Introduction
Globally in 2018, >90% of oral cancer patients
suffer from squamous cell carcinoma (SCC), a malignancy potentially
associated with lymph node metastasis (1). SCC starts with variation in mucosal
epithelial cells and results in cells with the essential
capabilities for malignant growth, loss of cell cycle control and
spread (2). Furthermore, the
epithelial dysplasia that occurs in SCC reduces the adhesion
between cells, which facilitates separation of cells from the tumor
and allows metastasis to other organs (3). The 5-year survival rate of SCC is ~50%
despite the availability of numerous treatment options, including
radiotherapy, chemotherapy and surgical excision (4). A new promising minimally invasive
treatment method for patients with SCC is needed due to the damage
to the human body, the unreliable efficacy and the side effects of
traditional clinical treatments (5).
Sonodynamic therapy (SDT) is an alternative approach
for treating patients with SCC that utilizes the synergistic
effects of low-intensity ultrasound and sonosensitizers to kill
cancer cells, resist bacteria and inhibit atherosclerotic plaque
progression (6–8). Hematoporphyrin monomethyl ether (HMME)
is an effective sonosensitizer in SDT with a stable structure,
lower dark toxicity and higher singlet oxygen yield to induce cell
apoptosis via the mitochondrial apoptotic pathway (9). Calcium ion (Ca2+) is a
secondary messenger which can regulate apoptosis and a sudden
increase of intracellular Ca2+ can induce oxidative
stress inside the cells (10).
During the process of SDT, the sonosensitizer is activated
releasing reactive oxygen species (ROS) that are the principle
mediators of cell apoptosis (11,12).
However, uncontrolled ROS activity in cells may induce the release
of ROS by adjacent mitochondria initiating a positive feedback loop
resulting in excessive ROS production leading to mitochondrial
injury (13).
Studies have also demonstrated that excessive ROS
levels generated during SDT stimulate apoptotic signaling pathways,
which include the proteins caspase-3, caspase-9 and Bax (14). During SDT, cell membrane integrity is
destroyed together with the loss of the mitochondrial membrane
potential (MMP) (15,16). This MMP loss leads to mitochondrial
membrane permeabilization (14) and
the release of cytochrome c, which activates caspase-9,
followed by caspase-3 and caspase-7 (17). Some studies have demonstrated that
following protoporphyrin IX mediated SDT, human tongue squamous
carcinoma SAS cells are arrested at the G2/M phase of
the cell cycle and upregulate p53, which can activate the Fas
apoptosis pathway eventually leading to cell death (18,19).
The present study investigated the effect of
HMME-mediated SDT on A-253 cells The findings of the present study
may facilitate the quest for a promising alternative approach for
treating patients with SCC.
Materials and methods
Cell culture
The A-253 cell line is derived from a human
submandibular gland epidermoid carcinoma (20). Human SCC A-253 cells were purchased
from the American Type Culture Collection and cultured in modified
McCoy's 5a medium (American Type Culture Collection) supplemented
with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA.) in an
incubator containing 5% CO2 at 37°C.
Treatment of cells with HMME
The A-253 cells were incubated in 96-well plates
with different concentrations of HMME (0–40 µg/ml at 5 µg/ml
intervals) for 90 min in the dark at 37°C. The sterile HMME
solution was supplied by Shanghai Xianhui Pharmaceutical Co.,
Ltd.
MTT assay
To investigate the cytotoxicity of HMME, cell
viability was measured using the MTT assay. Cells at the
exponential growth phase were used in each experiment. Overall, 10
µl MTT (5 mg/ml) was added to each well and incubated at 37°C
incubator for 4 h. After removal of the MTT, 1 ml DMSO was added to
dissolve the violet formazan crystals. Absorbance at 570 nm was
measured using the microplate reader Bio-Tek ELX800 (Biotek
Instruments, Inc.).
SDT treatment in vitro
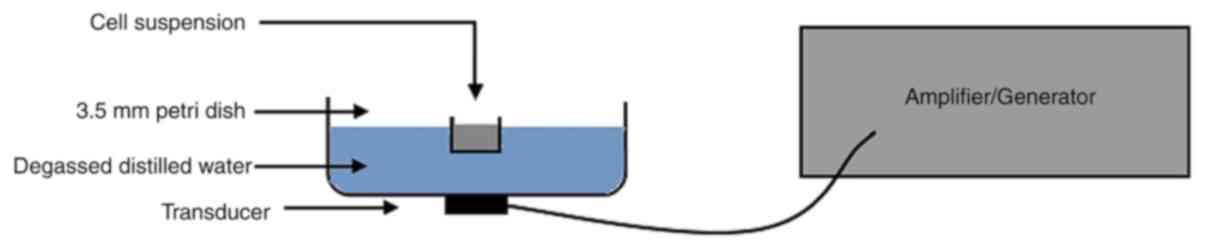
Cell suspension was added to a 3.5-mm petri dish and
later positioned in the tank of degassed distilled water ensuring
that the cells were 3 cm away from the ultrasound transducer
(Fig. 1). The ultrasonic generator,
amplifier and transducer utilized in this experiment were supplied
by the Harbin Institute of Technology. The ultrasound resonance
frequency was 1 MHz, with 30% duty factor and 100 Hz repetition
rate, and the ultrasound intensity was 1.5 W/cm2 as
measured by a needle hydrophone (HNC-1000; Onda Corp) inside the
well. The temperature change in the medium should not be >1°C
during the experiment. In the present study, MTT results
demonstrated that 10 µg/ml HMME was the optimal concentration to
use for in vitro SDT treatment. HMME (10 µg/ml) was then
applied in combination with different ultrasonic durations (0, 1,
3, 5, 10 and 15 min) to investigate the survival rate of A-253
cells.
Cell apoptosis and necrosis
analysis
To investigate cell apoptosis and necrosis following
SDT, the Annexin V-FITC apoptosis kit (Merck KGaA) was used for
flow cytometry analysis according to the manufacturer's
instructions. Subsequently, cells in McCoy's 5a medium were
randomly divided into 4 groups: The control group (PBS), the
ultrasound group (1 MHz; 1.5 W/cm2), the HMME group (10
µg/ml) and the SDT group (1 MHz; 1.5 W/cm2 ultrasound
combined with 10 µg/ml HMME). After incubation with HMME or PBS for
90 min at 37°C, both the ultrasound group and the SDT group were
exposed to ultrasound for 1 min at room temperature. After 3 h of
treatment, 1×106/ml cell per well were then incubated
with 5 µl Annexin V and 5 µl PI (Sigma-Aldrich; Merck KGaA) for 10
min at room temperature in the dark. Samples were detected using a
FACSCalibur flow cytometer (BD Biosciences). FlowJo version 10
(Tree Star, Inc.) was used to analyze data.
Cell apoptosis and necrosis were also investigated
using Hoechst 33258 and PI staining (Merck KGaA) followed by
fluorescence microscopy. Firstly, the treated samples were washed
with PBS 3 times and then stained according to the manufacturer's
protocols. In brief, samples were incubated with 10 µg/ml PI for 10
min at 37°C in the dark and stained with 5 µg/ml Hoechst 33258
(Merck KGaA)for 5 min. Following incubation, the samples were
washed with PBS twice and analyzed using the fluorescence
microscope (Zeiss GmbH) at the excitation wavelength of 330–385 nm
and the emission wavelength of 420–480 nm at ×200
magnification.
Intracellular ROS and Ca2+
measurements
The A-253 cells were incubated with 20 µΜ
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Merck KGaA) and 10 µM
fluo-3/acetoxymethylester (Μerck KGaA) for 30 min at 37°C to
measure the fluorescence of intracellular ROS and Ca2+,
respectively. Samples were then washed 3 times with PBS and
immediately surveyed using the fluorescence microscope. A total of
1×106 cells were collected and measured using a
fluorospectrophotometer (Varian Australia Pty, Ltd.) at 488 nm
excitation and 525 nm emission wavelengths.
Statistical analysis
All experiments were repeated three times
independently and all values were expressed as the mean ± standard
deviation. Differences between multiple groups were analyzed using
one-way ANOVA, followed by Tukey's post-hoc test. Statistical
analyses were performed using SPSS version 25.0 (IBM, Corp).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Increasing HMME concentration
decreases A-253 cell survival rates
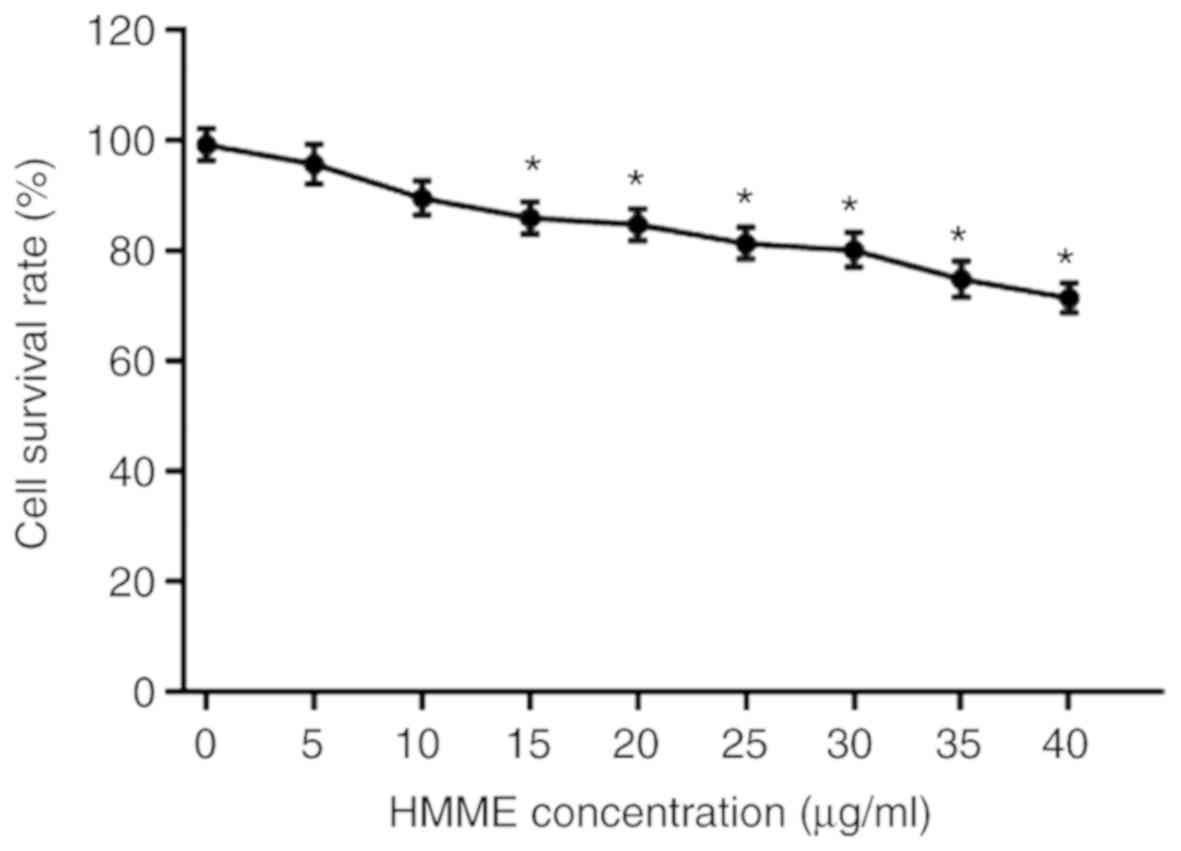
Cytotoxicity of HMME was measured using the MTT
assay. Fig. 2 represents the A-253
cell survival rates at different HMME concentrations. With
increasing HMME concentration, the cell survival rate decreased.
The lowest cell survival rate was 71% when 40 µg/ml HMME was used.
However, HMME concentrations of 5 and 10 µg/ml did not have
significant effects on cell survival rates.
Increased ultrasound exposure time
during SDT decreases A-253 cell survival rates
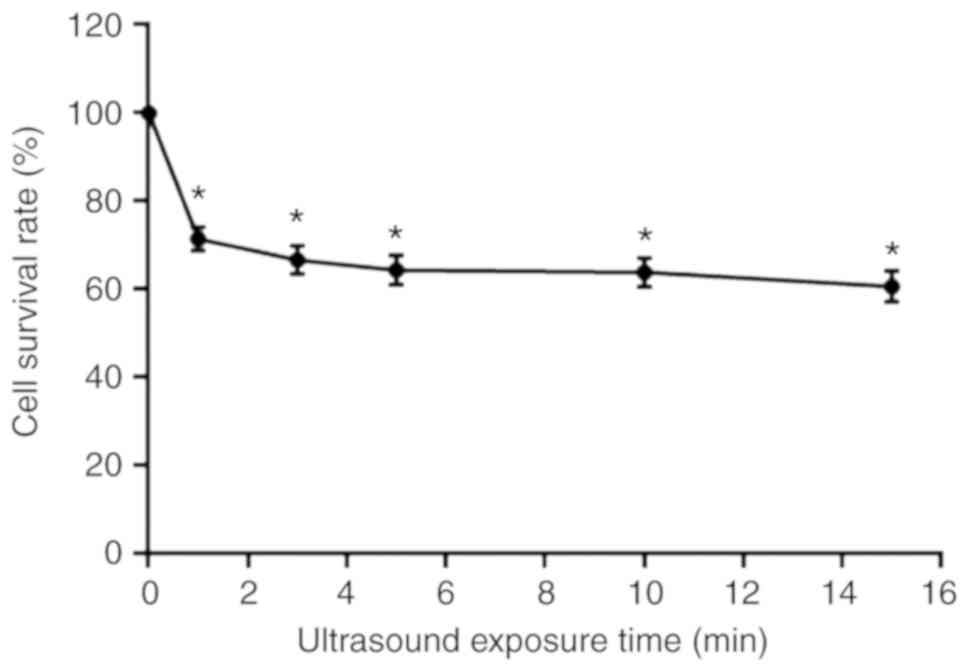
Cell survival rates after SDT treatment was also
detected by MTT assay. As represented by Fig. 3, the survival rates of A-253 cells
following SDT combined with HMME treatment depended on the duration
of ultrasound exposure. When the ultrasound exposure time was
increased from 1 to 15 min, the survival rate was significantly
decreased from 73 to 62% (Fig.
3).
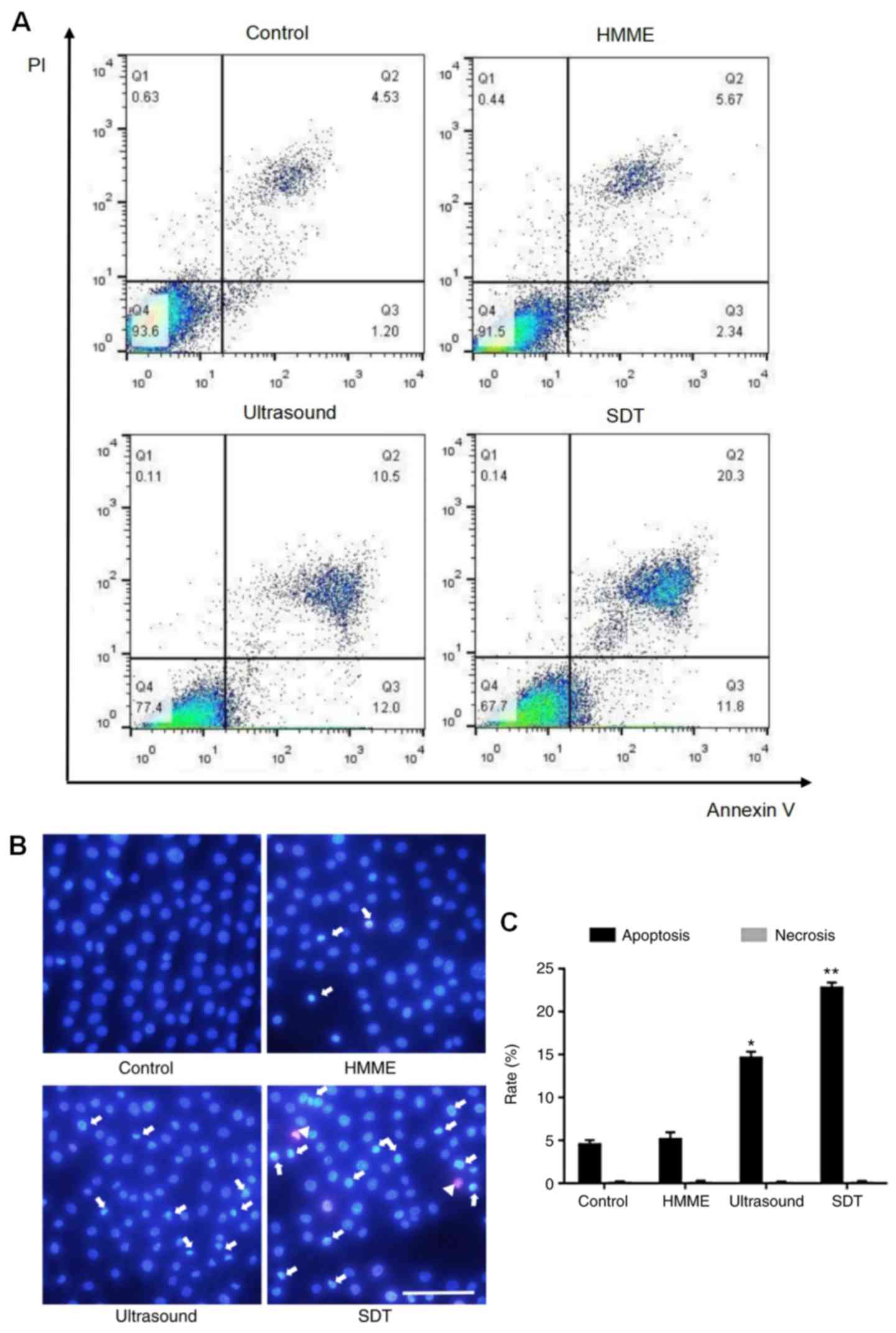
Apoptosis is induced by SDT
Flow cytometry results are represented in Fig. 4A. Cells in the right lower quadrant
(Annexin V+/PI−) represent early apoptotic
cells, those in the right upper quadrant(Annexin
V+/PI+) represent late apoptotic cells. The
apoptosis rates of A-253 cells were as follows: SDT treatment
(32.10%), HMME (8.01%) and ultrasound (22.50%) compared with the
control group (5.73%) (Fig. 4A). In
the double staining with Hoechst 33258 and PI, normal cells
displayed regular blue fluorescence, apoptotic cells bright blue
fluorescence and necrotic cells pink fluorescence (Fig. 4B). The percentages of apoptotic cells
were increased to 14.6% in the ultrasound group (P<0.05) and
22.8% in the SDT group (P<0.01), compared with those in the
control group (Fig. 4C). However,
the apoptotic rate in the HMME group demonstrated no significant
difference compared with the control group. According to the
results of fluorescence quantification, very few necrotic cells
were identified in all groups (Fig.
4C). Taken together, these results demonstrate that the rate of
apoptosis in the A-253 cell line was increased by SDT.
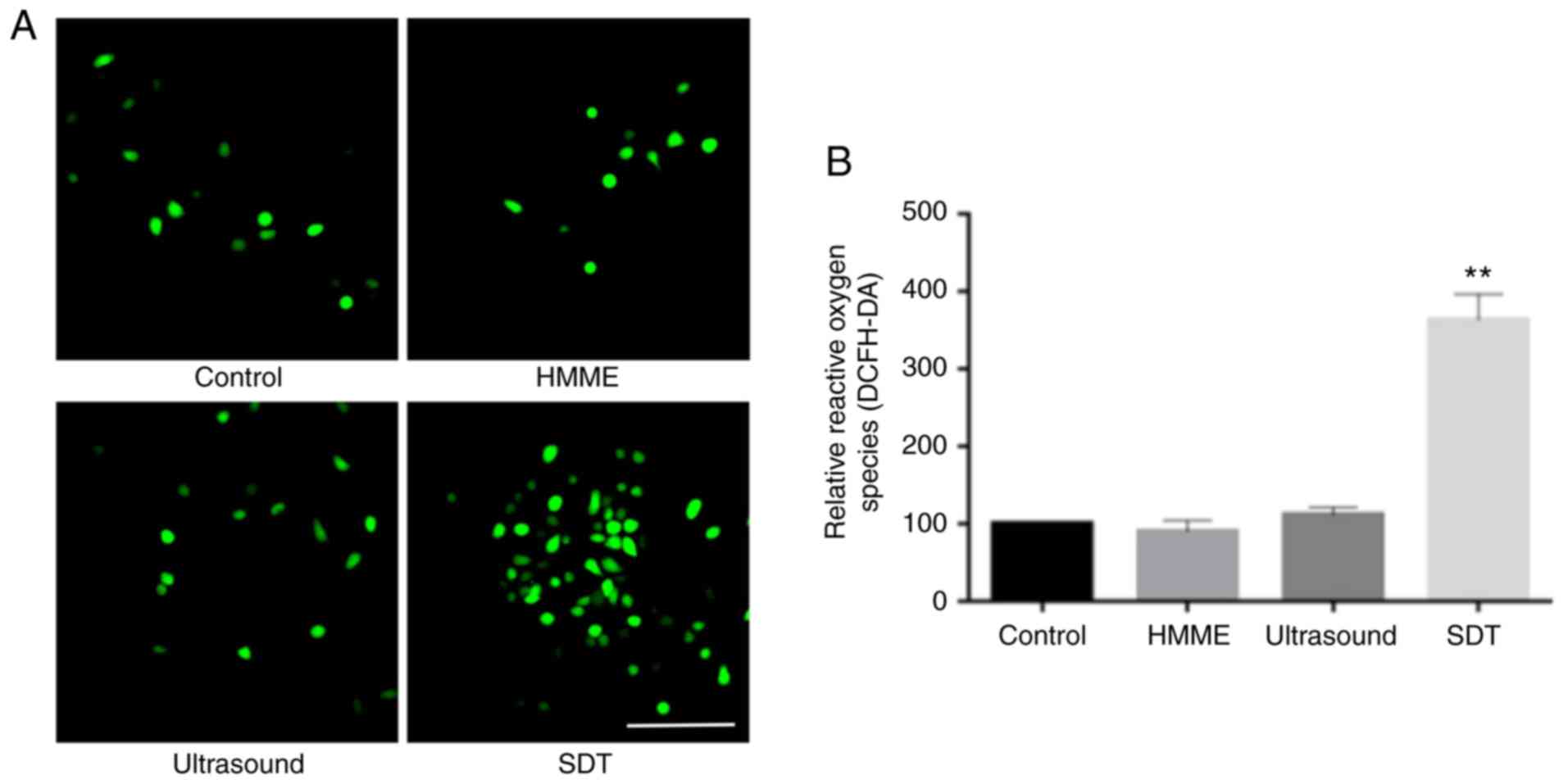
ROS generation increases with SDT
DCFH-DA fluorescence was observed mainly in the SDT
group compared with the other 3 groups (Fig. 5A). The ROS level in the SDT group was
significantly increased (363.1%; P<0.01), while showing no
significant difference in the ultrasound (113.6%; P>0.05) and
the HMME (92.5%; P>0.05) groups compared with that in the
control group (Fig. 5B).
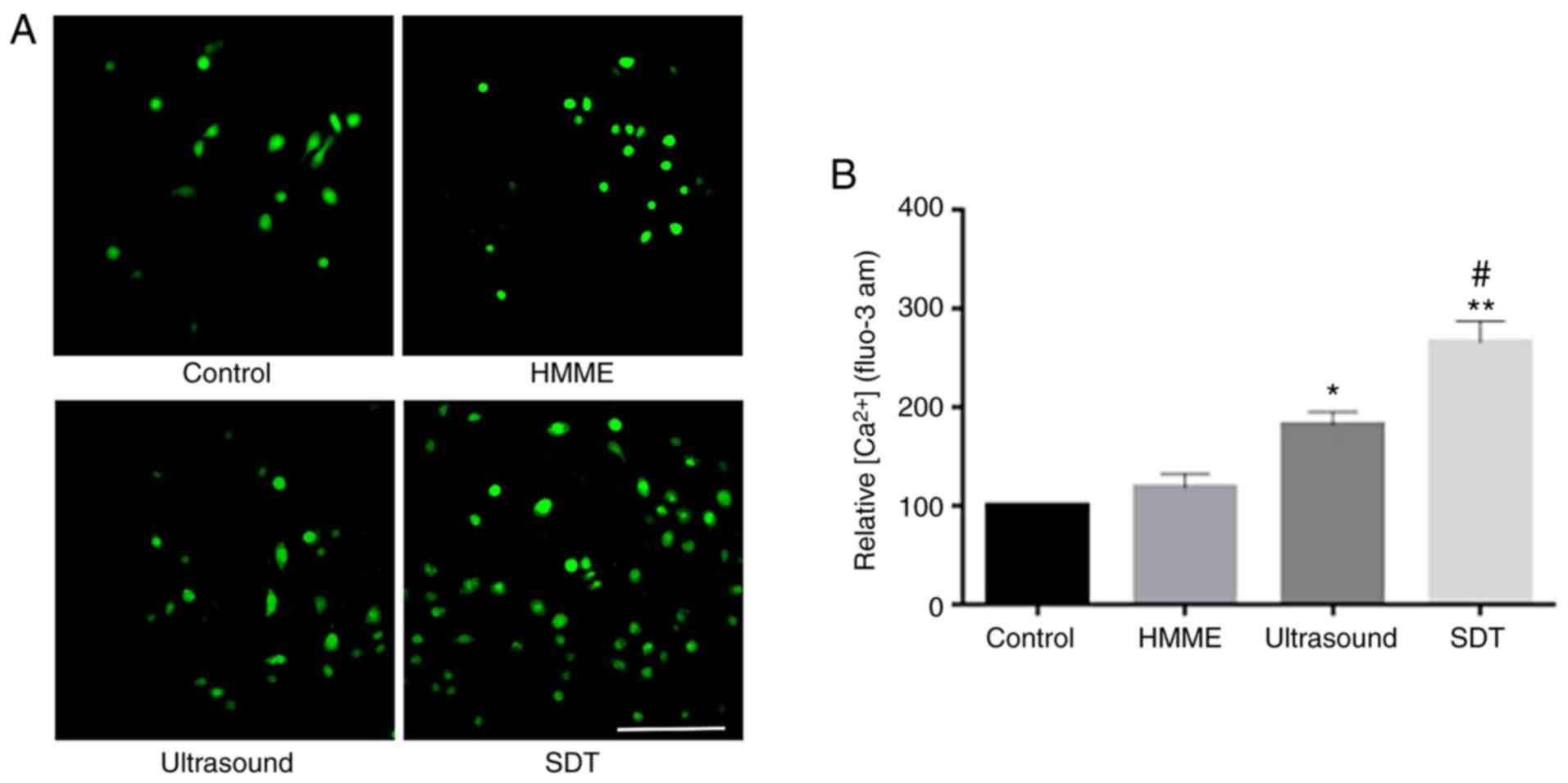
Calcium overload in A-253 cells
treated using SDT and ultrasound
Calcium fluorescence intensity was observed more in
the SDT group and to a lower extent in the ultrasound group
compared with that in the control group (Fig. 6A). Furthermore, Ca2+
levels were increased to 181.2% (P<0.05) and 268.7% (P<0.01)
in the ultrasound and SDT groups, respectively, compared with those
in the control group (Fig. 6B).
Together these results demonstrate that Ca2+ levels were
significantly increased in A-253 cells following SDT and
ultrasound.
Discussion
HMME, a second-generation sonosensitizer, has been
extensively utilized in SDT treatment due to its low toxicity and
high selectivity by highly metabolic tissues (21). The optimal concentration of HMME used
in the present study is consistent with the results of a previous
study that reported the highest cytotoxicity of SDT to U937 cells
in the presence of 10 µg/ml HMME (6). Hao et al (10) also reported the lowest viability of
C6 cells in the presence of 1 MHz ultrasound combined with 10 µg/ml
HMME.
It is well documented that apoptosis is the major
form of death in numerous types of cancer cells in response to SDT
(22,23), which is in accordance with the
results of the current study. In the present study, the apoptotic
rate in the SDT group was 32.10% (P<0.05), while the rates in
the HMME and the ultrasound treatment groups were 8.01 and 22.50%,
respectively. In addition, the Hoechst 33258 and PI assays
confirmed the results of the flow cytometry indicating that the
numbers of apoptotic cells were increased in the SDT group compared
with those in other groups. During the SDT process, the
sonosensitizer is activated and ROS is released; the imbalance
between ROS release and elimination may induce further ROS release
by the mitochondria (13). This
positive feedback results in excess ROS production resulting in
mitochondrial injury and apoptosis (24). The ROS level was significantly
increased in the SDT group but not in the ultrasound and HMME
groups, compared with that in the control group. The findings of
the present study indicated that HMME-SDT enhances ROS release and
affects cellular conditions of A-253 cells. Notably apoptosis
fluorescence was also observed in the ultrasound group confirming
previous findings (25). It is well
known that ultrasound alone can exert acoustic streaming and
cavitation, thereby inducing various biological effects such as
exerting shear stresses on the cell membrane, pore formation and
endocytosis, leading to induction of cell apoptosis (26).
Ca2+ serves a key role as a second
messenger in cellular transmission (27). Intracellular Ca2+ overload
may induce cell apoptosis or death (10). As a result, high intracellular
Ca2+ levels can be regarded as a signal of early
apoptosis (28). The findings of the
present study demonstrated that the Ca2+ levels were
increased in the ultrasound and SDT groups compared with those in
the control group. During the process of SDT, cavitation can also
occur. When the cell membrane is broken, molecules such as
Ca2+ can enter the cell by passive diffusion (29). ROS overload may regulate ion
channels, including the Ca2+ channel, which also induces
Ca2+ influx (30). These
findings may explain the phenomenon of Ca2+ overload in
both the SDT and ultrasound groups in the current study.
In conclusion, HMME-SDT significantly induces
apoptosis, leading to ROS generation and Ca2+ overload
in A-253 cells. HMME-SDT may be a promising alternative approach in
patients with SCC.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Natural
Science Foundation, Heilongjiang Province of China (no. H2017022)
and the Natural Science Foundation of China (no. 81670994).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and DZ conceived, designed and performed the
experiments. ZH, WC and LB analyzed the data. YZ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HMME
|
hematoporphyrin monomethyl ether
|
|
SDT
|
sonodynamic therapy
|
|
ROS
|
reactive oxygen species
|
|
SCC
|
squamous cell carcinoma
|
|
MMP
|
mitochondrial membrane potential
|
|
DCFH-DA
|
2′,7′-dichlorofluorescein
diacetate
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pérez-Sayáns M, Suárez-Peñaranda JM,
Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM and García-García
A: The role of p21Waf1/CIP1 as a Cip/Kip type cell-cycle regulator
in oral squamous cell carcinoma (Review). Med Oral Patol Oral Cir
Bucal. 18:e219–e225. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Somasundaram RT, Kaur J, Leong I,
MacMillan C, Witterick IJ, Walfish PG and Ralhan R: Subcellular
differential expression of Ep-ICD in oral dysplasia and cancer is
associated with disease progression and prognosis. BMC Cancer.
16:4862016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taghavi N and Yazdi I: Prognostic factors
of survival rate in oral squamous cell carcinoma: Clinical,
histologic, genetic and molecular concepts. Arch Iran Med.
18:314–319. 2015.PubMed/NCBI
|
|
5
|
Kerker FA, Adler W, Brunner K, Moest T,
Wurm MC, Nkenke E, Neukam FW and von Wilmowsky C: Anatomical
locations in the oral cavity where surgical resections of oral
squamous cell carcinomas are associated with a close or positive
margin-a retrospective study. Clin Oral Investig. 22:1625–1630.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su X, Wang P, Wang X, Cao B, Li L and Liu
Q: Apoptosis of U937 cells induced by hematoporphyrin monomethyl
ether-mediated sonodynamic action. Cancer Biother Radiopharm.
28:207–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhuang D, Hou C, Bi L, Han J, Hao Y, Cao W
and Zhou Q: Sonodynamic effects of hematoporphyrin monomethyl ether
on staphylococcus aureus in vitro. FEMS Microbiol Lett.
361:174–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Y, Liu Y, Chen X, Gong J, Huang Z,
Wang W, Shi Y, Wang Y, Yao J, Shen Z, et al: 5-aminolevulinic
acid-mediated sonodynamic therapy alleviates atherosclerosis via
enhancing efferocytosis and facilitating a shift in the Th1/Th2
balance toward Th2 polarization. Cell Physiol Biochem. 47:83–96.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilmin K, Kujawska T, Secomski W, Nowicki
A and Grieb P: 5-Aminolevulinic acid-mediated sonosensitization of
rat RG2 glioma cells in vitro. Folia Neuropathol. 54:234–240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao D, Song Y, Che Z and Liu Q: Calcium
overload and in vitro apoptosis of the C6 glioma cells mediated by
sonodynamic therapy (hematoporphyrin monomethyl ether and
ultrasound). Cell Biochem Biophys. 70:1445–1452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su X, Wang P, Yang S, Zhang K, Liu Q and
Wang X: Sonodynamic therapy induces the interplay between apoptosis
and autophagy in K562 cells through ROS. Int J Biochem Cell Biol.
60:82–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhou Q, Deng Z, Pan M, Liu X, Wu J,
Yan F and Zheng H: IR-780 Dye as a sonosensitizer for sonodynamic
therapy of breast tumor. Sci Rep. 6:259682016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bullon P, Cordero MD, Quiles JL, Morillo
JM, del Carmen Ramirez-Tortosa M and Battino M: Mitochondrial
dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide
as a possible link between cardiovascular disease and
periodontitis. Free Radic Biol Med. 50:1336–1343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Jia Y, Wang P, Liu Q and Zheng H:
Current status and future perspectives of sonodynamic therapy in
glioma treatment. Ultrason Sonochem. 37:592–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun X, Xu H, Shen J, Guo S, Shi S, Dan J,
Tian F and Tian Y and Tian Y: Real-time detection of intracellular
reactive oxygen species and mitochondrial membrane potential in
THP-1 macrophages during ultrasonic irradiation for optimal
sonodynamic therapy. Ultrason Sonochem. 22:7–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Wang P, Zhao P, Zhu S, Wang X and
Liu Q: Apoptosis induced by sonodynamic treatment by protoporphyrin
IX on MDA-MB-231 cells. Ultrasonics. 52:490–496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu ZR, Chen SQ, Zou YW, Wu XY, Li HY,
Wang XQ, Shi Y and Niu HX: Hypochlorite modified albumins promote
cell death in the tubule interstitium in rats via mitochondrial
damage in obstructive nephropathy and the protective effects of
antioxidant peptides. Free Radic Res. 52:616–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Sun M, Wang Y, Lv Y, Hu Z, Cao W,
Zheng J and Jiao X: Effect of cell cycle phase on the sensitivity
of SAS cells to sonodynamic therapy using low-intensity ultrasound
combined with 5-aminolevulinic acid in vitro. Mol Med Rep.
12:3177–3183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lv Y, Zheng J, Zhou Q, Jia L, Wang C, Liu
N, Zhao H, Ji H, Li B and Cao W: Antiproliferative and
apoptosis-inducing effect of exo-protoporphyrin IX based
sonodynamic therapy on human oral squamous cell carcinoma. Sci Rep.
7:409672017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sisto M, Lisi S, D'Amore M, Mitolo V and
Scagliusi P: Anti-Ro and anti-La autoantibodies induce TNF-alpha
production by human salivary gland cells: An in vitro study.
Reumatismo. 59:221–226. 2007.PubMed/NCBI
|
|
21
|
Li W, Fei JF, Yang Q, Li BL, Lin C, Yue Q
and Meng QG: Acute toxic effects of sonodynamic therapy on
hypertrophic scar fibroblasts of rabbit ears. Genet Mol Res.
14:4203–4214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie R, Xu T, Zhu J, Wei X, Zhu W, Li L,
Wang Y, Han Y, Zhou J and Bai Y: The combination of glycolytic
inhibitor 2-deoxyglucose and microbubbles increases the effect of
5-aminolevulinic acid-sonodynamic therapy in liver cancer cells.
Ultrasound Med Biol. 43:2640–2650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiong W, Wang P, Hu J, Jia Y, Wu L, Chen
X, Liu Q and Wang X: A new sensitizer DVDMS combined with multiple
focused ultrasound treatments: An effective antitumor strategy. Sci
Rep. 5:174852015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun H, Ge W, Gao X, Wang S, Jiang S, Hu Y,
Yu M and Hu S: Apoptosis-promoting effects of hematoporphyrin
monomethyl ether-sonodynamic therapy (HMME-SDT) on endometrial
cancer. PLoS One. 10:e01379802015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Gao Q, Guo S, Cheng J, Sun X, Li
Q, Wang T, Zhang Z, Cao W and Tian Y: The sonodynamic effect of
curcumin on THP-1 cell-derived macrophages. BioMed Res Int.
2013:7372642013.PubMed/NCBI
|
|
26
|
Qin P, Han T, Yu ACH and Xu L: Mechanistic
understanding the bioeffects of ultrasound-driven microbubbles to
enhance macromolecule delivery. J Control Release. 272:169–181.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Yue W, Huang Z, Chen ZQ, Zhan Q, Ren
FB, Liu JY and Fu SB: Calcium overload induces C6 rat glioma cell
apoptosis in sonodynamic therapy. Int J Radiat Biol. 87:1061–1066.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tian J, Gan Y, Pan C, Zhang M, Wang X,
Tang X and Peng X: Nerol-induced apoptosis associated with the
generation of ROS and Ca2+ overload in saprotrophic
fungus Aspergillus flavus. Appl Microbiol Biotechnol.
102:6659–6672. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tran TA, Roger S, Le Guennec JY, Tranquart
F and Bouakaz A: Effect of ultrasound-activated microbubbles on the
cell electrophysiological properties. Ultrasound Med Biol.
33:158–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lentacker I, De Cock I, Deckers R, De
Smedt SC and Moonen CT: Understanding ultrasound induced
sonoporation: Definitions and underlying mechanisms. Adv Drug Deliv
Rev. 72:49–64. 2014. View Article : Google Scholar : PubMed/NCBI
|