabolic
fingerprints of small molecules, from plasma metabolites of
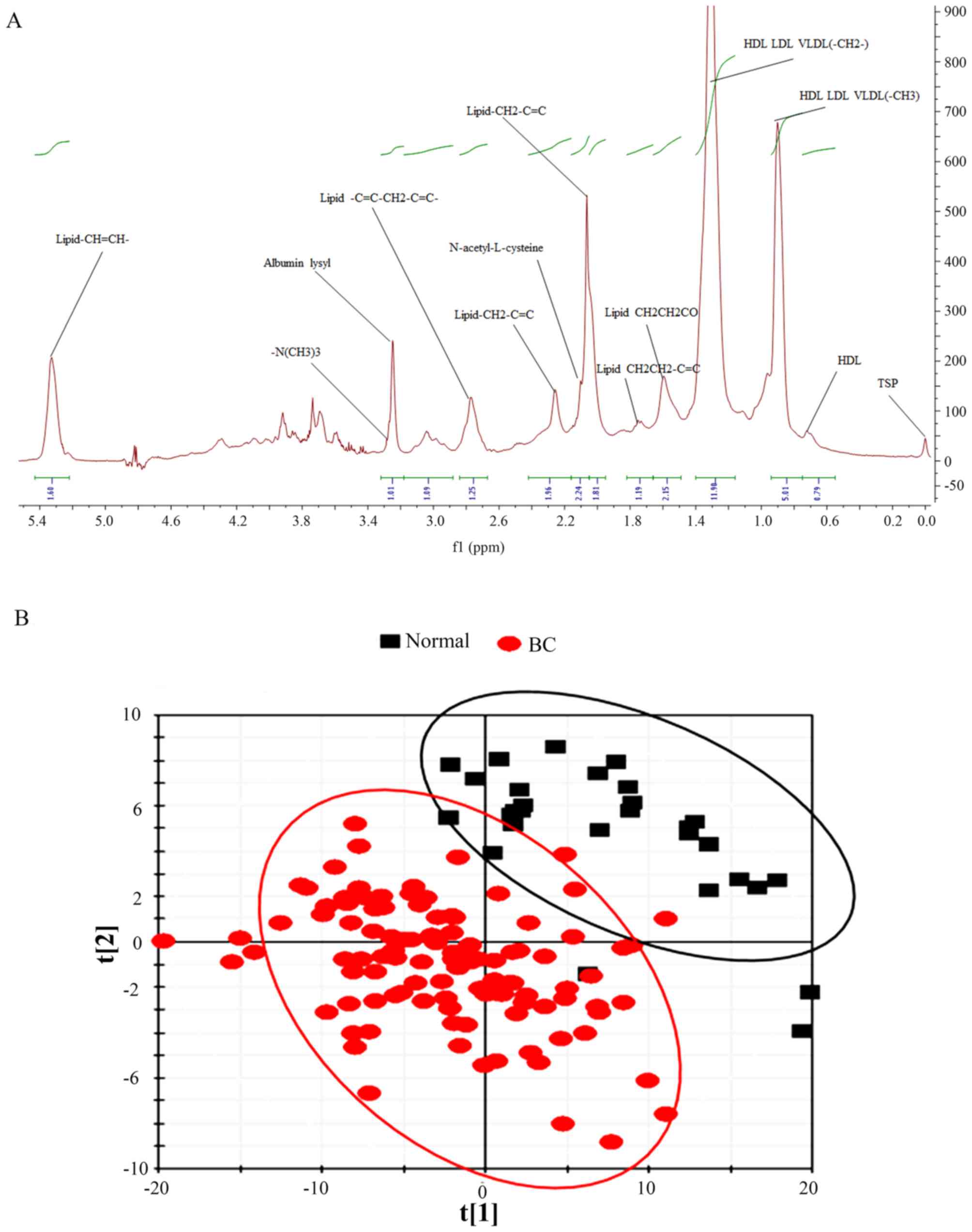
patients with BC and the control group are presented in Fig. 1A; while the LIPO 1H NMR
spectra, the metabolic fingerprints of large molecules, from plasma
metabolites of patients with BC and the control group are presented
in Fig. 2A. Chemical shift and peak
multiplicity were used to assign the specific plasma metabolite
(37–41).
The latent biochemical information from the
1H NMR spectra were analyzed by partial least squares
discriminant analysis. For the small molecules in the LMWM model,
there was a clear separation between patients with BC and the
control population based on score plots (Fig. 1B). For large molecules in the LIPO
model, the score plots also indicated a distinct difference between
patients with BC and the control population (Fig. 2B).
Numerous alterations in endogenous metabolites were
discovered in the 1H NMR spectra of plasma samples in
both the LMWM and LIPO models. Fig.
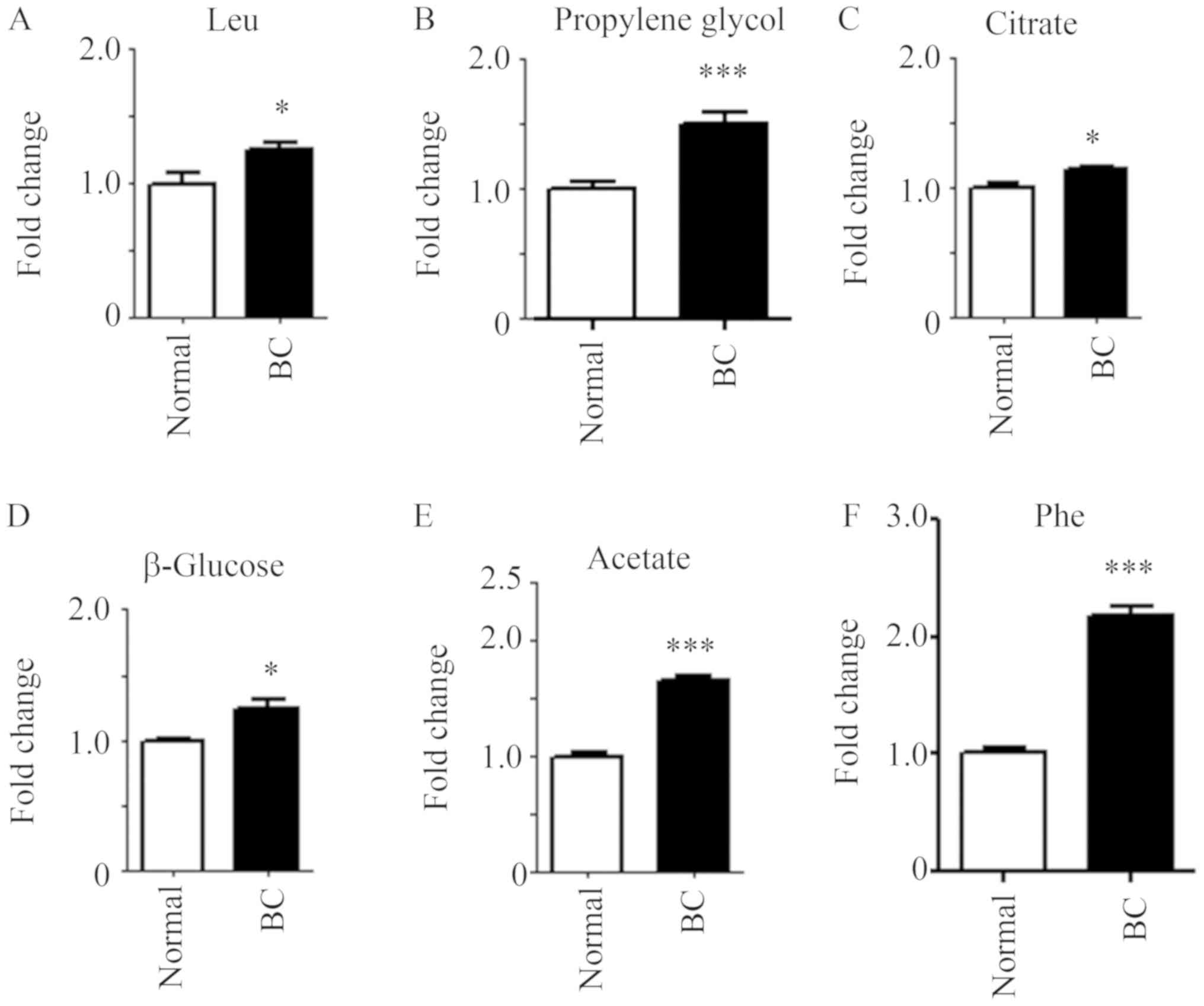
3 shows the prominent small molecules, whose presence was
greater in patients with BC in the LMWM model. Compared with the
control population, six metabolites, namely leucine, propylene
glycol, citrate, β-glucose, acetate and phenylalanine, were
significantly elevated in patients with BC (Fig. 3). There were seven metabolites,
namely arginine, glutamate, trimethylamine, lysine, α-glucose,
tyrosine and histidine, with significantly decreased levels in
patients with BC compared with that in the control group (Fig. 4).
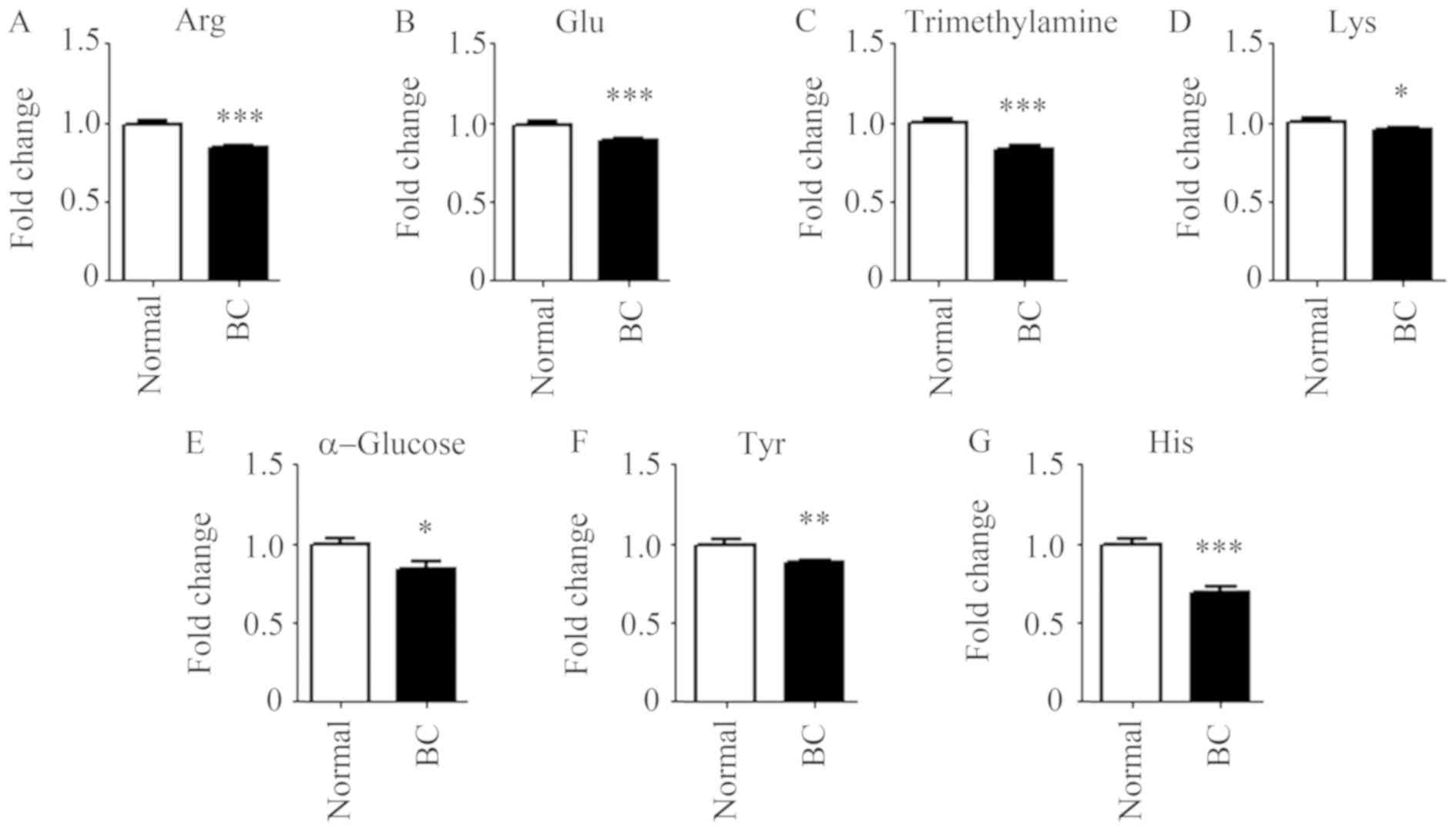 | Figure 4.Small molecule metabolites decreased
in the plasma of patients with BC. (A) arginine, (B) glutamate, (C)
trimethylamine, (D) lysine, (E) α-glucose, (F) tyrosine and (G)
histidine in the plasma of patients with BC and the control
population. n=35 for control population; n=105 for patients with
BC. *P<0.05, **P<0.01 and ***P<0.001 vs. normal. BC,
breast cancer; Arg, arginine; Glu, glutamate; Lys, lysine; Tyr,
tyrosine; His, histidine. |
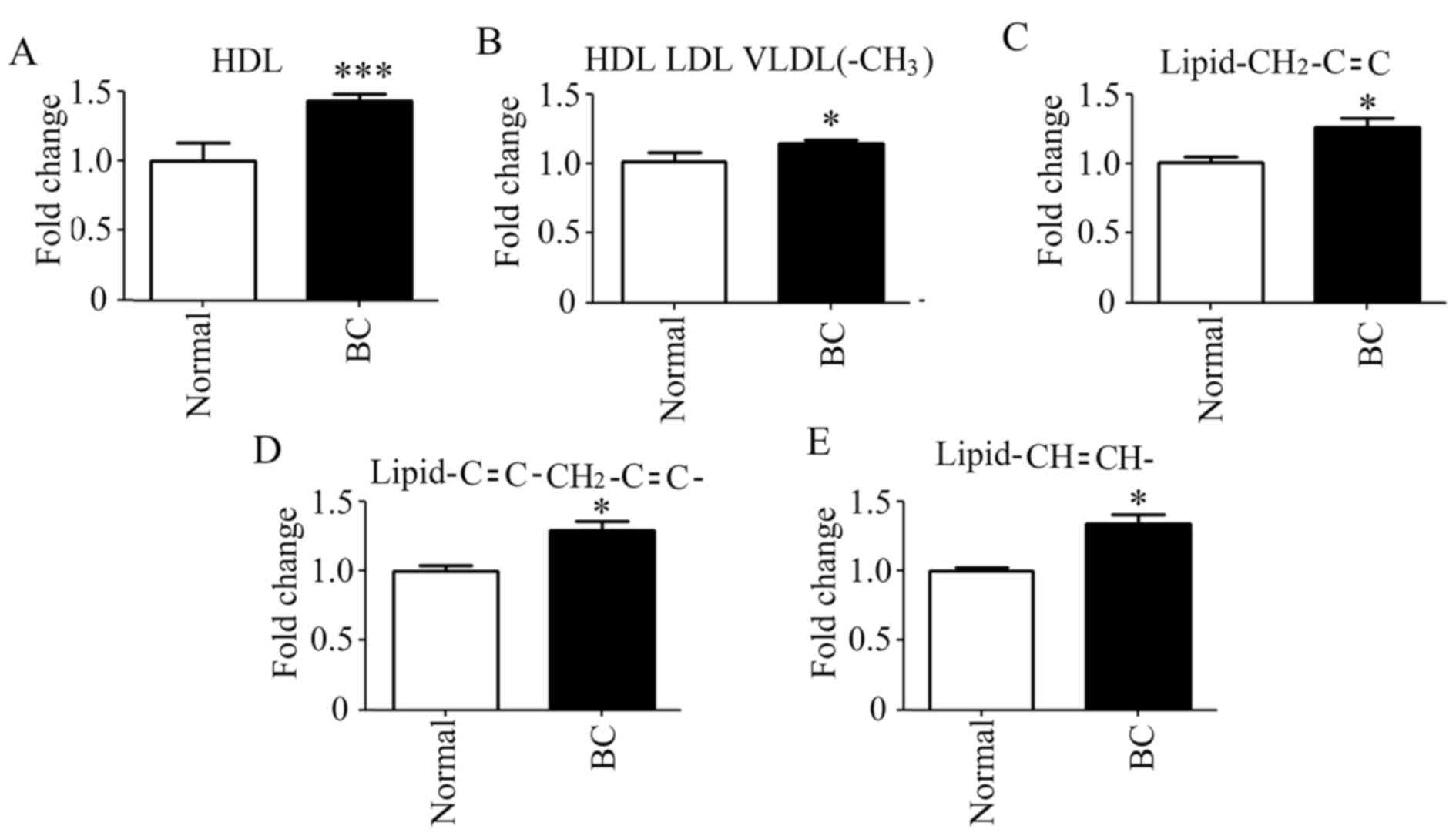
It is noteworthy that the prominent large molecules
(lipids or lipoproteins) were different in the LIPO model analysis
between patients with BC and the control population. The levels of
all prominent large molecules were increased (Fig. 5). These large molecules were
high-density lipoprotein (HDL), HDL low-density lipoprotein (LDL)
very low-density lipoprotein (VLDL)(-CH3),
lipid-CH2-C=C-, lipid-C=C-CH2-C=C- and
lipid-CH=CH-. In addition, HDL was significantly elevated in
patients with BC.
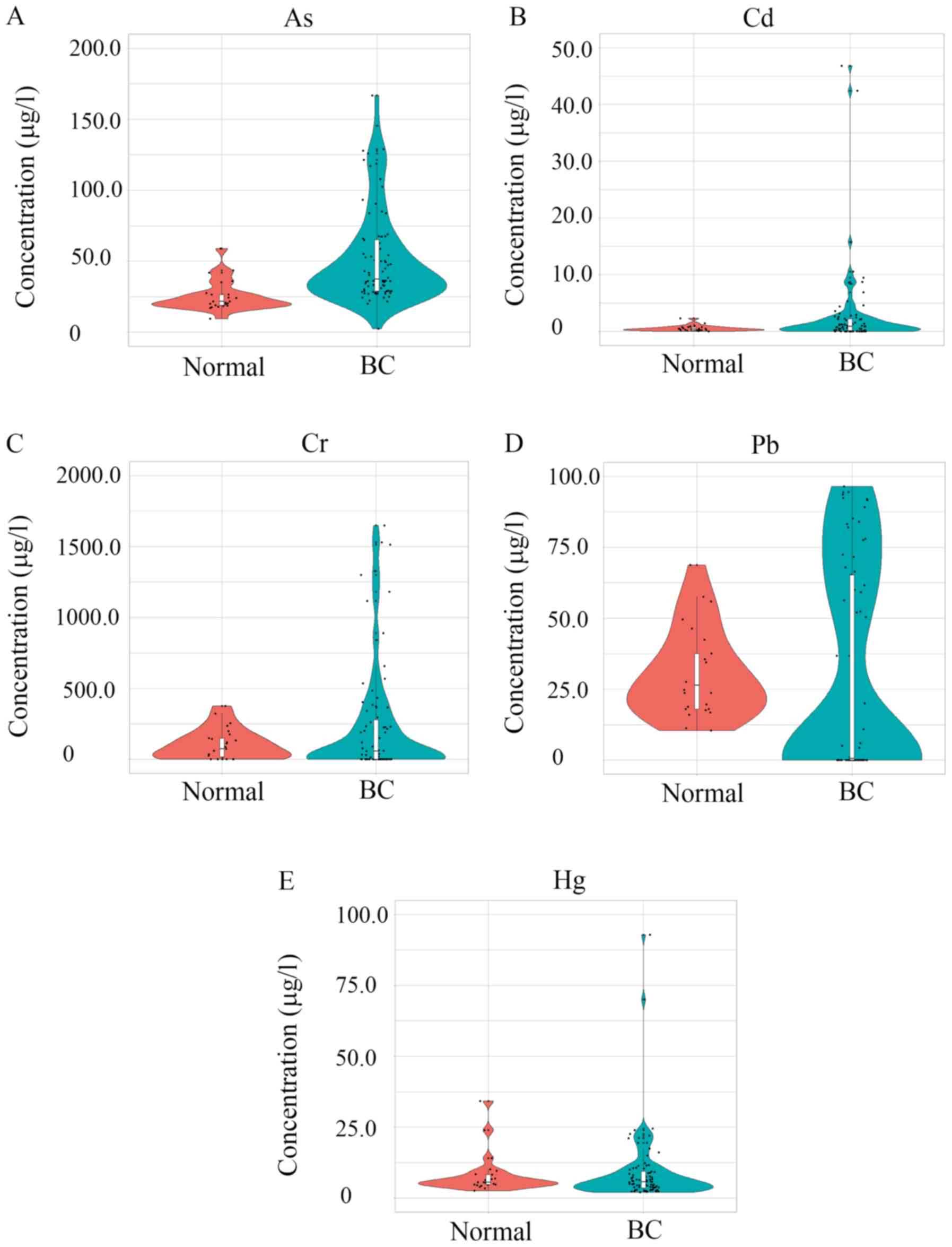
Levels of five heavy metals in the
blood
Five common heavy metals (those found at the highest
levels in Tengzhou) were measured in the blood samples, including
Cd, Cr, As, Hg, and Hg. The five heavy metals in order of relative
concentrations (µg/l) from low to high were as follows: Cd, Hg, As,
Pb and Cr. A total of 4 heavy metals, including As, Cd, Cr and Pb,
were significantly increased in patients with BC compared with
levels in the control population (Fig.
6). As showed the most significant (P=2.48×10−9)
increase in patients with BC. Cd showed the greatest increase
(14.91 fold) of the metals in patients with BC following by Cr
(3.24 fold), As (2.14 fold) and Pb (1.52 fold).
Discussion
BC is a common type of cancer affecting women
worldwide. A number of factors are associated with BC development,
including genetic background, diet, lifestyle, obesity, smoking,
alcohol consumption and environmental contamination (44–47).
Contamination of the local environment serves a vital role in BC
development. Environmental metallic compounds have been identified
as risk factors for development of BC (48) and a number of heavy metals have been
reported to be risk factors for numerous types of cancer, including
stomach and liver cancers (49–52). The
World Health Organization has classified As, Cd and Ni as Group 1
human carcinogens (53). Pb, Hg and
Cr have been established as human and animal carcinogens or
cocarcinogens (29,30). In the present study, it was found
that Cd levels were higher in patients with BC compared with those
in the control population (15 fold); at the same time, Cr, As and
Pb were also elevated in patients with BC by 3.24, 2.14 and 1.52
fold, respectively. This suggests that these four heavy metals may
be involved in BC development. Patients with BC, in addition to the
control population, were all situated in a local region area with
many mines and where heavy metal environmental contamination has
been reported to be high.
It has been reported over the last 10 years that
heavy metals cause a number of issues, such as immunodeficiency,
osteoporosis, neurodegeneration, organ failure and cancer (22). Previous studies have also reported
potential associations between heavy metals and estrogen-dependent
disorders, including pre-term deliveries, spontaneous abortions,
endometrial cancer and BC (22–24). The
general population is mainly exposed to heavy metals through
environmental contamination. Sources of environmental Cd exposure
to the general population include cigarette smoking, dietary
sources and drinking water (31,32). Cd
has been found in surface water and even ground water (54). Meanwhile, epidemiological studies
have found potential associations linking Cd exposure and BC
development (22–24). It is reported that Cr exposure is a
risk factor for BC development (55). In addition, Cr has been identified as
a potential risk factor for lung cancer, and cancer of the buccal
cavity, pharynx, esophagus and NHL, exclusively in women who smoke
tobacco, drink Cr-laden water and eat Cr-laden vegetables (56,57).
Exposure to As is mainly through food, water and inhalation of
sawdust or smoke from burning As-treated materials (58). Exposure of the general population to
As is associated with the development of breast, skin, lung,
bladder, liver and kidney cancer (54). It has been found that As is a
potential risk factor for the development of BC in patients with
the BRCA1 gene (33).
Another notable finding in the present study was the
elevation of plasma lipids in patients with BC compared with that
in the control population. Usually, lipids are responsible for
cardiovascular disease (11).
However, more recently it has been discovered that circulating
lipids are potential risk factors for BC development (11,12). The
cofactors of hyperlipidemia for BC include a short breastfeeding
period and mutations in the BRCA1 and BRCA2 genes
(59). Furthermore, it has been
reported that lipids are the risk factors for BC reoccurrence.
Overall, hyperlipidemia, high serum cholesterol, LDL-cholesterol
and triglyceride levels were found to be increased in patients with
BC compared with the levels in the control population (60).
Environmental heavy metal exposure serves a vital
role in the disturbance of lipid metabolism in humans (61). Elevated blood Cd concentration is
reported to be a potential risk factor for dyslipidemia (62). The association of blood Cd and
dyslipidemia is not dependent on lifestyle and BMI (62). Furthermore, blood Cd was found to be
a more valid biomarker for dyslipidemia compared with Cd in urine
(61). Blood Cd level is not only
associated with the increased prevalence of dyslipidemia, but also
with the elevated prevalence of high total cholesterol, high
triglyceride, high LDL-cholesterol and low HDL-C (62).
Epidemiological studies show that As exposure is
associated with cardiovascular diseases, including coronary heart
disease and peripheral arterial heart disease (62,63). As
can influence the blood concentration of apolipoproteins, which
indicates that As may be a potential risk factor for
dyslipidemia-associated diseases (63). Another study suggested that As can
mediate dyslipidemia and electrolyte retention in rats (64).
The findings of the present study suggest that
environmental exposure to heavy metals, such as Cd, As, Cr and Pb,
may influence blood lipid levels and other small molecule
metabolites, which in turn may be involved in BC development.
However, a limitation of this study was the small population size
for both the patients with BC and the control patients. Further
studies to examine urinary heavy metals are required to understand
the impact of heavy metals on metabolism and finally on BC
development.
Acknowledgements
Not applicable.
Funding
This study was supported by Shandong Province
Medical and Health Technology Development Project (grant no.
2016WS0627, Ethic no. 2017- Ethic review-03) and the Supporting
Fund for Teachers' research of Jining Medical University (grant no.
JY2016KJ041Y, Ethic no. 2017- Ethic review-04).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LL and MZ provided key intellectual input into the
conception and design of the study, and assisted in the writing of
the original manuscript. YM and WW performed the experiments. WZ
and YM analyzed the data and contributed to the writing of the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was performed in accordance to the
Institutional Ethical Committee and the Helsinki Declaration of
1975, as revised in 1983, and was approved by the Institutional
Review Board of the Affiliated Tengzhou Central People's Hospital
of Jining Medical University. Written informed consent was obtained
from the patients and controls in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lécuyer L, Victor Bala A, Deschasaux M,
Bouchemal N, Nawfal Triba M, Vasson MP, Rossary A, Demidem A, Galan
P, Hercberg S, et al: NMR metabolomics signatures reveal predictive
plasma metabolites associated with long-term risk of developing
breast cancer. Int J Epidemiol. 47:484–494. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I and Ervik M:
GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC
CancerBase No. 11. Lyon, France: International Agency for Research
on Cancer; 2013
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rzymski P, Tomczyk K, Rzymski P,
Poniedziałek B, Opala T and Wilczak M: Impact of heavy metals on
the female reproductive system. Ann Agric Environ Med. 22:259–264.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prüss-Üstün A and Corvalán C: Preventing
disease through healthy environments. Towards an estimate of the
environmental burden of disease. World Health Organization; France:
2006
|
|
6
|
Howell A, Anderson AS, Clarke RB, Duffy
SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM and
Harvie MN: Risk determination and prevention of breast cancer.
Breast Cancer Res. 16:4462014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Claudino WM, Quattronem A, Biganzoli L,
Pestrin M, Bertini I and Di Leo A: Metabolomics: Available results,
current research projects in breast cancer, and future
applications. J Clin Oncol. 25:2840–2846. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Griffin JL: The Cinderella story of
metabolic profiling: Does metabolomics get to go to the functional
genomics ball? Philos Trans R Soc London B Biol Sci. 361:147–161.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kruk J, Doskocz M, Jodłowska E,
Zacharzewska A, Łakomiec J, Czaja K and Kujawski J: NMR techniques
in metabolomic studies: A quick overview on examples of
utilization. Appl Magn Reson. 48:1–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Denkert C, Budczies J, Kind T, Weichert W,
Tablack P, Sehouli J, Niesporek S, Könsgen D, Dietel M and Fiehn O:
Mass spectrometry-based metabolic profiling reveals different
metabolite patterns in invasive ovarian carcinomas and ovarian
borderline tumors. Cancer Res. 66:10795–10804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bathen TF, Sitter B, Sjøbakk TE, Tessem MB
and Gribbestad IS: Magnetic resonance metabolomics of intact
tissue: A biotechnological tool in cancer diagnostics and treatment
evaluation. Cancer Res. 70:6692–6696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brennan L: NMR-based metabolomics: From
sample preparation to applications in nutrition research. Prog Nucl
Magn Reson Spectrosc. 83:42–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dunn WB, Goodacre R, Neyses L and Mamas M:
Integration of metabolomics in heart disease and diabetes research:
Current achievements and future outlook. Bioanalysis. 3:2205–2222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suhre K, Shin SY, Petersen AK, Mohney RP,
Meredith D, Wägele B, Altmaier E; CARDIoGRAM, ; Deloukas P, Erdmann
J, et al: Human metabolic individuality in biomedical and
pharmaceutical research. Nature. 477:54–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bictash M, Ebbels TM, Chan Q, Loo RL, Yap
IK, Brown IJ, de Iorio M, Daviglus ML, Holmes E, Stamler J, et al:
Opening up the ‘Black Box’: Metabolic phenotyping and
metabolome-wide association studies in epidemiology. J Clin
Epidemiol. 63:970–979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmes E, Loo RL, Stamler J, Bictash M,
Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, et al:
Human metabolic phenotype diversity and its association with diet
and blood pressure. Nature. 453:396–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Norvig P, Relman DA, Goldstein DB, et al:
2020 visions. Nature. 463:26–32. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Byrne C, Divekar SD, Storchan GB, Parodi
DA and Martin MB: Metals and breast cancer. J Mammary Gland Biol
Neoplasia. 18:63–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krizek M, Senft V and Motan J: Copper and
the human body. Cas Lek Cesk. 136:698–701. 1997.(In Czech).
PubMed/NCBI
|
|
20
|
Chan S, Gerson B and Subramaniam S: The
role of copper, molybdenum, selenium, and zinc in nutrition and
health. Clin Lab Med. 18:673–685. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christianson DW and Cox JD: Catalysis by
metal-activated hydroxide in zinc and manganese metalloenzymes.
Annu Rev Biochem. 68:33–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waalkes MP, Fox DA, States JC, Patierno SR
and McCabe MJ Jr: Metals and disorders of cell accumulation:
Modulation of apoptosis and cell proliferation. Toxicol Sci.
56:255–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Järup L: Hazards of heavy metals
contamination. Br Med Bull. 68:167–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rzymski P, Niedzielski P, Poniedziałek B
and Klimaszyk P: Bioaccumulation of selected metals in bivalves
(Unionidae) and Phragmites australis inhabiting a municipal water
reservoir. Environ Monit Assess. 186:3199–3212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hayes RB: The carcinogenicity of metals in
humans. Cancer Causes Control. 8:371–385. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Some metals and metallic compounds. IARC
Monogr Eval Carcinog Risk Chem Hum. 23:1–420. 1980.PubMed/NCBI
|
|
27
|
Gartell MJ, Craun JC, Podrebarae DS and
Gunderson EL: Pesticides, selected elements and other chemicals in
infant and toddler total diet samples. October 1980-March 1982. J
Assoc Off Anal Chem. 69:123–145. 1986.PubMed/NCBI
|
|
28
|
Gartell MJ, Craun JC, Podrebarae DS and
Gunderson EL: Pesticides, selected elements and other chemicals in
adult total diet samples, October 1980-March 1982. J Assoc Off Anal
Chem. 69:146–159. 1986.PubMed/NCBI
|
|
29
|
Szyczewski P, Siepak P, Niedzielski P and
Sobczyński T: Research on heavy metals in Poland. Pol J Environ
Stud. 8:755–768. 2009.
|
|
30
|
Antila E, Mussalo-Rauhamaa H, Kantola M,
Atroshi F and Westermarck T: Association of cadmium with human
breast cancer. Sci Total Environ. 186:251–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romaniuk A, Lyndin M, Moskalenko R,
Kuzenko Y, Gladchenko O and Lyndina Y: Pathogenetic mechanisms of
heavy metals effect on proapoptotic and proliferative potential of
breast cancer. Interv Med Appl Sci. 7:63–67. 2015.PubMed/NCBI
|
|
32
|
Zhang W, Zhao Y, Li F, Li L, Feng Y, Min
L, Ma D, Yu S, Liu J, Zhang H, et al: Zinc oxide nanoparticle
caused plasma metabolomic perturbations correlate with hepatic
steatosis. Front Pharmacol. 9:572018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soininen P, Kangas AJ, Würtz P, Tukiainen
T, Tynkkynen T, Laatikainen R, Järvelin MR, Kähönen M, Lehtimäki T,
Viikari J, et al: High-throughput serum NMR metabonomics for
cost-effective holistic studies on systemic metabolism. Analyst.
134:1781–1785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mäkinen VP, Soininen P, Forsblom C,
Parkkonen M, Ingman P, Kaski K, Groop PH; FinnDiane Study Group, ;
Ala-Korpela M: 1H NMR metabonomics approach to the disease
continuum of diabetic complications and premature death. Mol Syst
Biol. 4:1672008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan G, Huang Y, Bu Q, Lv L, Deng P, Zhou
J, Wang Y, Yang Y, Liu Q, Cen X and Zhao Y: Zinc oxide
nanoparticles cause nephrotoxicity and kidney metabolism
alterations in rats. J Environ Sci Health A Tox Hazard Subst
Environ Eng. 47:577–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan Q, He Q, Deng X, Hao F, Tang H and
Wang Y: Systemic metabolic responses of broiler chickens and
piglets to acute T-2 toxin intravenous exposure. J Agric Food Chem.
64:714–723. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Feng YN, Li L, Zhang HF, Zhang YN,
Zhang PF, Liu XQ, Zhang WD, Huang TT, Zhao L, et al:
Tissue-specific regulation of the contents and correlations of
mineral elements in hens by zinc oxide nanoparticles. Biol Trace
Elem Res. 177:353–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Men Y, Li L, Zhang F, Kong X, Zhang W, Hao
C and Wang G: Evaluation of heavy metals and metabolites in the
urine of patients with breast cancer. Oncol Lett. 19:1331–1337.
2020.PubMed/NCBI
|
|
39
|
Zimeri AM, Robb SW, Hassan SM, Hire RR and
Davis MB: Assessing heavy metal and PCB exposure from tap water by
measuring levels in plasma from sporadic breast cancer patients, a
pilot study. Int J Environ Res Public Health. 12:15683–15691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wood RY and Della-Monica NR: Psychosocial
factors influencing breast cancer risk appraisal among older women.
Qual Health Res. 21:783–795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Salhab M, Bismohun S and Mokbel K:
Risk-reducing strategies for women carrying BRCA1/2 mutations with
a focus on prophylactic surgery. BMC Womens Health. 10:282010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jevtic M, Velicki R, Popovic M,
Cemerlic-Adjic N, Babovic SS and Velicki L: Dietary influence on
breast cancer. J BUON. 15:455–461. 2010.PubMed/NCBI
|
|
43
|
Ebrahim AM, Eltayeb MA, Shaat MK, Mohmed
NM, Eltayeb EA and Ahmed AY: Study of selected trace elements in
cancerous and non-cancerous human breast tissues from Sudanese
subjects using instrumental neutron activation analysis. Sci Total
Environ. 383:52–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Florea AM and Büsselberg D: Metals and
breast cancer: Risk factors or healing agents? J Toxicol.
2011:1596192011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ilychova SA and Zaridze DG: Cancer
mortality among female and male workers occupationally exposed to
inorganic lead in the printing industry. Occup Environ Med.
69:87–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qu W, Tokar EJ, Kim AJ, Bell MW and
Waalkes MP: Chronic cadmium exposure in vitro causes acquisition of
multiple tumor cell characteristics in human pancreatic epithelial
cells. Environ Health Perspect. 120:1265–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheung MR: Blood lead concentration
correlates with all cause, all cancer and lung cancer mortality in
adults: A population based study. Asian Pac J Cancer Prev.
14:3105–3108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Person RJ, Tokar EJ, Xu Y, Orihuela R,
Ngalame NN and Waalkes MP: Chronic cadmium exposure in vitro
induces cancer cell characteristics in human lung cells. Toxicol
Appl Pharmacol. 273:281–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wadhwa SK, Kazi TG, Afridi HI, Talpur FN
and Naeemullah: Interaction between carcinogenic and
anti-carcinogenic trace elements in the scalp hair samples of
different types of Pakistani female cancer patients. Clin Chim
Acta. 439:178–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Núñez O, Fernández-Navarro P,
Martín-Méndez I, Bel-Lan A, Locutura JF and López-Abente G: Arsenic
and chromium topsoil levels and cancer mortality in Spain. Environ
Sci Pollut Res Int. 23:17664–17675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Welling R, Beaumont JJ, Petersen SJ,
Alexeeff GV and Steinmaus C: Chromium VI and stomach cancer: A
meta-analysis of the current epidemiological evidence. Occup
Environ Med. 72:151–159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Peralta-Videa JR, Lopez ML, Narayan M,
Saupe G and Gardea-Torresdey J: The biochemistry of environmental
heavy metal uptake by plants: Implications for the food chain. Int
J Biochem Cell Biol. 41:1665–1677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
ATSDR: Toxicological profile for arsenic.
Agency for Toxic Substances and Disease Registry (ATSDR). U.S.
Department of Health and Human Services; Atlanta: 2007
|
|
54
|
IARC Arsenic, metals, fibres and dusts.
IARC monographs on the evaluation of carcinogenic risks to humans.
100c. International Agency for Research on Cancer; Lyon: 2012
|
|
55
|
Hasija K and Bagga HK: Alterations of
serum cholesterol and serum lipoprotein in breast cancer of women.
Indian J Clin Biochem. 20:61–66. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Raza U, Asif MR, Rehman AB and Sheikh A:
Hyperlipidemia and hyper glycaemia in breast cancer patients is
related to disease stage. Pak J Med Sci. 34:209–214. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Laisupasain P, Thompat W, Sukarayodhin S,
Somprom A and Sudjaroen Y: Comparison of Serum lipid profiles
between normal control and breast cancer patients. J Lab Physician.
5:38–41. 2013. View Article : Google Scholar
|
|
58
|
Alexopoulos CG, Blatsios B and Avgerinos
A: Serum lipids and lipoprotein disorders in cancer patients.
Cancer. 60:3065–3070. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Santos-Gallego CG and Jialal I: Cadmium
and atherosclerosis: Heavy metal or singing the blues?
Atherosclerosis. 249:230–232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhou Z, Lu YH, Pi HF, Gao P, Li M, Zhang
L, Pei LP, Mei X, Liu L, Zhao Q, et al: Cadmium exposure is
associated with the prevalence of dyslipidemia. Cell Physiol
Biochem. 40:633–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ledda C, Iavicoli I, Bracci M, Avola R,
Senia P, Santarelli L, Pomara C and Rapisarda V: Serum lipid,
lipoprotein and apolipoprotein profiles in workers exposed to low
arsenic levels: Lipid profiles and occupational arsenic exposure.
Toxicol Lett. 282:49–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Waghe P, Sarkar SN, Sarath TS, Kandasamy
K, Choudhury S, Gupta P, Harikumar S and Mishra SK: Subchronic
arsenic exposure through drinking water alters lipid profile and
electrolyte status in rats. Biol Trace Elem Res. 176:350–354. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Cha YJ, Kim ES and Koo JS: Amino acid
transporters and glutamine metabolism in breast cancer. Int J Mol
Sci. 19:E9072018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
His M, Viallon V, Dossus L, Gicquiau A,
Achaintre D, Scalbert A, Ferrari P, Romieu I, Onland-Moret NC,
Weiderpass E, et al: Prospective analysis of circulating
metabolites and breast cancer in EPIC. BMC Med. 17:1782019.
View Article : Google Scholar : PubMed/NCBI
|