Introduction
Liver cancer is common malignancy with poor
prognosis. In the United States of America (USA), 42,220
individuals were diagnosed with liver cancer in 2018, resulting in
30,200 fatalities (1). In addition,
the 5-year relative survival rate for liver cancer is ~18% in the
USA (1). Due to recent advances in
liver transplantation technologies, patients with liver cancer
often demonstrate optimal survival rates if the cancer is diagnosed
at a localized stage (2). However,
the survival rates of patients with liver cancer with distant tumor
localization remains low (2).
Therefore, it is necessary to identify novel agents that may
improve the treatment of patients with liver cancer.
S-phase kinase associated protein 2 (Skp2) has been
demonstrated to control cellular growth, tumor development and
progression (3). Skp2 is a component
of the Skp1-Cul1-F-box protein E3 ubiquitin ligase that mediates
the ubiquitination and subsequent proteasomal degradation of
targeted substrates (4). Skp2 has
been characterized as an oncoprotein, and functions by facilitating
the deregulated ubiquitination and proteolysis of several tumor
suppressor proteins (4). Skp2 has
been demonstrated to enhance tumor progression and development via
the regulation of various biological processes, namely cell
proliferation, the cell cycle, apoptosis, invasion and metastasis
(4–6). In liver cancer, the expression of Skp2
is associated with histological grade and tumor size, suggesting
that Skp2 may be a prognostic biomarker for patients with liver
cancer (7,8). A recent study demonstrated that Skp2
promoted the proliferation, colony formation, migration and
invasion of liver cancer cells (8).
Therefore, Skp2 may be a potential target in the treatment of liver
cancer.
Paeoniflorin (PF) is a bioactive component isolated
from the paeony root, which exerts multiple immunoregulatory,
anti-hyperglycemic and anti-hypotensive effects (9,10). PF
has been suggested to possess anti-tumor functions in a variety of
human cancer types, including liver cancer (11–15).
However, PF did not affect normal hepatocyte growth, and was
demonstrated to induce apoptosis via endoplasmic reticulum (ER)
stress and activation of the mitochondria-dependent pathway
(16). However, the mechanisms of
PF-mediated tumor suppressive function have not been fully
identified. In the present study, PF-induced anti-tumor activity
was investigated in liver cancer cells. As Skp2 is an important
oncoprotein in liver cancer cells, whether PF inhibited Skp2
expression was determined. It was identified that PF inhibited the
expression levels of Skp2 in liver cancer cells. The data suggest
that inhibition of Skp2 by PF may be a novel therapeutic approach
for treating patients with liver cancer.
Materials and methods
Reagents
PF was purchased from the HuanYu Biotechnology
Development Company. MTT was purchased from Sigma-Aldrich; Merck
KGaA. The Annexin apoptosis assay kit was obtained from Beyotime
Institute of Biotechnology. The Transwell chambers and Matrigel
were purchased from BD Biosciences. Lipofectamine® 2,000
reagent was obtained from Invitrogen; Thermo Fisher Scientific,
Inc. The anti-Skp2 (1:2,000; cat. no. 4358), anti-tubulin (1:5,000;
cat. no. 2146), anti-β-actin (1:5,000; cat. no. 3700), anti-cyclin
dependent kinase inhibitor 1A (p21; 1:1,000; cat. no. 2491) and
anti-cyclin dependent kinase inhibitor 1C (p57; 1:1,000; cat. no.
2557) antibodies were obtained from Cell Signaling Technology, Inc.
Goat anti-mouse secondary antibody (1:5,000; cat. no. A-11031) and
goat anti-rabbit secondary antibody (1:5,000; cat. no. A-11034)
were purchased from Thermo Fisher Scientific, Inc.
Cell culture
The human liver cancer HepG2 cell line was purchased
from The Cell Bank of Type Culture Collection of Shanghai Chinese
Academy of Sciences. HepG2 cells were grown in Dulbecco's modified
eagle's medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc). The
cells were cultured under a humidified atmosphere of 5%
CO2 at 37°C.
MTT assay
The HepG2 cells were seeded into 96-well plates
(5×103 cells/well) and incubated overnight at 37°C
overnight. PF was dissolved in dimethyl sulfoxide (DMSO) and the
cells were exposed to different concentrations of PF (0, 25 and 50
µM) or 0.1% DMSO (control group) for 72 h. The MTT assay was
performed as previously described (17).
Cell apoptosis assay
The HepG2 cells (5×105 cells/well) were
seeded into 6-well plates and exposed to 25 µM PF for 48 h.
Subsequently, the cells were collected and resuspended in buffer
including 5 µl Annexin V-fluorescein isothiocyanate (FITC) and 5 µl
propidium iodide (PI) for 15 min at room temperature in the dark.
The levels of apoptosis were then determined as previously
described (17).
Wound healing assay
The HepG2 cells (5×105 cells/well) were
cultured in DMEM supplemented with 10% FBS at 37°C in 5%
CO2 to 90% confluence. A small sterile pipette tip was
used to create a rectangular lesion. Following treatment of the
cells with 25 µM of PF for 20 h at 37°C, optical microscopy images
were captured using an inverted light microscope (Olympus
Corporation; magnification, ×100).
Transwell invasion assay
The HepG2 cells were treated with 25 µM of PF and
cultured in the upper chambers of inserts with Matrigel using
serum-free medium at 37°C for 20 h. The bottom chambers were filled
with complete medium. Following incubation at 37°C for 20 h, the
upper cells in the chambers were cleaned and the bottom cells were
stained with 4 µg/ml of Calcein AM at 37°C for 1 h. The cells were
then analyzed by fluorescence microscopy (magnification, ×100).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.) and reverse transcribed
into cDNA using TaqMan® Reverse Transcription Reagents
(Thermo Fisher Scientific, Inc.). RT-qPCR was performed using Power
SYBR Green PCR Master mix (Thermo Fisher Scientific, Inc.).
Specifically, 2 µg of total RNA was subjected to first strand cDNA
synthesis in a total volume of 20 µl. The RT reaction was performed
as follows: 37°C for 1 h, followed by 95°C for 5 min. The following
primer sequences were used for qPCR: Skp2 forward,
5′-TGCTAAGCAGCTGTTCCAGA-3′ and reverse, 5′-AAGATTCAGCTGGGTGATGG-3′
and GAPDH forward, 5′-ACCCAGAAGACTGTGGATGG-3′ and reverse,
5′-CAGTGAGCTTCCCGTTCAG-3′. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 10 min; 45
cycles of 95°C for 15 sec, 60°C for 1 min; and a final extension at
72°C for 5 min. Relative expression levels were quantified using
the 2−ΔΔCq method as previously described (18,19), and
normalized to the internal reference gene GAPDH.
Western blot analysis
The cell lysates were extracted using RIPA buffer
(Cell Signaling Technology, Inc.) and western blot analysis was
performed as previously described (20). ImageJ software (version 1.52k;
National Institutes of Health) was used densitometric
quantification of the protein bands.
Transfection
HepG2 cells (5×105 cells/well) were
cultured in 6-well plates at 37°C. The cells were either treated
with 25 µM of PF, or transfected with 1 mg of Skp2 cDNA constructor
or 10 nM of Skp2 small interfering (si)RNA (both from Shanghai
Genechem Co, Ltd.) for 48 h using Lipofectamine® 2000,
as previously described (21). The
Skp2 siRNA sequences used were as follows: Sense,
5′-CCUAUCGAACUCAGUUAUATT-3′ and antisense,
5′-UAUAACUGAGUUCGAUAGGTC-3′.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5.0; GraphPad Software, Inc.). The results
are presented as the mean ± standard error of the mean. The
difference between groups was analyzed using analysis of variance
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
PF inhibits Skp2 expression in liver
cancer cells
Skp2 serves an important role as an oncoprotein in
liver cancer. The potential inhibitory effect of PF on Skp2
expression in liver cancer cells was investigated. RT-qPCR was
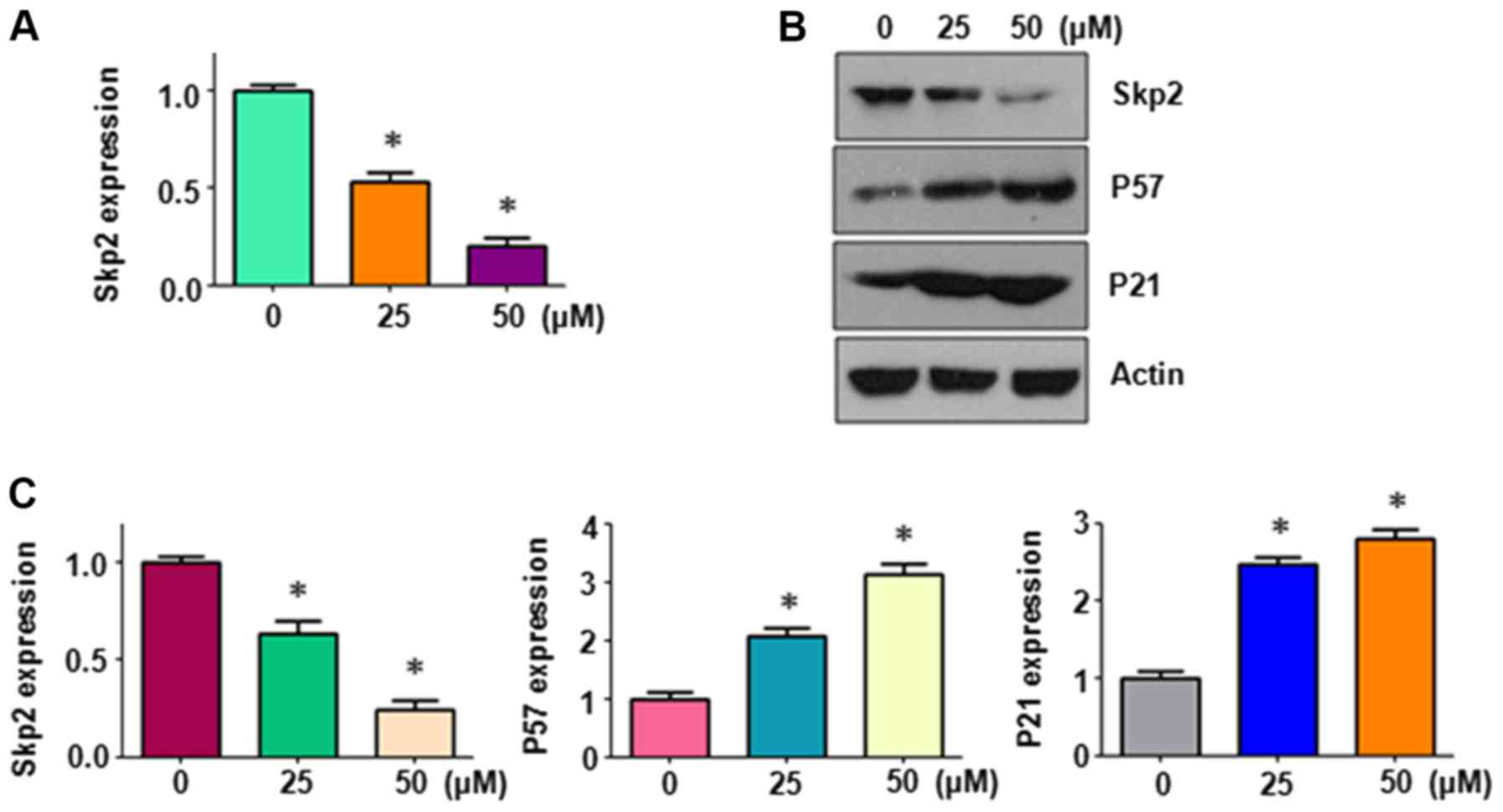
performed to quantify the mRNA levels of Skp2 in HepG2 cells. It
was revealed that Skp2 mRNA expression was significantly inhibited
by PF treatment (Fig. 1A). The
protein expression levels of Skp2 in PF-treated HepG2 cells were
measured using western blot analysis. PF treatment was demonstrated
to inhibit the expression of Skp2 protein in HepG2 cells (Fig. 1B and C). The downregulation of Skp2
expression by PF was evaluated by measuring the expression levels
of downstream genes of Skp2, namely p21 and p57 in HepG2 cells. The
western blot analysis data indicated that p57 and p21 expression
levels were increased following PF treatment (Fig. 1B and C). The present data indicated
that PF treatment inhibited the expression of Skp2 in liver cancer
cells.
Overexpression of Skp2 rescues
PF-induced cell viability inhibition and apoptosis
To further determine whether Skp2 is involved in
PF-mediated tumor growth inhibition, HepG2 cells were transfected
with Skp2 cDNA to overexpress Skp2, and were further treated with
PF. The MTT assay was used to measure the proliferation rate of
HepG2 cells following PF treatment, and transfection with the Skp2
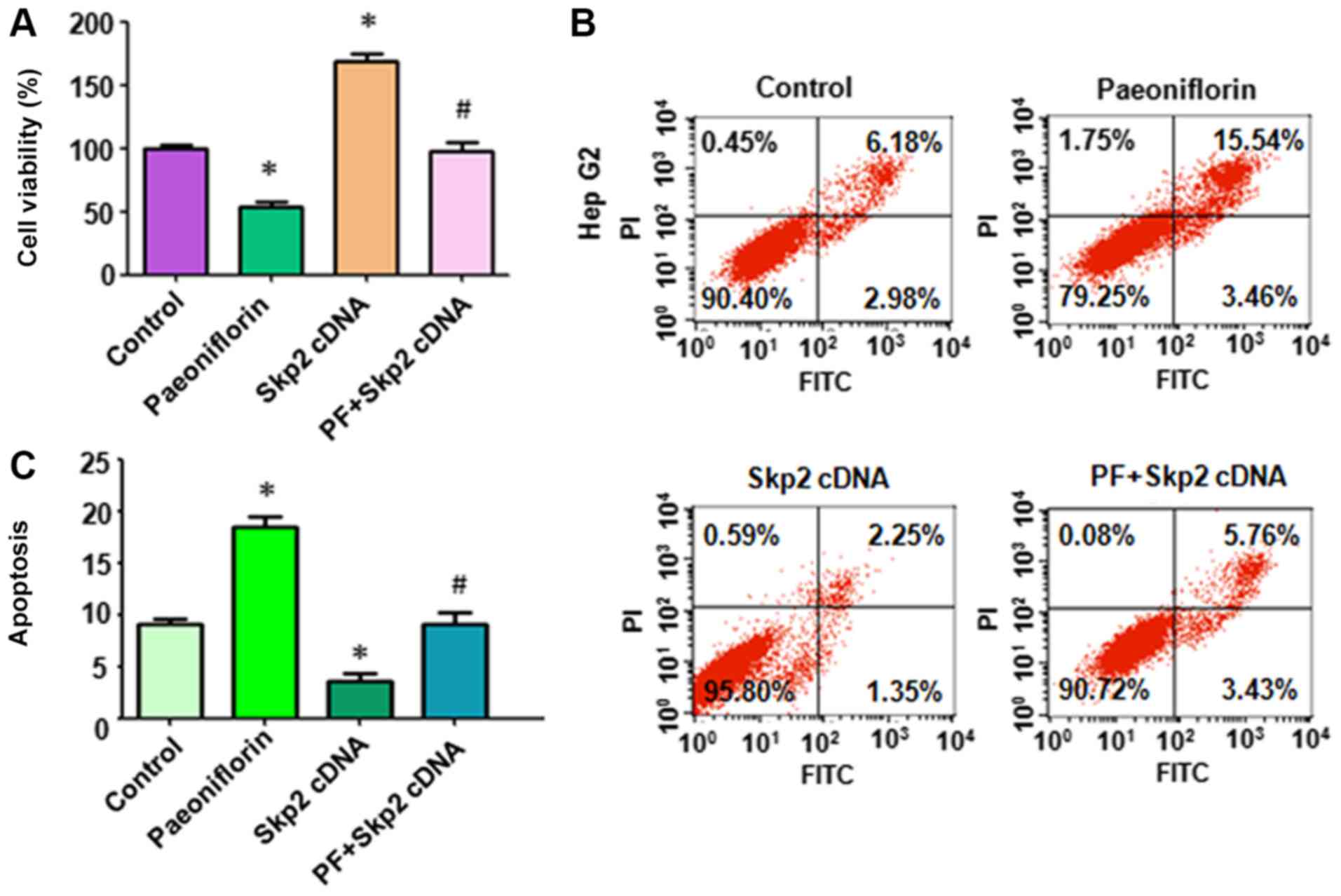
cDNA vector. It was identified that 25 µM PF significantly
inhibited HepG2 cell viability (Fig.
2A). Overexpression of Skp2 enhanced cell viability in HepG2
cells (Fig. 2A). Notably, the
overexpression of Skp2 abrogated PF-induced cell viability
inhibition in liver cancer cells (Fig.
2A). Next, the induction of apoptosis was measured using the
FITC/PI method in HepG2 cells following PF treatment and
transfection with Skp2 cDNA. PF exposure increased the level of
apoptosis (18.0%) in the treatment group compared with the control
group (9.16%) (Fig. 2B). The
overexpression of Skp2 resulted in a decrease in the levels of
apoptosis in the treatment group (3.6%) compared with the control
group (9.16%) (Fig. 2B). Notably,
the overexpression of Skp2 abolished PF-induced apoptosis in HepG2
cells (Fig. 2B). Taken together, the
data demonstrated that Skp2 is primarily involved in PF-mediated
cell viability inhibition and apoptosis.
Overexpression of Skp2 abolishes
PF-induced inhibition of migration and invasion
The effects of Skp2 on PF-inhibited cell motility
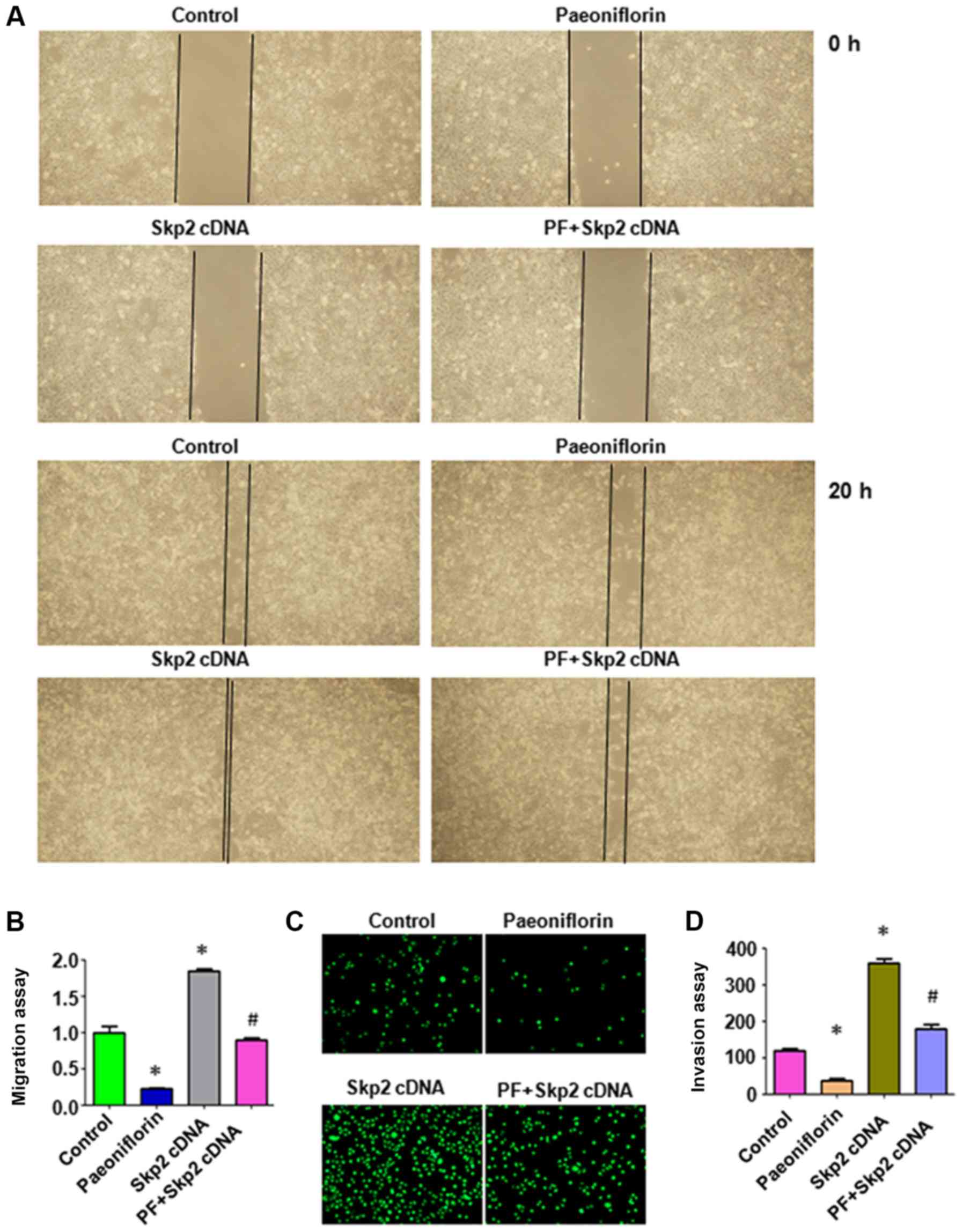
were further investigated in the liver cancer HepG2 cells. A wound
healing assay was used to measure the migration of HepG2 cells
following PF treatment and Skp2 cDNA vector transfection. PF
inhibited the migration of HepG2 cells (Fig. 3A and B), and overexpression of Skp2
increased cell migration in liver cancer cells and abolished
PF-induced inhibition of cell migration (Fig. 3A and B). The Transwell chamber assay
was used to measure the invasion of HepG2 cells following PF
exposure and Skp2 cDNA transfection. The data indicated that PF
inhibited invasion (Fig. 3C and D),
and overexpression of Skp2 abrogated the PF-mediated inhibition of
cell invasion in HepG2 cells (Fig. 3C
and D). These results indicated that PF inhibited cell
migration and invasion in part by targeting Skp2 in HepG2
cells.
Overexpression of Skp2 abolishes
PF-mediated induction of p57 and p21
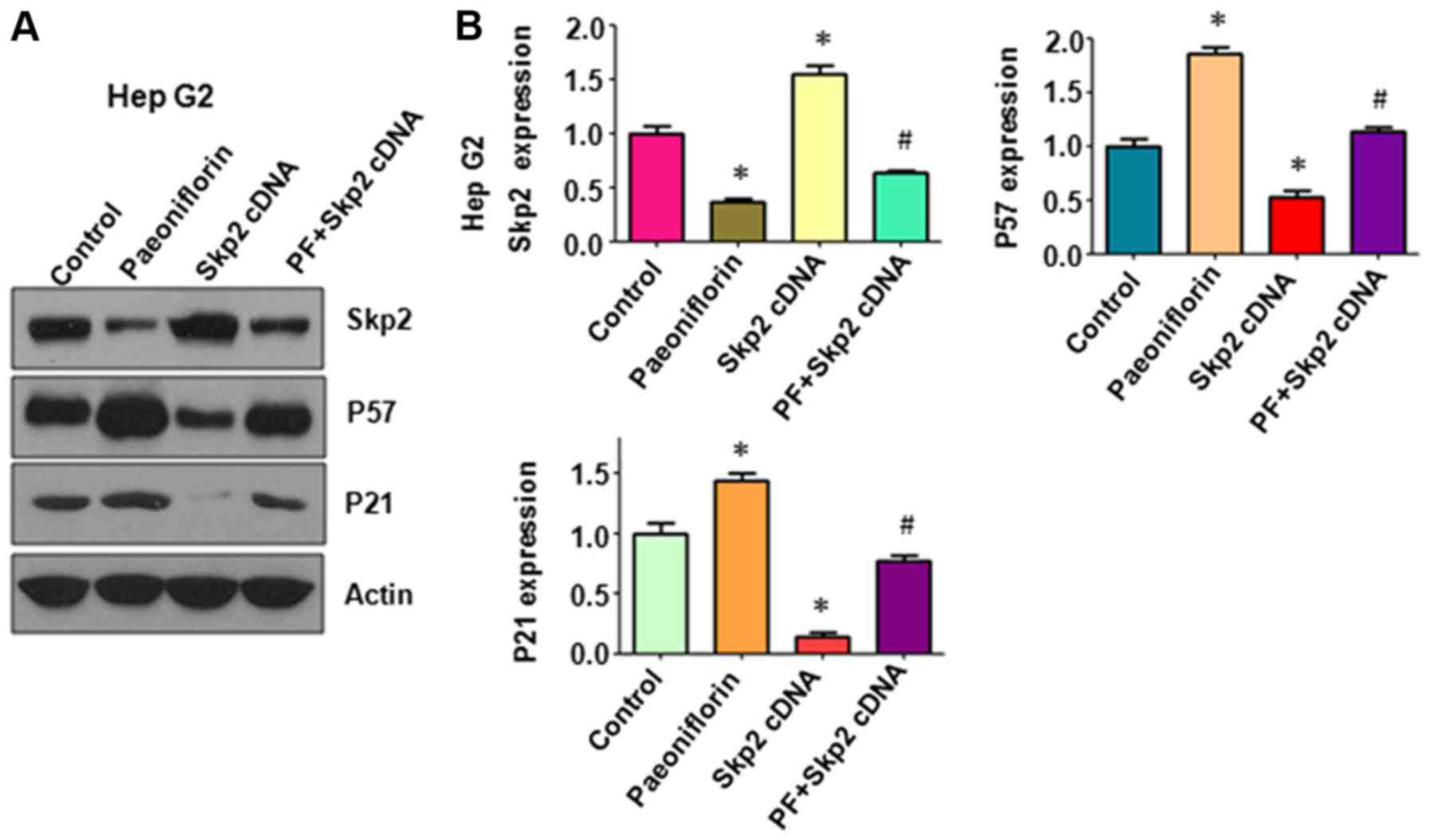
The effects of Skp2 overexpression on the expression
of its downstream genes p57 and p21 were investigated. The western
blot analysis results indicated that PF treatment inhibited Skp2
expression and increased the expression levels of p57 and p21 in
HepG2 cells (Fig. 4A and B).
Overexpression of Skp2 decreased the levels of p57 and p21 and
abolished the PF-mediated induction of their expression in the
HepG2 cells (Fig. 4A and B). Taken
together, these data suggested that PF exerted its tumor
suppressive function in part by the induction of p57 and p21
expression in liver cancer cells.
Downregulation of Skp2 promotes
PF-induced cell viability inhibition and apoptosis
The role of Skp2 in PF-mediated cell viability
inhibition was examined by Skp2 siRNA transfection in HepG2 cells,
followed by PF treatment. Skp2 siRNA transfection was demonstrated
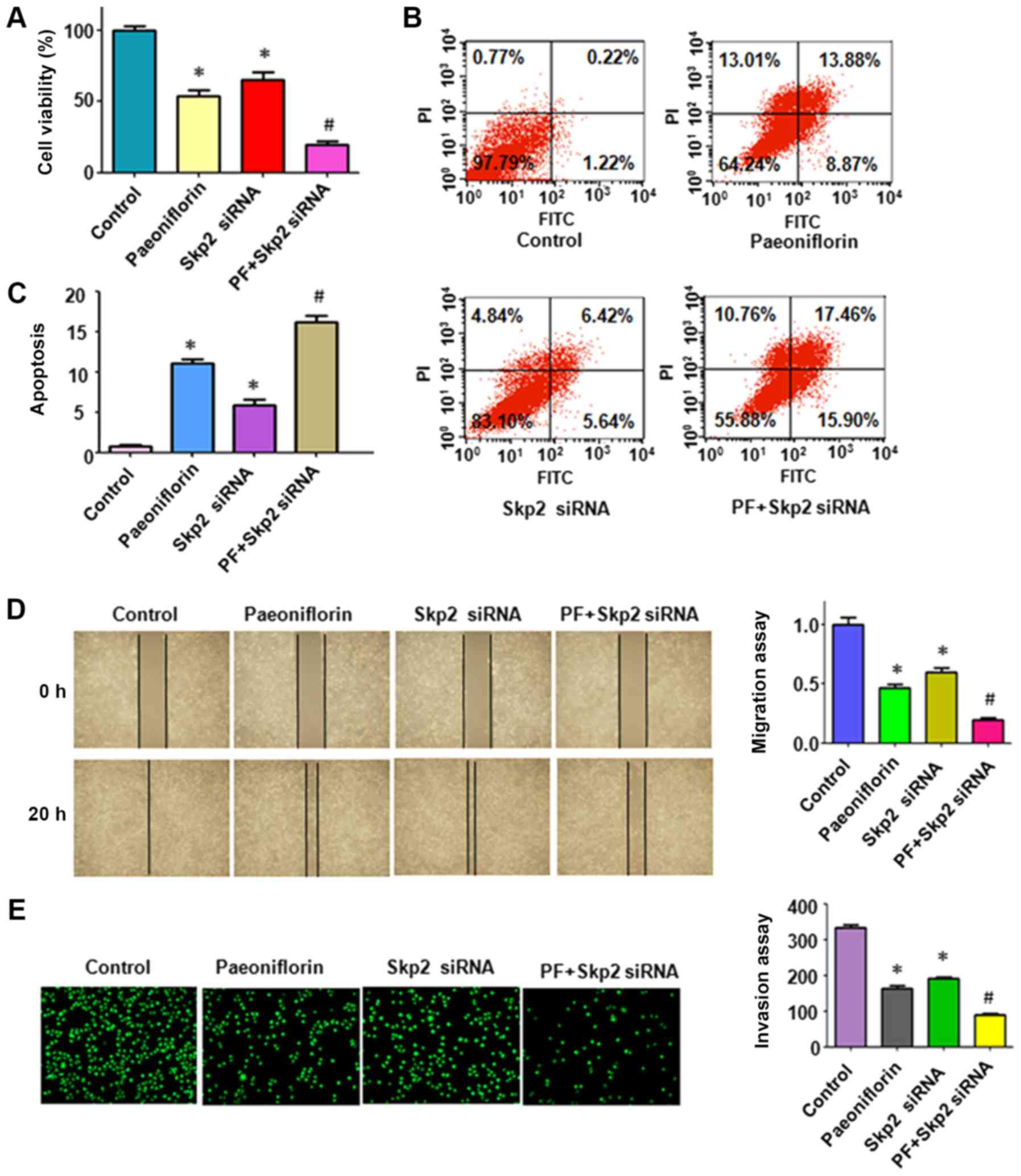
to inhibit the viability of HepG2 cells (Fig. 5A). Downregulation of Skp2 in the
presence of PF resulted in an increased rate of inhibition compared
with that observed in the presence of PF treatment alone, or siRNA
transfection alone (Fig. 5A). The
induction of apoptosis was also assessed in HepG2 cells following
Skp2 siRNA transfection and PF treatment. Downregulation of Skp2
induced apoptosis in HepG2 cells and enhanced PF-induced apoptosis
(Fig. 5B and C). Therefore, Skp2 was
determined to be an important oncoprotein involved in PF-induced
cell viability inhibition and apoptosis.
Downregulation of Skp2 enhances
PF-mediated cell invasion and migration
The role of Skp2 in the PF-triggered inhibition of
migration was further assessed using wound healing assays in HepG2
cells treated with PF and transfected with Skp2 siRNA. Skp2 siRNA
inhibited the migration of HepG2 cells (Fig. 5D). Notably, the downregulation of
Skp2 promoted the PF-mediated inhibition of cell migration
(Fig. 5D). Similar results were
observed in HepG2 cells following Skp2 siRNA transfection in the
presence of PF treatment. The Transwell chamber assay data
indicated that the downregulation of Skp2 increased PF-induced cell
invasion (Fig. 5E). Western blot
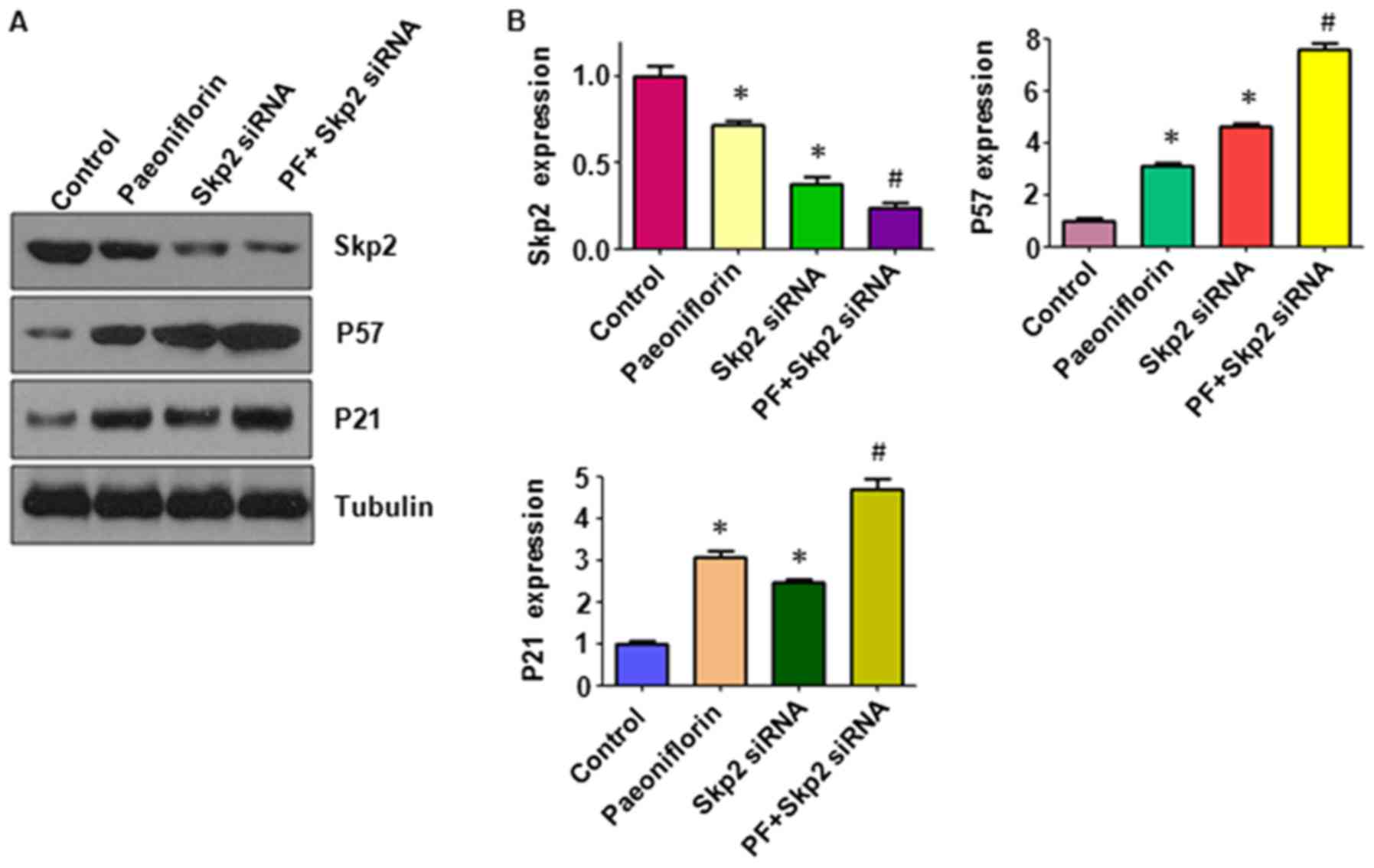
analysis data demonstrated that the downregulation of Skp2
increased the levels of p57 and p21 in HepG2 cells (Fig. 6A and B). The downregulation of Skp2
increased PF-mediated induction of p57 and p21 expression in HepG2
cells (Fig. 6A and B). Therefore, PF
was considered to exert its anti-tumor activity partly via
induction of p57 and p21 expression in liver cancer cells.
Discussion
Emerging evidence has demonstrated that Skp2 serves
a critical role in the development of liver cancer. For example,
the nuclear expression of Skp2 predicted poor prognosis in patients
with liver carcinoma (22). In
addition, the inhibition of Skp2 induced apoptosis and inhibited
cell proliferation in liver carcinoma cells via the upregulation of
cyclin dependent kinase inhibitor 1B (p27) (23). It has also been suggested that Skp2
functions with N-Rasv12 or Akt to induce liver tumor development in
mice (24). Recently, it was
demonstrated that Skp2 promoted liver carcinoma progression via
AMP-activated protein kinase-Skp2-CARM1 signaling, which led to the
regulation of nutrient-deprived autophagy induction (8). Notably, the Hippo signaling pathway can
suppress liver tumorigenesis via the Skp2 pathway (25). Skp2 and cyclin-dependent kinases
regulatory subunit 1 promoted the degradation of cell cycle
regulators and were associated with liver carcinoma prognosis
(26). The X protein of hepatitis B
virus (HBV) binds to Skp2 and inhibits the ubiquitination and
proteasomal degradation of c-Myc in human hepatoma cell lines
(27). Similarly, HBV core promoter
mutations enhanced cell proliferation via transcription factor
E2F1-mediated upregulation of Skp2 transcription (28,29).
Hepatocyte growth factor suppressed HepG2 cell proliferation by
increasing p27 expression and by decreasing Skp2 expression
(30). These studies have suggested
that Skp2 is an important oncoprotein participating in liver cancer
development, and that the inhibition of Skp2 may be used as a
strategy to treat liver cancer.
PF has been demonstrated to inhibit the growth of
HepG2 and Bel-7402 cells, and to decrease the invasion, adhesion
and metastasis of liver cancer cells (14). In concordance with these data, the
present study demonstrated that PF suppressed cell viability, and
inhibited the migration and invasion of liver cancer HepG2 cells. A
previous study indicated that PF induced apoptosis in liver cancer
HepG2 and SMMC-7721 cells by the inhibition of Bcl-2 and the
induction of Bax and cleaved caspase-3 expression, and the
downregulation of the prostaglandin E receptor EP2 subtype in liver
cancer cells (31). PF exposure
decreased the expression levels of matrix metalloprotein-9 (MMP-9)
and extracellular signal-regulated kinase, whereas it increased the
levels of E-cadherin in liver cancer cells (14). The data indicated that PF inhibited
the expression levels of Skp2 in liver cancer cells. Recently, it
was suggested that PF exerted antitumor effects by inactivating
Skp2 in glioma cells (32). This
suggested that PF may decrease the expression levels of Skp2 in
various types of human cancer, indicating that PF may be a
potential inhibitor of Skp2 in multiple cancer types.
Several agents have been identified to inhibit the
expression of Skp2 in human cancer. For example, troglitazone, an
agonist of peroxisome proliferator-activated receptor gamma, was
identified to downregulate the expression levels of Skp2 and to
induce p27-associated cell cycle arrest in liver cancer cells
(33,34). EB1089 induced Skp2-dependent p27
accumulation, resulting in inhibition of cell growth and cell cycle
G1 phase arrest in hepatoma cells (35). Longikaurin A, a natural kauranoid
diterpenoid, induced cell cycle arrest by inhibition of Skp2
expression in liver carcinoma cells (36). Simvastatin, an HMG-Co-enzyme A
reductase inhibitor, induced cell cycle arrest via suppression of
the STAT3/Skp2 axis in order to promote p27 and p21 accumulation in
liver carcinoma cells (37).
Curcumin has been demonstrated to inhibit the expression of Skp2 in
several human cancer types (17,38,39).
Rottlerin exhibited its anti-tumor activity by the inhibition of
Skp2 in breast cancer and pancreatic cancer cells (18,40). The
present study demonstrated that PF inhibited Skp2 expression in
liver cancer cells. It is important to note that the present study
had certain limitations, such as the use of only 1 cell line.
Future studies are required to determine the role of PF in multiple
cell lines. Regarding wound healing assays, serum-starvation is
applied to halt proliferation and ensure that the wound closure is
a direct result of migration. However, the present study used 10%
FBS in the wound healing assay as HepG2 cell viability is notably
decreased if cells are serum starved (41). Future studies should also examine
whether PF regulated the cell cycle via inhibition of Skp2. A
previous study reported that PF did not affect normal hepatocyte
growth in mice (16); however,
whether PF affects normal and healthy liver cells in human requires
further elucidation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL and LL designed the present study. HL, LZ, JZ and
ZW acquired and analyzed the data. LL supervised the present study,
and HL, LZ and LL drafted and edited the initial manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual Report to the Nation on the Status of Cancer, 1975–2014,
Featuring Survival. J Natl Cancer Inst. 109:2017. View Article : Google Scholar
|
|
3
|
Wang G, Chan CH, Gao Y and Lin HK: Novel
roles of Skp2 E3 ligase in cellular senescence, cancer progression,
and metastasis. Chin J Cancer. 31:169–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frescas D and Pagano M: Deregulated
proteolysis by the F-box proteins SKP2 and beta-TrCP: Tipping the
scales of cancer. Nat Rev Cancer. 8:438–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Z, Liu P, Inuzuka H and Wei W: Roles
of F-box proteins in cancer. Nat Rev Cancer. 14:233–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan CH, Morrow JK, Zhang S and Lin HK:
Skp2: A dream target in the coming age of cancer therapy. Cell
Cycle. 13:679–680. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu M, Ma J, Xue W, Cheng C, Wang Y, Zhao
Y, Ke Q, Liu H, Liu Y, Li P, et al: The expression and prognosis of
FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol Oncol
Res. 15:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei X, Li X, Yan W, Zhang X, Sun Y and
Zhang F: SKP2 promotes hepatocellular carcinoma progression through
nuclear AMPK-SKP2-CARM1 signaling transcriptionally regulating
nutrient-deprived autophagy induction. Cell Physiol Biochem.
47:2484–2497. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen A, Wang H, Zhang Y, Wang X, Yu L, Xu
W, Xu W and Lin Y: Paeoniflorin exerts neuroprotective effects
against glutamateinduced PC12 cellular cytotoxicity by inhibiting
apoptosis. Int J Mol Med. 40:825–833. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu P, Zhu L, Liu Y, Zhang L, Liu J and
Shen H: Protective effects of paeoniflorin on TNBS-induced
ulcerative colitis through inhibiting NF-kappaB pathway and
apoptosis in mice. Int Immunopharmacol. 50:152–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kapoor S: The emerging antineoplastic
effects of paeoniflorin. Anticancer Drugs. 24:4292013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Qin F, Gao S, Zhen Y, Huang D and
Dong L: Paeoniflorin exerts protective effect on radiation-induced
hepatic fibrosis in rats via TGF-β1/Smads signaling pathway. Am J
Transl Res. 10:1012–1021. 2018.PubMed/NCBI
|
|
13
|
Li JZ, Tang XN, Li TT, Liu LJ, Yu SY, Zhou
GY, Shao QR, Sun HP, Wu C and Yang Y: Paeoniflorin inhibits
doxorubicin-induced cardiomyocyte apoptosis by downregulating
microRNA-1 expression. Exp Ther Med. 11:2407–2412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu JT, He W, Song SS and Wei W:
Paeoniflorin inhibited the tumor invasion and metastasis in human
hepatocellular carcinoma cells. Bratisl Lek Listy. 115:427–433.
2014.PubMed/NCBI
|
|
15
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Z, Chen W, Yan X, Bi L, Guo S and
Zhan Z: Paeoniflorin protects cells from GalN/TNF-α-induced
apoptosis via ER stress and mitochondria-dependent pathways in
human L02 hepatocytes. Acta Biochim Biophys Sin (Shanghai).
46:357–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Ye X, Cai X, Su J, Ma R, Yin X,
Zhou X, Li H and Wang Z: Curcumin suppresses cell growth and
invasion and induces apoptosis by down-regulation of Skp2 pathway
in glioma cells. Oncotarget. 6:18027–18037. 2015.PubMed/NCBI
|
|
18
|
Yin X, Zhang Y, Su J, Hou Y, Wang L, Ye X,
Zhao Z, Zhou X, Li Y and Wang Z: Rottlerin exerts its anti-tumor
activity through inhibition of Skp2 in breast cancer cells.
Oncotarget. 7:66512–66524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Zhao M, Xu H, Wang K, Fu Z, Jiang
Y and Yao Z: RASAL2 down-regulation in ovarian cancer promotes
epithelial-mesenchymal transition and metastasis. Oncotarget.
5:6734–6745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Hou Y, Yin X, Su J, Zhao Z, Ye X,
Zhou X, Zhou L and Wang Z: Rottlerin inhibits cell growth and
invasion via down-regulation of Cdc20 in glioma cells. Oncotarget.
7:69770–69782. 2016.PubMed/NCBI
|
|
22
|
Shin E, Kim SH, Jeong HY, Jang JJ and Lee
K: Nuclear expression of S-phase kinase-associated protein 2
predicts poor prognosis of hepatocellular carcinoma. Apmis.
120:349–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi M, Liu D, Zhang S, Hu P and Sang T:
Inhibition of S-phase kinase-associated protein 2-mediated p27
degradation suppresses tumorigenesis and the progression of
hepatocellular carcinoma. Mol Med Rep. 11:3934–3940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Delogu S, Wang C, Cigliano A, Utpatel K,
Sini M, Longerich T, Waldburger N, Breuhahn K, Jiang L, Ribback S,
et al: SKP2 cooperates with N-Ras or AKT to induce liver tumor
development in mice. Oncotarget. 6:2222–2234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Chen Q, Liu Q, Li Y, Sun X, Hong
L, Ji S, Liu C, Geng J, Zhang W, et al: Hippo signaling suppresses
cell ploidy and tumorigenesis through Skp2. Cancer Cell.
31:669–684,e7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calvisi DF, Ladu S, Pinna F, Frau M,
Tomasi ML, Sini M, Simile MM, Bonelli P, Muroni MR, Seddaiu MA, et
al: SKP2 and CKS1 promote degradation of cell cycle regulators and
are associated with hepatocellular carcinoma prognosis.
Gastroenterology. 137:1816–1826,e1-10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalra N and Kumar V: The X protein of
hepatitis B virus binds to the F box protein Skp2 and inhibits the
ubiquitination and proteasomal degradation of c-Myc. FEBS Lett.
580:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Tai AW, Tong S and Lok AS: HBV
core promoter mutations promote cellular proliferation through
E2F1-mediated upregulation of S-phase kinase-associated protein 2
transcription. J Hepatol. 58:1068–1073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang Y, Tong S, Tai AW, Hussain M and Lok
AS: Hepatitis B virus core promoter mutations contribute to
hepatocarcinogenesis by deregulating SKP2 and its target, p21.
Gastroenterology. 141:1412–1421, e1-e5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Ozaki I, Mizuta T, Yoshimura T,
Matsuhashi S, Hisatomi A, Tadano J, Sakai T and Yamamoto K:
Mechanism of beta 1-integrin-mediated hepatoma cell growth involves
p27 and S-phase kinase-associated protein 2. Hepatology.
38:305–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu S, Sun W, Wei W, Wang D, Jin J, Wu J,
Chen J, Wu H and Wang Q: Involvement of the prostaglandin E
receptor EP2 in paeoniflorin-induced human hepatoma cell apoptosis.
Anticancer Drugs. 24:140–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ouyang J, Xu H, Li M, Dai X, Fu F, Zhang X
and Lan Q: Paeoniflorin exerts antitumor effects by inactivating S
phase kinase-associated protein 2 in glioma cells. Oncol Rep.
39:1052–1062. 2018.PubMed/NCBI
|
|
33
|
Zhong WB, Tsai YC, Chin LH, Tseng JH, Tang
LW, Horng S, Fan YC and Hsu SP: A synergistic anti-cancer effect of
troglitazone and lovastatin in a human anaplastic thyroid cancer
cell line and in a mouse xenograft model. Int J Mol Sci. 19(pii):
E18342018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motomura W, Takahashi N, Nagamine M,
Sawamukai M, Tanno S, Kohgo Y and Okumura T: Growth arrest by
troglitazone is mediated by p27Kip1 accumulation, which results
from dual inhibition of proteasome activity and Skp2 expression in
human hepatocellular carcinoma cells. Int J Cancer. 108:41–46.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo W, Chen Y, Liu M, Du K, Zheng G, Cai
T, Zhang W, Zhao F, Yao T, Yang R and Chen J: EB1089 induces
Skp2-dependent p27 accumulation, leading to cell growth inhibition
and cell cycle G1 phase arrest in human hepatoma cells. Cancer
Invest. 27:29–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao YJ, Bai HY, Li ZH, Zou J, Chen JW,
Zheng F, Zhang JX, Mai SJ, Zeng MS, Sun HD, et al: Longikaurin A, a
natural ent-kaurane, induces G2/M phase arrest via downregulation
of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in
hepatocellular carcinoma cells. Cell Death Dis. 5:e11372014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang ST, Ho HJ, Lin JT, Shieh JJ and Wu
CY: Simvastatin-induced cell cycle arrest through inhibition of
STAT3/SKP2 axis and activation of AMPK to promote p27 and p21
accumulation in hepatocellular carcinoma cells. Cell Death Dis.
8:e26262017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng S, Wang Y, Zhang R, Yang G, Liang Z,
Wang Z and Zhang G: Curcumin exerts its antitumor activity through
regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells.
Onco Targets Ther. 10:2377–2388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su J, Zhou X, Wang L, Yin X and Wang Z:
Curcumin inhibits cell growth and invasion and induces apoptosis
through down-regulation of Skp2 in pancreatic cancer cells. Am J
Cancer Res. 6:1949–1962. 2016.PubMed/NCBI
|
|
40
|
Su J, Wang L, Yin X, Zhao Z, Hou Y, Ye X,
Zhou X and Wang Z: Rottlerin exhibits anti-cancer effect through
inactivation of S phase kinase-associated protein 2 in pancreatic
cancer cells. Am J Cancer Res. 6:2178–2191. 2016.PubMed/NCBI
|
|
41
|
Dituri F, Mazzocca A, Fernando J, Peidrò
FJ, Papappicco P, Fabregat I, De Santis F, Paradiso A, Sabbà C and
Giannelli G: Differential inhibition of the TGF-β signaling pathway
in HCC cells using the small molecule inhibitor LY2157299 and the
D10 monoclonal antibody against TGF-β receptor Type II. PLoS One.
8:e671092013. View Article : Google Scholar : PubMed/NCBI
|