Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer in adults (1).
Patients are generally asymptomatic chiefly at the initial phases.
Specific early markers have not been identified, resulting in a
late diagnosis, frequently even when metastasis is already present
(1,2), and there are different histological
subtypes with distinct responses to therapies (3). All of the above lead to high mortality
rates (1). Hence, an experimental
model would be a valuable tool to study this disease and
particularly to understand the molecular mechanisms involved in the
early stages of renal carcinogenic process, which is almost
impossible to achieve in patients. The authors' group has
demonstrated that N-diethylnitrosamine (DEN)-initiated and
ferric nitrilotriacetate (FeNTA)-promoted renal tumors
histologically correspond to clear cell RCC (ccRCC), the most
common subtype occurring in patients (4) and that the exposure to FeNTA during
either one or two months, causes distinct pre-neoplastic lesions
and pro-carcinogenic molecular alterations, representing these
times of exposure, differential early stages of renal
carcinogenesis (5,6).
Nuclear factor kappa B (NF-κB) is a collective term
to refer to the family of dimeric Rel transcription factors, which
consists of five proteins: p65 (Rel A), c-Rel, RelB, NFκB1
(p105/p50) and NFκB2 (p100/p52) (7),
being considered p65/p50 the most classic dimer, where p65 is the
subunit responsible for the transactivation of its target genes
(8). In most cells, NF-κB is
retained in the cytoplasm by its inhibitory protein, IκB (7,8), which
blocks the factor's nuclear localization signal. IκB separation,
therefore, allows NF-κB nuclear translocation and its consequent
binding to κB sites in DNA. Additionally, NF-κB's activity is
controlled in the nucleus by IκB as well, where it induces the
factor's liberation from chromatin and its export to the cytoplasm
(9,10). The expression of numerous genes
related to various events such as cell proliferation, inflammation
and inhibition of apoptosis, is under the influence of this
transcription factor (7), its
deregulation being associated with almost all types of cancer
(11,12). Particularly, different authors have
reported an NF-κB overexpression in RCC tumors and cell lines
(12–15), and in certain publications this
overexpression has been correlated to tumor grade, invasion and
metastasis, proposing the factor as a target for RCC treatment
(2,3,14,16). In
fact, apoptosis-induction and/or growth-repression of different RCC
cell lines have been demonstrated by using a specific inhibitor of
NF-κB activation (Bay-11-7085) or by blocking tyrosine kinases
involved in NF-κB activation signaling (17,18). The
p65 subunit was studied in all the aforementioned reports apart
from Meteoglu et al (12) who
analyzed the p50 subunit. Furthermore, Ng et al (19) reported a decrease in the levels of
p50, p52 and c-Rel, as well as the absence of RelB, but an increase
in p65 expression in human RCC tumors.
On the other hand, the epidermal growth factor
receptor (EGFR) gene is a known target of NF-κB (20). The EGFR is a transmembrane protein of
the ErbB tyrosine kinases family (21) and when it is activated, multiple
signaling pathways are induced, thus modulating pleiotropic cell
responses, such as proliferation, migration and apoptosis (21). In the kidney, this receptor
participates in renal hemodynamics, electrolyte management,
magnesium reabsorption, phosphate transport regulation and proximal
tubule gluconeogenesis, as well as in renal reparation after
ischemia-induced damage (22).
However, dysregulation of EGFR has been associated with progressive
fibrotic renal damage, polycystic renal disease and RCC (22). In this last case, previous studies
have reported EGFR overexpression, but the clinical significance of
this increase and its subcellular localization are still
controversial (23–28).
Therefore, in the present study, the behavior of
NF-κB (p65), IκBα and EGFR was analyzed in renal tumors and at
different early stages of FeNTA-induced carcinogenesis, to
establish the equivalence between experimental and human neoplasms
in this regard and to investigate the probable participation of
these molecules in RCC development.
Materials and methods
Reagents and antibodies
All reagents were purchased from Sigma-Aldrich Merck
KGaA unless otherwise indicated. Primary antibodies against IκBα
(cat. no. sc-371), NF-κB p65 (cat. no. sc-8008), EGFR (cat. no.
sc-03-G), α-tubulin (cat. no. sc-5286), GAPDH (cat. no. sc-48167),
β-actin (cat. no. sc-1616) and histone H3 (cat. no. sc-10809) used
for western blotting (WB), immunohistochemistry (IHC), and the
supershift test of the electrophoretic mobility shift assay (EMSA),
as well as the secondary antibodies (anti-mouse and anti-rabbit,
cat. nos. sc-2005 and sc-2004, respectively) used for WB were
purchased from Santa Cruz Biotechnology, Inc., while the anti-IgG
secondary antibodies coupled with biotin (ABC kit
Vectastain®; cat. nos. PK-6101 and PK 6102) and the
peroxidase substrate kit DAB (cat. no. SK-4100) used for IHC were
purchased from Vector Laboratories, Inc.
Carcinogen preparation
FeNTA solution was prepared as previously described
(6).
Experimental protocol
All experiments involving animals were performed
according to the Mexican Official Norm NOM-062-ZOO-1999 and
approved by the Institutional Committee for the Use and Care of
Laboratory Animals (FQ/CICUAL/081/14). Experimental protocols were
carried out as previously described (6) adapted from other authors' studies
(29–31). Briefly, 67 male Wistar rats aged
between 31 and 36 days and weighing 70–80 g were housed in a
controlled temperature environment (21–23°C) with 55–62% humidity
and under 12 h light/dark cycles. Rats had free access to food and
water and were randomly distributed in two groups: 18 animals
treated with a vehicle were used as the control group (C) and 49
animals treated with a single intraperitoneal (i.p.) administration
of N-DEN (200 mgDEN/kgbw) and weekly
increasing i.p. doses of FeNTA (3–9
mgFe/kgbw) twice a week, maintaining the 9
mgFe/kgbw dose from the 4th to the 16th week
in the carcinogenesis group (DF). To analyze early stages of
carcinogenesis, 10 rats were decapitated after one month and 10
more after two months of FeNTA-exposure for DF group as well as 6
animals for the corresponding C group for each time of study.
Euthanasia was executed, in both cases, 48 h after the last
injection of the carcinogen to avoid its acute effects and without
anesthesia as the anesthetic may interfere in different manners
with the chemistry of renal tissue and other tissues of interest,
such as liver and brain, as well as blood samples. In the present
study specifically kidneys, liver and lungs were used. Organs were
removed immediately after sacrifice. The renal cortex was carefully
dissected and all tissues were immediately frozen in liquid
nitrogen storing them at −70°C until use. For the carcinogenesis
protocol, the remaining animals were euthanized two months after
the final FeNTA exposure, some of them by decapitation as described
above to obtain tumor samples for reverse transcription (RT)-PCR
assays, while others were anesthetized i.p. with sodium
pentobarbital (50 mg/kgbw) and the kidneys were perfused
with pH 7.4 Krebs-Ringer solution with 250 mM
ethylendiaminetetraacetic acid (EDTA), formalin-fixed at room
temperature for 24 h and prepared for IHC following standard
protocols (32). Animal welfare was
carefully monitored during the experimental protocol, twice a week
during the first 3 months and 4 times a week during the last three
months. Euthanasia was executed by carbon dioxide inhalation when
rats showed signs of health deterioration such as loosing 20% of
weight, or exhibiting prostration, mobility impairment and/or
difficulty eating, urinating or defecating.
IHC
Sections (5-µm thick; rotation micrometer,
Leica®; Leica Microsystems GmbH) from formalin-fixed
paraffin-embedded kidney tissue specimens were placed on slides,
deparaffinized with xylene and rehydrated with decreasing
concentrations of ethanol (100, 96, 80, 70 and 50%) for 3 min each.
The antigen was retrieved by heating at 100°C for 30 min in 0.01 M
sodium citrate buffer (pH 6.0) and subsequently incubated for 30
min with blocking solution (3% H2O2 in PBS)
to suppress the endogenous peroxidase activity. After rinsing with
PBS, the slides were incubated in 0.5% Triton X-100 at room
temperature for 30 min and non-specific immunoglobulin binding was
blocked by incubating sections in 5% albumin IgG free (Jackson
ImmunoResearch Laboratories, Inc.) in PBS at room temperature for
30 min. The slides were incubated overnight at 4°C in a humidified
chamber with the corresponding primary antibody at a 1:250
dilution, prepared with 3% Triton X-100 in PBS. Then, the unbound
primary antibody was removed by washing with PBS and the slides
were incubated at room temperature for 2 h with a 1:200 dilution of
anti-rabbit or anti-mouse (case dependent) IgG secondary antibody
coupled with biotin (ABC kit Vectastain®, Vector
Laboratories, Inc.). Sections were washed with PBS and incubated
for 1 h at room temperature in peroxidase-conjugated avidin-biotin
reagent (ABC kit Vectastain®, Vector Laboratories,
Inc.). The immunoreaction was detected with the DAB kit (Vector
Laboratories Inc.) and samples were counterstained with hematoxylin
at room temperature for 2 min and dehydrated with increasing
concentrations of ethanol (50, 70, 80, 96 and 100%), and solutions
of 1:1 ethanol-xylene and 100% xylene for 3 min each. Finally, four
random fields of each sample were analyzed utilizing a Nikon E600
light microscope; the staining intensity (mean grey value) of
immunopositive cells was evaluated with the ImageJ software version
1.44p (National Institutes of Health) (33), which converts the grey level to a
numerical value using a scale from 0 (white) to 255 (black), as
described previously (32).
mRNA extraction and RT-PCR
Semi-quantitative mRNA analysis was achieved by
RT-PCR. Total RNA was extracted with the Direct-zol™ RNA MiniPrep
kit according to the manufacturer's protocol (cat. no. R2052, Zymo
Research). RNA concentrations were determined
spectrophotometrically at 260 nm. RT-PCR was carried out starting
with 1 µg of total RNA following manufacturer's protocol with the
RevertAid First Strand cDNA Synthesis kit (cat. no. K1622; Thermo
Fisher Scientific, Inc.). The thermocycling conditions for cDNA
synthesis and amplification were: 1 cycle 55°C (30 min), 94°C (2
min), 94°C (30 sec); 10 cycles 94°C (30 sec), 60°C (30 sec), 72°C
(30 sec); 20 cycles 94°C (30 sec), 60°C (30 sec), 72°C (5–30 sec
with five seconds increases per cycle); 1 cycle 94°C (30 sec), 60°C
(30 sec) and 72°C (7 min). GAPDH was used as control. The
oligonucleotide sequences used were: (1) for EGFR, 5′-ACAGAGGACAACATAGATGAC-3′
(forward) and 5′-CTGGGCAGTGTTGAGATAC-3′ (reverse) (34); and (2)
for GAPDH, 5′-GGCTGAGAATGGGAAGCTGGTCAT-3′ (forward) and
5′-CAGCCTTCTCCATGGTGGTGAAGA-3′ (reverse) (35). Amplified fragments were analyzed on a
2% agarose gel stained with ethidium bromide at room temperature
for 30 min and visualized with ultraviolet light (Benchtop 2UV™
Transilluminator UVP). Bands were quantified by densitometry
analysis as described below.
Tissue homogenates and western blot
analysis
A total of 150 mg of renal cortex tissue were
homogenized (PT10/35GT homogenizer Polytron®; Kinematica
AG), in cold lysis buffer (1 mM DTT, 10 mM Tris-HCl, 1 mM EDTA, 1
mM sodium orthovanadate, 15 mM sodium azide, 1.0% Triton X-100 and
30% glycerol) supplemented with the commercial protease inhibitor
cOmplete™ Mini and phosphatase inhibitor PhosSTOP™ Cocktail tablets
(Roche Diagnostics GmbH). After homogenates' centrifugation (13,000
× g for 30 min at 4°C) (Thermo Fisher Scientific, Inc.; Legend RT+
centrifuge), supernatants were recovered and total protein was
quantified by the Bradford method (Bio-Rad Laboratories Inc.).
Equal protein amounts (60–80 µg) were electrophoresed in a 10%
SDS-PAGE polyacrylamide gel and transferred to an
Immobilon® PVDF membrane (EMD Millipore). Membranes were
blocked for 1 h at room temperature with 1% nonfat dry milk and
incubated with the primary antibodies against p65 (1:250), IκBα
(1:250), EGFR (1:250) or α-tubulin (1:1,000) overnight at 4°C.
Subsequently, membranes were washed and incubated with the
corresponding secondary antibody (1:30,000 for anti-rabbit and
anti-mouse). Immunoreactive signal bands were detected using
Immobilon™ Western Chemiluminescent HRP Substrate (EMD Millipore)
and were recorded on X-ray films and quantified by densitometry, as
described below.
Nuclear protein extraction and
EMSA
Nuclear protein extracts were prepared by
homogenizing 100 mg kidney cortex (PT10/35GT homogenizer
Polytron® Kinematica AG) for 8–20 sec in 800 µl buffer A
(10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM
DTT, 0.5 mM PMSF and a tablet of protease inhibitor cOmplete™ Mini
per 10 ml buffer). Homogenates were mixed with 5 µl Nonidet P-40
(NP-40) and incubated at 4°C for 20 min, stirring 10 times at
intervals of 2 min. Then samples were centrifuged for 15 min at
1,500 × g at 4°C (Thermo Fisher Scientific, Inc.; Legend RT+
centrifuge) and pellets were resuspended in 100 µl buffer B (20 mM
HEPES, pH 7.9, 420 mM NaCl, 25% glycerol, 1.5 mM MgCl2,
0.2 mM EDTA, 0.5 mM PMSF, 0.5 mM DTT and a tablet of protease
inhibitor cOmplete™ Mini for each 10 ml buffer). After 20 min of
incubation at 4°C, mixing by inversion every 2 min, tubes were
centrifuged for 10 min at 13,000 × g at 4°C (Thermo Fisher
Scientific, Inc.; Legend RT+ centrifuge) and the supernatants
containing the nuclear proteins were stored at −20°C until use.
EMSA assay and oligonucleotide digoxigenin labeling were carried
out using a commercial kit according to manufacturer's protocol
(DIG Gel Shift kit, 2nd generation, Roche Diagnostics GmbH).
Briefly, 60 µg of nuclear proteins were incubated with 2 ng of
labeled double-stranded consensus sequence recognized by NF-κB:
5′-AGTTGAGACTTTCCCGGGAGGC-3′ (Santa Cruz Biotechnology, Inc.).
DNA-protein complexes were resolved by 5% native polyacrylamide gel
electrophoresis with 0.5X TBE (50 mM Tris, 45 mM boric acid and 0.5
mM EDTA) for 120 min at 80 V. Samples were transferred to a
positively-charged nylon membrane (Roche Diagnostics GmbH) in 0.5X
TBE for 30 min at 300 mA (Trans-Blot® SD Semi-dry
Transfer cell, Bio-Rad Laboratories Inc.) and crosslinked for 60
sec (CL-1000 Ultraviolet Crosslinker, UVP). Finally, digoxigenin
immunodetection was performed and autoradiographs developed. The
specificity of binding and recognition of the shifted-bands'
identity was examined by: i) Assays without sample, ii) assay
without labeled oligonucleotide, iii) competition assay with
100-fold molar excess of unlabeled oligonucleotide and iv)
supershift assay performed by previously incubating the nuclear
extract with the anti-p65 antibody for 15 min at 37°C.
Cell fractionation
A total of 150 mg of renal cortex were homogenized
(PT10/35GT homogenizer Polytron® Kinematica) in Gough
buffer (10 mM Tris-HCl, 0.15 M NaCl, 1.5 mM MgCl2, 0.65%
NP-40, 0.5 mM PMSF and 1 mM DTT) and incubated on ice for 30 min
with agitation every 5 min. Homogenates were centrifuged at 13,000
× g for 2 min at 4°C (Thermo Fisher Scientific, Inc.; Legend RT+
centrifuge). Supernatants (cytoplasmic fraction) were recovered and
stored at 4°C and pellets were resuspended in 250 µl HEPES buffer
(20 mM HEPES, pH 7.9, 25% glycerol, 0.4 M NaCl, 1.5 mM
MgCl2, 0.2 mM EDTA, 0.5 mM PMSF and 1 mM DTT). After 2 h
of incubation at 4°C with constant agitation, samples were
centrifuged for 10 min at 12,000 × g at 4°C (Thermo Fisher
Scientific, Inc.; Legend RT+ centrifuge) and supernatants (nuclear
proteins) were transferred to tubes containing 400 µl buffer D (20
mM HEPES, pH 7.9, 20% glycerol, 50 M KCl, 0.2 M EDTA, 0.5 mM PMSF
and 1 mM DTT). Protein concentrations were determined by the
Bradford method (Bio-Rad Laboratories Inc.) to load the same amount
of protein from the cytoplasmic and nuclear fractions in
electrophoresis gels for WB analysis.
Densitometry and statistical
analysis
Densitometry analysis was performed using the ImageJ
Software version 1.44p (NIH) (33).
For WB and RT-PCR, the results from the experimental groups were
expressed as relative densitometric units with respect to the
control group (RDU/C), calculated by dividing the total
densitometric arbitrary units of each band corresponding to the
protein of interest, by the value of the band corresponding to the
protein used as loading control, obtained from the same sample in
the same membrane; and this result, in turn, was divided by the
value obtained from the control group. For EMSA, data were
calculated by dividing the densitometric units of the shifted
oligonucleotide band from problem samples by the mean densitometric
value obtained from control samples. Lastly, for WB of cell
fractions, results were expressed as the rate obtained by dividing
the densitometric value of the band corresponding to the protein of
interest by that of the loading control band (RDU).
All data are presented as the mean ± standard error
of the mean. Results were analyzed by one-way analysis of variance
followed by Tukey post hoc test on Fig.
1 and by unpaired t-test with Welch's correction in all other
figures. Statistical analysis was performed using GraphPad Prism
version 5.0b for Windows (GraphPad Software, Inc.). P≤0.05 was
considered to indicate a statistically significant difference.
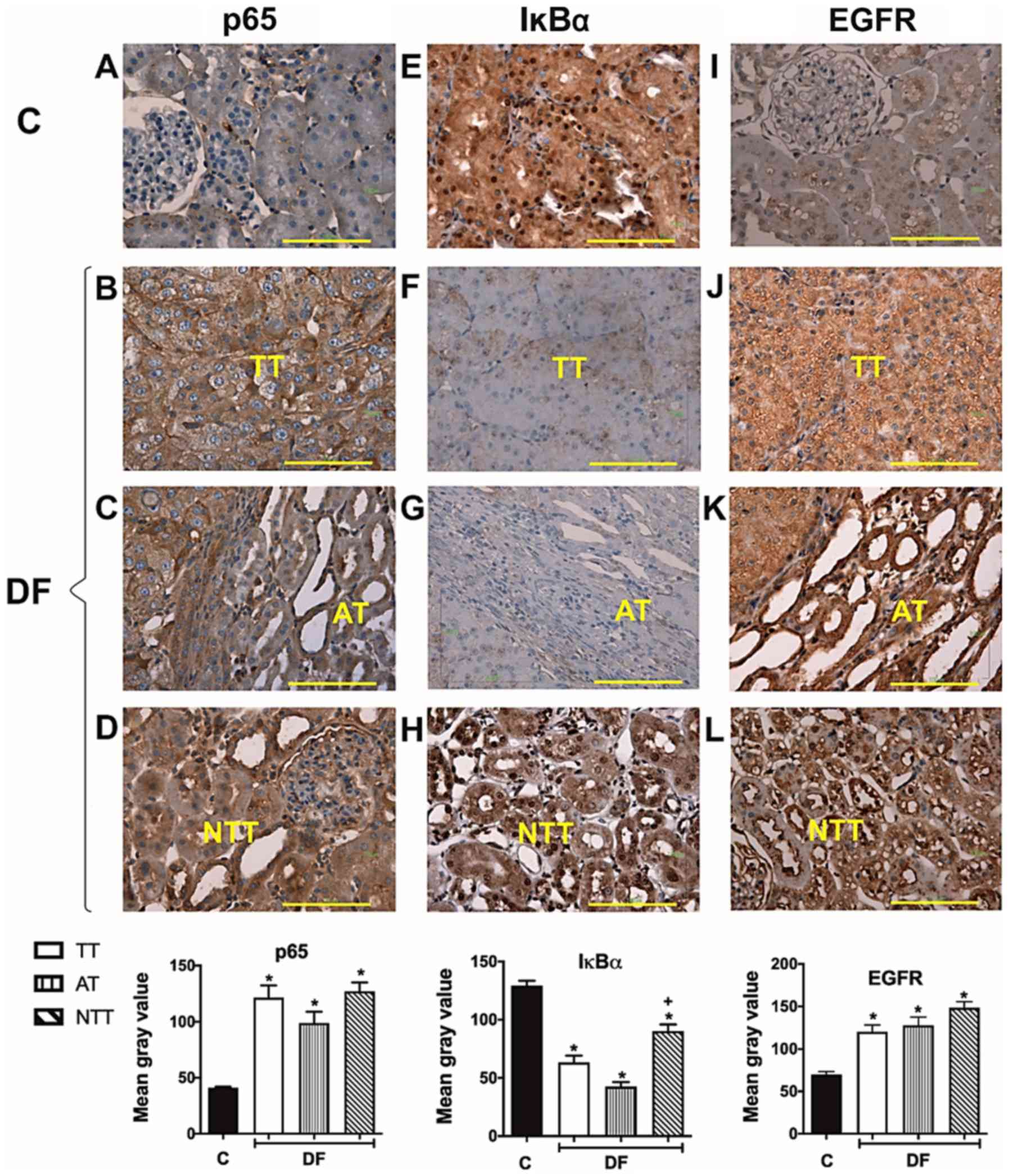 | Figure 1.Immunohistochemistry of NF-κB (p65),
IκBα and EGFR in renal samples obtained at the end of the
carcinogenesis protocol. (A-L) Representative kidney cortex
photomicrographs of these proteins are displayed from the C group
for (A) p65, (E) IκBα and (I) EGFR and DF exposed rats, as well as
the corresponding quantification histograms. NF-κB (p65) levels
increased (B) TT, (C) AT and (D) NTT in a similar way. IκBα levels
were abated in (F) TT, (G) AT and (H) NTT regions in the DF group,
although in this case the decrease in NTT was smaller than in TT
and AT. For its part, low EGFR expression in C rats was observed,
(I) which increased in kidneys from the DF group in the analyzed
areas in a comparable manner for (J) TT, (K) AT, and (L) NTT. AT
images (C, G and K) include a TT area as reference. Scale bar=100
µm. Magnification, ×400. A total of four random fields per sample
were analyzed, n=3 for the C group and 5 for the different areas of
DF group. Results are expressed as the mean gray value. Columns
represent the mean ± standard error of the mean. *P≤0.05 vs. C,
+P≤0.05 vs. TT and AT. C, control group; DF,
N-dietilnitrosamine + ferric nitrilotriacetate treated group; TT,
tumor tissues; AT, adjacent tissue; NTT, non-tumor tissue; NF-κB,
nuclear factor kappa B; EGFR, epidermal growth factor receptor. |
Results
p65, IκBα and EGFR in FeNTA-induced
renal tumors
Initially, to determine if FeNTA-induced tumors
exhibited similar alterations to those reported in human RCC, the
behavior of NF-κB (p65), IκBα and EGFR was analyzed in tumor tissue
(TT), as well as in adjacent tissue (AT) and non-TT (NTT) from the
DF group compared with the renal cortex from the C group. Fig. 1 displays IHC representative images
and histograms of the mean immunoreactivity quantification of these
three molecules in renal samples from C and DF groups. Unlike renal
tissue from C rats, where low NF-κB (p65) presence was detected
(Fig. 1A), a strong positive NF-κB
(p65) stain was observed in all kidney areas studied from the DF
group (Fig. 1B-D), i.e. the increase
in this protein was similar in TT, AT and NTT. In contrast, the
intensity of the IκBα positive stain observed in the C group
(Fig. 1E) decreased in rats exposed
to the carcinogen (Fig. 1F-H).
Interestingly, NTT exhibited an IκBα staining intensity that was
decreased compared with the C group, but higher than the TT and AT,
as well as a more frequent nuclear presence (Fig. 1H). Concerning EGFR, immunostaining
was similar in TT and AT with a heterogeneous behavior, showing
different levels of overexpression 63% of the studied samples
(Fig. 1J) as compared with C
(Fig. 1I); while 37% had no changes
or even presented a negative stain. However, the mean value of EGFR
levels for all analyzed specimens displayed an augment in TT as
well as in AT, and it was detected preferentially in the cytoplasm
in both areas; although the nuclear presence of this receptor was
higher in AT. In NTT, conversely, EGFR levels increased in all
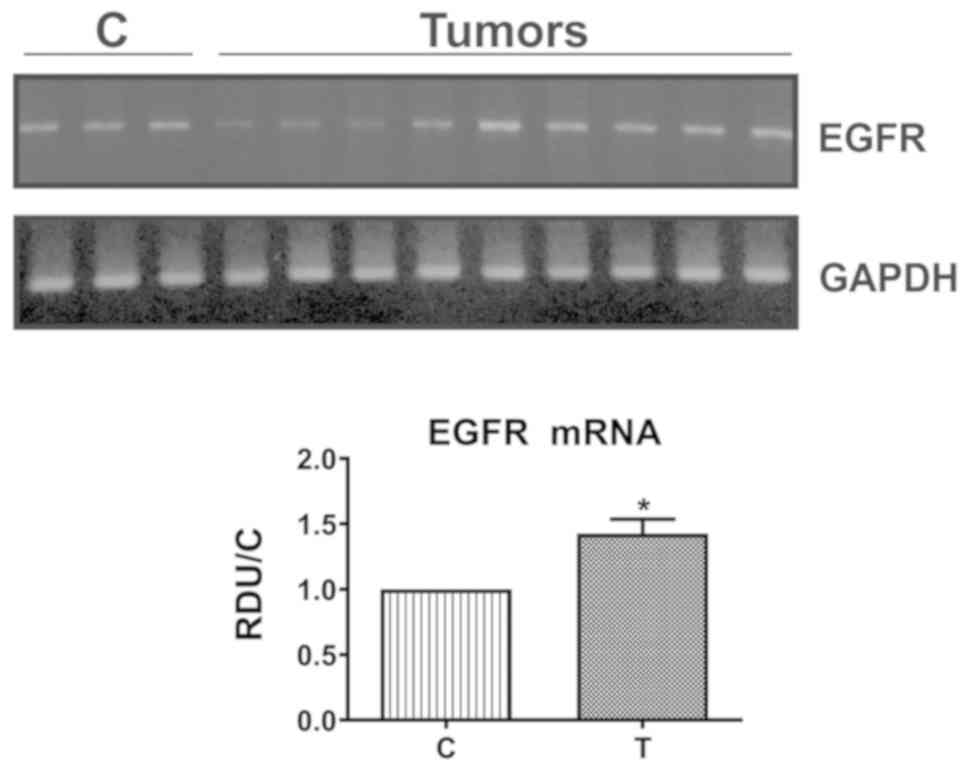
samples in the cytoplasm and in the nucleus (Fig. 1L), like in AT. Moreover, in
concordance with protein behavior, the mean EGFR mRNA levels were
also significantly enhanced in tumors, despite some samples showing
no changes or a decrease (39%) (Fig.
2).
p65, IκBα and EGFR at early stages of
FeNTA-induced renal carcinogenesis
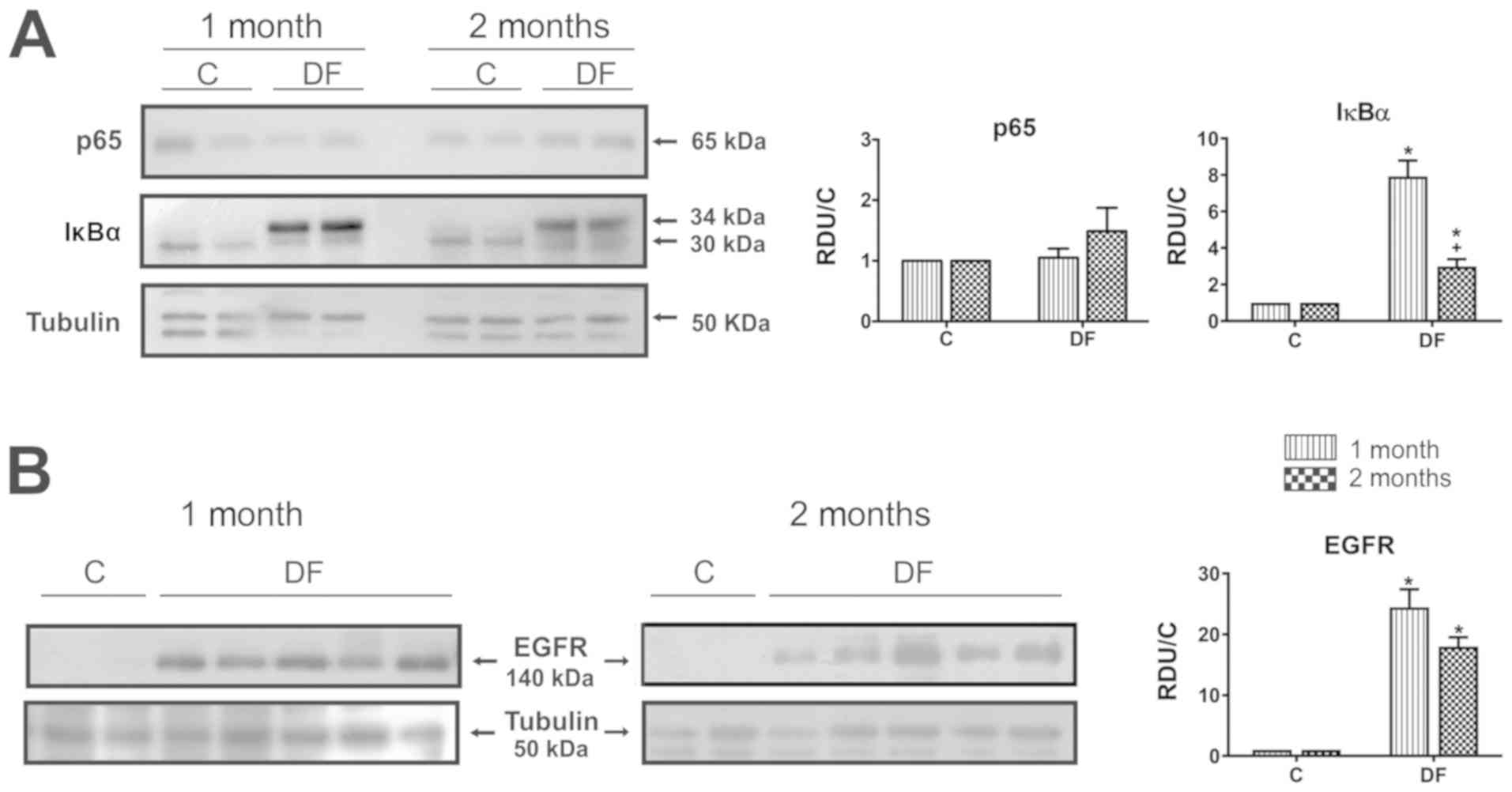
p65, IκBα and EGFR levels were determined in the
kidney cortex from FeNTA-exposed rats during either one or two
months, in order to study the probable participation of these
molecules in the carcinogenic process. WB representative images and
densitometry analysis of all samples examined are displayed on
Fig. 3. Results exhibited no changes
in p65 protein levels after either one or two months of
FeNTA-exposure (Fig. 3A). In the
case of IκBα (Fig. 3A), two bands
were observed, one being present mainly in the C group with an
electrophoretic shift corresponding to a molecular weight of ~32
kDa, while the other one was more intense in DF group, showing a
displacement that corresponds to ~34 kDa, which is closer to the
molecular weight reported for IκBα in rats, i.e. 35 kDa (Uniprot
#UniRef90_P2596). Due to the above, IκBα results were calculated
with the sum of the densitometric units from both bands, finding a
statistical augment at both times studied, which was more evident
after one month of carcinogen-exposure. On the other hand, EGFR
levels were significantly enhanced in the DF group at both times
studied (Fig. 3B) and, although the
rise seems to be more intense after one month of FeNTA-exposure, as
occurred for IκBα, there was no statistical difference between
them.
NF-κB activity and EGFR mRNA
expression at early stages of FeNTA-induced renal
carcinogenesis
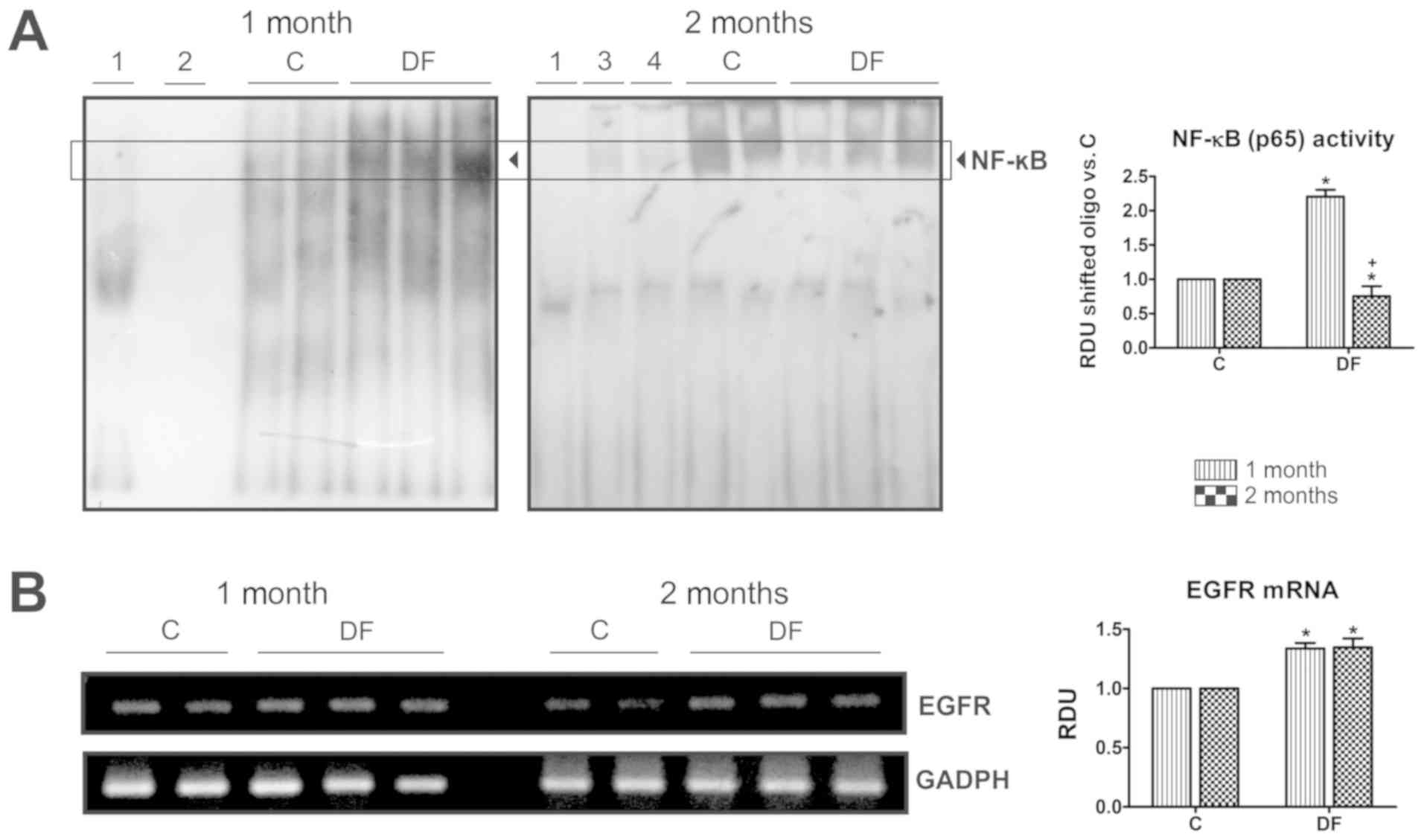
Given the preceding findings, EMSAs were carried out
in nuclear extracts to investigate NF-κB-DNA binding activity
(Fig. 4A). Different tests were
performed as technique controls: i) Assays without sample and ii)
without labeled-oligonucleotide, where no shifted band was detected
(lanes 1 and 2, respectively); iii) a competition assay with a
100-fold molar excess of unlabeled-oligonucleotide, to confirm the
identity of the observed band (lane 3); and vi) a supershift assay
using the anti-p65 antibody to verify the presence of this protein
in the NF-κB complex (lane 4). These two last tests were conducted
with the first control sample shown in the two months image and
where a notable decrease in the intensity of the corresponding band
can be appreciated. When samples were analyzed, a patent rise of
NF-κB binding to its consensus sequence was observed after one
month of FeNTA-exposure; whereas the factor's activity was abated
after two months' exposure revealed by a decrease in the intensity
of the band in samples from the DF group compared with that from
the C one. EGFR mRNA levels, however, remained significantly
heightened at both times studied (Fig.
4B).
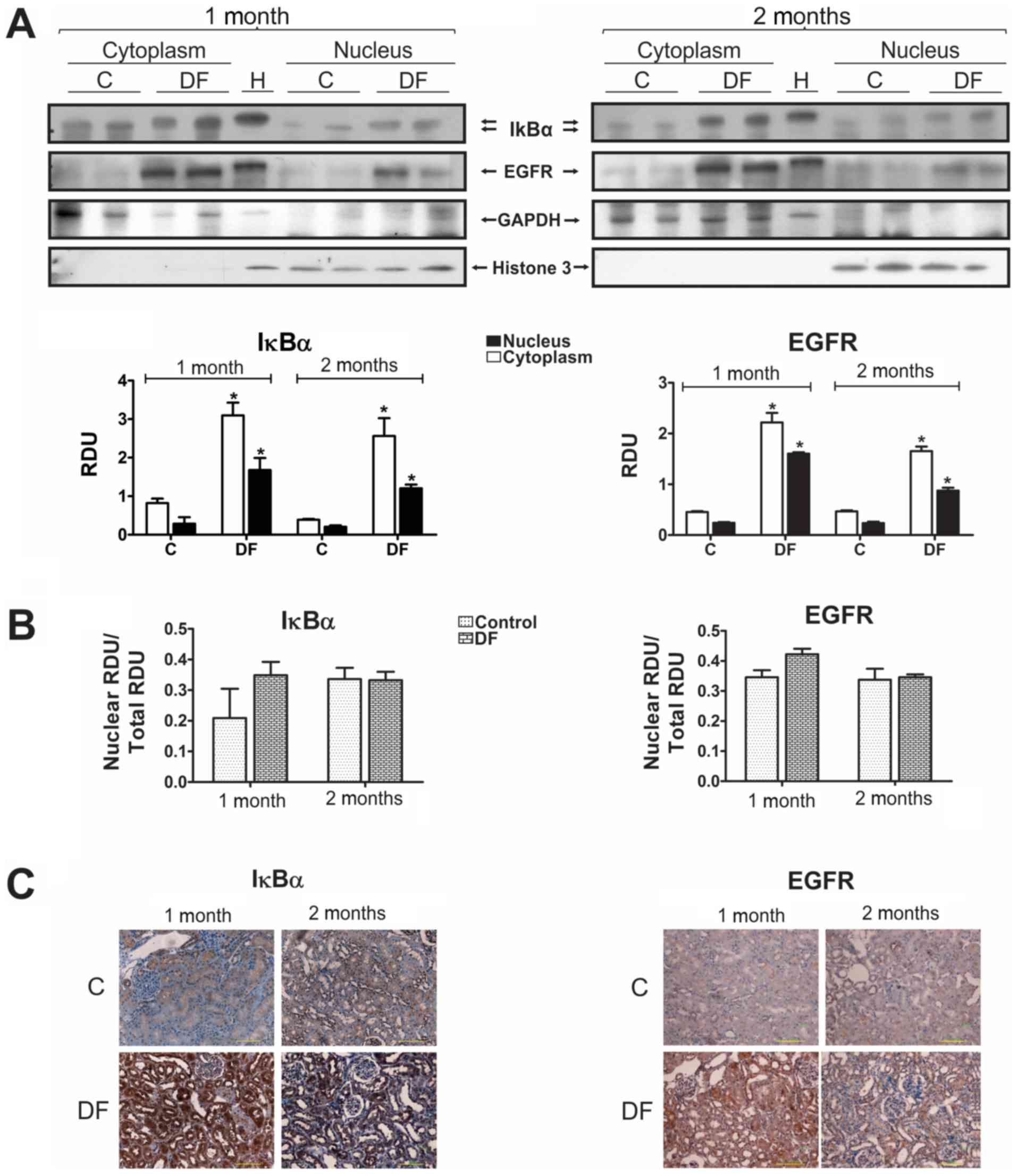
Subcellular localization of IκBα and
EGFR at early stages of FeNTA-induced renal carcinogenesis
Cytoplasmic and nuclear fractions were prepared to
investigate the subcellular distribution of IκBα and EGFR by WB
(Fig. 5A). For this specific
determination, relative densitometric units were not adjusted to
the values of the corresponding C groups, in order to facilitate
the assessment of the differences in both cell fractions of the
kidney cortex from C and DF treated animals. GAPDH and histone 3
were used as loading controls for cytoplasmic and nuclear extracts,
respectively. As it can be seen, IκBα and EGFR were present in the
cytoplasm as well as in the nucleus and displayed a statistically
significant increase in both cell fractions, which appears to be
higher after one month than after two, a behavior that coincides
with that observed when total extracts were analyzed (Fig. 3). However, no changes were detected
in the translocation rate of either of these proteins (Fig. 5B), calculated by dividing the nuclear
fraction by the total densitometric units, though a tendency to
accretion was observed in the first month. In addition, these
results were corroborated by IHC (Fig.
5C), observing both proteins (IκBα and EGFR) mostly in the
cytoplasm but also in the nucleus, especially IκBα.
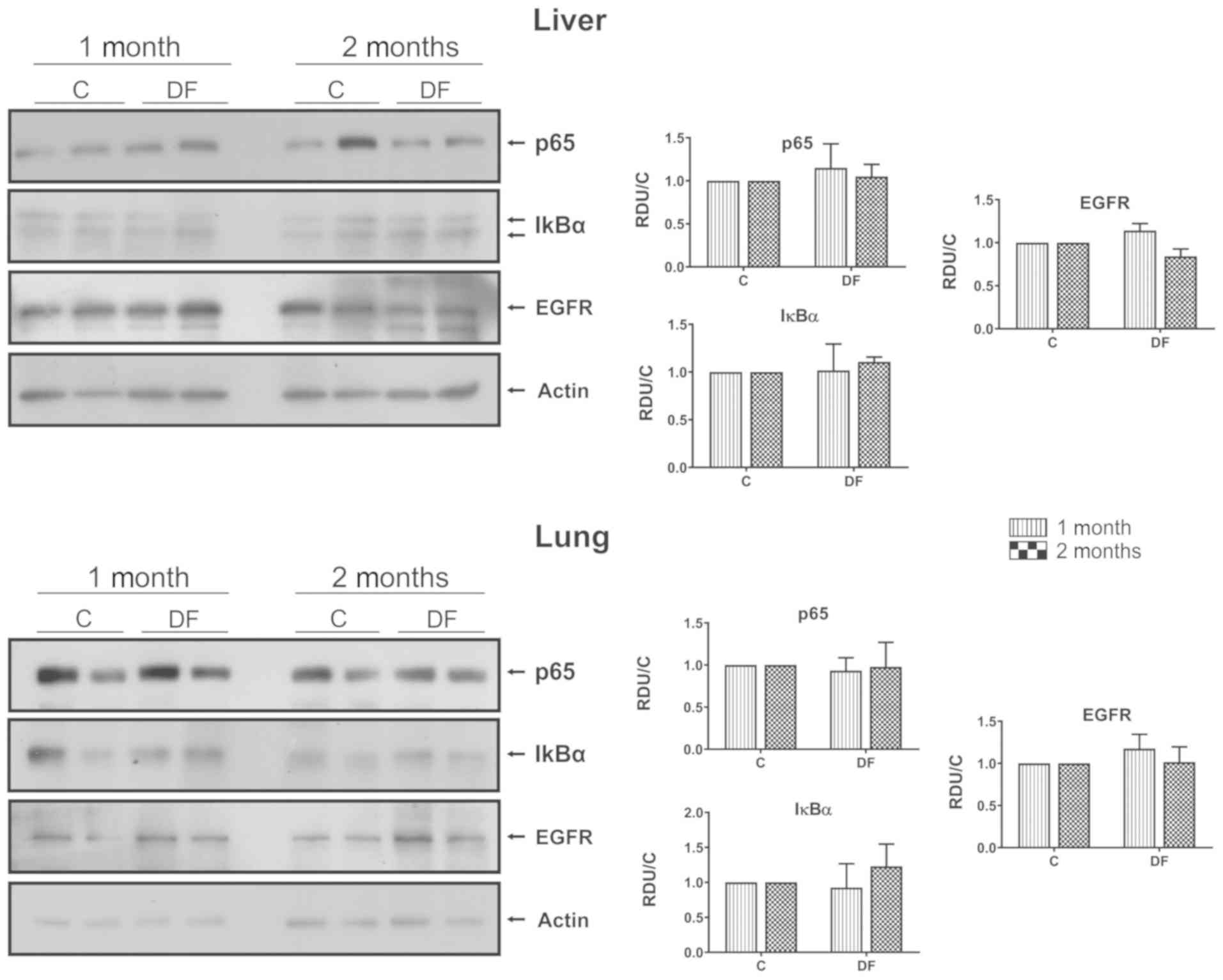
p65, IκBα and EGFR in hepatic and lung
tissues
Finally, the authors reported in a previous study
(6) that no primary tumors were
developed in liver or lungs using the scheme of FeNTA-exposure
followed in the present investigation; thus, in order to determine
if the alterations observed in p65, IκBα and EGFR behavior were
particularly associated with the kidney, and was not a generalized
response, the status of these proteins was determined in hepatic
and lung tissues, finding no differences between C and DF groups
(Fig. 6).
Discussion
RCC is the most common urological type of cancer in
adults and has a high mortality rate due to several complications,
such as a late diagnosis (1), which
additionally makes it very difficult to study events happening at
RCC's early phases, this being therefore feasible mostly in
experimental models. The research group implemented a FeNTA-induced
renal carcinogenesis protocol in rats and all obtained tumors were
histopathologically characterized as clear cell subtype of RCC
(6), the most frequent subtype in
patients (1,4), and even different Fuhrman grades were
identified. Also, pre-neoplastic lesions as well as different
pro-carcinogenic alterations have been found after either one or
two months of FeNTA-exposure and, consequently, these times of
exposure were established as distinct early stages of the
carcinogenesis process (5,6).
The present study found an overexpression of p65 in
FeNTA-induced renal tumors, in agreement with previous observations
for NF-κB in rat kidney after long term of FeNTA-exposure (36,37) and
similar to what happens in patients' clear cell subtype tumors
(14–16,19), as
well as in some RCC human cell lines (13). Moreover, NF-κB activity is
principally regulated by its inhibitory protein, IκBα, and a
substantial decrease, or even its absence, was observed in the
experimental tumors, which is consistent with the findings of Oya
et al (14) in human RCC.
Also, it is worth noting that IκBα was less reduced and its nuclear
presence was more frequent in NTT compared with TT and AT,
suggesting that it must be restraining NF-κB activity in this area,
where p65 was found to be increased in the same way as in TT, thus
IκBα might be at least one of the mechanisms by which these cells
are defended from malignant transformation. For its part, the mean
value of EGFR levels in FeNTA-induced tumors exhibited an
enhancement compared with renal tissue from C rats, despite some of
them presenting no changes or even a decrease, as described in
human tumors (23). Likewise, EGFR
mRNA levels increased in most experimental tumors (61%), suggesting
that the augment in the protein is transcriptionally induced and
very probably caused, at least in part, by NF-κB, since p65 also
augmented. Nevertheless, the clinical significance of the EGFR
enhancement is still controversial and seems to be associated to
its subcellular distribution (12,24,25,27,28). In
this respect, since EGFR was found preferentially in the cytoplasm
of the tumor cells, a bad prognosis would be expected according to
Kankaya et al (25), who
analyzed ccRCC subtype particularly.
Subsequently, the behavior of these molecules at
early stages of renal carcinogenesis was analyzed. After one month
of FeNTA-exposure, NF-κB activity was augmented even though p65
levels did not change and IκBα was increased. This could be
explained by p65 posttranslational modifications which have been
reported to increase the affinity of the transcription factor for
its target sequence or to promote its association with some
co-activators (38,39) and thus, IκBα might be playing other
roles than regulating NF-κB. In this latter case, for instance,
some authors have demonstrated that nuclear IκBα forms part of a
transcriptional complex that associates with the promoter of
different genes such as hox (40). Products of hox genes are a
family of transcription factors implicated in renal organogenesis,
and a change in their expression has been associated with ccRCC
development (41,42). In fact, the present results showed
augmented levels of IκBα in the cytoplasm but also in the nucleus
and, interestingly, a tendency to increase its nuclear
translocation was even noticed in the first month. On the other
hand, it has been established that, besides preventing NF-κB
translocation into the nucleus, IκBα is able to retain p53 in the
cytoplasm, thereby counteracting the tumor-suppressive functions of
p53 in other neoplasias (43).
Nowadays, however, the behavior of HOX proteins and their possible
role in the FeNTA-induced renal carcinogenesis are unknown and, to
the best of our knowledge, the association between IκBα and p53 has
not been investigated in RCC, which represent interesting
perspectives for future studies.
It is likewise important to point out that exposure
of rats to FeNTA during either one or two months, not only induced
a renal increase of IκBα, but a shift in its electrophoretic
mobility was detected, suggesting that a modified form of IκBα is
accumulating in response to the carcinogen. Moreover, this shifted
IκBα band increased more in the first month than at the second one.
Phosphorylation of IκBα gives rise to its ubiquitination, a
modification that is recognized by the 26S proteasome for the
inhibitor's degradation. It may therefore indicate that the
carcinogen provokes IκBα renal accumulation due to the inhibition
of the 26S proteasome, effect that has been demonstrated by Okada
et al (44). IκBα accretion
may then be an early alteration that leads to promotion of the
pro-carcinogenic mechanisms described above.
After two months of carcinogen exposure, in
contrast, renal NF-κB activity decreased and this was consistent
with the IκBα rise, suggesting that IκBα is carrying out its
classical inhibitory functions on the NF-κB transcription factor at
this more advanced early stage of renal carcinogenesis. Hence, the
behavior observed at the first month seems to be the primary
response to the carcinogen, whereas after two months, this effect
is being controlled probably in most renal cells as a defense
mechanism against malignant transformation, as was proposed above
for NTT. However, as mentioned earlier, a shifted band of IκBα was
also observed in WB assays after this time of FeNTA-exposure, so it
would be relevant to identify posttranslational modifications
possibly present in IκBα at the different stages of the
carcinogenic process and to investigate the roles this protein
might be playing in each stage. Intensive efforts are been
conducted in the author's laboratory in this respect. Furthermore,
a common therapeutic approach is to prevent the degradation of
IκBα, but the true contribution of this protein to cancer
pathogenesis is far from being understood (43).
Regarding EGFR, protein levels increased similarly
after either one or two months of FeNTA exposure, as well as those
of its mRNA, indicating a transcriptional regulation. Nevertheless,
the increase in the receptor's protein levels was notably higher
(around 25–30 times vs. C) than that of its mRNA (1.4 times vs. C),
hence translational and/or posttranslational events may be involved
too. Furthermore, although to a lesser extent, EGFR protein levels
also increased more than its mRNA levels in the experimental tumors
(2 vs. 1.4 times, respectively). EGFR, for example, may have a
greater stability, as a result of a decrease in its degradation.
This hypothesis is supported by Zhou and Yang (45), who demonstrated deficient EGFR
degradation in human ccRCC cell lines induced by an accumulation of
HIF2α, which suppress rabaptin-5 gene expression, thus delaying
EGFR lysosomal degradation, since this protein mediates
endosome-lysosome fusion. Results from the author's laboratory,
soon to be published, also indicate an increase of HIF2α renal
levels after two months of FeNTA-exposure as well as in the induced
tumors. This mechanism may explain the cytoplasmic localization of
EGFR found in the present study too. It is unknown, however, if
this mechanism takes place in rats.
In the first month, the increase of EGFR mRNA levels
coincided with the rise found in NF-κB activity, suggesting that
this transcriptional factor is at least one of the causes of the
receptor's response. After two months, however, while EGFR mRNA
levels were still high, NF-κB's activity decreased, so the
receptor's increment could be a consequence of other factors. For
example, HIF2α, which aside from participating in EGFR
stabilization as previously discussed, is likewise involved in EGFR
gene expression and preliminary results from the authors' research
group suggest an increase in the HIF2α transcription factor after
two months' exposure, but not at the first one, contrary to that
observed for NF-κB. Therefore, EGFR mRNA levels accretion may be
the response to NF-κB's activity after one month of FeNTA-exposure
and to HIF2α's activity at the second one. Another transcription
factor probably involved as well in the noticed EGFR behavior is
AP-1, since there are 7 target sequences for it (c-Jun) in the gene
promoter of the receptor (46) and
an increase of AP-1 activity (c-Jun EMSA) at the early stages of
FeNTA-induced renal carcinogenesis has been observed (still
unpublished results of our group). On the other hand, although
EGFR's most studied and reported function is as a cell membrane
receptor transducing extracellular signals, its nuclear
translocation has also been demonstrated, where it acts as a
transcription co-factor linked to other proteins including STAT3,
STAT5 and E2F1 (47,48), or stimulates DNA repair by
associating with the catalytic subunit of DNA-dependent protein
kinases when DNA is damaged (49–51).
Therefore, EGFR subcellular location was determined, finding that
this receptor is present and increased in the cytoplasm, but also
in the nucleus after either one or two months of FeNTA-exposure,
while in tumors the vast majority was in the cytoplasm. Hence, at
the early stages of carcinogenesis, EGFR may be participating in
renal cells' transformation by stimulating signaling pathways
associated with cell proliferation, angiogenesis, invasion and
apoptosis inhibition, but also may be playing anti-oncogenic roles
such as DNA repair promotion as a protective mechanism against
FeNTA-induced oxidative damage (52); moreover, EGFR may also be playing
these anti-oncogenic roles in the non-transformed renal tissue
areas (NTT and AT), where this receptor was observed in the nucleus
more frequently compared with tumors. In contrast, in the
neoplastic tissue, EGFR could be preferentially participating in
tumor maintenance by inducing, for example, angiogenic signaling
pathways, as this receptor was preferentially localized in the
cytoplasm. Nevertheless, what EGFR is indeed doing at each stage of
carcinogenesis should be investigated in future studies.
Finally, whether the alterations in p65, IκBα and
EGFR observed in kidney were also present in liver and lungs, as it
is known that FeNTA and DEN may cause hepatic and/or pulmonary
damage was determined (53). Data
indicated no alterations in either of these organs, so the behavior
observed in p65, IκBα and EGFR is very probably kidney specific.
Since the authors previously reported that, using the scheme of
FeNTA-exposure followed in the present study, no primary tumors
were developed in the liver or lungs (5,6), these
observations strongly support the participation of the molecules
analyzed in the current study in renal carcinogenesis.
It is worth pointing out that the present results,
together with the great similarity with histological and mitogen
associated protein kinase's behavior previously reported (5,6),
reinforce the resemblance between the experimental and human renal
carcinoma, and strengthen the usefulness of the experimental model
to study this type of cancer, particularly in its early stages,
which are almost impossible to analyze in patients.
In conclusion, according to the evidence obtained in
the present work, NF-κB, IκBα and EGFR are probably implicated in
the carcinogenic process, but these molecules undergo different
adjustments as it evolves. In summary, a classical combination of
changes was observed in tumors, suggesting that these molecules may
be important for maintenance of the malignant phenotype, as has
been proposed in human RCC. On the contrary, in the two early
stages assessed, distinctive non-conventional combinations of
changes and subcellular distributions were observed, suggesting
that their behavior could be related to different signaling
pathways and therefore to particular effects depending on the phase
of the carcinogenic process. Given the similarity of experimental
and human neoplasms, this could also happen in human carcinoma
development. Therefore, novel insights were provided in the present
study to continue searching for mechanisms other than the classic
roles of NF-κB, IκBα and EGFR, which may be responsible for the
renal cells' malignant transformation, and then to identify
molecular markers that lead to an opportune detection and to
develop more effective therapeutic and/or preventive strategies
against RCC.
Acknowledgements
The authors would like to thank Dr Lucía Macías
Rosales, (Animal Experimentation Unit, Faculty of Chemistry,
National Autonomous University of Mexico), for her assistance in
animal care and treatment. Also, the authors acknowledge Dr Elena
Martínez-Klimova, (Department of Biology, Faculty of Chemistry,
National Autonomous University of Mexico), for her contribution to
the language editing of the manuscript.
Funding
The present study was supported by the Universidad
Nacional Autónoma de México through Dirección General de Asuntos
del Personal Académico-Programa de Apoyo a Proyectos de
Investigación e Innovación Tecnológica (UNAM-DGAPA-PAPIIT; grant
nos. IN227010, IN221313 and IN228716), the Consejo Nacional de
Ciencia y Tecnología (CONACyT) through Fondo Sectorial de
Investigación para la Educación (grant no. 284155) and the Facultad
de Química through Programa de Apoyo a la Investigación y el
Posgrado (PAIP; grant no. 5000-9109), given to MEIR. TPP, FAA, CVO
and PCM received a fellowship from CONACyT. Also special thanks to
Programa de Apoyo a los Estudios de Posgrado (PAEP) for the
donation received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TPP participated in the experimental design,
carcinogenesis protocol execution, all the results generation,
analysis and interpretation, and is a main contributor of the
manuscript preparation. FAA participated in the carcinogenesis
protocol execution, electrophoretic-mobility shift assay and
reverse transcription-PCR techniques standardization, data analysis
and interpretation, figures design, and manuscript drafting and
critical revision. JDS participated in the carcinogenesis protocol
execution, different techniques advising, as well as data analysis
and interpretation. CVO established the carcinogenesis protocol in
our laboratory and participated in western blotting results
analysis and interpretation. PCM participated in the carcinogenesis
protocol execution, data analysis and interpretation, figures
design, and manuscript critical revision. CAMR participated in IHC
technique advising, standardization and images acquisition,
processing, analysis and interpretation. DTH contributed in IHC
assays execution and results generation. MEIR followed and advised
throughout the project, participated in experimental conception and
design, samples extraction, data analysis and interpretation, and
is a main contributor of the manuscript preparation. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments involving animals were performed
according to the Mexican Oficial Norm NOM-062-ZOO-199 and with the
approval of the Institutional Committee for the Use and Care of
Laboratory Animals (FQ/CICUAL/081/14). Ethical approval for the
animal experiments conducted in the study was obtained
non-retrospectively. In addition, tumor burden did not exceed the
recommended dimensions and rats were anesthetized and sacrificed by
acceptable methods.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ccRCC
|
clear cell renal cell carcinoma
|
|
FeNTA
|
ferric nitrilotriacetate
|
|
DEN
|
N-dietilnitrosamine
|
|
DF
|
DEN+FeNTA
|
|
NTT
|
non-tumor tissue
|
|
TT
|
tumor tissue
|
|
AT
|
adjacent tissue
|
|
WB
|
western blotting
|
|
IHC
|
immunohistochemistry
|
|
EMSA
|
electrophoretic mobility shift
assay
|
References
|
1
|
Makhov P, Joshi S, Ghatalia P, Kutikov A,
Uzzo RG and Kolenko VM: Resistance to systemic therapies in clear
cell renal cell carcinoma: Mechanisms and management strategies.
Mol Cancer Ther. 17:1355–1364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peri S, Devarajan K, Yang DH, Knudson AG
and Balachandran S: Meta-analysis identifies NF-κB as a therapeutic
target in renal cancer. PLoS One. 8:e767462013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morais C, Gobe G, Johnson DW and Healy H:
The emerging role of nuclear factor kappa B in renal cell
carcinoma. Int J Biochem Cell Biol. 43:1537–1549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muglia VF and Prando A: Renal cell
carcinoma: Histological classification and correlation with imaging
findings. Radiol Bras. 48:166–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aguilar-Alonso FA, Solano JD,
Vargas-Olvera CY, Pacheco-Bernal I, Pariente-Pérez TO and
Ibarra-Rubio ME: MAPKs' status at early stages of renal
carcinogenesis and tumors induced by ferric nitrilotriacetate. Mol
Cell Biochem. 404:161–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vargas-Olvera CY, Sánchez-González DJ,
Solano JD, Aguilar-Alonso FA, Montalvo-Muñoz F, Martínez-Martínez
CM, Medina-Campos ON and Ibarra-Rubio ME: Characterization of
N-diethylnitrosamine-initiated and ferric
nitrilotriacetate-promoted renal cell carcinoma experimental model
and effect of a tamarind seed extract against acute nephrotoxicity
and carcinogenesis. Mol Cell Biochem. 369:105–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitchell S, Vargas J and Hoffmann A:
Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med.
8:227–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayden MS and Ghosh S: Regulation of NF-κB
by TNF family cytokines. Semin Immunol. 26:253–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desterro JM, Rodriguez MS and Hay RT:
SUMO-1 modification of IκBα inhibits NF-kappaB activation. Mol
Cell. 2:233–239. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renard P, Percherancier Y, Kroll M, Thomas
D, Virelizier JL, Arenzana F and Bachelerie F: Inducible NF-kappaB
activation is permitted by simultaneous degradation of nuclear
IkappaBalpha. J Biol Chem. 275:15193–15199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lua J, Qayyum T, Edwards J and Roseweir
AK: The prognostic role of the non-canonical nuclear factor-kappa B
pathway in renal cell carcinoma patients. Urol Int. 101:190–196.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meteoglu I, Erdogdu IH, Meydan N, Erkus M
and Barutca S: NF-kappaB expression correlates with apoptosis and
angiogenesis in clear cell renal cell carcinoma tissues. J Exp Clin
Cancer Res. 27:532008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morais C, Pat B, Gobe G, Johnson DW and
Healy H: Pyrrolidine dithiocarbamate exerts anti-proliferative and
pro-apoptotic effects in renal cell carcinoma cell lines. Nephrol
Dial Transplant. 21:3377–3388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oya M, Takayanagi A, Horiguchi A, Mizuno
R, Ohtsubo M, Marumo K, Shimizu N and Murai M: Increased nuclear
factor-κB activation is related to the tumor development of renal
cell carcinoma. Carcinogenesis. 24:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sourbier C, Danilin S, Lindner V, Steger
J, Rothhut S, Meyer N, Jacqmin D, Helwig JJ, Lang H and Massfelder
T: Targeting the nuclear factor-κB rescue pathway has promising
future in human renal cell carcinoma therapy. Cancer Res.
67:11668–11676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Đorđević G, Matušan-Ilijaš K, Sinožić E,
Damante G, Fabbro D, Grahovac B, Lučin K and Jonjić N: Relationship
between vascular endothelial growth factor and nuclear factor-κB in
renal cell tumors. Croat Med J. 49:608–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An J and Rettig MB: Epidermal growth
factor receptor inhibition sensitizes renal cell carcinoma cells to
the cytotoxic effects of bortezomib. Mol Cancer Ther. 6:61–69.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oka D, Nishimura K, Shiba M, Nakai Y, Arai
Y, Nakayama M, Takayama H, Inoue H, Okuyama A and Nonomura N:
Sesquiterpene lactone parthenolide suppresses tumor growth in a
xenograft model of renal cell carcinoma by inhibiting the
activation of NF-kappaB. Int J Cancer. 120:2576–2581. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng KL, Yap NY, Rajandram R, Small D,
Pailoor J, Ong TA, Razack AH, Wood ST, Morais C and Gobe GC:
Nuclear factor-kappa B subunits and their prognostic
cancer-specific survival value in renal cell carcinoma patients.
Pathology. 50:511–518. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thornburg NJ and Raab-Traub N: Induction
of epidermal growth factor receptor expression by Epstein-barr
virus latent membrane protein 1 C-terminal-activating region 1 is
mediated by NF-κB p50 Homodimer/Bcl-3 complexes. J Virol.
81:12954–12961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Staruschenko A, Palygin O, Ilatovskaya DV
and Pavlov TS: Epidermal growth factors in the kidney and
relationship to hypertension. Am J Physiol Renal Physiol.
305:F12–F20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng F, Singh AB and Harris RC: The role
of the EGF family of ligands and receptors in renal development,
physiology and pathophysiology. Exp Cell Res. 315:602–610. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen D, Lane B, Jin T, Magi-Galluzzi C,
Finke J, Rini BI, Bukowski RM and Zhou M: The prognostic
significance of epidermal growth factor receptor expressionin
Clear-cell renal cell carcinoma: A call for standardized methods
for immunohistochemical evaluation. Clin Genitourinary Cancer.
5:264–270. 2007. View Article : Google Scholar
|
|
24
|
Kallio JP, Hirvikoski P, Helin H,
Kellokumpu-Lehtinen P, Luukkaala T, Tammela TL and Martikainen PM:
Membranous location of EGFR immunostaining is associated with good
prognosis in renal cell carcinoma. Br J Cancer. 89:1266–1269. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kankaya D, Kiremitci S, Tulunay O and
Baltaci S: Prognostic impact of epidermal growth factor receptor on
clear cell renal cell carcinoma: Does it change with different
expression patterns? Indian J Pathol Microbiol. 59:35–40.
2016.PubMed/NCBI
|
|
26
|
Merseburger AS, Hennenlotter J, Simon P,
Kruck S, Koch E, Horstmann M, Kuehs U, Küfer R, Stenzl A and Kuczyk
MA: Membranous expression and prognostic implications of epidermal
growth factor receptor protein in human renal cell cancer.
Anticancer Res. 25:1901–1907. 2005.PubMed/NCBI
|
|
27
|
Mock H, Sauter G, Buchholz N, Gasser TC,
Bubendorf L, Waldman FM and Mihatsch MJ: Epidermal growth factor
receptor expression is associated with rapid tumor cell
proliferation in renal cell carcinoma. Hum Pathol. 28:1255–1259.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pu YS, Huang CY, Kuo YZ, Kang WY, Liu GY,
Huang AM, Yu HJ, Lai MK, Huang SP, Wu WJ, et al: Characterization
of membranous and cytoplasmic EGFR expression in human normal renal
cortex and renal cell carcinoma. J Biomed Sci. 16:82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Athar M and Iqbal M: Ferric
nitrilotriacetate promotes N-diethylnitrosamine-induced renal
tumorigenesis in the rat: Implications for the involvement of
oxidative stress. Carcinogenesis. 19:1133–1139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebina Y, Okada S, Hamazaki S, Ogino F, Li
JL and Midorikawa O: Nephrotoxicity and renal cell carcinoma after
use of Iron- and aluminum-nitrilotriacetate complexes in Rats2. J
Natl Cancer Inst. 76:107–113. 1986.PubMed/NCBI
|
|
31
|
Jahangir T and Sultana S: Modulatory
effects of shape pluchea lanceolata against chemically induced
oxidative damage, hyperproliferation and Two-stage renal
carcinogenesis in wistar rats. Mol Cell Biochem. 291:175–185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mendoza-Rodrıguez CA, Monroy-Mendoza MG,
Morimoto S and Cerbón MA: Pro-apoptotic signals of the bcl-2 gene
family in the rat uterus occurs in the night before the day of
estrus and precedes ovulation. Mol Cell Endocrinol. 208:31–39.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ledeganck KJ, Boulet GA, Horvath CA,
Vinckx M, Bogers JJ, Van Den Bossche R, Verpooten GA and De Winter
BY: Expression of renal distal tubule transporters TRPM6 and NCC in
a rat model of cyclosporine nephrotoxicity and effect of EGF
treatment. Am J Physiol Renal Physiol. 301:F486–F493. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai FG, Xiao JS and Ye QF: Effects of
ischemic preconditioning on cyclinD1 expression during early
ischemic reperfusion in rats. World J Gastroenterol. 12:2936–2940.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rehman MU, Tahir M, Khan AQ, Khan R,
Lateef A, Oday OH, Qamar W, Ali F and Sultana S: Chrysin suppresses
renal carcinogenesis via amelioration of hyperproliferation,
oxidative stress and inflammation: Plausible role of NF-κB. Toxicol
Lett. 216:146–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Siddiqi A, Hasan SK, Nafees S, Rashid S,
Saidullah B and Sultana S: Chemopreventive efficacy of hesperidin
against chemically induced nephrotoxicity and renal carcinogenesis
via amelioration of oxidative stress and modulation of multiple
molecular pathways. Exp Mol Pathol. 99:641–653. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Christian F, Smith EL and Carmody RJ: The
regulation of NF-κB subunits by phosphorylation. Cells. 5(pii):
E122016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perkins ND: Post-translational
modifications regulating the activity and function of the nuclear
factor kappa B pathway. Oncogene. 25:6717–6730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Espinosa L, Bigas A and Mulero MC: Novel
functions of chromatin-bound IκBα in oncogenic transformation. Br J
Cancer. 111:1688–1692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cantile M, Schiavo G, Franco R, Cindolo L,
Procino A, D'Armiento M, Facchini G, Terracciano L, Botti G and
Cillo C: Expression of lumbosacral HOX genes, crucial in kidney
organogenesis, is systematically deregulated in clear cell kidney
cancers. Anticancer Drugs. 22:392–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mulero MC, Ferres-Marco D, Islam A,
Margalef P, Pecoraro M, Toll A, Drechsel N, Charneco C, Davis S,
Bellora N, et al: Chromatin-bound IκBα regulates a subset of
polycomb target genes in differentiation and cancer. Cancer Cell.
24:151–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morotti A, Crivellaro S, Panuzzo C, Carrà
G, Guerrasio A and Saglio G: IκB-α: At the crossroad between
oncogenic and tumor-suppressive signals. Oncol Lett. 13:531–534.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okada K, Wangpoengtrakul C, Osawa T,
Toyokuni S, Tanaka K and Uchida K: 4-Hydroxy-2-nonenal-mediated
impairment of intracellular proteolysis during Oxidative Stress:
Identification of proteasomes as target molecules. J Biol Chem.
274:23787–23793. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou L and Yang H: The von Hippel-lindau
tumor suppressor protein promotes c-Cbl-independent
poly-ubiquitylation and degradation of the activated EGFR. PLoS
One. 6:e239362011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Johnson AC, Murphy BA, Matelis CM,
Rubinstein Y, Piebenga EC, Akers LM, Neta G, Vinson C and Birrer M:
Activator protein-1 mediates induced but not basal epidermal growth
factor receptor gene expression. Mol Med. 6:17–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Brand TM, Iida M, Luthar N, Starr MM,
Huppert EJ and Wheeler DL: Nuclear EGFR as a molecular target in
cancer. Radiother Oncol. 108:370–377. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharmila R and Sindhu G: Evaluate the
antigenotoxicity and anticancer role of β-sitosterol by determining
oxidative DNA damage and the expression of phosphorylated
Mitogen-activated Protein Kinases', C-fos, C-jun, and endothelial
growth factor receptor. Pharmacogn Mag. 13:95–101. 2017.PubMed/NCBI
|
|
49
|
Bandyopadhyay D, Mandal M, Adam L,
Mendelsohn J and Kumar R: Physical Interaction between epidermal
growth factor receptor and DNA-dependent protein kinase in
mammalian cells. J Biol Chem. 273:1568–1573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Goodwin JF and Knudsen KE: Beyond DNA
repair: DNA-PK function in cancer. Cancer Discov. 4:1126–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liccardi G, Hartley JA and Hochhauser D:
EGFR nuclear translocation modulates DNA repair following cisplatin
and ionizing radiation treatment. Cancer Res. 71:1103–1114. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Toyokuni S, Mori T and Dizdaroglu M: DNA
base modifications in renal chromatin of wistar rats treated with a
renal carcinogen, ferric nitrilotriacetate. Int J Cancer.
57:123–128. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ansar S, Iqbal M and Athar M:
Nordihydroguairetic acid is a potent inhibitor of
ferric-nitrilotriacetate-mediated hepatic and renal toxicity, and
renal tumour promotion, in mice. Carcinogenesis. 20:599–606. 1999.
View Article : Google Scholar : PubMed/NCBI
|