Introduction
Pancreatic ductal adenocarcinoma (PDAC) arises from
the ductal cells of the exocrine pancreas and comprises the vast
majority of all types of pancreatic cancer (1). PDAC is ranked the 7th leading cause of
cancer-associated mortality worldwide, predominantly due to poor
diagnosis (2) and the incidence of
PDAC was 12.3/100,000 in the United States in 2011 (3). It has been reported that only 10–20% of
patients are surgically resectable at the time of diagnosis, and
PDAC has a global 5-year survival rate of <5% (4). Currently, only a limited number of
chemotherapeutic agents have been demonstrated to be effective
against PDAC, including gemcitabine and fluorouracil (5). Despite advancements in the treatment of
pancreatic cancer, therapies for PDAC are inadequate and the
prognosis of PDAC remains unoptimistic due to late detection, drug
resistance, and high recurrence and metastatic rates (6,7). Thus,
there is a critical need to improve survival rates by identifying
novel treatment targets and prognostic factors for patients with
PDAC.
With the development of modern biomedicine, growing
evidence has demonstrated that multiple gene alterations, such as
those in KRAS, cyclin-dependent kinase inhibitor 2A (CDKN2A) and
TP53, may act as key determinants of tumor fate within the pancreas
(8), and that several cellular
signaling pathways are closely associated with the occurrence and
progression of PDAC (9,10). Next-generation sequencing (NGS)
technology has been widely applied as a notable tool in early
cancer diagnosis, cancer grading and progression prediction
(11). Furthermore, a substantial
amount of biological and clinical data for PDAC cases has been
generated with the development of NGS analysis, and these available
datasets provide opportunities to systemically investigate and
analyze a wide range of expression patterns and molecular
signatures for PDAC (12).
The present study aimed to improve the understanding
of the molecular basis of PDAC and to identify novel prognostic
factors for PDAC. The GSE46234 dataset was downloaded from the Gene
Expression Omnibus (GEO) database, in order to determine the
upregulated differentially expressed genes (DEGs) in the tumor
tissues of patients with PDAC. Subsequently, Gene Ontology (GO)
functional enrichment and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analyses were performed. Following identification of
the hub genes via protein-protein interaction (PPI) network
screening, their relevance with overall survival (OS) and
disease-free survival (DFS) time in patients with PDAC was
evaluated. Taken together, the results of the present study provide
further insight into the potential candidate biomarkers for the
progression and prognosis of PDAC at the molecular level.
Materials and methods
Study design and source of data
In the present study, GEO profiles with raw data of
the CEL file type based on the GPL570 platform (Affymetrix Human
Genome U133 Plus 2.0 Array), with information on the probe ID, Gene
Symbol and Entrez Gene ID were included. The gene expression
profiles of the GSE46234 dataset were downloaded from the GEO
database (ncbi.nlm.nih.gov/gds/), which comprised data from four
healthy and four PDAC tissue samples.
Identification of DEGs
The GEO2R online tool (ncbi.nlm.nih.gov/geo/geo2r) within R software version
3.6.2 (13) was used to determine
the DEGs between tumor tissues in patients with PDAC and normal
tissues in healthy subjects. An unpaired Student's t-test was
performed to identify the DEGs, and the DEGs were considered to be
statistically significant according to the cut-off criteria of
|logFC|≥2 and adjusted P<0.01 (14). Furthermore, visual hierarchical
cluster analysis was applied to identify the upregulated DEGs using
Morpheus online analysis software (software.broadinstitute.org/morpheus). A heat map was
constructed to validate these results.
GO functional enrichment and KEGG
pathway analyses of the DEGs
The Database for Annotation, Visualization and
Integrated Discovery version 6.7 (DAVID) (david-d.ncifcrf.gov/) was used to perform GO
functional enrichment and KEGG pathway analyses GO analysis is
widely used to annotate specific genes and gene products for
high-throughput genome and transcriptome data (14). In the present study, GO analysis was
performed to predict the potential functions of the DEGs based on
biological process (BP), molecular function (MF) and cellular
component (CC). For both analyses, P<0.05 was considered to
indicate statistical significance.
PPI network construction
The Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (15) was used to assess and integrate the
PPIs, including direct (physical) and indirect (functional)
associations among the DEGs. The PPI network for the DEGs was
constructed using the Molecular Complex Detection plugin within
Cytoscape software version 3.7.1 (16). PPI network modules were screened, and
scores >3 and nodes >4 were used as the selection criteria.
P<0.05 was considered to indicate a statistical
significance.
Survival analysis and expression
levels of the hub genes
The present study set out to identify candidate
biomarkers for PDAC prognosis. Genes with the highest scores in the
PPI network were selected as the hub genes, and the OS and DFS
outcomes based on the hub gene expression levels were depicted
according to the data downloaded from the Gene Expression Profiling
Interactive Analysis database (17).
The expression levels of the hub genes in patients at different
tumor stages were downloaded from the interactive web resource,
UALCAN (ualcan.path.uab.edu/index.html). The hub genes
associated with both shorter OS and DFS were identified for further
study, and their expression levels were compared in both PDAC
tissues and normal adjacent tissues.
Patients and samples
The subsequent experiments were approved and
authorized by The Ethics Committee of Taizhou Hospital of Zhejiang
Province, and written informed consent was obtained from all
patients prior to study commencement. PDAC tissues and normal
adjacent tissues were provided by the Biobank of Taizhou Hospital
of Zhejiang province (Taizhou, China). Samples obtained from six
patients initially diagnosed by two pathologists of pathology
department in Taizhou hospital were resected during surgery and
immediately frozen in liquid nitrogen for 3 months. Clinical
characteristics, including age, sex, tumor location, TNM stage
(18) and differentiation are
presented in Table I.
 | Table I.Clinical characteristics of the six
patients with pancreatic ductal adenocarcinoma. |
Table I.
Clinical characteristics of the six
patients with pancreatic ductal adenocarcinoma.
| Sex | Age, years | Location of
tumor | TNM
stagea |
Differentiation |
|---|
| Male | 60 |
Head | T2N0M0 |
Poor |
| Male | 57 |
Body and tail | T2N1M0 |
Poor |
| Female | 55 |
Head | T3N1M0 |
Moderate |
| Male | 70 |
Head | T3N1M0 |
Moderate |
| Male | 46 |
Body and tail | T1N1M0 |
Moderate |
| Female | 67 |
Head | T2N0M0 |
Well |
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the six PDAC and normal
adjacent tissue samples using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The purity and concentration of total RNA
were measured using a NanoDrop ND-1000 spectrophotometer (Thermo
Fisher Scientific, Inc.). A total of 1 µg total RNA was reverse
transcribed into cDNA using M-MuLV Reverse Transcriptase (Toyobo
Life Science) at a total volume of 20 µl, according to the
manufacturer's protocol. The primer sequences (Genewiz, Inc.) used
for qPCR are presented in Table II.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 3 min followed by 40 cycles of
denaturation at 95°C for 10 sec and annealing and elongation at
60°C for 30 sec. The relative expression levels of abnormal
spindle-like microcephaly-associated protein (ASPM), mitotic
checkpoint serine/threonine-protein kinase BUB1 β (BUB1B)
and protein spindly (SPDL1) were determined using the
2−ΔΔCq method (19) and
normalized to the internal reference gene, GAPDH. The difference in
mRNA expression level between the matched tumor and nontumor tissue
samples was assessed using a paired Student's t-test within
GraphPad Prism (version 7.0; GraphPad Software). P<0.05 was
considered to indicate a statistically significant difference.
 | Table II.Primer sequences used for reverse
transcription-quantitative PCR analysis. |
Table II.
Primer sequences used for reverse
transcription-quantitative PCR analysis.
| Gene | Forward sequence
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| ASPM |
GGCCCTAGACAACCCTAACGA |
AGCTTGGTGTTTCAGAACATC |
| BUB1B |
AAATGACCCTCTGGATGTTTGG |
GCATAAACGCCCTAATTTAAGCC |
| SPDL1 |
CGAGAGCTAGCTGAGCGAAT |
CAGCCTCTTTGAGCCTGCAT |
| GAPDH |
AAGCCTGCCGGTGACTAAC |
GTTAAAAGCAGCCCTGGTGAC |
Results
Differential gene analysis at the mRNA
level
Gene expression profiles from the GSE46234 dataset
were downloaded from the GEO database; the significantly altered
genes were screened to determine the DEGs in PDAC tissues. A total
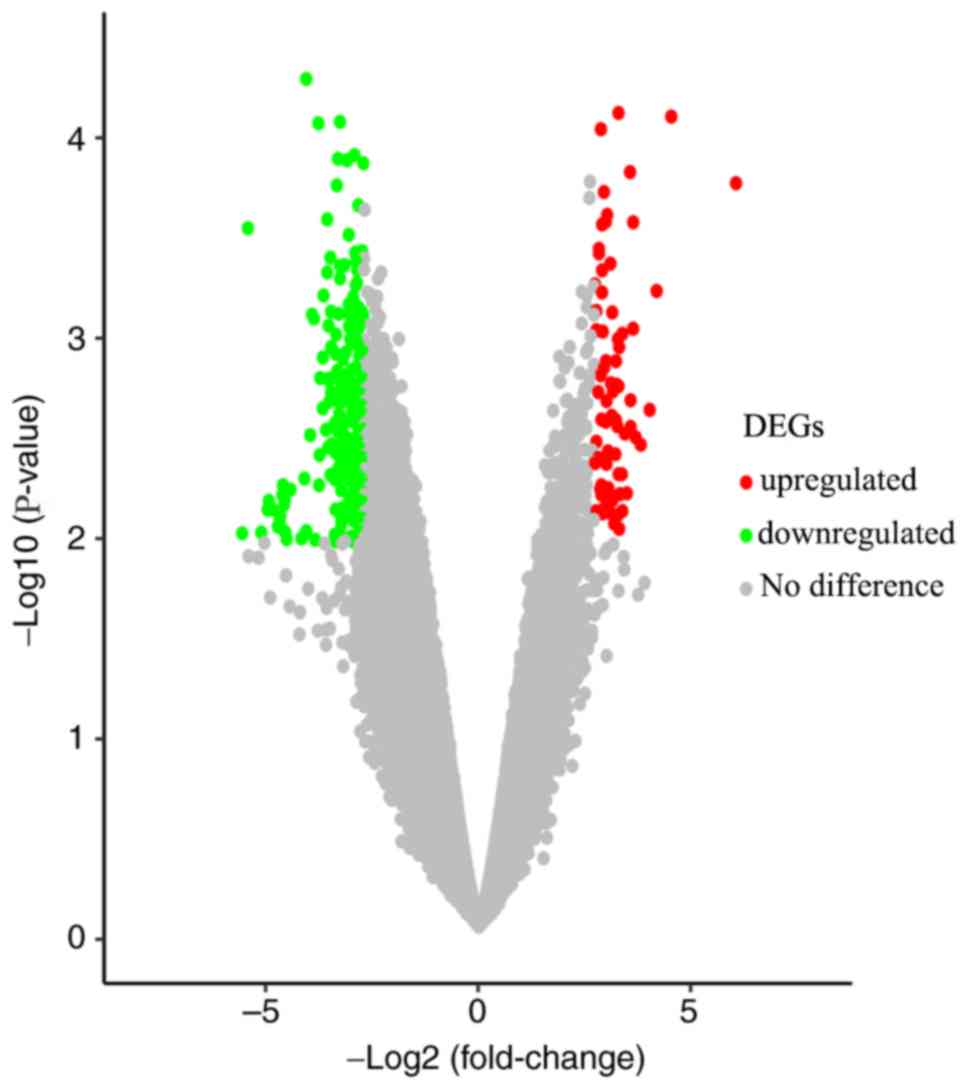
of 217 DEGs were identified via GEO2R analysis, including 65
upregulated genes and 152 downregulated genes (Fig. 1), and the top 10 most upregulated and
downregulated DEGs are presented in Table III. The results were further
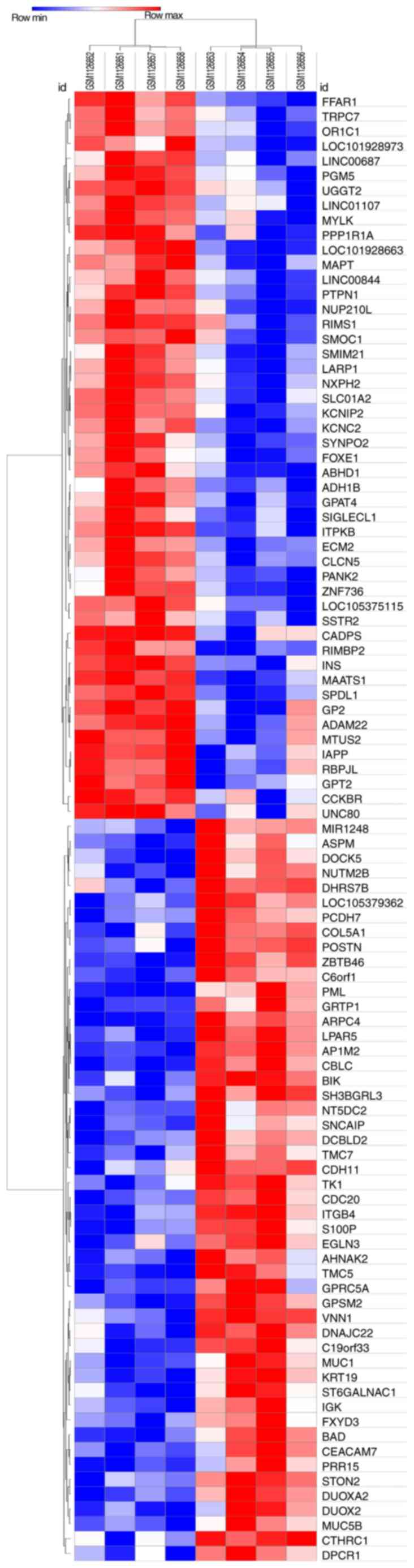
validated using the Morpheus online tool, and the DEGs (including
the top 50 most upregulated genes) are presented in a hierarchical
clustering heat map (Fig. 2).
 | Table III.Top 10 most upregulated and
downregulated differentially expressed genes in the GSE46234
dataset. |
Table III.
Top 10 most upregulated and
downregulated differentially expressed genes in the GSE46234
dataset.
| A, Upregulated |
|---|
|
|---|
| Gene symbol | Fold change | P-value |
|---|
| IAPP | 6.61 |
2.33×10−4,b |
| FFAR1 | 4.94 |
3.93×10−5,b |
| NUP210L | 4.78 |
6.57×10−4,b |
| CADPS | 4.71 |
6.87×10−4,b |
| LOC101928663 | 4.60 |
6.64×10−5,b |
| SMIM21 | 4.54 |
1.41×10−3,a |
| LINC00844 | 4.47 |
1.10×10−3,a |
| INS | 4.34 |
2.10×10−4,b |
| MAPT | 4.34 |
3.99×10−4,b |
| SLCO1A2 | 4.31 |
7.53×10−4,b |
|
| B,
Downregulated |
|
| Gene
symbol | Fold
change | P-value |
|
| S100P | −7.35 |
1.37×10−4,b |
| DUOXA2 | −5.50 |
6.17×10−5,b |
| C19orf33 | −5.08 |
4.99×10−4,b |
| ST6GALNAC1 | −4.89 |
2.06×10−3,a |
| POSTN | −4.41 |
2.19×10−4,b |
| MUC5B | −4.40 |
7.80×10−4,b |
| DPCR1 | −4.33 |
1.84×10−3,a |
| BIK | −4.32 |
1.20×10−4,b |
| TMC7 | −4.09 |
8.32×10−4,b |
| IGK | −4.01 |
9.70×10−4,b |
GO functional enrichment and KEGG
pathway analyses
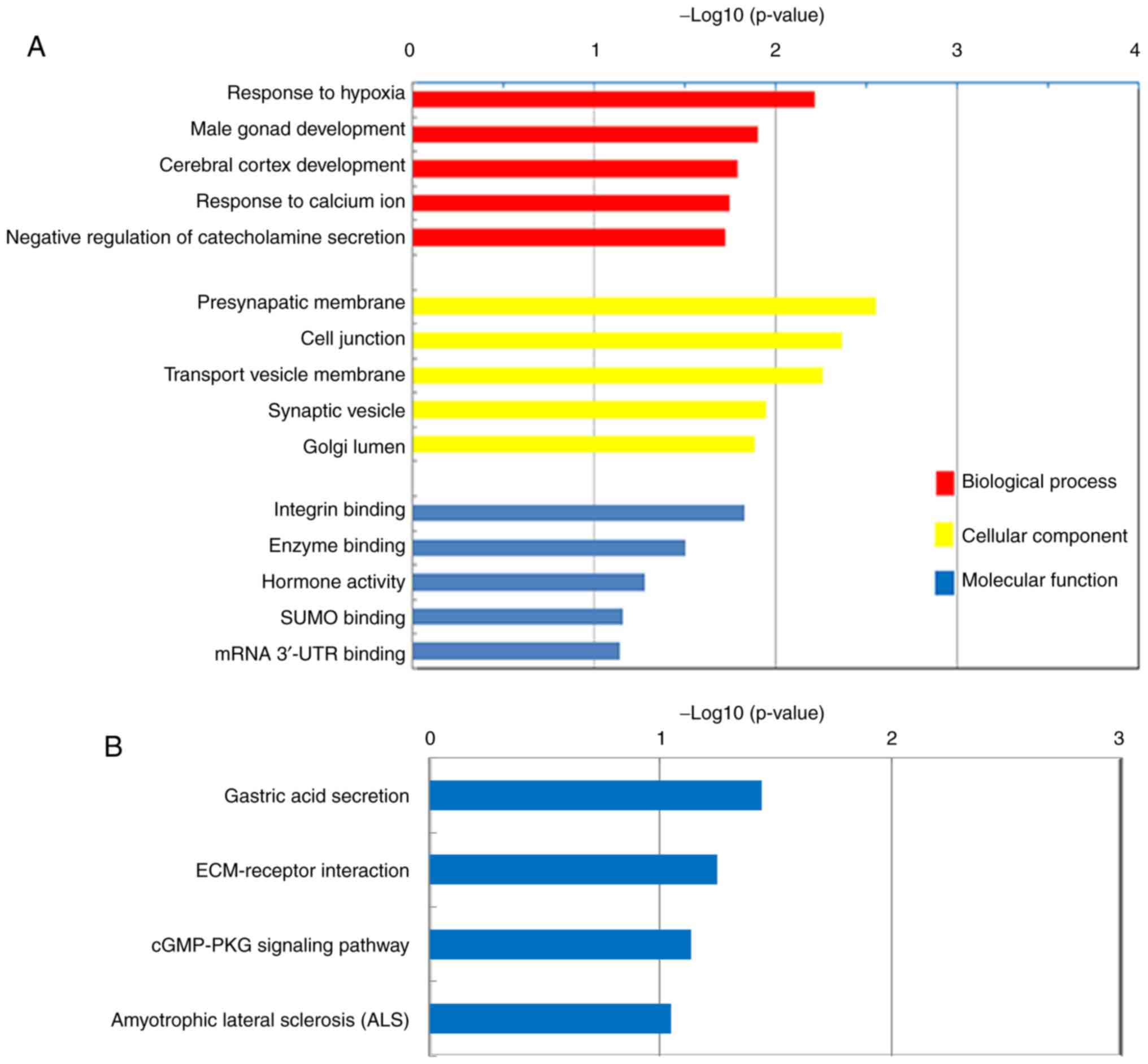
The top five BPs, CCs and MFs of the upregulated
DEGs in PDAC, according to GO analysis, are presented in Fig. 3A. The majority of the DEGs were
demonstrated to be significantly enriched in CCs, including the
‘Presynaptic membrane’, ‘Cell junction’, ‘Transport vesicle
membrane’, ‘Synaptic vesicle’ and ‘Golgi lumen’. These DEGs were
also demonstrated to be associated with BPs, including the
‘Response to hypoxia’, ‘Cerebral cortex development’, ‘Response to
calcium ion’ and the ‘Negative regulation of catecholamine
secretion’. Furthermore, the DEGs were indicated to exert MFs,
including the regulation of ‘Integrin binding’, ‘Enzyme binding’
and ‘Hormone activity’. KEGG pathway analysis demonstrated that the
upregulated DEGs were enriched in ‘Gastric acid secretion’,
‘ECM-receptor interaction’, ‘cGMP-PKG signaling pathway’ and
‘Amyotrophic lateral sclerosis’ (Fig.
3B).
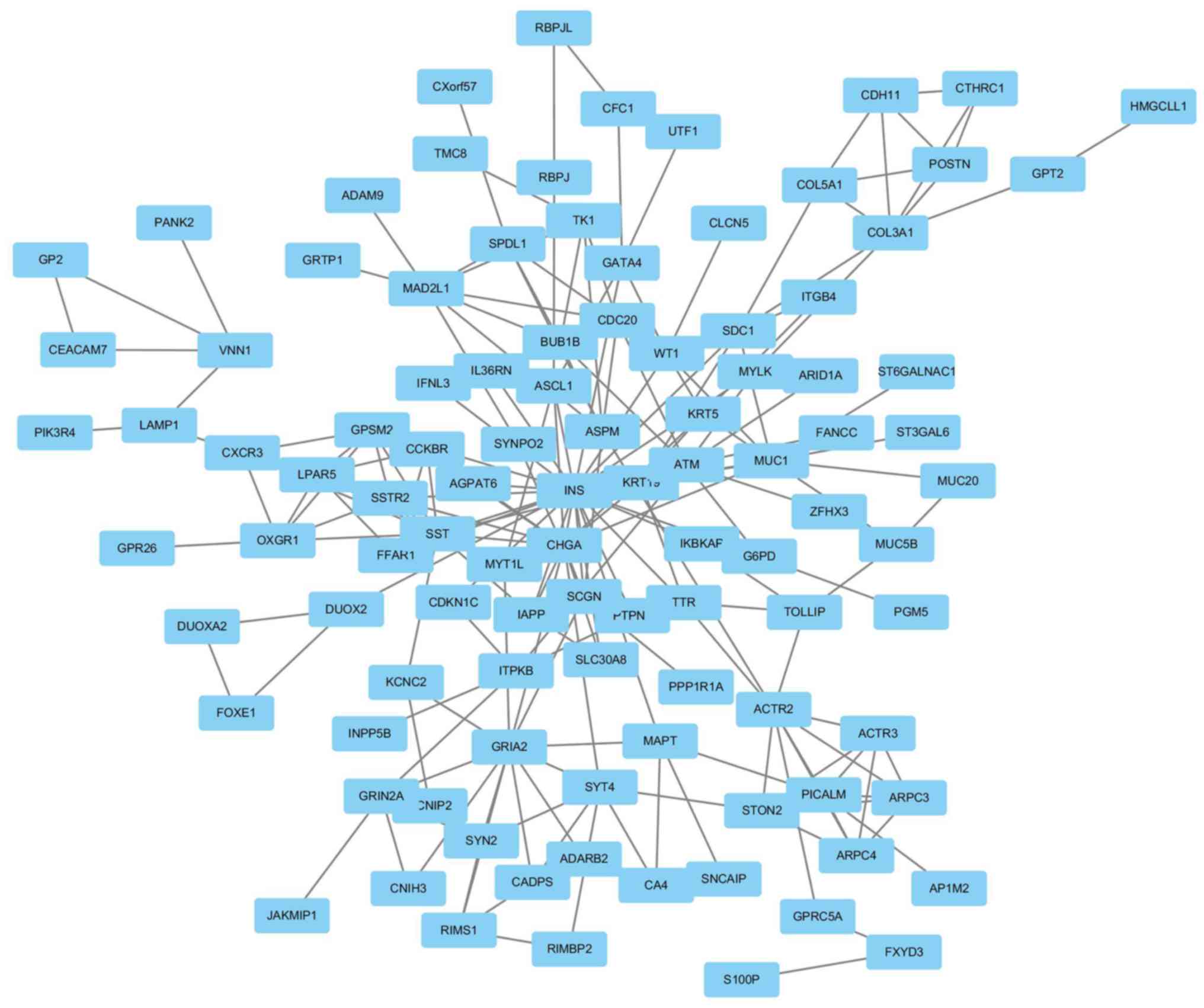
Module screening of the PPI network
and hub gene identification
A PPI network was constructed using Cytoscape
software based on the DEGs identified from the GSE46234 dataset.
Genes with the highest scores were screened and considered hub
genes, which were more likely to be associated with PDAC. The top
18 hub genes included ACTR2, ACTR3, ARPC3, ARPC4, ASPM, BUB1B,
CDC20, CXCR3, GPSM2, LPAR5, MAD2L1, OXGR1, PICALM, SPDL1, SST,
SSTR2, STON2 and TK1 (Fig. 4).
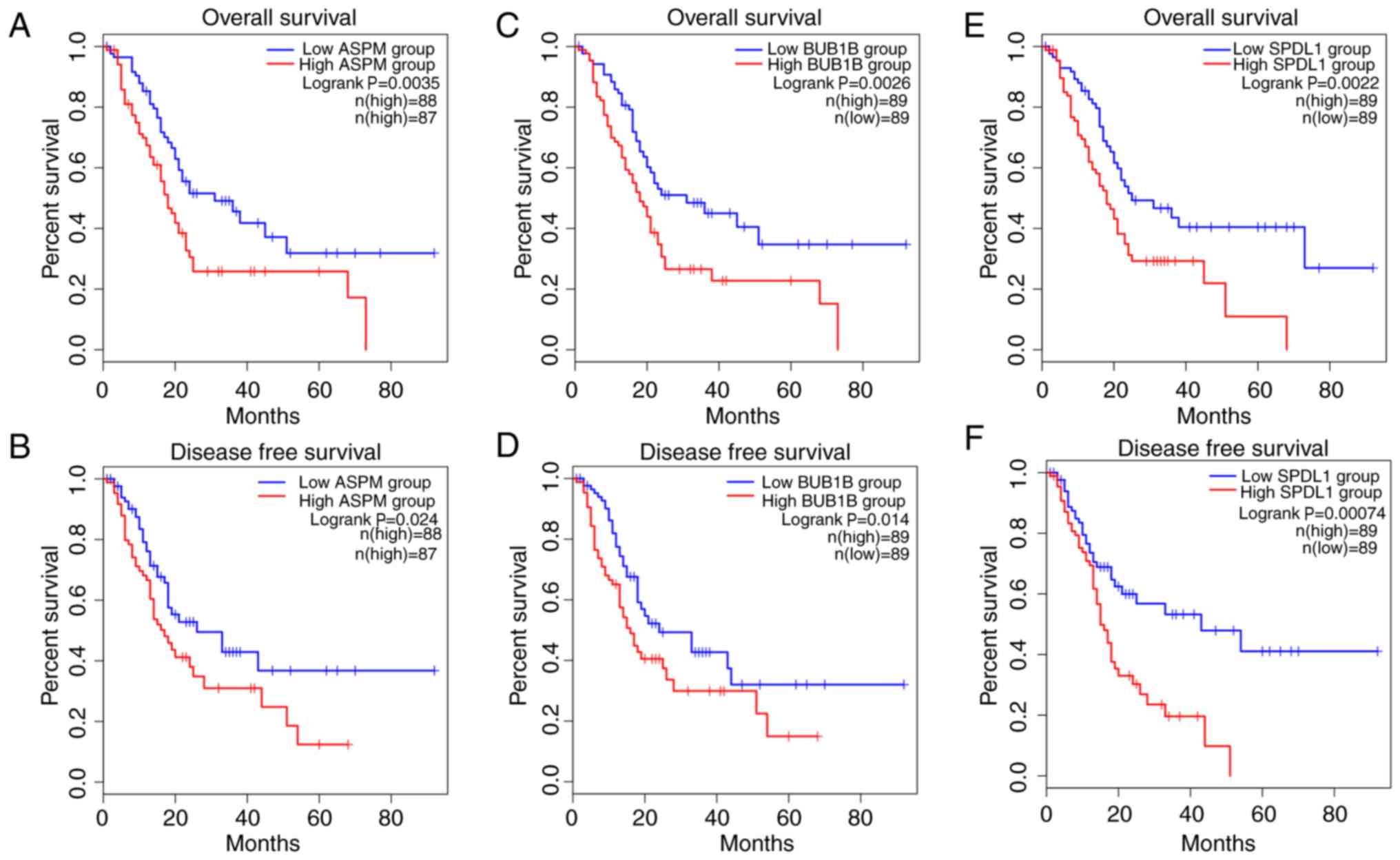
Upregulation of ASPM, BUB1B and SPDL1
predicts poor survival outcome in patients with PDAC
In order to identify candidate prognostic
biomarkers, the top 18 hub genes identified in the PPI network were
analyzed, and only the genes which were associated with both
shorter OS and DFS were regarded as potential biomarkers for PDAC
prognosis. Among the 18 hub genes, ASPM, BUB1B, CDC20, MAD2L1,
SPDL1 and TK1 demonstrated association with prognosis. The results
demonstrated that patients with PDAC with upregulated expression of
ASPM, BUB1B, MAD2L1 and SPDL1 in tumors had significantly worse OS,
while the upregulation of ASPM, BUB1B, CDC20 and SPDL1 was
associated with significantly decreased DFS. Furthermore,
upregulation of only ASPM, BUB1B and SPDL1 in tumors was
significantly associated with both poor OS and DFS in patients with
PDAC (all P<0.05; Fig. 5).
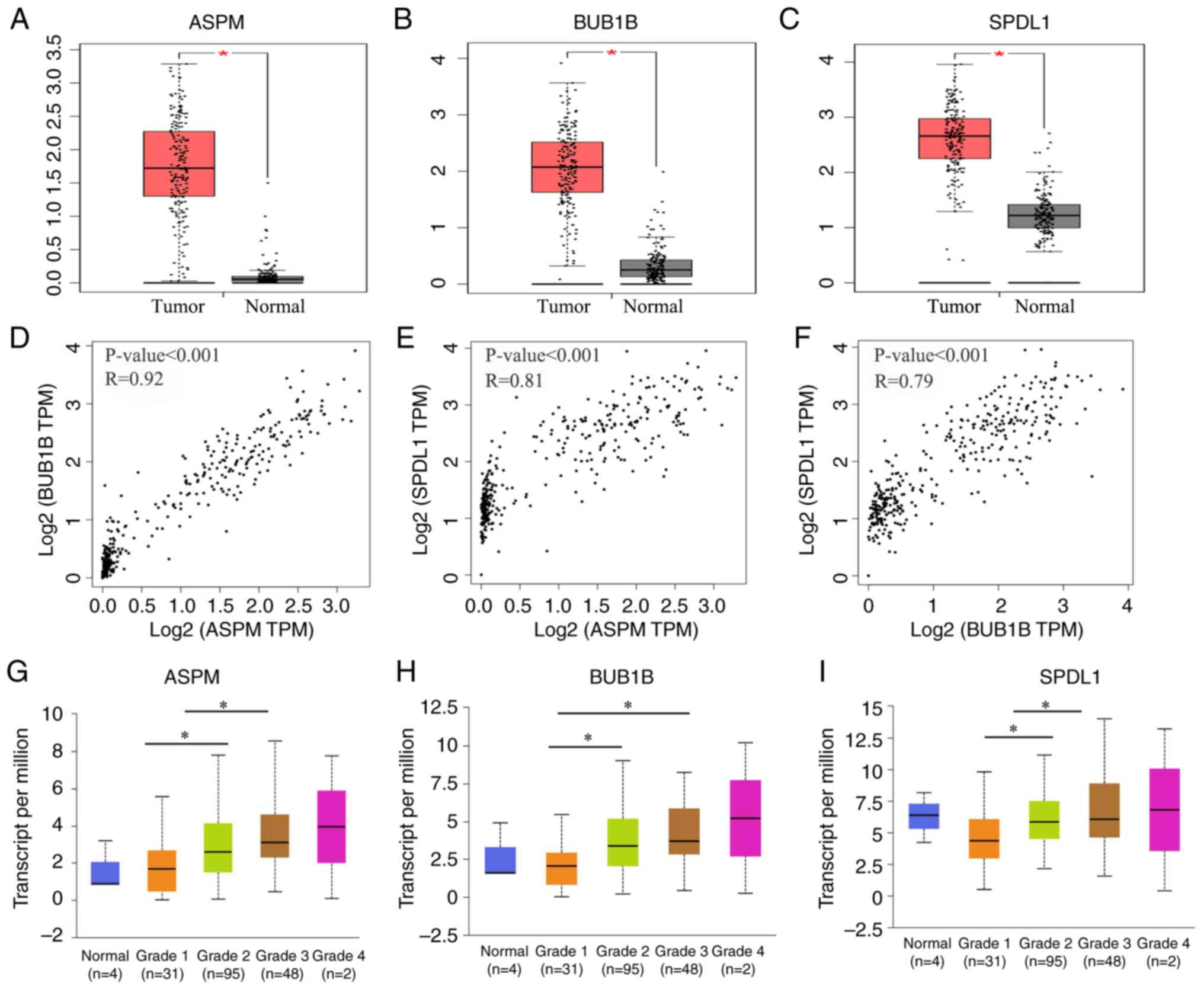
ASPM, BUB1B and SPDL1 were selected for further
analysis. The expression of these three genes was demonstrated to
be significantly higher in tumor tissues compared with normal
adjacent tissues (all P<0.05; Fig.
6A-C). Furthermore, these three genes were also significantly
upregulated in patients with grade G2 and G3 neoplasms compared
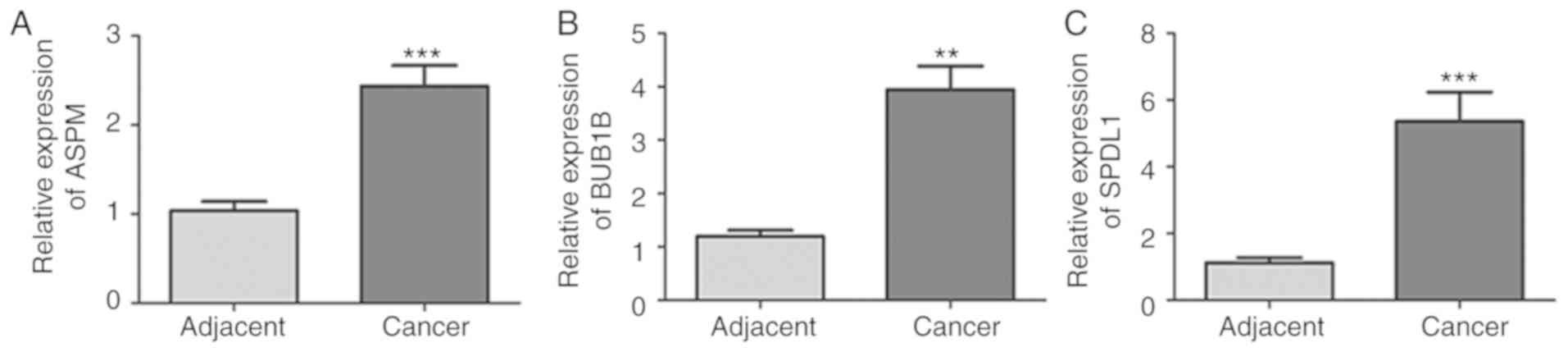
with patients with G1 neoplasms (all P<0.05; Fig. 6G-I. Consistent with the results of
the database analysis, mRNA expression of ASPM, BUB1B and SPDL1 was
significantly higher in PDAC tissues compared with normal adjacent
tissues (all P<0.01; Fig. 7).
Discussion
Compared with all other malignancies, PDAC has the
poorest prognosis, with a 5% 5-year survival rate and a mean life
expectancy of <6 months, which is predominantly due to
resistance to standard therapy (20,21).
Commonly used tumor markers, such as CA19-9 and CEA, are
ineffective for the early detection of PDAC (22). Furthermore, they fail to provide
information on the exact location of the cancer lesions, thus
making it difficult to determine a therapeutic strategy (23,24).
Therefore, identifying prognostic factors for patients with PDAC
remains critical, with the aim to develop novel therapeutic
approaches and select adequate treatment strategies.
In the present study, 217 DEGs were screened,
including 65 upregulated and 152 downregulated genes, in the PDAC
tissue samples of the GSE46234 dataset. GO analysis indicated that
the 65 upregulated genes were significantly enriched in functions
involving the regulation of ‘Integrin binding’, ‘Enzyme binding’
and ‘Hormone activity’. Previous studies have demonstrated that the
interactions between ligands and integrins, such as αvβ6, could
affect the proliferation, invasion, metastasis and angiogenesis of
PDAC cells (5,25,26).
Furthermore, hormone inhibitors, such as angiotensin system
inhibitors were applied to improve the prognosis of PDAC (27). KEGG pathway analysis demonstrated
that the upregulated genes were predominantly involved in
‘ECM-receptor interaction’ and the ‘cGMP-PKG signaling pathway’,
which has been identified as a key signaling pathway in different
types of cancer, including bladder cancer (28). A total of 18 hub genes were
discovered following construction of the PPI network, in which
three key genes (ASPM, BUB1B and SPDL1) were demonstrated to be
enriched in the cell cycle biological processes/pathways, and were
significantly associated with both shorter OS and DFS in patients
with PDAC. The results of the present study were unable to be
compared with the findings of the original study as it is
unavailable. Thus, TCGA cohort analysis was performed; the results
demonstrated high ASPM, BUB1B and SPDL1 expression levels in
patients with PDAC, which was further validated by RT-qPCR
analysis. Furthermore, upregulation of ASPM, BUB1B and SPDL1 was
associated with the histological grade of the neoplasm. This
suggests that high ASPM, BUB1B and SPDL1 expression levels may
contribute to PDAC progression and a worse prognosis, indicating
the prognostic value of ASPM, BUB1B and SPDL1 for patients with
PDAC.
ASPM was originally identified as a
centrosomal protein that regulates neurogenesis and brain size
(29), and was reported to be
extensively expressed in a variety of malignant tissues, such as
gliomas, ovarian and hepatocellular cancer tissues (30–32).
Bikeye et al (33) reported
that ASPM is upregulated in recurrent tumors and demonstrates a
positive association with the pathological grading of gliomas.
Upregulation of ASPM has also been reported to be associated with
the pathological grading and poor survival of patients with ovarian
cancer and hepatocellular carcinoma (31,34). In
the present study, ASPM expression was demonstrated to be higher in
tumor tissues compared with normal adjacent tissues, and was
associated with poor survival of patients with PDAC. These results
validate the findings of previous studies, which suggest that
ASPM may enhance PDAC tumor progression by promoting Wnt
activity to regulate cancer stemness (32–35).
The results of the present study suggest that high
BUB1B expression may play a key role in the tumorigenesis
and progression of PDAC; this is consistent with the findings of
previous bioinformatics analyses, which suggest that BUB1B
demonstrates high connectivity degrees to PDAC, and may be useful
as a therapeutic target (32,36,37).
BUB1B upregulation is also reportedly associated with chromosomal
instability in other malignancies, including bladder cancer, breast
cancer and kidney carcinomas (38–40).
Furthermore, BUB1B upregulation is also associated with the
progression and recurrence of gastric cancer (37). SPDL1 is required for efficient
chromosome congression and mitotic checkpoint regulation, and is
also involved at the spindle checkpoint during mitosis (41). High SPDL1 expression is an
independent prognostic indicator for cancer-specific survival and
has been associated with increased cellular proliferation in oral
cancer, highlighting its potential value in therapy (42). Hence, these data support the
hypothesis that ASPM, BUB1B and SPDL1 may be candidate molecular
markers for PDAC progression and prognosis, and thus may be useful
as therapeutic targets.
In the present study, the DEGs between PDAC and
normal tissue samples in the GSE46234 dataset were determined, and
the hub genes among the DEGs were demonstrated to be associated
with the prognosis of patients with PDAC patients. Furthermore,
ASPM, BUB1B and SPDL1 were identified as potential candidate
biomarkers for OS and DFS in patients with PDAC. High ASPM, BUB1B
and SPDL1 mRNA expression levels were validated by TCGA cohort
analysis and subsequent RT-qPCR analysis, which may preliminarily
uncover the pathophysiological role of these hub genes in PDAC at
the molecular level. However, the sample size for microarray
analysis in the present study was small, thus further molecular and
biological studies with larger sample sizes are required to
validate these findings, and to confirm the functions of the key
genes in PDAC.
Taken together, the results of the present study
demonstrate that the upregulation of ASPM, BUB1B and SPDL1 in tumor
tissues is closely associated with tumor development and poor OS
and DFS in patients PDAC. This indicates their potential prognostic
values and use as therapeutic targets.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science
Foundation of Zhejiang Province, China (grant no. LQ19H200001).
Availability of data and materials
The datasets analyzed in the present study are
available in the GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE46234)
and TCGA (https://cancergenome.nih.gov) databases.
Authors' contributions
XT and NW participated in the study design, data
collection and analysis. XT contributed to the collection of
samples, RT-qPCR analysis and the writing of the article. NW
critically reviewed the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Taizhou Hospital of Zhejiang Province (Taizhou, China)
and written informed consent was obtained from all patients prior
to study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Howlader N, Noone AM, Krapcho M, Miller D,
Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS,
Feuer EJ and Cronin KA: SEER Cancer Statistics Review, 1975–2011.
National Cancer Institute; Bethesda, MD: 2019
|
|
4
|
Pozios I, Knösel T, Zhao Y, Assmann G,
Pozios I, Müller MH, Bruns CJ, Kreis ME and Seeliger H: Expression
of phosphorylated estrogen receptor beta is an independent negative
prognostic factor for pancreatic ductal adenocarcinoma. J Cancer
Res Clin Oncol. 144:1887–1897. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kasuga A, Hamamoto Y, Takeuchi A, Kawasaki
K, Suzuki T, Hirata K, Sukawa Y, Takaishi H and Kanai T: Positive
relationship between subsequent chemotherapy and overall survival
in pancreatic cancer: Meta-analysis of postprogression survival for
first-line chemotherapy. Cancer Chemother Pharmacol. 79:595–602.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karakas Y, Lacin S and Yalcin S: Recent
advances in the management of pancreatic adenocarcinoma. Expert Rev
Anticancer Ther. 18:51–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruckert MT, de Andrade PV, Santos VS and
Silveira VS: Protein tyrosine phosphatases: Promising targets in
pancreatic ductal adenocarcinoma. Cell Mol Life Sci. 76:2571–2592.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Biankin AV, Waddell N, Kassahn KS, Gingras
MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J,
et al: Pancreatic cancer genomes reveal aberrations in axon
guidance pathway genes. Nature. 491:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heiser PW, Cano DA, Landsman L, Kim GE,
Kench JG, Klimstra DS, Taketo MM, Biankin AV and Hebrok M:
Stabilization of beta-catenin induces pancreas tumor formation.
Gastroenterology. 135:1288–1300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Wu X, Wang G, Hu S, Zhang Y and
Zhao S: CALD1, CNN1, and TAGLN identified as potential prognostic
molecular markers of bladder cancer by bioinformatics analysis.
Medicine (Baltimore). 98:e138472019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tell RW and Horvath CM: Bioinformatic
analysis reveals a pattern of STAT3- associated gene expression
specific to basal-like breast cancers in human tumors. Proc Natl
Acad Sci USA. 111:12787–12792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gentleman R and Ihaka R: R: A language and
environment for statistical computing. Computing. 1:12–21.
2011.
|
|
14
|
Saura M, Marquez S, Reventun P,
Olea-Herrero N, Arenas MI, Moreno-Gómez-Toledano R, Gómez-Parrizas
M, Muñóz-Moreno C, González-Santander M, Zaragoza C and Bosch RJ:
Oral administration of bisphenol A induces high blood pressure
through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB
J. 28:4719–4728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allen PJ, Kuk D, Castillo CF, Basturk O,
Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide
V, He J, et al: Multi-institutional validation study of the
American Joint Commission on Cancer (8th Edition) changes for T and
N staging in patients with pancreatic adenocarcinoma. Ann Surg.
265:185–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanemura M, Miyoshi E, Nagano H, Eguchi H,
Matsunami K, Taniyama K, Hatanaka N, Akamatsu H, Mori M and Doki Y:
Cancer immunotherapy for pancreatic cancer utilizing α-gal
epitope/natural anti-Gal antibody reaction. World J Gastroenterol.
21:11396–11410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Zhao X and You S: Identification of
key regulators of pancreatic ductal adenocarcinoma using
bioinformatics analysis of microarray data. Medicine (Baltimore).
98:e140742019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakamoto T, Saito H, Amisaki M, Tokuyasu
N, Honjo S and Fujiwara Y: Combined preoperative
platelet-to-lymphocyte ratio and serum carbohydrate antigen 19-9
level as a prognostic factor in patients with resected pancreatic
cancer. Hepatobiliary Pancreat Dis Int. 18:278–284. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Piao J, Zhu L, Sun J, Li N, Dong B, Yang Y
and Chen L: High expression of CDK1 and BUB1 predicts poor
prognosis of pancreatic ductal adenocarcinoma. Gene. 701:15–22.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin X, Wang M, Wang H, Deng H, He T, Tan
Y, Zhu Z, Wu Z, Hu S and Li Z: Evaluation of neurotensin receptor 1
as a potential imaging target in pancreatic ductal adenocarcinoma.
Amino Acids. 49:1325–1335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda M, Fukushima T, Ogawa K, Kimura H,
Ono M, Yamaguchi T, Ikehara Y and Saji H: Synthesis and evaluation
of a radioiodinated peptide probe targeting αvβ6 integrin for the
detection of pancreatic ductal adenocarcinoma. Biochem Biophys Res
Commun. 445:661–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Principe M, Borgoni S, Cascione M,
Chattaragada MS, Ferri-Borgogno S, Capello M, Bulfamante S,
Chapelle J, Di Modugno F, Defilippi P, et al: Alpha-enolase (ENO1)
controls alpha v/beta 3 integrin expression and regulates
pancreatic cancer adhesion, invasion, and metastasis. J Hematol
Oncol. 10:162017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Naxerova K, Pinter M, Incio J, Lee
H, Shigeta K, Ho WW, Crain JA, Jacobson A, Michelakos T, et al: Use
of angiotensin system inhibitors is associated with immune
activation and longer survival in nonmetastatic pancreatic ductal
adenocarcinoma. Clinical Cancer Res. 23:5959–5969. 2017. View Article : Google Scholar
|
|
28
|
Tang F, He Z, Lei H, Chen Y, Lu Z, Zeng G
and Wang H: Identification of differentially expressed genes and
biological pathways in bladder cancer. Mol Med Rep. 17:6425–6434.
2018.PubMed/NCBI
|
|
29
|
Kouprina N, Pavlicek A, Collins NK, Nakano
M, Noskov VN, Ohzeki J, Mochida GH, Risinger JI, Goldsmith P,
Gunsior M, et al: The microcephaly ASPM gene is expressed in
proliferating tissues and encodes for a mitotic spindle protein.
Hum Mol Genet. 14:2155–2165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raman P, Maddipati R, Lim KH and Tozeren
A: Pancreatic cancer survival analysis defines a signature that
predicts outcome. PLoS One. 13:e02017512018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brüning-Richardson A, Bond J, Alsiary R,
Richardson J, Cairns DA, McCormack L, Hutson R, Burns P, Wilkinson
N, Hall GD, et al: ASPM and microcephalin expression in epithelial
ovarian cancer correlates with tumor grade and survival. Br J
Cancer. 104:1602–1610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang WY, Hsu CC, Wang TY, Li CR, Hou YC,
Chu JM, Lee CT, Liu MS, Su JJ, Jian KY, et al: A gene expression
signature of epithelial tubulogenesis and a role for ASPM in
pancreatic tumor progression. Gastroenterology. 145:1110–1120.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bikeye SN, Colin C, Marie Y, Vampouile R,
Ravassard P, Rousseau A, Boisselier B, Idbaih A, Calvo CF, Leuraud
P, et al: ASPM-associated stem cell proliferation is involved in
malignant progression of gliomas and constitutes an attractive
therapeutic target. Cancer Cell Int. 10:12010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin SY, Pan HW, Liu SH, Jeng YM, Hu FC,
Peng SY, Lai PL and Hsu HC: ASPM is a novel marker for vascular
invasion, early recurrence, and poor prognosis of hepatocellular
carcinoma. Clin Cancer Res. 14:4814–4820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu M, Ting DT, Stott SL, Wittner BS,
Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, et
al: RNA sequencing of pancreatic circulating tumor cells implicates
WNT signaling in metastasis. Nature. 487:510–513. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Long J, Zhang Z, Liu Z, Xu Y and Ge C:
Identification of genes and pathways associated with pancreatic
ductal adenocarcinoma by bioinformatics analyses. Oncol Lett.
11:1391–1397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong S, Huang F, Zhang H and Chen Q:
Overexpression of BUB1B, CCNA2, CDC20, and CDK1 in tumor tissues
predicts poor survival in pancreatic ductal adenocarcinoma. Biosci
Rep. 39:2019. View Article : Google Scholar
|
|
38
|
Pinto M, Vieira J, Ribeiro FR, Soares MJ,
Henrique R, Oliveira J, Jerónimo C and Teixeira MR: Overexpression
of the mitotic checkpoint genes BUB1 and BUBR1 is associated with
genomic complexity in clear cell kidney carcinomas. Cell Oncol.
30:389–395. 2008.PubMed/NCBI
|
|
39
|
Scintu M, Vitale R, Prencipe M, Gallo AP,
Bonghi L, Valori VM, Maiello E, Rinaldi M, Signori E, Rabitti C, et
al: Genomic instability and increased expression of BUB1B and
MAD2L1 genes in ductal breast carcinoma. Cancer Lett. 254:298–307.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yamamoto Y, Matsuyama H, Chochi Y, Okuda
M, Kawauchi S, Inoue R, Furuya T, Oga A, Naito K and Sasaki K:
Overexpression of BUBR1 is associated with chromosomal instability
in bladder cancer. Cancer Genet Cytogenet. 174:42–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barisic M, Sohm B, Mikolcevic P, Wandke C,
Rauch V, Ringer T, Hess M, Bonn G and Geley S: Spindly/CCDC99 is
required for efficient chromosome congression and mitotic
checkpoint regulation. Mol Biol Cell. 21:1968–1981. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silva PMA, Delgado ML, Ribeiro N, Florindo
C, Tavares ÁA, Ribeiro D, Lopes C, do Amaral B, Bousbaa H and
Monteiro LS: Spindly and Bub3 expression in oral cancer: Prognostic
and therapeutic implications. Oral Dis. 25:1291–1301. 2019.
View Article : Google Scholar : PubMed/NCBI
|