Introduction
Colon cancer is the third most commonly diagnosed
cancer and poses a serious threat to public health (1). Worldwide, 608,700 individuals succumb
to colon cancer and there are ~1.4 million incident cases each year
(2,3). The pathogenesis of colon cancer is
complicated; therefore, despite efforts to understand its
pathogenesis to improve treatment options, prognosis and survival
within 60 months have remained relatively unchanged. Understanding
the pathogenesis of colon cancer may assist in developing effective
therapeutics for patients.
Long-term exposure to high concentrations of glucose
may lead to glycoxidation of tissue proteins and lipids to produce
advanced glycation end products (AGEs) (4–6).
Recently, several studies have demonstrated that AGEs and receptor
AGEs (RAGEs) serve important roles in proliferation, invasion and
epithelial-mesenchymal transition of cells by activating multiple
signaling pathways (7–10). AGEs and RAGE may enhance the levels
of proliferation and migration of breast cancer through the
IKK/NF-κB signaling pathway in mice (11). Qin et al (12) identified that AGEs promoted cell
proliferation and migration through RAGEs and the PI3K/AKT pathway.
Conversely, Li et al (13)
revealed that AGEs and RAGEs decreased cell proliferation through
the PI3K/AKT signaling pathway (13). Therefore, the effects of AGEs and
RAGEs on proliferation and migration of cells are
controversial.
Epithelial-mesenchymal transition (EMT) of cancer
cells results in an increase in migration and invasion of cancer
cells (14). The PI3K/AKT, IκB
kinase (IKK)/NF-κB and Erk pathways were demonstrated to contribute
to EMT (15–17). Therefore, the hypothesis of the
present study was that AGEs may affect proliferation, invasion and
EMT in human SW480 colon cancer cells through the PI3K/AKT
signaling pathway. The aim of the present study was to determine
the mechanism underlying the AGEs-mediated induction of
proliferation, invasion and EMT in SW480, and potentially highlight
novel therapeutic targets for treating patients with colon
cancer.
Materials and methods
Reagents
FBS, RPMI-1640 medium, PBS and penicillin were
obtained from Hyclone; GE Healthcare Life Sciences. LY294002, a
commonly used broad-spectrum inhibitor of PI3K, was obtained from
Selleck Chemicals. AGEs were obtained from Shanghai Yuanmu
Biotechnology, Co., Ltd.
Cell culture
SW480 colon cancer cells were purchased from the
American Type Culture Collection. Cells were cultured in RPMI-1640
medium (cat. no. SH30809.01B) supplemented with 10% FBS (cat. no.
SH30087.01) and 1% penicillin (cat. no. SH30010) with 5%
CO2 at 37°C. At 100% confluence, SW480 cells were
passaged using trypsin-EDTA.
MTT assay
Cell proliferation was evaluated using a CellTiter
96® AQueous One Solution Cell Proliferation assay (MTT
assay), purchased from Promega Corporation. SW480 cells were seeded
in a 96-well plate at a density of 1×104 cells per well
and incubated in a 37°C incubator for 12 h. Subsequently, cells
were washed with PBS twice and treated with AGEs as aforementioned.
The proliferation of SW480 cells was detected on days 0, 1, 2, and
3. A total of 10 µl MTT reagent was added to each well of the
96-well plate for 4 h. Cell proliferation was measured at a
wavelength of 490 nm at the different time points using a
microplate reader (Thermo Fisher Scientific, Inc.). The inhibition
rate was calculated as: 1-[optical density (OD) value of
experimental group/OD value of control group] ×100, and the
proliferation ratio was calculated as follows: [(Mean OD value at
time point/mean OD at day 0)-1] ×100.
Cell cycle progression and
apoptosis
Following transfection for 48 h, SW480 cells were
washed twice with pre-cooled PBS and fixed with pre-cooled 70%
ethanol overnight at 4°C. Subsequently, cells were resuspended in
500 µl PBS containing propidium iodide (PI; 50 µg/ml) staining
solution with 0.2% Triton X-100 and RNase A (100 µg/ml), and
incubated for 30 min at 4°C in the dark. Cell cycle distribution
was measured using a BD FACScalibur™ flow cytometer (BD
Biosciences) and ModFit LT v.4.0 (BD Biosciences). To measure
apoptosis, 1.25 µl Annexin V-fluorescein isothiocyanate were added
to 500 µl 1X binding buffer, and cells were incubated with this
solution in the dark and at room temperature for 15 min.
Eventually, 10 µl PI was added to the cells that were left in the
dark at room temperature for 15 min. Apoptosis was measured using
flow cytometry as aforementioned immediately.
Transwell invasion assay
Transwell invasion assays were used to determine
cell invasion. BD Matrigel™ (BD Biosciences), diluted 1:3 in
RPMI-1640 medium, was added to cold Transwell chambers (BD
Biosciences) and incubated at 37°C for 2 h. Following transfection,
SW480 cells were resuspended in 100 µl serum-free medium and added
to the upper chamber of a Transwell insert, and allowed to incubate
at 5% CO2 at 37°C for 24 and 48 h. Cells in the upper
chamber were fixed in 4% paraformaldehyde for 15 min. Following
fixing, the cells were washed in PBS, stained with crystal violet
for 10 min and counted at magnification, ×400 on an inverted
optical microscope imaging system.
Wound healing assay
Cell migration was determined using a wound healing
assay. SW480 cells were seeded in a 6-well plate at a density of
1×106 cells per well and incubated with 5%
CO2 at 37°C. At 80% confluence, SW480 cells were
scratched to create a cell-free area using a sharp edge. Cells were
washed with PBS, serum-free medium was added and the cells were
incubated at 37°C for 0, 24 and 48 h. Cell migration into the
scratched area was imaged every 24 h following scratching using an
inverted optical microscope (magnification, ×100). The width of the
scratched area was measured using Image Pro-Plus version 6.0 (Media
Cybernetics, Inc.). The migration rate was calculated using the
following formula: 1-(wound width at indicated time/wound width at
day 0) ×100.
Western blot analysis
Total protein was extracted from cultured SW480
cells using RIPA buffer (RIPA: 50 mM Tris-HCL PH 7.4; 150 mM NaCL;
1 mM EDTA; 1% Triton X-100; 0.5% sodium deoxycholate; 0.1% SDS; 100
mM PMSF) containing phosphatase and protease inhibitor cocktail
(100X; cat. no. 539131; Calbiochem; Merck KGaA) 4°C for 15 min.
Protein concentration was measured using Bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.). A total of 40 µg protein was
loaded on a 12% SDS-PAGE gel and transferred to a PVDF membrane
(EMD Millipore). Membranes were blocked with 5% non-fat milk in
TBS-Tween [TBST; 150 mmol/l NaCl, 10 mmol/l Tris-HCl (pH 8.0) and
0.1% Tween-20] for 1 h at room temperature. Membranes were washed 3
times with TBST and incubated with one of the following primary
antibodies: AKT (Abcam; cat. no. ab8805; 1:1,000), PI3K (Abcam;
cat. no. ab151549; 1:1,000), epithelial cadherin (E-cadherin;
Abcam; cat. no. ab194982; 1:1,000) and GAPDH (Abcam; cat. no.
ab8245; 1:1,000) at 4°C overnight. Membranes were washed 3 times
with TBST, and subsequently incubated for 1 h at room temperature
with either an immunoglobulin G horseradish peroxidase
conjugated-anti-rabbit or anti-rat secondary antibodies (cat. no.
BA1058, Wuhan Boster Biological Technology, Ltd.; 1:2,000).
Following incubation with the secondary antibody, the membranes
were washed with TBST, and signals were visualized using Immobilon
Western Chemiluminescent Substrate (EMD Millipore).
Statistical analysis
Comparisons between the control and the experimental
groups were performed using a one-way analysis of variance followed
by Student-Newman-Keuls post hoc test for pairwise comparison
between groups using SPSS v.20.0 (IBM Corp.). Data are presented as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of AGEs on proliferation of
SW480 cells
SW480 cells were divided into three groups: i)
Control; ii) cells treated with AGEs alone; and iii) cells treated
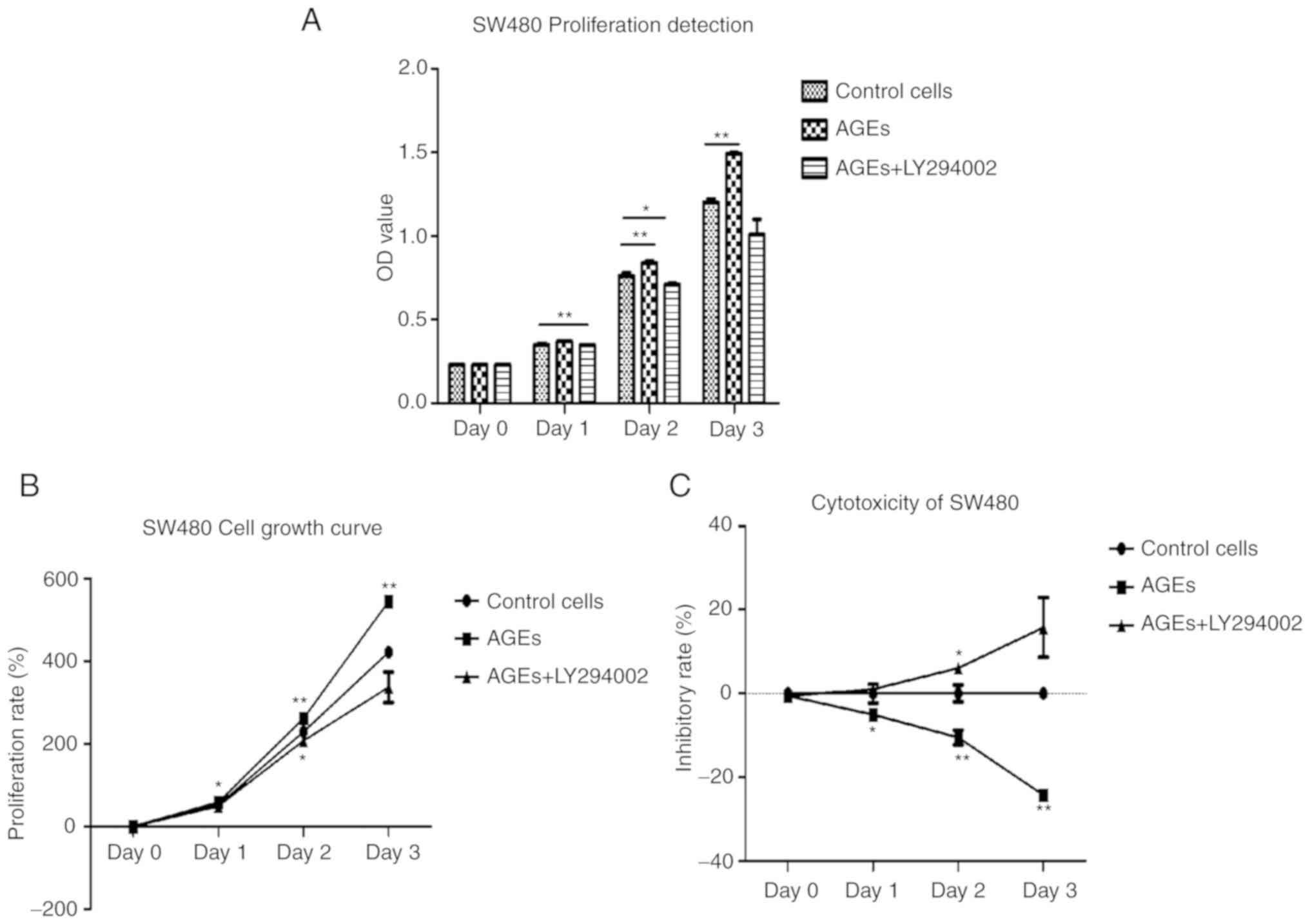
with AGEs and LY294002 (Fig. 1).
Compared with the control group, the absorbance values of cells
treated with AGEs was significantly increased, particularly on days
2 (P<0.001) and 3 (P<0.001). The absorbance values of cells
treated with AGEs and LY294002 was significantly decreased compared
with cells treated with AGEs alone on day 1 (P<0.001) and 2
(P<0.05) (Fig. 1A). The
proliferation ratio of cells treated with AGEs alone was also
significantly increased on days 1 (P<0.05) and 2 (P<0.001)
and 3 (P<0.001) compared with the control group. The
proliferation ratio of cells treated with AGEs and LY294002 was
also significantly increased on days 2 (P<0.05) compared with
the control group (Fig. 1B).
Conversely, the proliferation ratio of cells treated with AGEs and
LY294002 was significantly decreased compared with cells treated
with AGEs alone on day 3 (Fig. 1B).
As demonstrated in Fig. 1C, the
inhibition rate of cells treated with AGEs alone was significantly
decreased compared with the inhibition rate of cells treated AGEs
and LY294002. These results suggest that AGEs may enhance
proliferation of SW480 cells through the PI3K signaling
pathway.
Effect of AGEs on cell cycle
distribution and apoptosis in SW480 cells
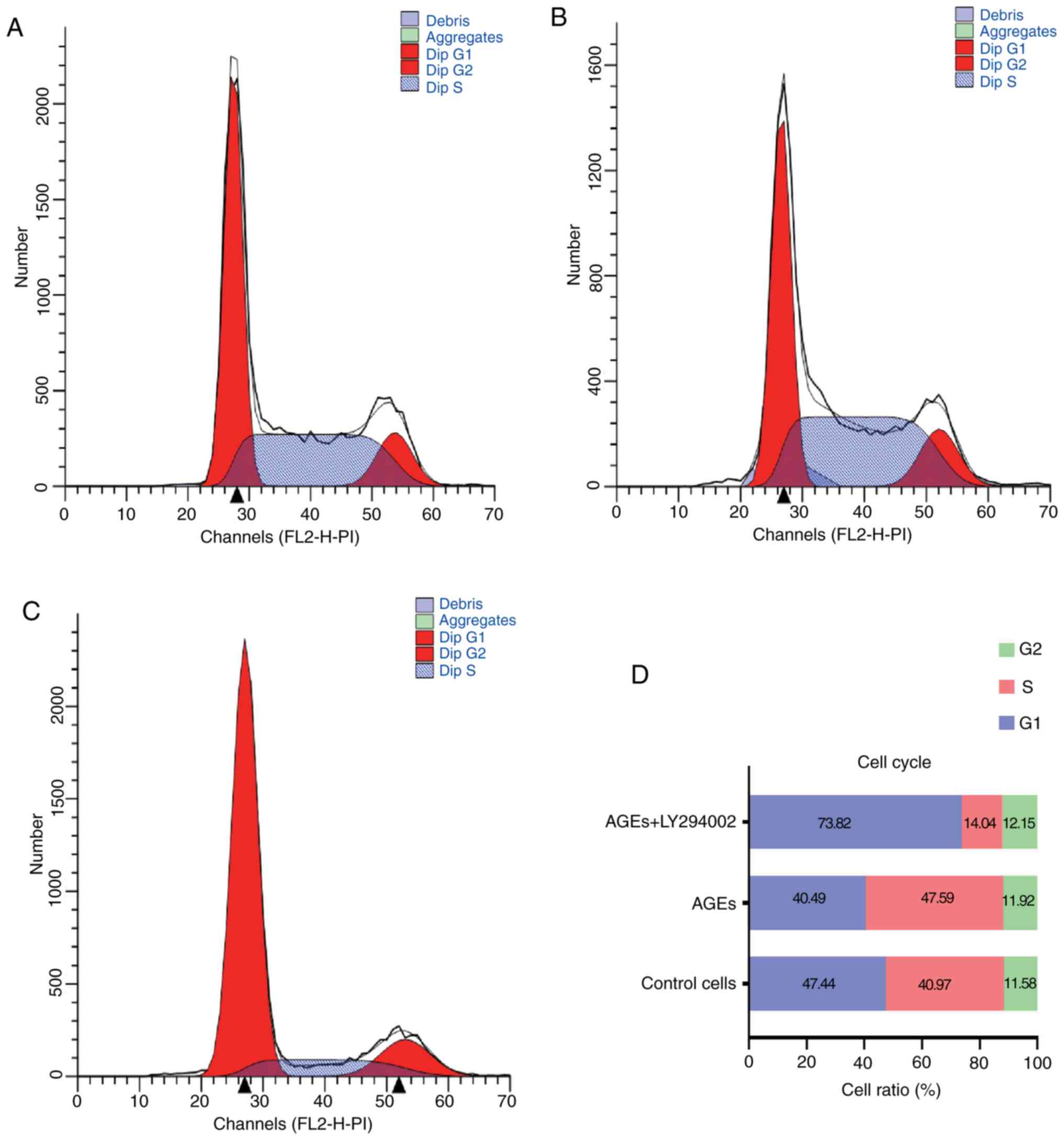
Figs. 2 and 3 demonstrate the effects of AGEs on cell
cycle distribution and apoptosis in SW480 cells. Compared with the
control group, in cells treated with AGEs alone there was a
decrease in the percentage of cells in G1-phase and increase in the
proportion of cells in S-phase. Treatment with LY294002 abrogated
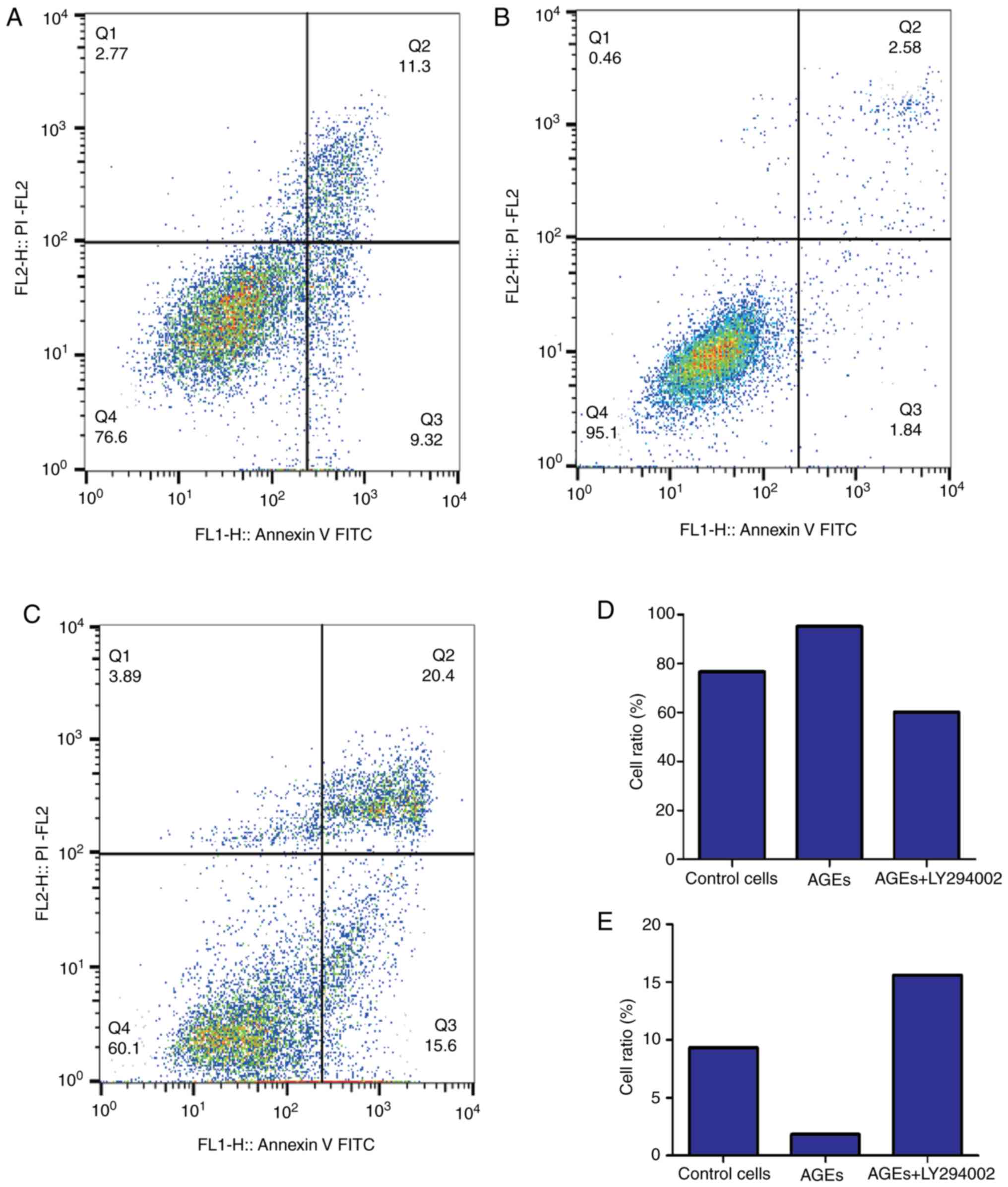
the effects of AGEs on cell cycle distribution (Fig. 2). As indicated in Fig. 3, treatment with AGEs alone decreased
the apoptotic rate in cells, and similar to the effects on cell
cycle distribution, LY294002 reversed this effect. These data
suggest that AGEs decreased the number of cells arrested at G1/S
phase and decreased apoptosis through the PI3K signaling
pathway.
AGEs increases the migratory and
invasive capacity of SW480 cells
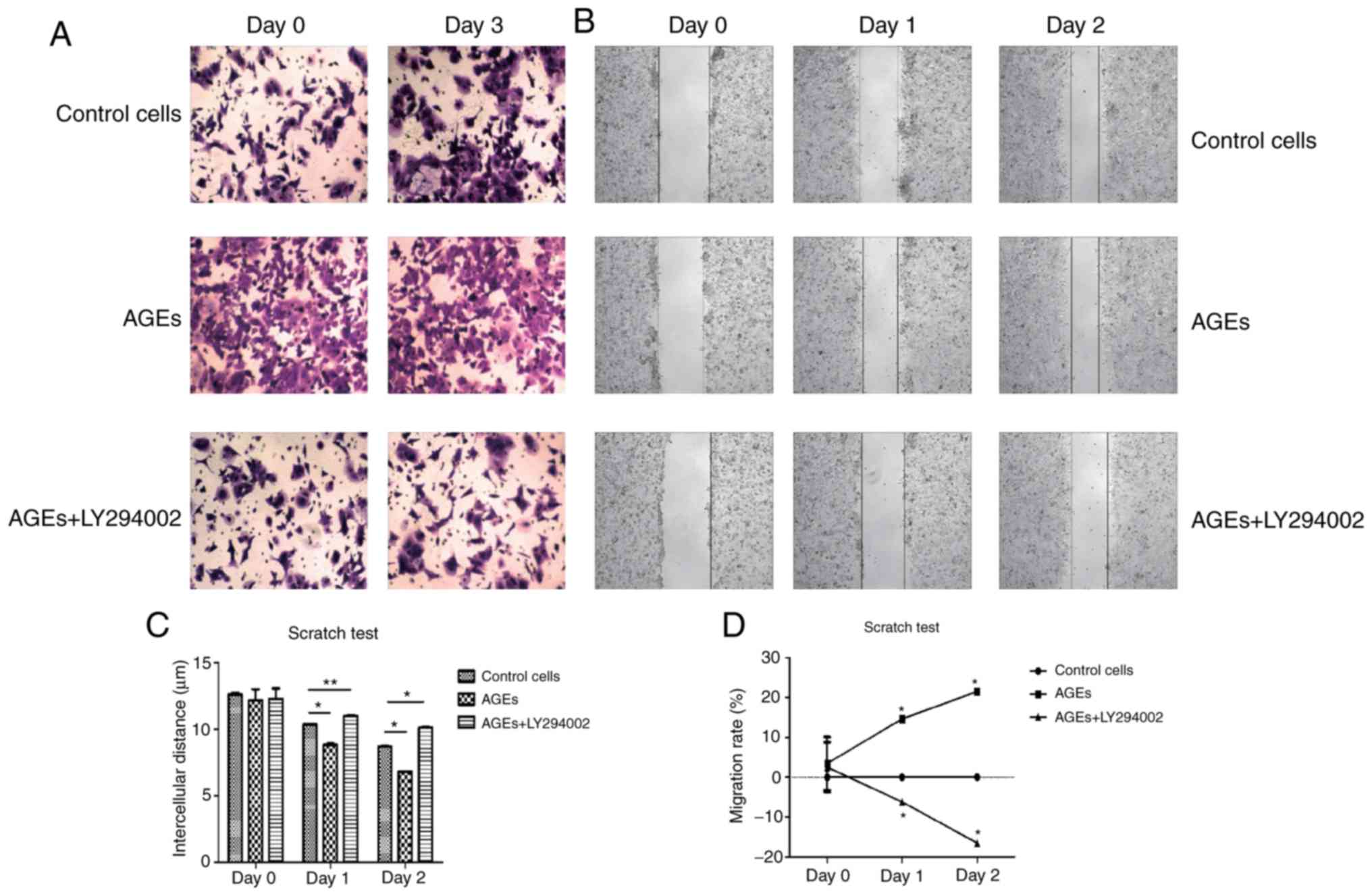
The results from the Transwell invasion assay
suggested that treatment with AGEs increased the invasive capacity
of cells compared with the control group, and LY294002 abrogated
the effects of AGEs on invasion (Fig.
4A). Similarly, wound closure was increased in cells treated
with AGEs compared with the control cells and cells treated with
AGEs and LY294002 (P<0.001) on days 1 and 2 (Fig. 4B and C). As demonstrated in Fig. 4D, the migratory rate cells treated
with AGEs was significantly increased compared with the other two
groups on days 1 and 2 (P<0.001). Conversely, the migratory rate
of cells treated with AGEs and LY294002 significantly decreased
compared with the two groups on days 1 and 2 (P<0.001). The
results of the invasion and wound healing assays suggest that AGEs
enhanced the invasive and migratory capacity of cells through the
PI3K signaling pathway.
Analysis of the effects of AGEs on
EMT
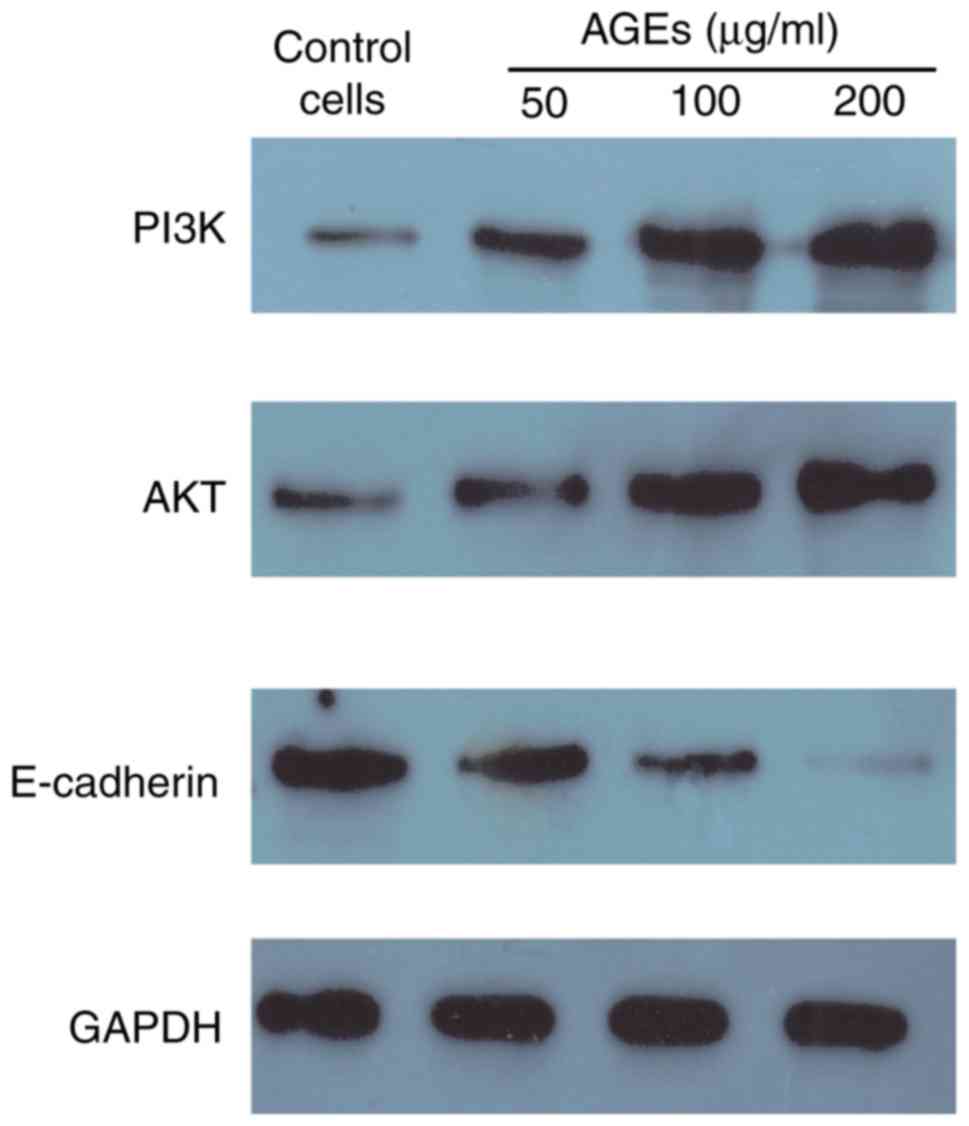
The protein expression levels of PI3K, AKT and
E-cadherin were assessed to determine the effects of AGEs on EMT
through the PI3K/AKT signaling pathway in SW480 cells. AGEs
increased the expression of PI3K and AKT, and decreased the
expression of E-cadherin in a concentration dependent manner
(Fig. 5). These results suggest that
AGEs may induce EMT in SW480 cells through the PI3K/AKT signaling
pathway.
Discussion
The effect of AGEs on cancer development has
garnered increasing interest in recent years. In vitro
assays in a number of studies have indicated that AGEs increase
proliferation, invasion and migration in colon (18,19),
pancreatic (20), liver (21), breast (22,23) and
renal cancer (24). Data from
epidemiological and animal studies have also suggested that AGEs
increase the risk of cancer development (25–28). In
the present study, the results demonstrated that AGEs increased
proliferation, invasion and migration, and decreased apoptosis in
SW480 cells. PI3K is a central protein involved in PI3K/AKT
signaling. PI3K activates lipids on cell membranes through a number
of signals, including hormones, growth factors and extracellular
matrix components (29). LY294002 is
the first synthetic molecule known to inhibit PI3Kα/β/δ function
(12) and in cells treated with
LY294002, Akt phosphorylation was decreased, resulting in
inhibition of cell proliferation (30). A previous study demonstrated that
LY294002 significantly inhibited growth and induced apoptosis of
colon cancer cells in vitro, by decreasing phosphorylation
of Akt on Ser473 (31). In addition,
LY294002 inhibits proliferation of ovarian cancer cells by inducing
marked nuclear pyknosis and decreasing cytoplasmic volume in the
tumor cells (32). The results of
the present study indicated that the effects of AGEs on
proliferation, invasion, migration and apoptosis were abrogated by
treatment with LY294002 in the SW480 cells. Therefore, PI3K/AKT
signaling may underlie the mechanism by which AGEs regulates
proliferation, invasion, migration and apoptosis in SW480
cells.
The PI3K/AKT signaling pathway is a significant
intracellular signaling pathway involved in regulation of a number
of cellular behaviors, including proliferation, metabolism and
apoptosis (33–35), and is frequently activated in cancer
cells (36). Boudot et al
(37) and Granado et al
(38) demonstrated that AKT
regulated cell migration through enhancing AKT signaling.
Consistent with Boudot et al (37) and Granado et al (37,38) the
expression of AKT protein was increased in SW480 cells treated with
AGEs in the present study. Western blot analysis demonstrated that
the expression of PI3K protein was also increased in SW480 cells
treated with AGEs. These results suggest that AGEs may increase the
protein expression levels of AKT by increasing the expression of
PI3K, thereby inhibiting apoptosis and promoting proliferation in
SW480 cells. Furthermore, expression of E-cadherin was decreased in
SW480 cells following treatment with various concentrations of
AGEs.
E-cadherin is a commonly used marker of EMT. EMT is
a complex biochemical process underlying increased migration and
invasion in cancer cells (39).
Prieto-García et al (40)
demonstrated that EMT increased migration in epithelial tumor
cells. If expression of E-cadherin is decreased, breast cancer
cells undergo EMT through activation of the NF-кB signaling pathway
(41). In the present study, the
western blot analysis data indicated that E-cadherin expression was
decreased following treatment with AGEs. Therefore, it is
hypothesized that the PI3K/AKT signaling pathway serves an
important role in AGEs-mediated activation of EMT through
decreasing expression of E-cadherin.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that AGEs induce
proliferation, invasion and EMT in SW480 cells through the PI3K/AKT
signaling pathway. AGEs increased the expression of PI3K and AKT,
which resulted in increased levels of proliferation, invasion and
EMT, thus identifying an association between AGEs and colon cancer,
and highlighting potentially novel therapeutic targets for
treatment of colon cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key Projects of
Guangxi Natural Science Foundation (grant no. 2013GXNSFDA019019);
the ‘139’ plan for Training High Level Cadre Talents in Guangxi
Medicine; Guangxi Special Fund Project for Cultivating Academic and
Technical Leaders in the New Century; The Second Level in Guangxi
New Century Ten, Hundred and Thousand Talents Project.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the author on reasonable request.
Authors' contributions
HL was involved in the conception and design of this
study, drafting the article and revising the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang KA, Piao MJ, Ryu YS, Hyun YJ, Park
JE, Shilnikova K, Zhen AX, Kang HK, Koh YS, Jeong YJ and Hyun JW:
Luteolin induces apoptotic cell death via antioxidant activity in
human colon cancer cells. Int J Oncol. 51:1169–1178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brownlee M: Advanced protein glycosylation
in diabetes and aging. Annu Rev Med. 46:223–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hori O, Yan SD, Ogawa S, Kuwabara K,
Matsumoto M, Stern D and Schmidt AM: The receptor for advanced
glycation end-products has a central role in mediating the effects
of advanced glycation end-products on the development of vascular
disease in diabetes mellitus. Nephrol Dial Transplant. 11 (Suppl
5):S13–S16. 1996. View Article : Google Scholar
|
|
6
|
Schmidt AM, Yan SD, Wautier JL and Stern
D: Activation of receptor for advanced glycation end products: A
mechanism for chronic vascular dysfunction in diabetic vasculopathy
and atherosclerosis. Circ Res. 84:489–497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao JM, He MY, Liu YW, Lu YJ, Hong YQ, Luo
HH, Ren ZL, Zhao SC and Jiang Y: AGE/RAGE/Akt pathway contributes
to prostate cancer cell proliferation by promoting Rb
phosphorylation and degradation. Am J Cancer Res. 5:1741–1750.
2015.PubMed/NCBI
|
|
8
|
Lee KJ, Yoo JW, Kim YK, Choi JH, Ha TY and
Gil M: Advanced glycation end products promote triple negative
breast cancer cells via ERK and NF-κB pathway. Biochem Biophys Res
Commun. 495:2195–2201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neviere R, Yu Y, Wang L, Tessier F and
Boulanger E: Implication of advanced glycation end products (Ages)
and their receptor (Rage) on myocardial contractile and
mitochondrial functions. Glycoconj J. 33:607–617. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun L, Huang T, Xu W, Sun J, Lv Y and Wang
Y: Advanced glycation end products promote VEGF expression and thus
choroidal neovascularization via Cyr61-PI3K/AKT signaling pathway.
Sci Rep. 7:149252017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nasser MW, Wani NA, Ahirwar DK, Powell CA,
Ravi J, Elbaz M, Zhao H, Padilla L, Zhang X, Shilo K, et al: RAGE
mediates S100A7-induced breast cancer growth and metastasis by
modulating the tumor microenvironment. Cancer Res. 75:974–985.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin Q, Niu J, Wang Z, Xu W, Qiao Z and Gu
Y: Heparanase induced by advanced glycation end products (AGEs)
promotes macrophage migration involving RAGE and PI3K/AKT pathway.
Cardiovasc Diabetol. 12:372013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li G, Xu J and Li Z: Receptor for advanced
glycation end products inhibits proliferation in osteoblast through
suppression of Wnt, PI3K and ERK signaling. Biochem Biophys Res
Commun. 423:684–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karin M, Yamamoto Y and Wang QM: The IKK
NF-kB system: A treasure trove for drug development. Nat Rev Drug
Discov. 3:17–26. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimomoto T, Luo Y, Ohmori H, Chihara Y,
Fujii K, Sasahira T, Denda A and Kuniyasu H: Advanced glycation end
products (AGE) induce the receptor for AGE in the colonic mucosa of
azoxymethane-injected Fischer 344 rats fed with a high-linoleic
acid and high-glucose diet. J Gastroenterol. 47:1073–1083. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen H, Wu L, Li Y, Meng J, Lin N, Yang D,
Zhu Y, Li X, Li M, Xu Y, et al: Advanced glycation end products
increase carbohydrate responsive element binding protein expression
and promote cancer cell proliferation. Mol Cell Endocrinol.
395:69–78. 2015. View Article : Google Scholar
|
|
20
|
Grote VA, Nieters A, Kaaks R, Tjønneland
A, Roswall N, Overvad K, Nielsen MR, Clavel-Chapelon F,
Boutron-Ruault MC, Racine A, et al: The associations of advanced
glycation end products and its soluble receptor with pancreatic
cancer risk: A case-control study within the prospective EPIC
cohort. Cancer Epidemiol Biomarkers Prev. 21:619–628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takino J, Yamagishi S and Takeuchi M:
Glycer-AGEs-RAGE signaling enhances the angiogenic potential of
hepatocellular carcinoma by upregulating VEGF expression. World J
Gastroenterol. 18:1781–1788. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Metformin inhibits advanced glycation end products
(AGEs)-induced growth and VEGF expression in MCF-7 breast cancer
cells by suppressing AGEs receptor expression via AMP-activated
protein kinase. Horm Metab Res. 45:387–390. 2013.PubMed/NCBI
|
|
23
|
Chen H, Li Y, Zhu Y, Wu L, Meng J, Lin N,
Yang D, Li M, Ding W, Tong X and Su Q: Advanced glycation end
products promote ChREBP expression and cell proliferation in liver
cancer cells by increasing reactive oxygen species. Medicine
(Baltimore). 96:e74562017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Liu K, Wang Z, Liu C, Han Z, Tao J,
Lu P, Wang J, Wu B, Huang Z, et al: Advanced glycation end products
accelerate arteriosclerosis after renal transplantation through the
AGE/RAGE/ILK pathway. Exp Mol Pathol. 99:312–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Foster D, Spruill L, Walter KR, Nogueira
LM, Fedarovich H, Turner RY, Ahmed M, Salley JD, Ford ME, Findlay
VJ and Turner DP: AGE metabolites: A biomarker linked to cancer
disparity? Cancer Epidemiol Biomarkers Prev. 23:2186–2191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vlassara H and Uribarri J: Advanced
glycation end products (AGE) and diabetes: Cause, effect, or both?
Curr Diab Rep. 14:4532014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duan Z, Chen G, Chen L,
Stolzenberg-Solomon R, Weinstein SJ, Mannisto S, White DL, Albanes
D and Jiao L: Determinants of concentrations of
N(ε)-carboxymethyl-lysine and soluble receptor for advanced
glycation end products and their associations with risk of
pancreatic cancer. Int J Mol Epidemiol Genet. 5:152–163.
2014.PubMed/NCBI
|
|
28
|
Yang S, Pinney SM, Mallick P, Ho SM,
Bracken B and Wu T: Impact of oxidative stress biomarkers and
carboxymethyllysine (an advanced glycation end product) on prostate
cancer: A prospective study. Clin Genitourin Cancer. 13:e347–e351.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xin M and Deng X: Nicotine inactivation of
the proapoptotic function of Bax through phosphorylation. J Biol
Chem. 280:10781–10789. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Semba S, Itoh N, Ito M, Harada M and
Yamakawa M: The in vitro and in vivo effects of
2-(4-morpholinyl)-8-phenyl-chromone (LY294002), a specific
inhibitor of phosphatidylinositol 3′-kinase, in human colon cancer
cells. Clin Cancer Res. 8:1957–1963. 2002.PubMed/NCBI
|
|
32
|
Hu L, Zaloudek C, Mills GB, Gray J and
Jaffe RB: In vivo and in vitro ovarian carcinoma growth inhibition
by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin
Cancer Res. 6:880–886. 2000.PubMed/NCBI
|
|
33
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zheng J, Koh X, Hua F, Li G, Larrick JW
and Bian JS: Cardioprotection induced by Na+/K+-ATPase activation
involves extracellular signal-regulated kinase 1/2 and
phosphoinositide 3-kinase/Akt pathway. Cardiovasc Res. 89:51–59.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Q, Turner KM, Alfred Yung WK, Chen K
and Zhang W: Role of AKT signaling in DNA repair and clinical
response to cancer therapy. Neuro Oncol. 16:1313–1323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blume-Jensen P and Hunter T: Oncogenic
kinase signalling. Nature. 411:355–365. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boudot C, Saidak Z, Boulanouar AK, Petit
L, Gouilleux F, Massy Z, Brazier M, Mentaverri R and Kamel S:
Implication of the calcium sensing receptor and the
Phosphoinositide 3-kinase/Akt pathway in the extracellular
calcium-mediated migration of RAW 264.7 osteoclast precursor cells.
Bone. 46:1416–1423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Granado MH, Gangoiti P, Ouro A, Arana L,
González M, Trueba M and Gómez-Muñoz A: Ceramide 1-phosphate (C1P)
promotes cell migration: Involvement of a specific C1P receptor.
Cell Signal. 21:405–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wallesch M, Pachow D, Blücher C, Firsching
R, Warnke JP, Braunsdorf WEK, Kirches E and Mawrin C: Altered
expression of E-Cadherin-related transcription factors indicates
partial epithelial-mesenchymal transition in aggressive
meningiomas. J Neurol Sci. 380:112–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Prieto-García E, Díaz-García CV,
García-Ruiz I and Agulló-Ortuño MT: Epithelial-to-mesenchymal
transition in tumor progression. Med Oncol. 34:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-кB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|