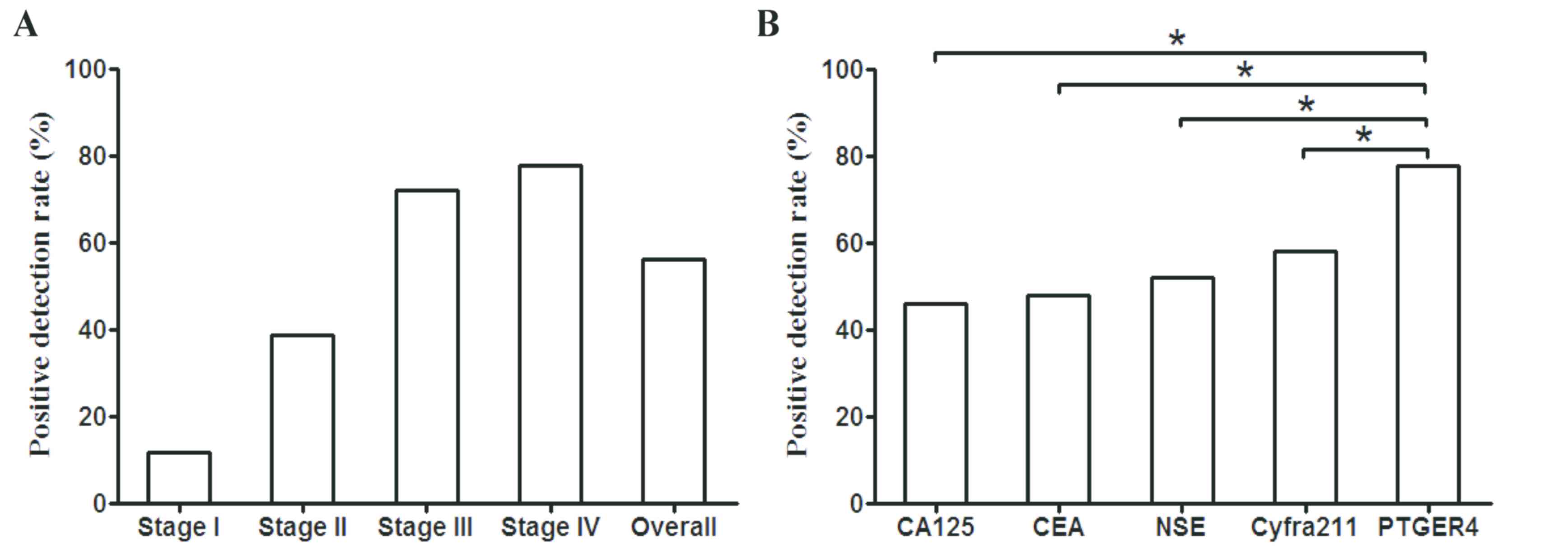
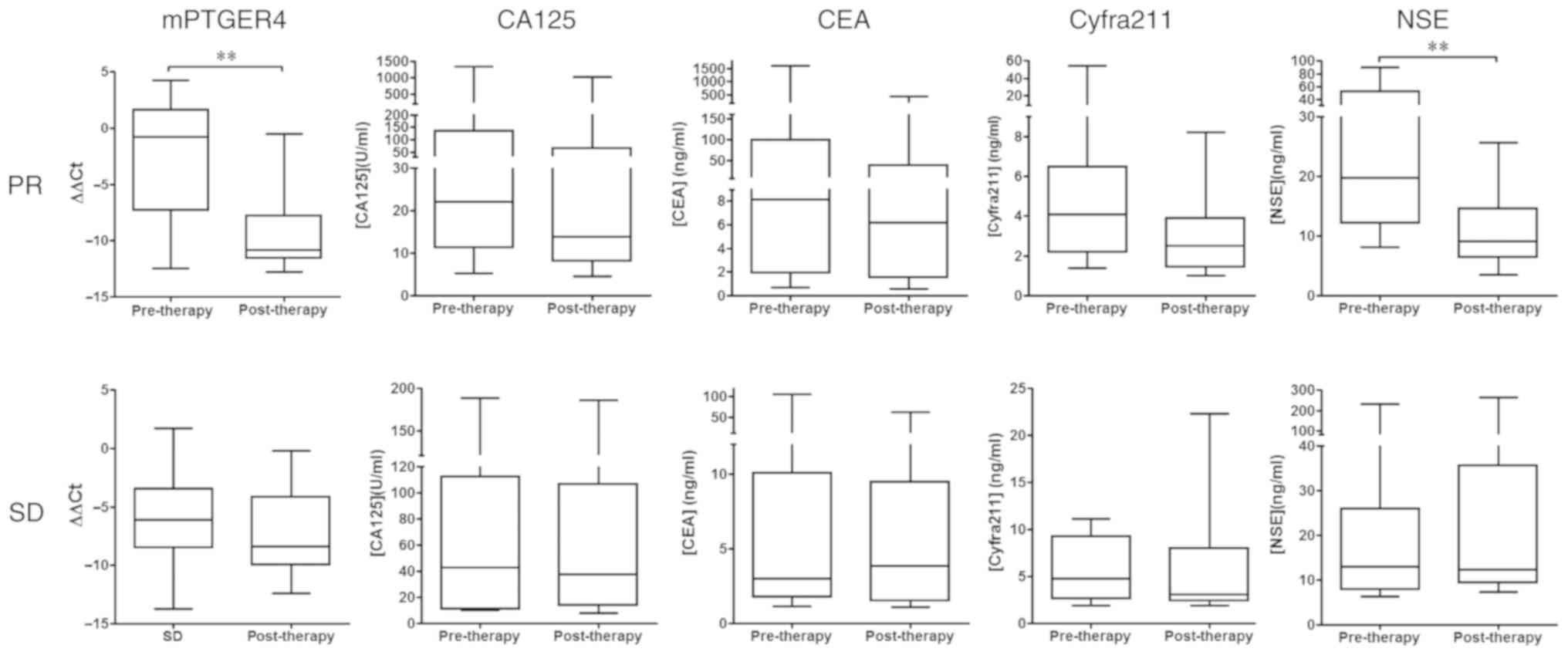
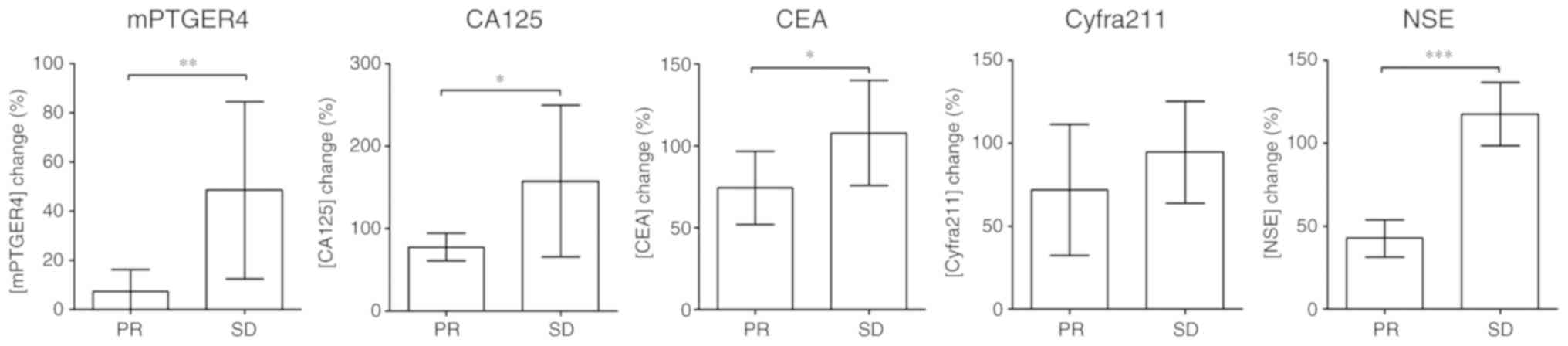
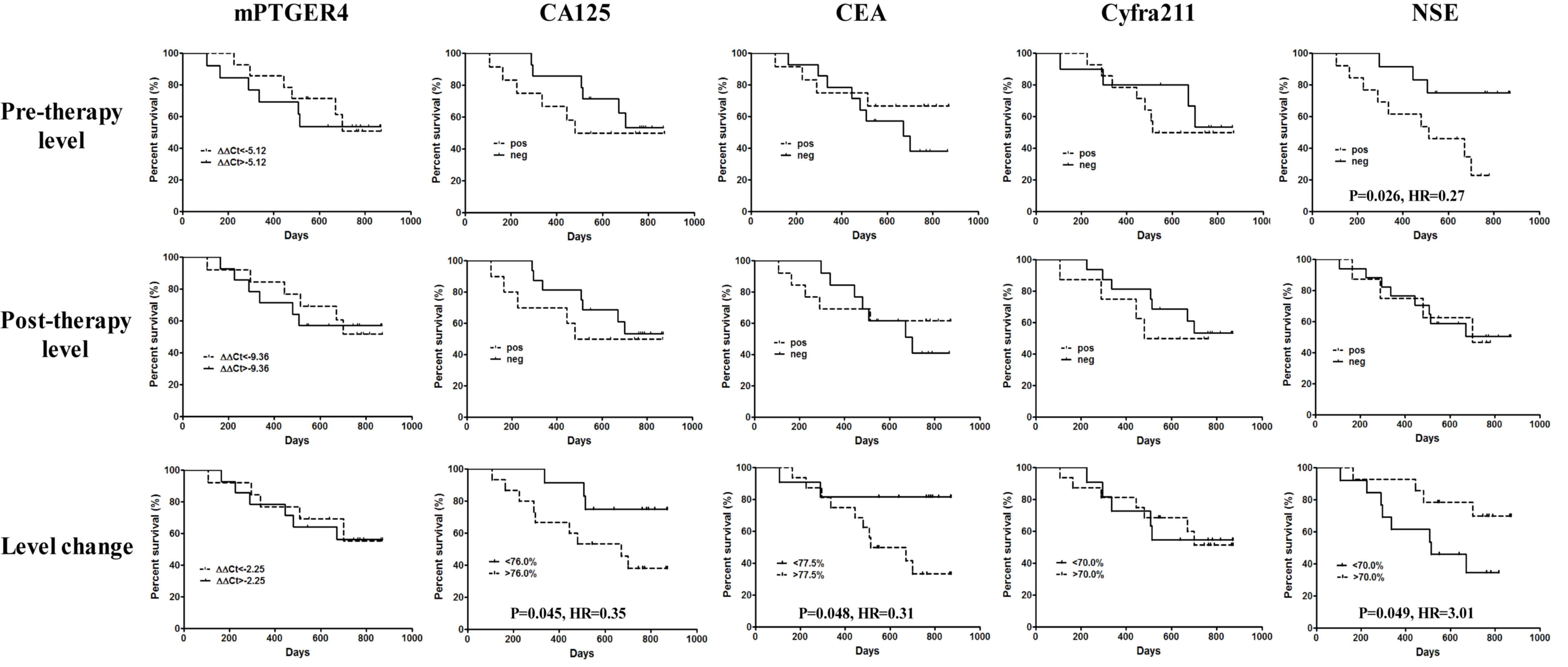
|
1
|
National Lung Screening Trial Research
Team, ; Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan
F, Fagerstrom RM, Gareen IF, Gierada DS, et al: Results of initial
low-dose computed tomographic screening for lung cancer. N Engl J
Med. 368:1980–1991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Belloni E, Veronesi G, Rotta L, Volorio S,
Sardella D, Bernard L, Pece S, Di Fiore PP, Fumagalli C, Barberis
M, et al: Whole exome sequencing identifies driver mutations in
asymptomatic computed tomography-detected lung cancers with normal
karyotype. Cancer Genet. 208:152–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uchida J, Kato K, Kukita Y, Kumagai T,
Nishino K, Daga H, Nagatomo I, Inoue T, Kimura M, Oba S, et al:
Diagnostic accuracy of noninvasive genotyping of EGFR in lung
cancer patients by deep sequencing of plasma cell-free DNA. Clin
Chem. 61:1191–1196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin X, Chen Y, Chen H, Fei S, Chen D, Cai
X, Liu L, Lin B, Su H, Zhao L, et al: Evaluation of tumor-derived
exosomal miRNA as potential diagnostic biomarkers for early-stage
non-small cell lung cancer using next-generation sequencing. Clin
Cancer Res. 23:5311–5319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leng Q, Lin Y and Jiang F, Lee CJ, Zhan M,
Fang H, Wang Y and Jiang F: A plasma miRNA signature for lung
cancer early detection. Oncotarget. 8:111902–111911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye M, Li S, Huang W, Wang C, Liu L, Liu J,
Liu J, Pan H, Deng Q, Tang H, et al: Comprehensive targeted
super-deep next generation sequencing enhances differential
diagnosis of solitary pulmonary nodules. J Thorac Dis. 10 (Suppl
7):S820–S829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Y, Feng G, Lu X, Qian W, Ye J,
Manrique CA, Ma C and Lu Y; written on behalf of the AME Lung
Cancer Collaborative Group, : Exploratory analysis of introducing
next-generation sequencing-based method to treatment-naive lung
cancer patients. J Thorac Dis. 10:5904–5912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oellerich M, Schütz E, Beck J and Walson
PD: Circulating cell-free DNA-diagnostic and prognostic
applications in personalized cancer therapy. Ther Drug Monit.
41:115–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim M, Kim CJ, Sunkara V, Kim MH and Cho
YK: Liquid biopsy in lung cancer: Clinical applications of
circulating biomarkers (CTCs and ctDNA). Micromachines (Basel).
9:E1002018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayo-de-Las-Casas C, Garzón Ibáñez M,
Jordana-Ariza N, García-Peláez B, Balada-Bel A, Villatoro S,
Malapelle U, Karachaliou N, Troncone G, Rosell R and Molina-Vila
MA: An update on liquid biopsy analysis for diagnostic and
monitoring applications in non-small cell lung cancer. Expert Rev
Mol Diagn. 18:35–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weiss G, Schlegel A, Kottwitz D, König T
and Tetzner R: Validation of the SHOX2/PTGER4 DNA methylation
marker panel for plasma-based discrimination between patients with
malignant and nonmalignant lung disease. J Thorac Oncol. 12:77–84.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu X, Zhao W and Wang B: DNA methylation
detection of SHOX2 and PTGER4 in plasma contributes to differential
diagnosis of pulmonary nodule patients. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 35:357–361. 2019.(In Chinese). PubMed/NCBI
|
|
13
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmidt B, Beyer J, Dietrich D, Bork I,
Liebenberg V and Fleischhacker M: Quantification of cell-free
mSHOX2 Plasma DNA for therapy monitoring in advanced stage
non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients.
PLoS One. 10:e01181952015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng XM, Liu XL, Xu L, Li Y, Wang H, Song
L and Xiao W: The mSHOX2 is capable of assessing the therapeutic
effect and predicting the prognosis of stage IV lung cancer. J Thor
Dis. 11:2458–2469. 2019. View Article : Google Scholar
|
|
17
|
Duffy MJ and O'Byrne K: Tissue and blood
biomarkers in lung cancer: A review. Adv Clin Chem. 86:1–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang B, Li X, Ren T and Yin Y:
Autoantibodies as diagnostic biomarkers for lung cancer: A
systematic review. Cell Death Discov. 5:1262019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin J, Zeng N, Yang T, Wan C, Chen L, Shen
Y and Wen F: Diagnostic value of autoantibodies in lung cancer: A
systematic review and meta-analysis. Cell Physiol Biochem.
51:2631–2646. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Epi proLung. Instruction for use.
Epigenomics AG; Berlin: 2018, https://www.epigenomics.com/wp-content/uploads/2018/08/IFU_0018_GB_rev4_Instructions_for_Use_Epi_proLung.pdfAugust
6–2018
|
|
22
|
Song L, Guo S, Wang J, Peng X, Jia J, Gong
Y, Yang B, Xiao W, Dong C, Liu H and Li Y: The blood mSEPT9 is
capable of assessing the surgical therapeutic effect and the
prognosis of colorectal cancer. Biomark Med. 12:961–973. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song L, Yu H, Jia J and Li Y: A systematic
review of the performance of the SEPT9 gene methylation assay in
colorectal cancer screening, monitoring, diagnosis and prognosis.
Cancer Biomark. 18:425–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jahr S, Hentze H, Englisch S, Hardt D,
Fackelmayer FO, Hesch RD and Knippers R: DNA fragments in the blood
plasma of cancer patients: Quantitations and evidence for their
origin from apoptotic and necrotic cells. Cancer Res. 61:1659–1665.
2001.PubMed/NCBI
|
|
25
|
Dietrich D, Hasinger O, Liebenberg V,
Field JK, Kristiansen G and Soltermann A: DNA methylation of the
homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell
lung cancer patients. Diagn Mol Pathol. 21:93–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bergheim J, Semaan A, Gevensleben H,
Groening S, Knoblich A, Dietrich J, Weber J, Kalff JC, Bootz F,
Kristiansen G and Dietrich D: Potential of quantitative SEPT9 and
SHOX2 methylation in plasmatic circulating cell-free DNA as
auxiliary staging parameter in colorectal cancer: A prospective
observational cohort study. Br J Cancer. 118:1217–1228. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song L, Jia J, Yu H, Peng X, Xiao W, Gong
Y, Zhou G, Han X and Li Y: The performance of the mSEPT9 assay is
influenced by algorithm, cancer stage and age, but not sex and
cancer location. J Cancer Res Clin Oncol. 143:1093–1101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song L, Jia J, Peng X, Xiao W and Li Y:
The performance of the SEPT9 gene methylation assay and a
comparison with other CRC screening tests: A meta-analysis. Sci
Rep. 7:30322017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Shen L, Geng J, Wu Y, Xiao H,
Zhang F and Si H: Prognostic value of cancer antigen −125 for lung
adenocarcinoma patients with brain metastasis: A random survival
forest prognostic model. Sci Rep. 8:56702018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Isaksson S, Jönsson P, Monsef N,
Brunnström H, Bendahl PO, Jönsson M, Staaf J and Planck M: CA 19-9
and CA 125 as potential predictors of disease recurrence in
resectable lung adenocarcinoma. PLoS One. 12:e01862842017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grunnet M and Sorensen JB:
Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung
Cancer. 76:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu Z, Zhang G, Yang M, Zhang S, Zhao B,
Shen G and Chai Y: Systematic review of CYFRA 21-1 as a prognostic
indicator and its predictive correlation with clinicopathological
features in non-small cell lung cancer: A meta-analysis.
Oncotarget. 8:4043–4050. 2017.PubMed/NCBI
|
|
33
|
Xue F, Zhu L, Wang L and Wang Q: Serum
neuron specific enolase levels correlate with patient prognosis for
advanced lung cancer. Int J Clin Exp Med. 8:9498–9504.
2015.PubMed/NCBI
|
|
34
|
Liu X, Zhang W, Yin W, Xiao Y, Zhou C, Hu
Y and Geng S: The prognostic value of the serum neuron specific
enolase and lactate dehydrogenase in small cell lung cancer
patients receiving first-line platinum-based chemotherapy. Medicine
(Baltimore). 96:e82582017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kalemkerian GP, Loo BW, Akerley W, Attia
A, Bassetti M, Boumber Y, Decker R, Dobelbower MC, Dowlati A,
Downey RJ, et al: NCCN guidelines insights: Small cell lung cancer,
version 2.2018. J Natl Compr Canc Netw. 16:1171–1182. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roviello G: The distinctive nature of
adenocarcinoma of the lung. Onco Targets Ther. 8:2399–2406. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Updated analysis of KEYNOTE-024: Pembrolizumab versus
platinum-based chemotherapy for advanced non-small-cell lung cancer
with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol.
37:537–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paz-Ares L, Luft A, Vicente D, Tafreshi A,
Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et
al: Pembrolizumab plus chemotherapy for squamous non-small-cell
lung cancer. N Engl J Med. 379:2040–2051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ravegnini G, Zolezzi Moraga JM, Maffei F,
Musti M, Zenesini C, Simeon V, Sammarini G, Festi D, Hrelia P and
Angelini S: Simultaneous analysis of SEPT9 promoter methylation
status, micronuclei frequency, and folate-related gene
polymorphisms: The potential for a novel blood-based colorectal
cancer biomarker. Int J Mol Sci. 16:28486–28497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma Y, Chen Y and Petersen I: Expression
and promoter DNA methylation of MLH1 in colorectal cancer and lung
cancer. Pathol Res Pract. 213:333–338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan SX, Hu RC, Liu JJ, Tan YL and Liu WE:
Methylation of PRDM2, PRDM5 and PRDM16 genes in lung cancer cells.
Int J Clin Exp Pathol. 7:2305–2311. 2014.PubMed/NCBI
|