Introduction
According to the 2018 Global Cancer Statistics, lung
cancer is the leading cause of cancer-associated mortality
worldwide (1) and was so in China in
2014 (2). However, the cure for lung
cancer remains elusive due to the aggressive and disseminative
nature of tumors which restricts the efficacy of cancer treatments
(3). The brain is a preferential
site of metastasis in lung cancer (4). Among patients with lung cancer, ~30–50%
develop brain metastasis (5–7), which substantially affects their
quality of life. However, in the present study, even in the absence
of brain metastasis, some individuals continued to exhibit
paraneoplastic neurological syndromes, such as behavioral, memory
and movement disorders as well as hypomnesis, dizziness and
headaches (8). A number of previous
studies have hypothesized that the pathophysiology underlying
paraneoplastic syndromes is associated with the secretion of
cytokines by the primary tumor (9–12). This
may induce injury to distant organs and cause microstructural
changes like those in the brain, which cause paraneoplastic
syndromes or alter the homing and proliferation of metastatic
cancer cells (9–13). However, the underlying mechanisms
remain unclear.
Due to the potential ability to assess the
characteristics of brain tissue noninvasively, magnetic resonance
imaging (MRI) is currently the main method used for diagnosis,
treatment guidance and monitoring of brain metastasis (14,15).
However, identifying early changes in the cerebral tissue of
patients with lung cancer without brain metastasis is difficult
(16). Fortunately, MRI images of
cerebral tissues contain a wealth of information invisible to the
human eye that can accessed by texture analysis (TA) techniques
(17). Texture is a visual stimulus
generated by repetitive image patterns, which can be depicted as
regular or irregular, smooth or rough and fine or coarse. Although
they can exhibit very complex patterns, some textures can be
extracted relatively easily even by visual assessment due to the
regular visual appearance of the patterns. However, most textures
display random patterns because textural primitives are random,
making them difficult to recognize and interpret. In medical
images, these types of random textures are more common than regular
patterns. As texture is an image feature defined by both pixel
locations and brightness value, TA enables mathematical calculation
of the patterns. Therefore, texture features provide the potential
to distinguish and characterize properties of cerebral tissue
(17).
The aim of the present study was to use texture
analysis (TA) methods to describe early cerebral tissue damage
caused by lung cancer without brain metastasis.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University (Jinan, China). Written informed consent was obtained
from all patients prior to the study start. Patients with lung
cancer were recruited from the Shandong Provincial Hospital between
December 2017 and October 2018. The TA study included 50
consecutive patients (36 males, 14 females; mean age, 60 years; age
range 47–75 years) who were all diagnosed by pathology and met the
diagnostic criteria for primary lung cancer according to the World
Health Organization (18). In
addition to a detailed review of pathological specimens by
pathologists upon admission to the hospital, all patients with lung
cancer underwent a careful physical examination performed by
experienced oncologists and neurologists from Shandong Provincial
Hospital Affiliated to Shandong University, in order to determine
the clinical stage, Karnofsky Performance Scale (KPS) score
(19) and numeric rating scale score
(NRS) (20). The demographic
information and clinical characteristics of the patients are
presented in Table I. The inclusion
criteria were as follows: i) Age, 18–75 years; ii) histological
type of small cell carcinoma, squamous cell carcinoma,
adenocarcinoma and others; iii) KPS score >60 and iv) no
neurological abnormalities. The exclusion criteria were as follows:
i) Age, <18 years or >75 years; ii) systemic illness such as
systemic lupus erythematosus, serious vascular disease, head
trauma, epilepsy (healthy controls) or claustrophobia; iii)
long-term use of psychoactive medication and iv) refusal to undergo
MRI. The control group (CG) comprised of 57 age- and sex-matched
healthy volunteers. The individuals in this group reported no types
of malignant tumor or any systemic, neurological or psychiatric
illnesses known to affect cerebral structure or function such as a
long history of psychoactive medication use, head trauma, serious
vascular disease, current depression, epilepsy or alcoholism.
 | Table I.Clinical characteristics of the
studied groups. |
Table I.
Clinical characteristics of the
studied groups.
|
Characteristics | Patient, n=50 | Control, n=57 |
|---|
| Age in years, mean
± standard deviation | 60.0 ± 7.2 | 59.1 ± 6.5 |
| Age range,
years | 47–75 | 48–75 |
| Gender, Female vs.
Male | 36/14 | 37/20 |
| Histological type,
n |
|
|
|
Adenocarcinoma | 29 | – |
|
Squamous | 17 | – |
| Small
cell | 4 | – |
| Clinical stage |
|
|
|
Early | 18 | – |
|
Advanced | 32 | – |
| Karnofsky
performance scale |
|
|
|
≥70 | 49 | – |
|
<70 | 1 | – |
| Numeric rating
scales |
|
|
| ≤3 | 37 | – |
|
>3 | 13 | – |
MRI examinations
Data were acquired within 2 weeks of diagnosis of
lung cancer primarily due to some patients being diagnosed in other
hospitals and then transferred to Shandong Provincial Hospital for
further antitumor treatment. Following diagnosis, all patients
underwent conventional MRI using a 3.0-T scanner (Philips Medical
Systems B.V.) equipped with an 8-channel head and neck coil. The
MRI machine was included in a detailed quality control plan
requiring daily, monthly and quarterly inspections. The radio
frequency amplifier properties and main magnetic field homogeneity
of the MRI machine were measured and controlled quarterly. The
following conventional MRI sequences were employed: Sagittal,
coronal and axial T2-weighted imaging (T2WI), T1-weighted imaging
(T1WI), fluid-attenuated inversion recovery imaging,
diffusion-weighted imaging, apparent diffusion coefficient and
enhanced T1WI. The sequences employed in the MRI protocol are
presented in Table II.
 | Table II.MRI sequences included in the
magnetic resonance protocol of the present study. |
Table II.
MRI sequences included in the
magnetic resonance protocol of the present study.
| Sequence | TR | TE | TI | Slice/gap | Matrix | FOV | Flip angle |
|---|
| Axial T2WI | 4,000 | 100 | 0 | 6.0/1.0 | 384×384 | 230 | 90 |
| Axial TIWI–IR | 3,000 | 43 | 1,150 | 6.0/1.0 | 264×264 | 230 | – |
| Axial FLAIR | 9,000 | 148 | 2,500 | 6.0/1.0 | 284×284 | 230 | – |
| DWI | 2,257 | 71 | 0 | 6.0/1.0 | 152×152 | 230 | 90 |
| Axial T1W-STIR | 3,000 | 43 | 1,150 | 6.0/1.0 | 264×264 | 230 | – |
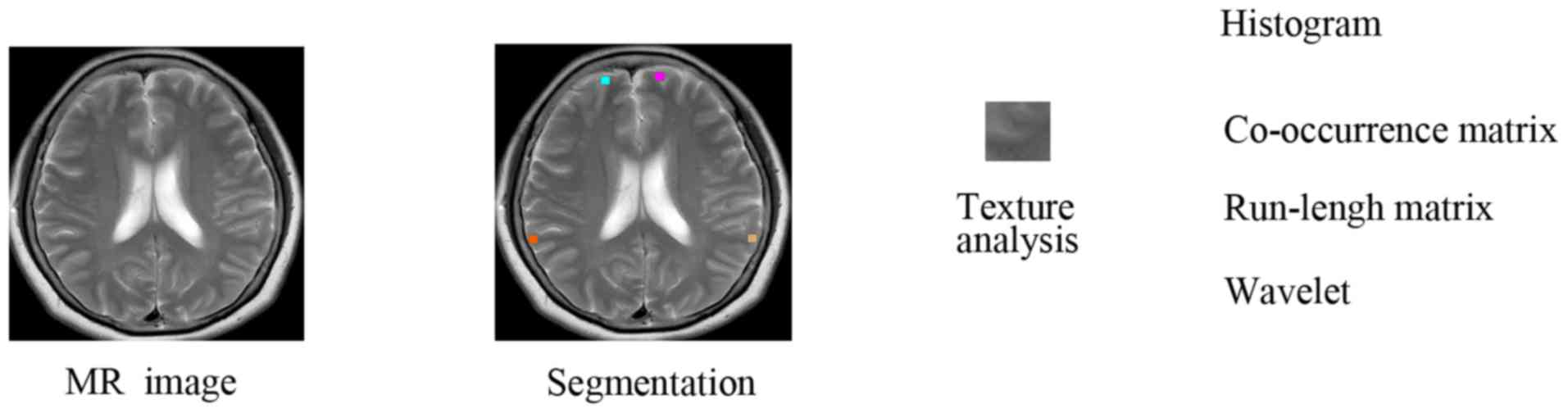
MR image segmentation
Image selection was carried out using a DICOM viewer
(version 3.0; Philips Medical Systems B.V.). For each MR image,
regions of interest (ROIs) were drawn symmetrically by hand on
bilateral sides of the hemispheres at each level of interest
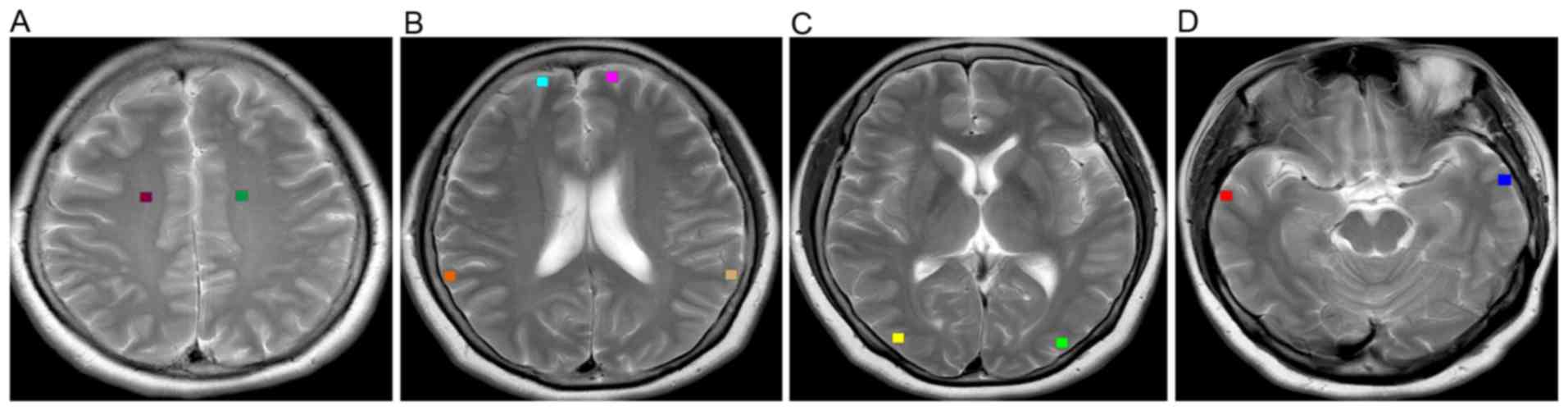
(21,22). In the present study, 16×16-pixel ROIs
were manually drawn on the frontoparietal white matter in the
centrum semiovale (Fig. 1A), frontal
cortices and parietal cortices at the level of the body of the
lateral ventricles (Fig. 1B),
occipital cortices at the level of the basal ganglia (Fig. 1C) and temporal cortices at the level
of the midbrain (Fig. 1D). The ROIs
were carefully placed by two experienced neuroradiologists from
Shandong Provincial Hospital Affiliated to Shandong University, to
avoid overlap with any areas of microhemorrhage, hyperintensity or
macroscopic hemosiderin depositions which may occur in some
patients. Fig. 2 presents an array
of texture blocks extracted from the cerebral tissues of a patient
with lung cancer, without metastasis and a healthy volunteer.
TA analysis technique
The axial T2WI sequence was selected from the whole
MRI study for TA as T2-weighted MR imaging has been demonstrated to
be sensitive to tissue abnormalities in the human brain (23). Only single slices were evaluated to
obtain a maximum in-plane resolution by avoiding the lower
resolution or interpolation, typically caused by 3-dimensional TA
(24). Further analysis was
performed using 4 MRI image slices selected from the T2WI sequence
at 4 interest levels. The texture features in 10 ROIs were assessed
based on the histogram, gray-level co-occurrence matrix (GLCM),
gray-level run length matrix (GLRLM) and wavelet transform, as
summarized in Table III (17,25,26). The
wavelet transform of the three dimensional image was the
decomposition of the image along the x, y and z directions using
low-pass filter (L) and high-pass filter (H), and each layer of
wavelet decomposition decomposed the image data into eight
different frequency bands (LLH, HLL, HLH, HHL, LHL, LHH, HHH and
LLL), which included seven high frequency bands (LLH-HHH) and one
low frequency band (LLL).
 | Table III.List of texture features. |
Table III.
List of texture features.
| Histogram | Gray-level
co-occurrence matrix | Gray-level run
length matrix | Wavelet |
|---|
| Mean | Joint energy | Long run
emphasis | HHH_kurtosis |
| Skewness | Inverse
difference | Run length
non-uniformity | HHL_maximum |
| Deviation | Correlation | Low gray-level run
emphasis | HLH_skewness |
| Variance | Difference
average | Short run low
gray-level emphasis | LLL_10
percentile |
| Kurtosis | Difference
entropy | Long run low
gray-level emphasis | LLL_median |
|
| Inverse difference
normalized | Short run high
gray-level emphasis | LLL_minimum |
|
| Joint entropy | Long run high
gray-level emphasis | LLL_variance |
|
| Sum average | Short run
emphasis |
|
|
| Sum entropy | Run gray-level
non-uniformity |
|
|
|
| Run percentage |
|
|
|
| High gray-level run
emphasis |
|
The first order statistics image properties derived
from the gray-scale frequency histogram depend solely on individual
pixel values. Second order statistics are properties of pixel pairs
and describe the relationship between pixels and their grey levels.
However, the first and second order statistics of images in texture
analysis only contained the amplitude information of the image,
ignoring the phase information, thus the wavelet transform of the
image was also analyzed. TA was performed using the Python language
(version 3.6) on the JetBrains PyCharm platform (version 2018.1.3).
Feature calculations were performed within the Pyradiomics package
(version 2.2.0), which is an open-source Python package designed
for the extraction of radiomic features from medical images
(27). As the Fisher criterion can
produce an array of features with highly discriminative power, a
feature selection method was applied using the Fisher coefficient
provided by the Python package, in order to identify the six
texture features with the highest discriminatory potential for
classification (17).
Statistical analysis
In total, 32 texture parameters were tested to
determine which and how many features in each ROI demonstrated
significant differences between patients with lung cancer, without
brain metastasis and healthy controls. Statistical analyses were
performed for each texture feature. Differences in texture features
between the two groups were analyzed via the Wilcoxon rank sum test
or Mann-Whitney U test, using SPSS software (version 22; IBM
Corp.). The Fisher coefficient was used to select the six most
discriminative parameters. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis workflow
Given brain MRI data sets of patients with lung
cancer without brain metastasis and healthy controls, four interest
levels were selected, and then texture blocks of 16×16 pixels were
manually placed in a symmetrical manner on bilateral sides of the
hemispheres at each level of interest. Finally, texture features
were calculated based on the image histogram, the co-occurrence
matrix, the run-length matrix and wavelets. The system diagram
presented in Fig. 3 represents the
process followed for MRI analysis.
Brain MRI of patients with lung cancer
and healthy controls
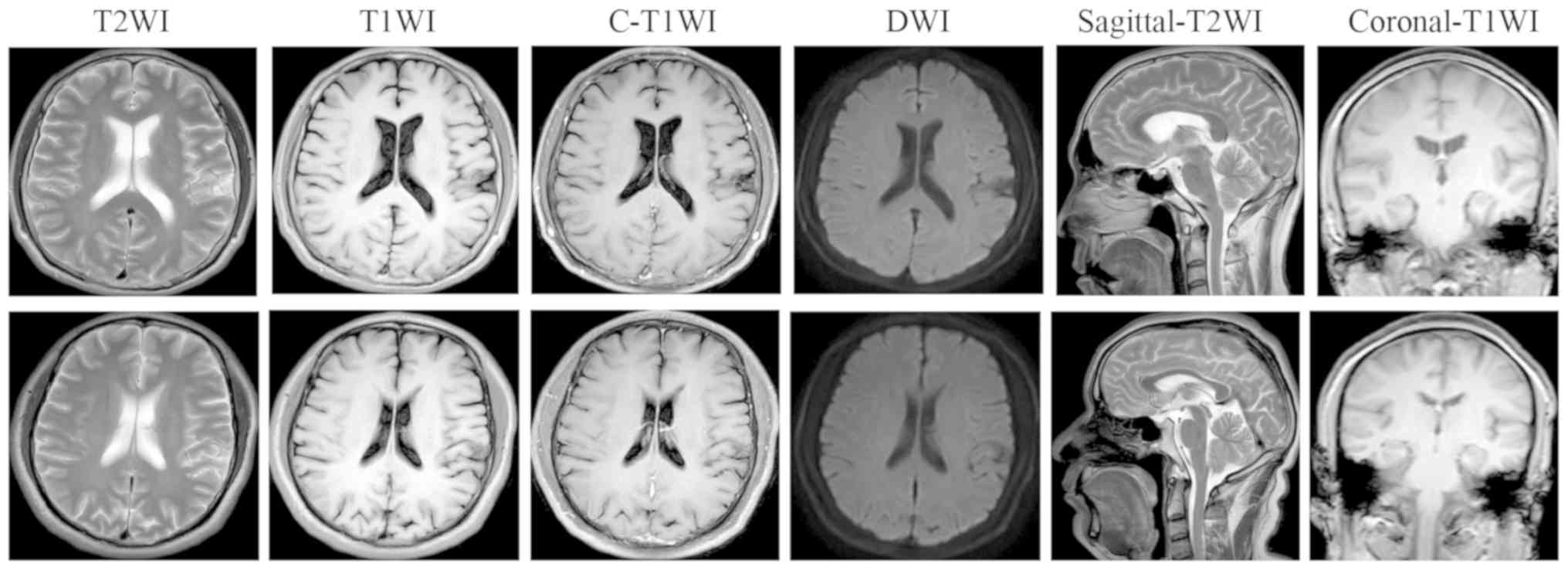
In this study, conventional MRI enabled detection of
lesions related to brain metastasis or primary brain tumors only
when gross structural abnormalities could be visually detected.
Based on the conventional MR images, no significantly different
signals were found in any sequence in patients with lung cancer
without metastasis compared with healthy volunteers (Table II). Fig.
4 shows conventional MR images of a 45-year-old female patient
who presented with lung cancer without brain metastasis (top row)
and a 54-year-old male healthy control (bottom row).
Comparison of texture parameters in
bilateral hemispheres between patients with lung cancer without
brain metastasis and healthy controls
For the comparison of bilateral hemispheres of 10
ROIs, 32 features were tested for each texture block to determine
which and how many of the parameters differed statistically between
patients with primary lung cancer without brain metastasis and
healthy controls. The number of texture parameters (n=32) which
were significantly different were analyzed using the Wilcoxon test
(Table IV). The feature vector
included mean and variance features from histogram statistics,
energy and correlation features from a co-occurrence matrix,
short-run and long-run emphasis features from a run-length matrix
and phase information features from wavelet transform. The 32
texture parameters included; Five histogram-based parameters, nine
GLCM-based parameters, 11 GLRLM-based parameters and seven
wavelet-based parameters. Among the five histogram-based
parameters, which depended solely on individual pixel gray-level
values, the following four parameters; mean, skewness, deviation
and variance were significantly different (P<0.05), and only the
kurtosis parameter demonstrated no significant difference
(P=0.686). Among the nine GLCM-based parameters, seven parameters
were significantly different; however, the parameters for
correlation (P=0.435) and normalized inverse difference (P=0.156)
demonstrated no significant differences. All 11 GLRLM-based
parameters were significantly different (P<0.05). Among the
seven wavelet-based parameters, five parameters were significantly
different (P<0.05), whereas wavelet-HHH_kurtosis and
wavelet-HLH_skewness failed to demonstrate statistically
significant differences (P>0.05, respectively) (Table IV). There were 27 texture parameters
with a statistically significant difference including 4
histogram-based parameters, 7 GLCM-based parameters, 11 GLRLM-based
parameters and 5 wavelet-based parameters. The feature parameters
with statistically significant differences were in first order
features, second order features and wavelets. For instance, mean
indicated the average density among the texture blocks; deviation
calculated the statistical variability of density among the pixels
in the blocks; sum average indicated the total number of correlated
pixel pairs with the same sum in the block; gray-level
non-uniformity referred to the orderliness or randomness of the
pixel densities among the blocks which could denote the degree of
structure, and gray-level run length emphasis measured consecutive
pixels of the same density along particular orientations as another
representation of structure.
 | Table IV.Comparison of texture features
between patients with lung cancer and healthy controls. |
Table IV.
Comparison of texture features
between patients with lung cancer and healthy controls.
| Feature | Patient, mean
(SD) | Control, mean
(SD) | P-value |
|---|
| Kurtosis | 3.649 (1.88) | 3.707 (2.57) | 0.686 |
| Mean | 544.176
(167.18) | 688.647
(209.26) | <0.001 |
|
Skewness | 0.614 (0.61) | 0.465 (0.67) | 0.001 |
|
Deviation | 64.877 (42.55) | 78.146 (47.07) | <0.001 |
|
Variance | 6,016.221
(460.30) | 8,319.324
(434.89) | <0.001 |
| Correlation | 0.806 (0.14) | 0.800 (0.15) | 0.435 |
| Difference
average | 0.873 (0.43) | 1.063 (0.51) | <0.001 |
| Difference
entropy | 1.584 (0.41) | 1.749 (0.45) | <0.001 |
| Inverse
difference | 0.674 (0.09) | 0.631 (0.09) | <0.001 |
| Inverse difference
normalized | 0.941 (0.01) | 0.939 (0.01) | 0.156 |
| Joint
energy | 0.073 (0.06) | 0.057 (0.06) | <0.001 |
| Joint
entropy | 4.772 (1.16) | 5.193 (1.22) | <0.001 |
| Sum
average | 11.151 (5.34) | 14.097 (8.38) | <0.001 |
| Sum
entropy | 3.791 (0.85) | 4.044 (0.90) | <0.001 |
| Gray-level
non-uniformity | 22.665 (9.16) | 21.697 (10.55) | <0.001 |
| High gray-level
run emphasis | 52.799 (65.99) | 86.091
(127.81) | <0.001 |
| Long run
emphasis | 4.592 (2.71) | 3.605 (1.98) | <0.001 |
| Long run high
gray-level emphasis | 135.634
(99.71) | 195.944
(295.67) | <0.001 |
| Long run low
gray-level emphasis | 0.640 (0.94) | 0.403 (0.59) | <0.001 |
| Low gray-level
run emphasis | 0.108 (0.08) | 0.086 (0.07) | <0.001 |
| Run length
non-uniformity | 80.196 (33.18) | 96.709 (34.77) | <0.001 |
| Run
percentage | 0.618 (0.11) | 0.673 (0.10) | <0.001 |
| Short run
emphasis | 0.694 (0.10) | 0.741 (0.09) | <0.001 |
| Short run high
gray-level emphasis | 43.297 (60.87) | 71.963
(107.37) | <0.001 |
| Short run low
gray-level emphasis | 0.067 (0.04) | 0.058 (0.04) | <0.001 |
|
wavelet-HHH_kurtosis | 8.636 (7.28) | 8.702 (7.19) | 0.932 |
|
wavelet-HHL_maximum | 35.940 (30.20) | 45.996 (40.29) | <0.001 |
|
wavelet-HLH_skewness | −0.224 (1.21) | −0.263 (1.34) | 0.930 |
| wavelet-LLL_10
percentile | 1,358.451
(402.93) | 1,689.975
(524.57) | <0.001 |
|
wavelet-LLL_median | 1,544.083
(19.83) | 1,927.296
(585.72) | <0.001 |
|
wavelet-LLL_minimum | 1,257.445
(375.73) | 1,538.135
(509.09) | <0.001 |
|
wavelet-LLL_variance | 46,637.723
(84568.84) | 64,229.389
(80765.98) | <0.001 |
Identification of the six most
discriminative parameters between patients with lung cancer without
brain metastasis and healthy controls
When the texture parameters of 10 ROIs were compared
in bilateral hemispheres between the lung cancer group and the
healthy control group, 27 of the 32 were significantly different.
Among the 27 significantly different parameters, six texture
features were identified using the Fisher coefficient provided by
the Python package. The Fisher criterion could produce a set of
features with a high discriminatory potential for separation and
classification which were also highly associated with each other.
Based on the Fisher coefficient and the values obtained by the
Wilcoxon test, the six most discriminative parameters for
differences between patients and healthy controls were selected.
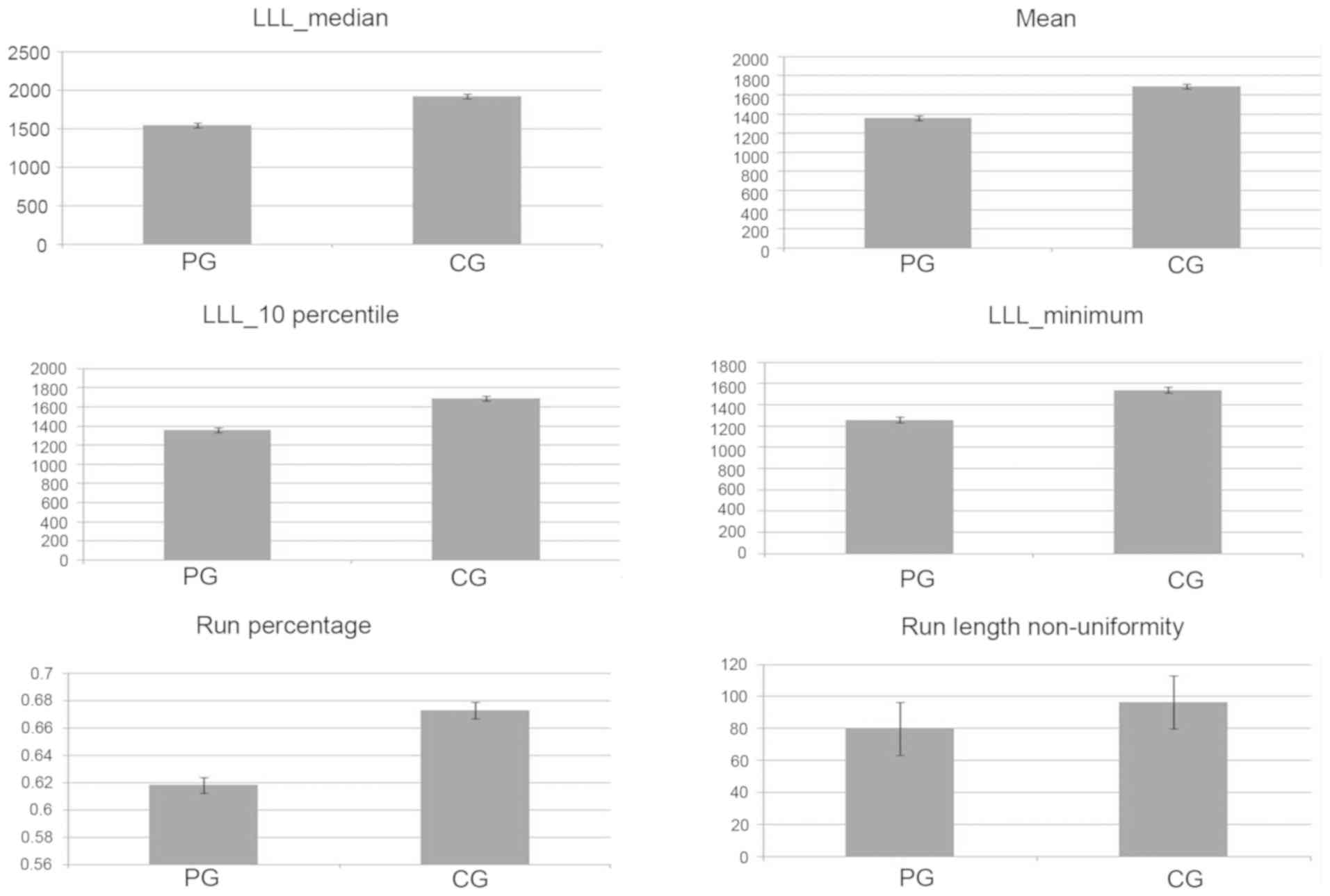
These six texture parameters were the wavelet-LLL_median, mean,
wavelet-LLL_ 10 percentile, wavelet-LLL_ minimum, run percentage,
and run length non-uniformity. The six most discriminative texture
features were extracted from the first-order statistics,
second-order statistics and wavelets. However, the six texture
features were mainly based on the wavelets and GLRLM features
(Fig. 5).
Comparison of the top six texture
parameters between the left and right hemispheres in patients with
lung cancer without brain metastasis and healthy controls
The comparison of bilateral hemispheres of patients
with lung cancer and healthy controls, proved that TA had a high
discriminatory potential, but its stability needed to be further
confirmed. Firstly, the difference between bilateral hemispheres in
the controls was compared using the six most discriminative
parameters (Table V). For these six
texture parameters, there were no significant differences between
the bilateral hemispheres based on the Wilcoxon test. Subsequently,
the six most discriminative parameters were used to find out if
primary lung cancer had the same impact on the bilateral
hemispheres. For these six texture parameters, there were no
significant differences between the bilateral hemispheres in
patients with lung cancer (Table
V).
 | Table V.Comparison of the top six texture
features between the left and right hemispheres in patients with
lung cancer, without brain metastasis and healthy controls. |
Table V.
Comparison of the top six texture
features between the left and right hemispheres in patients with
lung cancer, without brain metastasis and healthy controls.
| Feature | Patients Right,
mean (SD) | Left, mean
(SD) | P-value | Controls Right,
mean (SD) | Left, mean
(SD) | P-value |
|---|
| LLL_median | 1,526.338
(442.99) | 1,561.828
(478.29) | 0.492 | 1,906.664
(580.25) | 1,947.927
(591.44) | 0.364 |
| Mean | 548.260
(160.31) | 560.092
(173.88) | 0.554 | 681.227
(206.92) | 696.062
(211.67) | 0.375 |
| LLL_10
percentile | 1,344.940
(387.64) | 1,371.958
(417.95) | 0.468 | 1,668.751
(520.21) | 1,711.199
(528.96) | 0.284 |
| LLL_minimum | 1,246.636
(365.12) | 1,268.253
(386.43) | 0.528 | 1,536.064
(488.56) | 1,540.206
(529.67) | 0.590 |
| Run percentage | 0.616 (0.11) | 0.621 (0.11) | 0.665 | 0.672 (0.10) | 0.673 (0.10) | 0.890 |
| Run length
non-uniformity | 19.620 (32.86) | 80.760 (33.54) | 0.692 | 96.930 (35.30) | 96.470 (34.29) | 0.875 |
Comparison of the top six texture
parameters between gray and white matter in patients with lung
cancer without brain metastasis and healthy controls
The top six texture parameters were tested to find
out how many and which of them differed statistically between gray
and white matter. The texture features of gray and white matter in
patients with lung cancer and healthy controls were also analyzed
using the Wilcoxon test and based on the top six texture
parameters. An identical trend between gray and white matter was
observed in the patient and control groups. Among the six
parameters, four parameters were significantly different between
the patient and control groups. These four parameters were
wavelet-LLL_median, wavelet-LLL_ 10 percentile, wavelet-LLL_
minimum, and run length non-uniformity. The parameters of mean and
run percentage showed no significant difference (Table VI).
 | Table VI.Comparison of the top six texture
features between gray matter and white matter in patients with lung
cancer, without brain metastasis and healthy controls. |
Table VI.
Comparison of the top six texture
features between gray matter and white matter in patients with lung
cancer, without brain metastasis and healthy controls.
|
| Patients | Controls |
|---|
|
|
|
|
|---|
| Feature | Gray matter, mean
(SD) | White matter, mean
(SD) | P-value | Gray matter, mean
(SD) | White matter, mean
(SD) | P-value |
|---|
| LLL_median | 1,578.194
(470.68) | 1,407.642
(392.67) | 0.001 | 1,974.210
(599.28) | 1,739.639
(486.80) | <0.001 |
| Mean | 567.995
(170.78) | 498.900
(139.48) | <0.001 | 706.957
(213.88) | 615.395
(171.85) | <0.001 |
| LLL_10
percentile | 1,362.905
(410.33) | 1,340.638
(373.17) | 0.608 | 1,694.792
(538.49) | 1,670.707
(466.43) | 0.661 |
| LLL_minimum | 1,250.471
(379.24) | 1,285.339
(361.70) | 0.389 | 1,521.724
(522.89) | 1,603.779
(445.74) | 0.124 |
| Run percentage | 0.647 (0.09) | 0.504 (0.09) | <0.001 | 0.703 (0.08) | 0.549 (0.09) | <0.001 |
| Run length
non-uniformity | 88.046 (30.90) | 48.790 (21.37) | <0.001 | 106.039
(30.97) | 59.380 (21.65) | <0.001 |
Comparison of texture parameters
between patients with lung cancer without brain metastasis and
healthy controls in cerebral gray and white matter
The aforementioned six most discriminative
parameters were used to determine which differed statistically
between patients with lung cancer without brain metastasis and
healthy controls in different cerebral regions. In white matter
regions, all six texture parameters analyzed with Wilcoxon test
showed significant differences between patients and controls
(Table VII). The six most
discriminative parameters were subsequently tested to determine
whether primary lung cancer has the same effect on the gray matter
regions. For these six texture parameters, all showed significant
differences between patients and controls (Table VII).
 | Table VII.Comparison of the top six texture
features between cerebral gray and white matter in patients with
lung cancer, without brain metastasis and healthy controls. |
Table VII.
Comparison of the top six texture
features between cerebral gray and white matter in patients with
lung cancer, without brain metastasis and healthy controls.
|
| Gray matter | White matter |
|---|
|
|
|
|
|---|
| Feature | Patients, mean
(SD) | Controls, mean
(SD) | P-value | Patients, mean
(SD) | Controls, mean
(SD) | P-value |
|---|
| LLL_median | 1,578.194
(470.68) | 1,974.210
(599.28) | <0.001 | 1,407.642
(392.67) | 1,739.639
(486.80) | <0.001 |
| Mean | 567.995
(170.78) | 706.957
(213.88) | <0.001 | 498.900
(139.48) | 615.395
(171.85) | <0.001 |
| LLL_10
percentile | 1,362.905
(410.33) | 1,694.792
(538.49) | <0.001 | 1,340.638
(373.17) | 1,670.707
(466.43) | <0.001 |
| LLL_minimum | 1,250.471
(379.24) | 1,521.724
(522.89) | <0.001 | 1,285.339
(361.70) | 1,603.779
(445.74) | <0.001 |
| Run percentage | 0.647 (0.09) | 0.703 (0.08) | <0.001 | 0.504 (0.09) | 0.549 (0.09) | <0.001 |
| Run length
non-uniformity | 88.046 (30.90) | 106.039
(30.97) | <0.001 | 48.790 (21.37) | 59.380 (21.65) | <0.001 |
Comparison of the texture features by
clinical stage in patients with lung cancer without brain
metastasis
To investigate the relationship between the texture
parameters of brain MR images and clinical lung cancer stages,
which was a revised 2017 edition of the American Joint Committee on
Cancer (AJCC) Cancer Staging Manual (28,29),
patients with lung cancer, without brain metastasis were divided
into two groups: Stage I+II and stage III+IV. The top six texture
parameters were tested to find out how many and which of them
differed statistically between the 2 patient groups. All of the six
texture parameters were significantly different between the patient
groups (Table VIII). Compared to
the stage I+II group, all six parameters were significantly greater
in the stage III+IV group (Table
VIII).
 | Table VIII.Comparison of the top six texture
features between different clinical stages in patients with lung
cancer, without brain metastasis. |
Table VIII.
Comparison of the top six texture
features between different clinical stages in patients with lung
cancer, without brain metastasis.
| Feature | Stage I+II, mean
(SD) | Stage III+IV, mean
(SD) | P-value |
|---|
| LLL_median | 1,348.190
(196.51) | 1,649.503
(525.46) | <0.001 |
| Mean | 482.068.995
(72.08) | 592.987
(190.12) | <0.001 |
| LLL_10
percentile | 1,181.089
(183.65) | 1,453.300
(455.26) | <0.001 |
| LLL_minimum | 1,093.485
(171.46) | 1,344.760
(425.07) | <0.001 |
| Run percentage | 0.580 (0.10) | 0.636 (0.11) | <0.001 |
| Run length
non-uniformity | 70.024 (27.21) | 85.705 (34.94) | <0.001 |
Comparison of the top six texture
features by histological type
The main histological types of lung cancer are
squamous cell carcinoma, adenocarcinoma and small cell lung cancer
(SCLC). The top six texture parameters were compared among these
groups to establish associations between the brain MRI texture
parameters and the histological types (Table IX). The top six texture parameters
showed no significant differences between the squamous cell and
adenocarcinoma groups but were all significantly different between
the SCLC group compared with the other groups. Compared to the
adenocarcinoma group, the SCLC group showed significantly increased
texture parameters; similar results were observed between the
squamous cell carcinoma and SCLC groups.
 | Table IX.Comparison of the top six texture
features between different histological types in patients with lung
cancer, without brain metastasis. |
Table IX.
Comparison of the top six texture
features between different histological types in patients with lung
cancer, without brain metastasis.
|
| Mean (SD) | P-value |
|---|
|
|
|
|
|---|
| Feature | Ad | Sq | Sm | Ad vs. Sq | Ad vs. Sm | Sq vs. Sm |
|---|
| LLL_median | 1,450.823
(18.75) | 1,544.848
(449.86) | 2,565.255
(451.93) | 0.334 | <0.001 | <0.001 |
| Mean | 519.909
(124.32) | 555.842
(164.23) | 921.111
(158.10) | 0.234 | <0.001 | <0.001 |
| LLL_10
percentile | 1,274.496
(300.34) | 1,362.517
(378.48) | 2,268.574
(413.46) | 0.141 | <0.001 | <0.001 |
| LLL_minimum | 1,176.569
(281.63) | 1,267.834
(350.97) | 2,084.727
(425.21) | 0.051 | <0.001 | <0.001 |
| Run percentage | 0.603 (0.11) | 0.623 (0.09) | 0.759 (0.06) | 0.145 | <0.001 | <0.001 |
| Run length
non-uniformity | 75.889 (31.78) | 80.426 (30.69) | 126.191
(28.12) | 0.158 | <0.001 | <0.001 |
Discussion
In light of recent advances in the acquisition and
analysis of MR images for the high-throughput extraction of imaging
feature information, medical imaging can now be used to quantify
the distinguishing features of tumor tissues (30–32).
This approach is distinct from prior subjective or qualitative
methods (14). Applying TA to
medical imaging features has been a significant field of research
and has generated an extensive body of literature (33–35). It
has been demonstrated that MR images include tissue-specific
texture features that can be extracted by mathematical methods
(30). TA can be used to distinguish
pathological from healthy human cerebral tissue and to classify
different types of cerebral tissue (15,36,37). TA
enables the discrimination of multiple sclerosis lesions from
normal and normal-appearing white matter (38). However, previous studies have mainly
focused on detecting small nodules, that is, the lesions
themselves. This study is distinct in that it primarily
characterizes early cerebral tissue damage caused by lung cancer
before metastasis by applying TA methods. The TA of MRI features
can also be used for classifying different cerebral tissues and
structures in brain MR images (36,39).
MR images were selected in the current study to
detect textural differences in cerebral tissue between patients
with primary lung cancer and healthy controls. Of a total of 32
texture parameters tested, 27 parameters were significantly
different between patients with lung cancer and healthy controls.
The results of this study indicate that in patients with lung
cancer, there are differences in the texture parameters of brain MR
images of cerebral tissues. It can therefore be inferred that lung
cancer can cause early brain tissue damage prior to metastasis.
Among the 27 significantly different parameters, six
texture features that best discriminated between patients and
healthy controls were identified by calculating Fisher
coefficients. These texture parameters were the LLL_median, mean,
LLL_percentile, LLL_minimum, run percentage and run length
non-uniformity. Using these six parameters, the two cerebral
hemispheres and gray and white matter were compared between and
within the participant groups. First, the left and right
hemispheres of cerebral tissue were compared in the controls, as
well as the gray and white matter. It is well known that the human
brain is symmetrical in structure and with respect to gray and
white matter, there are obvious differences in both composition and
function. Gray matter is mainly composed of the cell bodies of
neurons, but white matter is principally surrounded by the myelin
sheaths of neurons. In regard to functionality, the former contains
nerve centers, which play a major role in generating neural
activity; the latter, on the other hand, represents the main
transmission pathways carrying the neural signals generated by the
brain and the gray matter of the spinal cord (40,41). The
results of the present study show that the texture parameters were
not significantly different between the 2 hemispheres, but 4 of 6
texture parameters were significantly different between gray and
white matter. It can therefore be concluded that TA applied to
brain MR images is stable and reliable. This analysis was repeated
in the patient group and an identical trend was observed, which may
indicate that primary lung lesions can cause similar early damage
in both hemispheres and that TA can be used to classify different
structures. By comparison of different regions of gray and white
matter between patients with lung cancer and healthy controls, all
six texture parameters were significantly different in both gray
matter and white matter. It can be assumed that the overt texture
changes are caused by the lung cancer and this leads to similar
impairments in both gray and white matter.
Clinical stage is used to describe the severity and
range of involvement of malignant tumors according to the original
tumor and degree of spread in an individual, which is the basis for
understanding the extent of the disease. This information can help
doctors develop appropriate treatment plans and understand the
prognosis and outcome of the disease. The numerically higher the
stage, the more advanced the tumor progression and the worse the
prognosis (42). The present study
analyzed associations between the clinical stages of lung cancer
and texture parameters of MR images. The results revealed that,
compared with the early-stage group (stage I+II), the
advanced-stage group (stage III+IV) showed a more significant
increase in all six parameters. This may indicate that increased
parameters are related to more advanced clinical stages of lung
cancer.
In addition to the clinical stage, the choice of
tumor treatment is also related to the pathological type of the
tumor (18,28,43–46).
Associations with the histological type were also analyzed in this
study. The results show that compared to adenocarcinoma group, the
SCLC group showed significantly increased texture parameters;
similar results were found between the squamous cell carcinoma and
SCLC groups. However, the texture parameters assessed in the
adenocarcinoma and squamous cell carcinoma groups were not
significantly different. It is necessary to note that compared with
adenocarcinoma and squamous cell carcinoma, SCLC has a higher
degree of malignancy and a worse prognosis (47,48). The
significant differences in texture parameters between SCLC and
adenocarcinoma or squamous cell carcinoma are possibly attributable
to these differences. It can be assumed that greater texture
changes are caused by greater degrees of malignancy.
There are several limitations of this study.
Firstly, as this is a preliminary experiment, the sample size was
relatively small. Furthermore, a limited set of features, rather
than a larger set of multiple hundreds or thousands (compound) of
features was selected for analysis because the selected texture
features have consistently been shown to be effective in the
analysis of medical images (25,38,49–51) and
robust against variations between scanners and protocol parameters
(52) especially after normalization
(53). Finally, in the absence of
confirmation of the early damage to human brain tissue, further
pathological investigation using an animal model is required.
In conclusion, this small pilot study indicates that
TA with a variable set of texture features can serve as an adjuvant
diagnostic tool to traditional MRI to detect early damage in
patients with lung cancer without brain metastasis. TA may serve as
a new method for the study of tumor metastasis and paraneoplastic
syndromes.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81670046).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX acquired the data, performed the analyses and
wrote the initial manuscript. XC and BW performed texture analysis.
GW, MH, RL and YQ acquired the data. JX and QY selected ROIs of the
MR images. ZL and MH made substantial contributions to the
conception and design of the present study. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Shandong Provincial Hospital Affiliated to Shandong
University (Jinan, China). Written informed consent was obtained
from all patients prior to the study start.
Patient consent for publication
All patients signed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr..Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, et al: Systemic therapy for stage iv non-small-cell lung
cancer: American Society of clinical oncology clinical practice
guideline update. J Clin Oncol. 33:3488–3515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sul J and Posner JB: Brain metastases:
Epidemiology and pathophysiology. Cancer Treat Res. 136:1–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited-the role of tumor-stroma interactions in
metastasis to different organs. Int J Cancer. 128:2527–2535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1998. CA Cancer J Clin. 48:6–29. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawaya R: Considerations in the diagnosis
and management of brain metastases. Oncology. 15:1145–1163.
2001.
|
|
8
|
Leypoldt F and Wandinger KP:
Paraneoplastic neurological syndromes. Clin Exp Immunol.
175:336–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gassmann P, Haier J, Schlüter K,
Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider
SW, Homey B and Müller A: CXCR4 regulates the early extravasation
of metastatic tumor cells in vivo. Neoplasia. 11:651–661. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erler JT, Bennewith KL, Cox TR, Lang G,
Bird D, Koong A, Le QT and Giaccia AJ: Hypoxia-induced lysyl
oxidase is a critical mediator of bone marrow cell recruitment to
form the premetastatic niche. Cancer Cell. 15:35–44. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Erler JT and Weaver VM: Three-dimensional
context regulation of metastasis. Clin Exp Metastasis. 26:35–49.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiratsuka S, Goel S, Kamoun WS, Maru Y,
Fukumura D, Duda DG and Jain RK: Endothelial focal adhesion kinase
mediates cancer cell homing to discrete regions of the lungs via
E-selectin up-regulation. Proc Natl Acad Sci USA. 108:3725–3730.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta GP and Massague J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aerts HJ, Velazquez ER, Leijenaar RT,
Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R,
Haibe-Kains B, Rietveld D, et al: Decoding tumour phenotype by
noninvasive imaging using a quantitative radiomics approach. Nat
Commun. 5:40062014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdullah N, Ngah UK and Aziz SA: Image
classification of brain MRI using support vector machine. 2011 IEEE
International Conference on Imaging Systems and Techniques.
242–247. 2011. View Article : Google Scholar
|
|
16
|
Connell JJ, Chatain G, Cornelissen B,
Vallis KA, Hamilton A, Seymour L, Anthony DC and Sibson NR:
Selective permeabilization of the blood-brain barrier at sites of
metastasis. J Natl Cancer Inst. 105:1634–1643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Holli KK, Harrison L, Dastidar P, Waljas
M, Liimatainen S, Luukkaala T, Ohman J, Soimakallio S and Eskola H:
Texture analysis of MR images of patients with mild traumatic brain
injury. BMC Med Imaging. 10:82010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health organization
classification of lung tumors: Impact of Genetic, Clinical and
Radiologic Advances Since the 2004 Classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chernov MF, Nakaya K, Izawa M, Hayashi M,
Usuba Y, Kato K, Muragaki Y, Iseki H, Hori T and Takakura K:
Outcome after radiosurgery for brain metastases in patients with
low Karnofsky performance scale (KPS) scores. Int J Radiat Oncol
Biol Phys. 67:1492–1498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karcioglu O, Topacoglu H and Dikme O and
Dikme O: A systematic review of the pain scales in adults: Which to
use? Am J Emerg Med. 36:707–714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimny A, Szmyrka-Kaczmarek M, Szewczyk P,
Bladowska J, Pokryszko-Dragan A, Gruszka E, Wiland P and Sasiadek
M: In vivo evaluation of brain damage in the course of systemic
lupus erythematosus using magnetic resonance spectroscopy,
perfusion-weighted and diffusion-tensor imaging. Lupus. 23:10–19.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bladowska J, Zimny A, Knysz B, Malyszczak
K, Koltowska A, Szewczyk P, Gasiorowski J, Furdal M and Sasiadek
MJ: Evaluation of early cerebral metabolic, perfusion and
microstructural changes in HCV-positive patients: A pilot study. J
Hepatol. 59:651–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Loizou CP, Petroudi S, Seimenis I,
Pantziaris M and Pattichis CS: Quantitative texture analysis of
brain white matter lesions derived from T2-weighted MR images in MS
patients with clinically isolated syndrome. J Neuroradiol.
42:99–114. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayerhoefer ME, Szomolanyi P, Jirak D,
Berg A, Materka A, Dirisamer A and Trattnig S: Effects of magnetic
resonance image interpolation on the results of texture-based
pattern classification: a phantom study. Invest Radiol. 44:405–411.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker AS, Schneider MA, Wurnig MC, Wagner
M, Clavien PA and Boss A: Radiomics of liver MRI predict metastases
in mice. Eur Radiol Exp. 2:112018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao J, Dwyer A, Summers RM and Mollura DJ:
Computer-aided diagnosis of pulmonary infections using texture
analysis and support vector machine classification. Acad Radiol.
18:306–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Griethuysen JJM, Fedorov A, Parmar C,
Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC,
Pieper S and Aerts HJWL: Computational radiomics system to decode
the radiographic phenotype. Cancer Res. 77:e104–e107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nasim F, Sabath BF and Eapen GA: Lung
Cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kay FU, Kandathil A, Batra K, Saboo SS,
Abbara S and Rajiah P: Revisions to the Tumor, Node, Metastasis
staging of lung cancer (8th edition): Rationale, radiologic
findings and clinical implications. World J Radiol. 9:269–279.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Castellano G, Bonilha L, Li LM and Cendes
F: Texture analysis of medical images. Clin Radiol. 59:1061–1069.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Napel S, Mu W, Jardim-Perassi BV, Aerts
HJWL and Gillies RJ: Quantitative imaging of cancer in the
postgenomic era: Radio(geno)mics, deep learning, and habitats.
Cancer. 124:4633–4649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallego-Ortiz C and Martel AL: Using
quantitative features extracted from T2-weighted MRI to improve
breast MRI computer-aided diagnosis (CAD). PLoS One.
12:e01875012017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nachimuthu DS and Baladhandapani A:
Multidimensional texture characterization: On analysis for brain
tumor tissues using MRS and MRI. J Digit Imaging. 27:496–506. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Galm BP, Martinez-Salazar EL, Swearingen
B, Torriani M, Klibanski A, Bredella MA and Tritos NA: MRI texture
analysis as a predictor of tumor recurrence or progression in
patients with clinically non-functioning pituitary adenomas. Eur J
Endocrinol. 179:191–198. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holli-Helenius K, Salminen A, Rinta-Kiikka
I, Koskivuo I, Bruck N, Bostrom P and Parkkola R: MRI texture
analysis in differentiating luminal A and luminal B breast cancer
molecular subtypes-a feasibility study. BMC Med Imaging. 17:692017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herlidou-Meme S, Constans JM, Carsin B,
Olivie D, Eliat PA, Nadal-Desbarats L, Gondry C, Le Rumeur E,
Idy-Peretti I and de Certaines JD: MRI texture analysis on texture
test objects, normal brain and intracranial tumors. Magn Reson
Imaging. 21:989–993. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kjaer L, Ring P, Thomsen C and Henriksen
O: Texture analysis in quantitative MR imaging. Tissue
characterisation of normal brain and intracranial tumours at 1.5 T.
Acta Radiol. 36:127–135. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mougiakakou SG, Valavanis IK, Nikita A and
Nikita KS: Differential diagnosis of CT focal liver lesions using
texture features, feature selection and ensemble driven
classifiers. Artif Intell Med. 41:25–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lerski RA, Straughan K, Schad LR, Boyce D,
Bluml S and Zuna I: MR image texture analysis-an approach to tissue
characterization. Magn Reson Imaging. 11:873–887. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cristofori I, Zhong W, Chau A, Solomon J,
Krueger F and Grafman J: White and gray matter contributions to
executive function recovery after traumatic brain injury.
Neurology. 84:1394–1401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barbey AK, Colom R, Solomon J, Krueger F,
Forbes C and Grafman J: An integrative architecture for general
intelligence and executive function revealed by lesion mapping.
Brain. 135:1154–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Woodard GA, Jones KD and Jablons DM: Lung
cancer staging and prognosis. Cancer Treat Res. 170:47–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Derks JL, Leblay N, Thunnissen E, van
Suylen RJ, den Bakker M, Groen HJM, Smit EF, Damhuis R, van den
Broek EC, Charbrier A, et al: Molecular subtypes of pulmonary
large-cell neuroendocrine carcinoma predict chemotherapy treatment
outcome. Clin Cancer Res. 24:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schwartz AM and Rezaei MK: Diagnostic
surgical pathology in lung cancer: Diagnosis and management of lung
cancer, 3rd ed: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest. 143 (5 Suppl):e251S–e262S.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society:
International multidisciplinary classification of lung
adenocarcinoma: Executive summary. Proc Am Thorac Soc. 6:244–285.
2011.
|
|
46
|
Travis WD, Brambilla E and Riely GJ: New
pathologic classification of lung cancer: Relevance for clinical
practice and clinical trials. J Clin Oncol. 31:992–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7(pii): 1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Walters S, Maringe C, Coleman MP, Peake
MD, Butler J, Young N, Bergstrom S, Hanna L, Jakobsen E, Kolbeck K,
et al: Lung cancer survival and stage at diagnosis in Australia,
Canada, Denmark, Norway, Sweden and the UK: A population-based
study, 2004–2007. Thorax. 68:551–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dennie C, Thornhill R, Sethi-Virmani V,
Souza CA, Bayanati H, Gupta A and Maziak D: Role of quantitative
computed tomography texture analysis in the differentiation of
primary lung cancer and granulomatous nodules. Quant Imaging Med
Surg. 6:6–15. 2016.PubMed/NCBI
|
|
50
|
Wibmer A, Hricak H, Gondo T, Matsumoto K,
Veeraraghavan H, Fehr D, Zheng J, Goldman D, Moskowitz C, Fine SW,
et al: Haralick texture analysis of prostate MRI: Utility for
differentiating non-cancerous prostate from prostate cancer and
differentiating prostate cancers with different Gleason scores. Eur
Radiol. 25:2840–2850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
MacKay JW, Murray PJ, Low SB, Kasmai B,
Johnson G, Donell ST and Toms AP: Quantitative analysis of tibial
subchondral bone: Texture analysis outperforms conventional
trabecular microarchitecture analysis. J Magn Reson Imaging.
43:1159–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mayerhoefer ME, Szomolanyi P, Jirak D,
Materka A and Trattnig S: Effects of MRI acquisition parameter
variations and protocol heterogeneity on the results of texture
analysis and pattern discrimination: An application-oriented study.
Med Phys. 36:1236–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Collewet G, Strzelecki M and Mariette F:
Influence of MRI acquisition protocols and image intensity
normalization methods on texture classification. Magn Reson
Imaging. 22:81–91. 2004. View Article : Google Scholar : PubMed/NCBI
|