Oral squamous cell carcinoma (OSCC) is the leading
cause of death among all oral cancers. This tumor originates from
the oral mucosa and accounted for over 350,000 new diagnoses and
more than 175,000 recorded deaths worldwide in 2018 (1). It was widely demonstrated that the
development of tumors, including that of OSCC, is sustained by
several risk factors and predisposing conditions such as fibers,
chemicals, pesticides and heavy metals able to induce pro-oncogenic
genetic and epigenetic alterations (2–6). Other
factors, including oral injuries, inflammatory diseases,
infections, and bacterial dysbiosis are now recognized as risk
factors for cancer development (7–10).
Regarding OSCC, it was demonstrated that its
development is strictly influenced by host-related and lifestyle
factors mainly represented by smoking, alcohol abuse, tobacco and
tobacco-derivate chewing and oral virus infections (HPV) (11,12). In
addition, it was demonstrated that the combination of smoke,
alcohol drinking and poor oral hygiene increase the risk of oral
cancer onset due to chronic inflammation and infection which
constitute the principal factors involved in cancer pathogenesis,
influencing the resident microbiota that are involved in the
homeostasis of the oral environment (13–17).
Importantly, a precise distinction must be made between microbiota
and microbiome: the former is comprised of all the bacteria species
hosted within the oral cavity, while with the latter term is used
to define the collective genomes of microorganisms inhabiting the
oral mucosa (18–20).
Oral microbiota changes are able to modulate the
connection between oral bacteria and humans causing diseases
(21,22). Oral microbiota seems to influence
OSCC through the carcinogenetic modulation of cell metabolism (such
as modifying the concentration of nutrients and vitamins), thereby
promoting the production of different cytokines known to be
involved in several pathological conditions (23–27).
Over 600 bacterial species constitute the oral
microbiota. However, the majority of these species are uncultivated
(19). The availability of new
sequencing technology allowed the identification of bacterial
communities that harbor the oral cavity and that are involved in
human health (28) (Table I).
Researchers have investigated the possible
association between microbes and the alteration of physiological
conditions. In this context, it was demonstrated that gut
microbiota predisposes individuals for the development of different
diseases including celiac disease, neurological disorders and blood
pressure alterations (29–31). All these pathological conditions are
involved in the development of severe health conditions mainly
represented by neurovascular disorders, chronic degenerative
diseases, and cancer (32–46). Among all cancers, oral cancer is
particularly related with oral and gut microbiota composition as
widely demonstrated. Among the different bacteria strongly
associated with OSCC, Fusobacterium nucleatum, Porphyromonas
gingivalis, and Prevotella intermedia are considered the
most represented bacteria types (47–51).
Moreover, other bacterial genera, such as Actinomyces,
Clostridium, Enterobacteriaceae, Fusobacterium, Haemophilus,
Porphyromonas, Prevotella, Streptococcus spp. and
Veillonella are associated with pre-cancerous lesions and
oral cancer (52). According to
other studies, a high bacterial load of Prevotella
melaninogenica, Streptococcus mitis and Capnocytophaga
gingivalis are identifiable in saliva samples of OSCC patients
(Table II) (50,51).
Although the association between some species and
oral cancer was already established, the complexity of the
relationship occurring between cancer and oral microbiota remains
unexplained and cannot be limited to the evaluation of a single
pathogen (53). Moreover, there are
no concordant analytical protocols for the analysis of oral
microbiota and microbiome. Therefore, it is difficult to establish
oral cavity-associated microbial patterns in cancer patients and
healthy subjects. Consequently, there is a lack of novel microbial
biomarkers for the early identification of oral carcinoma (54,55).
Previous findings demonstrated that a strong
association between oral microbiota and oral cancer exists.
Starting from this supposition, a number of studies focused on the
prevention of neoplastic transformation and retardation of cancer
progression by modulating the carcinogenic or protective
microbiome. In this context, probiotics administration was recently
considered a good cancer preventive strategy due to the
immunological effects (8,9,25). The
beneficial effects of lactic ferments and probiotics were
identified in the 19th century by Dr Ilya Metchnikoff.
Nevertheless, only in recent years have these products been widely
used for the treatment of several diseases (56,57).
Numerous studies have shown the potential positive effects of
probiotics on cancer through several mechanisms that include immune
modulation, the prevention of pathogen infections, inflammatory
modulation, reduced cancer formation and metastatic process
(9,25). To the best of our knowledge, few data
have been generated about the effects of probiotics in oral cancer
development. Of note, the results of a previous study demonstrated
that the administration of Lactobacillus rhamnosus GG (LGG)
was able to increase the effects of geniposide, an anticancer
molecule tested on human oral squamous carcinoma cells (HSC-3),
demonstrating the beneficial role of LGG as potential adjuvant of
geniposide treatment (58).
The aim of this review was to describe the
scientific evidence collected during the years pertaining to oral
microbiota and neoplastic transformation with special attention for
OSCC. Finally, a brief overview on the anti-tumoral effect of
probiotics and their applications in oral cancer was reported.
Observational studies have shown a link among oral
cancer and infrequent tooth brushing, infrequent dental visits and
loss of or missing teeth (59–62).
These findings, however, pertain only to non-smokers and
non-drinkers (13–14). Another study revealed that
periodontal illnesses are correlated with an increased risk for
oral tumors (63). Furthermore,
research performed on 51 tongue cancer patients and 54 normal
controls cases revealed that chronic periodontal inflammation is a
cancer risk factor (64). In
addition, periodontitis patients showed an increased risk for OSCC
compared to healthy controls (65).
Another observational study conducted on a wide cohort of
individuals in the USA investigated the use of dental care and oral
cancer risk. The analysis of covariates and dental care
appointments demonstrated that individuals with a dental
appointment during the past 12 months had a lower (62%) oral cancer
risk compared with subjects that had not used dental care
procedures in the past year (66).
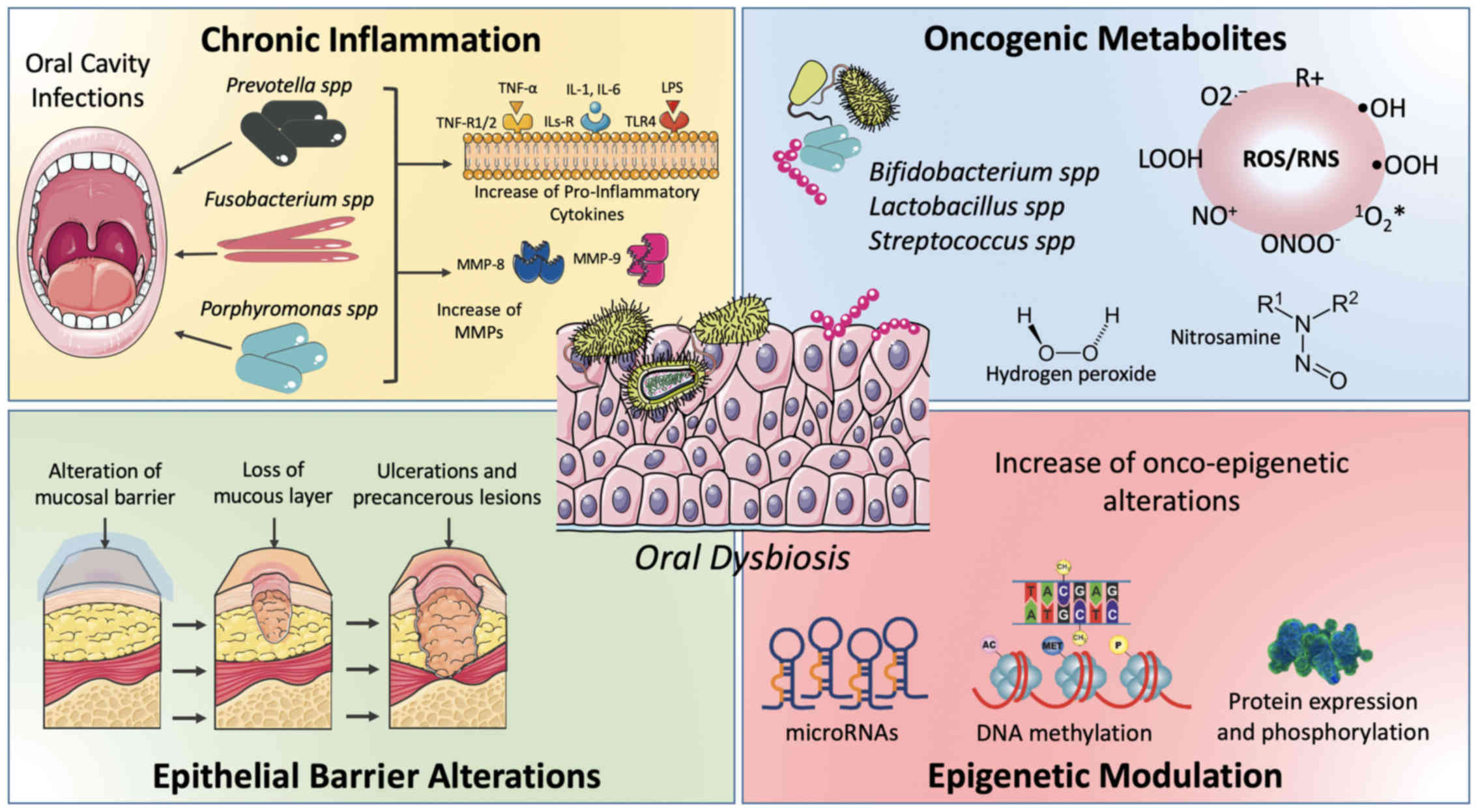
Finally, it was recently demonstrated that
microbiota and oral mucosa dysbiosis lead to the accumulation of
different epigenetic alterations predisposing for neoplastic
transformation (Fig. 1) (81).
Chronic inflammation. According to data reported in
the literature, approximately 25% of human cancer shares chronic
inflammation as a risk factor, indicating that inflammation is one
of the most important hallmarks of cancer (82). Several processes including cell
proliferation, angiogenesis, mutagenesis and oncogene activation
may be caused or facilitated by chronic inflammatory mediators that
alter the normal homeostasis of cells and tissues (82).
The upregulation of cytokines and other inflammatory
factors lead to the alteration of different molecular pathways,
including metabolic pathways, responsible for the modulation of
cell metabolism and proliferation. For example, RAGE protein
expression changes significantly after periodontal diseases
mediated by oral microbiota alterations, leading to carcinogenesis
(88). Moreover, gram-negative
bacteria release a pro-inflammatory lipopolysaccharide endotoxin
(LPS) able to stimulate the production of IL1-β, IL-6 ant TNF-α by
binding the TLR receptor of leucocytes (89,90). In
particular, these inflammatory cytokines lead to the overexpression
of other pro-inflammatory proteins stimulating the release of
phospholipase A2, prostaglandins (PG) and acute phase proteins
(91,92). Other studies demonstrated that high
IL-1 levels favor a pro-angiogenetic microenvironment, supporting
tumor spread (93–95). Simultaneously, IL-1 induces MMP-9,
which has been associated with more aggressive phenotypes of
carcinoma, higher invasiveness, and low patient survival (96,97).
Other studies demonstrated that tumor spread is also
sustained by the overexpression of IL-6, which in turn leads to the
upregulation of matrix-metalloproteinases, adhesion molecules and
endothelial leukocyte adhesion molecules (98,99). All
these data showed that interleukins, and in particular IL-6, are
strictly involved in neoplastic transformation (100). Besides interleukins, altered levels
of TNF-α, mediated by the alteration of Wnt and NF-κB pathways,
were associated with the development of tumors (101,102).
These data further corroborate the importance of NF-κB in cancer.
Indeed, NF-κB acts as an immunostimulant factor against neoplastic
cells; however, its protein expression is increased in several
cancers acting as an oncogene (103,104).
The abovementioned evidence suggested that
dysbiosis-associated cancer may rely on the abnormal activation of
NF-κB (68). Thus, a fundamental
role is played also by the immune system, which in the presence of
pathogens stimulates the production of NF-κB (105).
Oncogenic substances production. Numerous substances
produced by bacteria have been suggested to possess a carcinogenic
action. Bacterial metabolism leads to the production of sulfur
compounds, acids and free radicals, mainly nitric and oxygen
reactive species, able to induce pro-tumoral genetic damage.
Furthermore, several bacteria have an alcoholic metabolism
responsible for the production of acetaldehyde, which sustains
neoplastic transformation (90).
Regarding reactive oxygen species (ROS) and reactive
nitrogen species (RNS), it is well established that alteration of
NADPH oxidase and nitric oxide synthase (NOS) activity leads to the
accumulation of these harmful substances which promote chronic
inflammation and, as described in the above chapter, cancer
development (106,107). Bacteria also play fundamental roles
in these processes. Some peroxygenase oral microorganisms are
involved in this process producing hydrogen peroxide
(H2O2) and include Bifidobacterium
adolescentis, Lactobacillus acidophilus, L. fermentum, L. jensenii,
L. minutus (90,108), Streptococcus gordonii, S. mitis,
S. oligofermentans, S. oralis, and S. sanguinis
(109).
Other oncogenic substances produced by oral bacteria
are represented by sulfides and nitrosamines. Bacteria including
Bacteroides and Firmicutes species are capable to
ferment the host excessive protein into sulfides and nitrosamines.
These harmful substances are able to induce DNA damage in the
oncogene or onco-suppressor genes (110,111).
Other studies highlighted the importance of
superoxide dismutase (SOD) activity and its expression through the
analysis of microbiomes detected in cancer samples and normal
mucosa of oral cancer patients (117). The SOD activity is fundamental for
inhibiting the detrimental effects of O2.−.
In particular, it was shown that in tumor samples the presence of
Fe2+ reacts with H2O2 leading to
the production of harmful reactive species that promote neoplastic
transformation by inducing DNA mutations affecting key genes
involved in the regulation of cell cycle (118).
Moreover, as aforementioned, pro-inflammatory
conditions sustained by microbial alterations contribute to
epithelial barrier alteration. According to Virchow (1881)
(129), the inflammatory events are
linked to microbiota and cancer. In addition, inflammation may
modify the bacteria population, favor microbial translocation and
induce the growth of specific bacteria (126,129,130).
Specific microbial metabolites (i.e., ROS and
hydroxyl radical) and toxins [such as cytolethal distending toxin
(CDT)] generate genomic damages inducing the neoplastic
transformation of epithelial cells. Moreover, bacteria activate
several signal transduction pathways through virulence genes, e.g.,
AvrA virulence factor. Another factor stimulated by bacteriocins
and bacterial proteins is the transforming growth factor β (TGF-β),
which induces abnormal cell proliferation (76,131).
Furthermore, TGF-β plays a role as an
immunomodulating factor inhibiting dendritic cells (DCs);
T-receptor cells thus act as tumor-promoter factors (132). Notably, the mechanism of TGF-β
signaling in promoting tumorigenesis is also associated with the
dysregulated inflammation microenvironment actuated by microbiota
(76).
It has been widely demonstrated that environmental
factors, including diet, lifestyle habits, and natural compounds,
are responsible for both genetic and epigenetic alterations
predisposing the development of several diseases, and have
beneficial effects in preventing the development of
chronic-degenerative disorders (133–140).
In particular, dietary intake and food consumption modulate several
cellular and molecular processes acting in a multifactorial manner
(141–143). Indeed, the consumption of specific
food and nutrients may modulate inflammatory and cell cycle
regulatory pathways to maintain the optimal cell homeostasis,
preventing the development of diseases (144,145).
On the contrary, imbalances of nutrient consumption and/or
absorption lead to epigenetic changes associated with certain
pathologies, including cancer. The way by which food and nutrient
intake is able to influence the onset of certain pathologies
remains to be determined. However, findings have shown how foods
can change the individual's redox state, the oral and gut
microbiota, the DNA methylation status and the alteration of
microRNA (miRNAs) expression levels (146–149).
In particular, the two latter epigenetic events are
now been recognized as key mechanisms of neoplastic transformation
and a plethora of diseases (12,87,150).
In this context, recent studies have identified different miRNAs, a
class of non-coding RNA of 20–22 nucleotides, associated with the
development and progression of different tumors (151–157).
In addition, numerous studies have demonstrated the presence of a
dual relationship between microbiota and host microRNAs (miRNAs)
and vice versa (158–160). Liu and co-workers showed that fecal
miRNAs produced by epithelial cells and Hopx-positive cells were
able to penetrate bacteria (F. nucleatum and E coli)
modulating the gene expressions of bacteria and altering the
microbiota composition and bacterial cell growth (161). These preliminary observations,
obtained in Dicer1-deficient mice, allowed the researchers to
conclude that fecal miRNAs exert an important role in the
regulation of gut microbiota and microbiome suggesting their
possible use as novel therapeutic strategies. Fecal miRNAs are not
derived only from intestinal cells. Different studies demonstrated
that fecal miRNAs can be derived from foods and can be absorbed by
intestinal epithelia modulating the expression levels of host genes
(162,163). These miRNAs are mainly planted
exosome-derived miRNAs; however, several studies showed that
milk-derived miRNAs play key roles (164–166).
All these food-derived miRNAs can presumably
interact with oral and gut microbiota (167,168).
On this basis, exogenous miRNAs may act as bacterial small RNA to
interfere with bacterial gene expression modulating the entire
microbiome (169,170). However, further studies are needed
to deepen the knowledge on interactions between host-miRNAs and
oral/gut microbiota. On the other hand, even the microbiota
resident in the oral and intestinal mucosa may modulate the
expression of specific miRNAs, thus highlighting a dual
relationship between miRNAs and microbiota and their ability to
influence each other (171).
One of the mechanisms by which microbiota alters the
expression levels of host-epithelial miRNAs is the production of
different metabolites leading to significant changes in host-cell
metabolism resulting in the alteration of the gene and miRNA
expressions (172–174). Pang et al investigated the
association among dysbiosis, dysfunction of epithelial barrier and
alteration of immune system to evaluate how dysbiosis stimulates
carcinogenesis (76).
In recent years, it was widely demonstrated that
the consumption of healthy foods enriched with probiotic acid
lactic bacteria has positive effects regarding tumor prevention.
Probiotics are able to reduce the mutagenic effects of harmful
substances while modulating the expression of proteins involved in
cell proliferation, apoptosis, inflammation, or immune system
activation (188). Several in
vitro experiments performed on cancer cells showed that
probiotics possess anti-proliferative and pro-apoptotic effects in
these tumor models (189). Lee and
co-workers demonstrated that the cytoplasmic elements of
Bifidobacterium longum, L. acidophilus and L. casei
exert anti-neoplastic effects in different tumor in vitro
models (190). Besides these
probiotics, Bacillus polyfermenticus (191), Lactobacillus acidophilus 606
(192), LGG/Bb12 (193), LGG/Bifidobacterium animalis
subsp. lactis (194), and
Lactobacillus rhamnosus GG (9) possessed anti-neoplastic effects in
colorectal cancer cell lines.
Few data are available on the effects of probiotics
on oral cancer. In a recent study, HSC-3 OSCC cell lines were used
to determine the effects of Lactobacillus rhamnosus GG (LGG)
in increasing the antiblastic effects of geniposide, a derivate of
Gardenia jasminoides which in preclinical studies showed
important anticancer effects (58,195).
The results obtained by the authors showed that the combined
treatment with geniposide and LGG increased the apoptotic rate of
HSC-3 cells. In particular, in a synergistic manner, LGG
intensified the antineoplastic action of geniposide, supporting the
tentative use of this combined therapy also in clinical practice.
In another study, Asoudeh-Fard et al (196) demonstrated that Lactobacillus
plantarum was able to inhibit and activate the MAPKs and PTEN
pathways, respectively, playing a potential role in the regulation
of cancer. Indeed, it is well known that PTEN and MAPKs are
associated with the inhibition and the initiation of cancer
development, respectively (196).
Consequently, a possible use of L. plantarum for probiotics
cancer therapy was proposed.
This review widely discussed how the dysregulation
of oral microbiota and oral mucosa homeostasis may represent
modifiable risk factors associated with the development of OSCC. In
addition, it was shown that certain bacterial strains may play a
protective role against oral neoplastic transformation suggesting
the possible use of probiotics administration as novel preventive
and therapeutic strategies. In this scenario, several species have
been strongly correlated with oral carcinoma, such as
Capnocytophaga gingivalis, Fusobacterium sp.,
Streptococcus sp., Peptostreptococcus sp.,
Porphyromonas gingivalis and Prevotella sp., due to
the fact that these bacteria may promote inflammation, cell
proliferation and the production of some oncogenic substances
(90). Recent findings have shown
that the evaluation of oral microbiota and microbiome may provide
important information on oral cancer oncogenesis, outcome
prediction and therapeutic response (including immunotherapy)
(198,199). In addition, thanks to the new
high-throughput molecular technologies it was possible to define
the precise composition and gene expression (microbiome) of oral
bacteria in OSCC patients and normal controls identifying specific
strains associated with an increased risk of OSCC development
(1,200). Therefore, the analysis of
circulating biomarkers (miRNAs, circulating DNA, specific proteins)
represents a good approach for the assessment of oral cancer risk
(201–203).
In this respect, the use of probiotics that in
appropriate amounts give a health benefit to the host, including
anti-tumoral effects, could be useful to promote cancer therapies
representing a new step of the evolution of anticancer
pharmacological treatments (190,204).
Although in vitro and in vivo experiments
demonstrated the beneficial effects probiotics (7–9), few
data are available about the efficacy of probiotics in oral cancer.
It is reasonable to hypothesize that the beneficial effects exert
by probiotics in intestinal and colorectal cancer is similar to
those acted in the oral mucosa. Starting from this assumption,
future studies are required to explore the involvement of oral
microbiota and its relationship with oral cancer.
Not applicable.
No funding was received.
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
GRMLR, DN and MS conceived the study. GG, EP
provided the information about microbiota and oral cancer. GRMLR,
GG and EP organized the Tables. GG and DN were involved in the
preparation of the figure. GRMLR, EP, ER and MS were involved in
the preparation of the original draft of the manuscript, while DN,
ER and MS reviewed and edited the article. All authors have read
and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fenga C, Gangemi S, Di Salvatore V,
Falzone L and Libra M: Immunological effects of occupational
exposure to lead (Review). Mol Med Rep. 15:3355–3360.
2017.(Review). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rapisarda V, Ledda C, Matera S, Fago L,
Arrabito G, Falzone L, Marconi A, Libra M and Loreto C: Absence of
t(14;18) chromosome translocation in agricultural workers after
short-term exposure to pesticides. Mol Med Rep. 15:3379–3382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rapisarda V, Salemi R, Marconi A, Loreto
C, Graziano AC, Cardile V, Basile MS, Candido S, Falzone L,
Spandidos DA, et al: Fluoro-edenite induces fibulin-3
overexpression in non-malignant human mesothelial cells. Oncol
Lett. 12:3363–3367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falzone L, Marconi A, Loreto C, Franco S,
Spandidos DA and Libra M: Occupational exposure to carcinogens:
Benzene, pesticides and fibers (Review). Mol Med Rep. 14:4467–4474.
2016.(Review). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malfa GA, Tomasello B, Sinatra F,
Villaggio G, Amenta F, Avola R and Renis M: ‘Reactive’ response
evaluation of primary human astrocytes after methylmercury
exposure. J Neurosci Res. 92:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vivarelli S, Salemi R, Candido S, Falzone
L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G and Libra
M: Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers
(Basel). 11:E382019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banna GL, Torino F, Marletta F, Santagati
M, Salemi R, Cannarozzo E, Falzone L, Ferraù F and Libra M:
Lactobacillus rhamnosus GG: An overview to explore the
rationale of its use in cancer. Front Pharmacol. 8:6032017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vivarelli S, Falzone L, Basile MS,
Nicolosi D, Genovese C, Libra M and Salmeri M: Benefits of using
probiotics as adjuvants in anticancer therapy. World Ac Sci J.
1:125–135. 2019.(Review).
|
|
10
|
Garozzo A, Falzone L, Rapisarda V, Marconi
A, Cinà D, Fenga C, Spandidos DA and Libra M: The risk of HCV
infection among health-care workers and its association with
extrahepatic manifestations (Review). Mol Med Rep. 15:3336–3339.
2017.(Review). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ng JH, Iyer NG, Tan MH and Edgren G:
Changing epidemiology of oral squamous cell carcinoma of the
tongue: A global study. Head Neck. 39:297–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Falzone L, Lupo G, La Rosa GRM, Crimi S,
Anfuso CD, Salemi R, Rapisarda E, Libra M and Candido S:
Identification of novel micrornas and their diagnostic and
prognostic significance in oral cancer. Cancers (Basel).
11:E6102019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graham S, Dayal H, Rohrer T, Swanson M,
Sultz H, Shedd D and Fischman S: Dentition, diet, tobacco, and
alcohol in the epidemiology of oral cancer. J Natl Cancer Inst.
59:1611–1618. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marshall JR, Graham S, Haughey BP, Shedd
D, O'Shea R, Brasure J, Wilkinson GS and West D: Smoking, alcohol,
dentition and diet in the epidemiology of oral cancer. Eur J Cancer
B Oral Oncol. 28B:9–15. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal BB, Vijayalekshmi RV and Sung B:
Targeting inflammatory pathways for prevention and therapy of
cancer: Short-term friend, long-term foe. Clin Cancer Res.
15:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Read SA and Douglas MW: Virus induced
inflammation and cancer development. Cancer Lett. 345:174–181.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Turnbaugh PJ, Ley RE, Hamady M,
Fraser-Liggett CM, Knight R and Gordon JI: The human microbiome
project. Nature. 449:804–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dewhirst FE, Chen T, Izard J, Paster BJ,
Tanner AC, Yu WH, Lakshmanan A and Wade WG: The human oral
microbiome. J Bacteriol. 192:5002–5017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolenbrander PE, Andersen RN, Blehert DS,
Egland PG, Foster JS and Palmer RJJ Jr: Communication among oral
bacteria. Microbiol Mol Biol Rev. 66:486–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Maweri SA, Warnakulasuriya S and Samran
A: Khat (Catha edulis) and its oral health effects: An
updated review. J Investig Clin Dent. 9:2018, https://doi.org/10.1111/jicd.12288 View Article : Google Scholar
|
|
22
|
Leonardi R, Loreto C, Barbato E, Polimeni
A, Caltabiano R and Lo Muzio L: A histochemical survey of the human
temporomandibular joint disc of patients with internal derangement
without reduction. J Craniofac Surg. 18:1429–1433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petralia MC, Mazzon E, Fagone P, Falzone
L, Bramanti P, Nicoletti F and Basile MS: Retrospective follow-up
analysis of the transcriptomic patterns of cytokines, cytokine
receptors and chemokines at preconception and during pregnancy, in
women with post-partum depression. Exp Ther Med. 18:2055–2062.
2019.PubMed/NCBI
|
|
24
|
Vesty A, Gear K, Biswas K, Radcliff FJ,
Taylor MW and Douglas RG: Microbial and inflammatory-based salivary
biomarkers of head and neck squamous cell carcinoma. Clin Exp Dent
Res. 4:255–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meurman JH: Oral microbiota and cancer. J
Oral Microbiol. 2:22010. View Article : Google Scholar
|
|
26
|
Pennisi M, Malaguarnera G, Bartolo GD,
Lanza G, Bella R, Chisari EM, Cauli O, Vicari E and Malaguarnera M:
Decrease in Serum Vitamin D Level of Older Patients with Fatigue.
Nutrients. 11:E25312019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pennisi M, Di Bartolo G, Malaguarnera G,
Bella R, Lanza G, Malaguarnera M and Vitamin D: Vitamin D serum
levels in patients with statin-induced musculoskeletal pain. Dis
Markers. 2019:35494022019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Human Microbiome Project Consortium: A
framework for human microbiome research. Nature. 486:215–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marasco G, Di Biase AR, Schiumerini R,
Eusebi LH, Iughetti L, Ravaioli F, Scaioli E, Colecchia A and Festi
D: Gut microbiota and celiac disease. Dig Dis Sci. 61:1461–1472.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quigley EMM: Microbiota-brain-gut axis and
neurodegenerative diseases. Curr Neurol Neurosci Rep. 17:942017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang T and Zubcevic J: Gut-brain axis in
regulation of blood pressure. Front Physiol. 8:8452017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vinciguerra L, Lanza G, Puglisi V, Pennisi
M, Cantone M, Bramanti A, Pennisi G and Bella R: Transcranial
Doppler ultrasound in vascular cognitive impairment-no dementia.
PLoS One. 14:e02161622019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Puglisi V, Bramanti A, Lanza G, Cantone M,
Vinciguerra L, Pennisi M, Bonanno L, Pennisi G and Bella R:
Impaired cerebral haemodynamics in vascular depression: insights
from transcranial doppler ultrasonography. Front Psychiatry.
9:3162018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lanza G, Cantone M, Musso S, Borgione E,
Scuderi C and Ferri R: Early-onset subcortical ischemic vascular
dementia in an adult with mtDNA mutation 3316G>A. J Neurol.
265:968–969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bordet R, Ihl R, Korczyn AD, Lanza G,
Jansa J, Hoerr R and Guekht A: Towards the concept of
disease-modifier in post-stroke or vascular cognitive impairment: A
consensus report. BMC Med. 15:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lanza G, Bramanti P, Cantone M, Pennisi M,
Pennisi G and Bella R: Vascular cognitive impairment through the
looking glass of transcranial magnetic stimulation. Behav Neurol.
2017:14213262017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bella R, Cantone M, Lanza G, Ferri R,
Vinciguerra L, Puglisi V, Pennisi M, Ricceri R, Di Lazzaro V and
Pennisi G: Cholinergic circuitry functioning in patients with
vascular cognitive impairment - no dementia. Brain Stimul.
9:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pennisi M, Lanza G, Cantone M, Ricceri R,
Spampinato C, Pennisi G, Di Lazzaro V and Bella R: Correlation
between motor cortex excitability changes and cognitive impairment
in vascular depression: Pathophysiological insights from a
longitudinal TMS study. Neural Plast. 2016:81549692016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pennisi G, Bella R and Lanza G: Motor
cortex plasticity in subcortical ischemic vascular dementia: What
can TMS say? Clin Neurophysiol. 126:851–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lanza G, Papotto M, Pennisi G, Bella R and
Ferri R: Epileptic seizure as a precipitating factor of vascular
progressive supranuclear palsy: A case report. J Stroke Cerebrovasc
Dis. 23:e379–e381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Concerto C, Lanza G, Cantone M, Pennisi M,
Giordano D, Spampinato C, Ricceri R, Pennisi G, Aguglia E and Bella
R: Different patterns of cortical excitability in major depression
and vascular depression: A transcranial magnetic stimulation study.
BMC Psychiatry. 13:3002013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bella R, Ferri R, Lanza G, Cantone M,
Pennisi M, Puglisi V, Vinciguerra L, Spampinato C, Mazza T,
Malaguarnera G, et al: TMS follow-up study in patients with
vascular cognitive impairment-no dementia. Neurosci Lett.
534:155–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bella R, Ferri R, Cantone M, Pennisi M,
Lanza G, Malaguarnera G, Spampinato C, Giordano D, Raggi A and
Pennisi G: Motor cortex excitability in vascular depression. Int J
Psychophysiol. 82:248–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bella R, Ferri R, Pennisi M, Cantone M,
Lanza G, Malaguarnera G, Spampinato C, Giordano D, Alagona G and
Pennisi G: Enhanced motor cortex facilitation in patients with
vascular cognitive impairment-no dementia. Neurosci Lett.
503:171–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pennisi G, Ferri R, Cantone M, Lanza G,
Pennisi M, Vinciguerra L, Malaguarnera G and Bella R: A review of
transcranial magnetic stimulation in vascular dementia. Dement
Geriatr Cogn Disord. 31:71–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bella R, Pennisi G, Cantone M, Palermo F,
Pennisi M, Lanza G, Zappia M and Paolucci S: Clinical presentation
and outcome of geriatric depression in subcortical ischemic
vascular disease. Gerontology. 56:298–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Atanasova KR and Yilmaz O: Looking in the
Porphyromonas gingivalis cabinet of curiosities: The
microbium, the host and cancer association. Mol Oral Microbiol.
29:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yilmaz O: The chronicles of
Porphyromonas gingivalis: The microbium, the human oral
epithelium and their interplay. Microbiology. 154:2897–2903. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Katz J, Onate MD, Pauley KM, Bhattacharyya
I and Cha S: Presence of Porphyromonas gingivalis in
gingival squamous cell carcinoma. Int J Oral Sci. 3:209–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nagy KN, Sonkodi I, Szöke I, Nagy E and
Newman HN: The microflora associated with human oral carcinomas.
Oral Oncol. 34:304–308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mager DL, Haffajee AD, Devlin PM, Norris
CM, Posner MR and Goodson JM: The salivary microbiota as a
diagnostic indicator of oral cancer: A descriptive, non-randomized
study of cancer-free and oral squamous cell carcinoma subjects. J
Transl Med. 3:272005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu X, Zhang Q, Hua H and Chen F: Changes
in the salivary microbiota of oral leukoplakia and oral cancer.
Oral Oncol. 56:e6–e8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee WH, Chen HM, Yang SF, Liang C, Peng
CY, Lin FM, Tsai LL, Wu BC, Hsin CH, Chuang CY, et al: Bacterial
alterations in salivary microbiota and their association in oral
cancer. Sci Rep. 7:165402017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pepe MS, Etzioni R, Feng Z, Potter JD,
Thompson ML, Thornquist M, Winget M and Yasui Y: Phases of
biomarker development for early detection of cancer. J Natl Cancer
Inst. 93:1054–1061. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lucs A, Saltman B, Chung CH, Steinberg BM
and Schwartz DL: Opportunities and challenges facing biomarker
development for personalized head and neck cancer treatment. Head
Neck. 35:294–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Meurman JH and Stamatova I: Probiotics:
Contributions to oral health. Oral Dis. 13:443–451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stamatova I and Meurman JH: Probiotics:
Health benefits in the mouth. Am J Dent. 22:329–338.
2009.PubMed/NCBI
|
|
58
|
Cheng Z, Xu H, Wang X and Liu Z:
Lactobacillus raises in vitro anticancer effect of
geniposide in HSC-3 human oral squamous cell carcinoma cells. Exp
Ther Med. 14:4586–4594. 2017.PubMed/NCBI
|
|
59
|
Chang JS, Lo HI, Wong TY, Huang CC, Lee
WT, Tsai ST, Chen KC, Yen CJ, Wu YH, Hsueh WT, et al: Investigating
the association between oral hygiene and head and neck cancer. Oral
Oncol. 49:1010–1017. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rosenquist K, Wennerberg J, Schildt EB,
Bladström A, Göran Hansson B and Andersson G: Oral status, oral
infections and some lifestyle factors as risk factors for oral and
oropharyngeal squamous cell carcinoma. A population-based
case-control study in southern Sweden. Acta Otolaryngol.
125:1327–1336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Divaris K, Olshan AF, Smith J, Bell ME,
Weissler MC, Funkhouser WK and Bradshaw PT: Oral health and risk
for head and neck squamous cell carcinoma: The Carolina Head and
Neck Cancer Study. Cancer Causes Control. 21:567–575. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Garrote LF, Herrero R, Reyes RM,
Vaccarella S, Anta JL, Ferbeye L, Muñoz N and Franceschi S: Risk
factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J
Cancer. 85:46–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Michaud DS, Liu Y, Meyer M, Giovannucci E
and Joshipura K: Periodontal disease, tooth loss, and cancer risk
in male health professionals: A prospective cohort study. Lancet
Oncol. 9:550–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Tezal M, Sullivan MA, Reid ME, Marshall
JR, Hyland A, Loree T, Lillis C, Hauck L, Wactawski-Wende J and
Scannapieco FA: Chronic periodontitis and the risk of tongue
cancer. Arch Otolaryngol Head Neck Surg. 133:450–454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tezal M, Sullivan MA, Hyland A, Marshall
JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, et
al: Chronic periodontitis and the incidence of head and neck
squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
18:2406–2412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Holmes L Jr, desVignes-Kendrick M, Slomka
J, Mahabir S, Beeravolu S and Emani SR: Is dental care utilization
associated with oral cavity cancer in a large sample of
community-based United States residents? Community Dent Oral
Epidemiol. 37:134–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Börnigen D, Ren B, Pickard R, Li J, Ozer
E, Hartmann EM, Xiao W, Tickle T, Rider J, Gevers D, et al:
Alterations in oral bacterial communities are associated with risk
factors for oral and oropharyngeal cancer. Sci Rep. 7:176862017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vogelmann R and Amieva MR: The role of
bacterial pathogens in cancer. Curr Opin Microbiol. 10:76–81. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lim Y, Totsika M, Morrison M and
Punyadeera C: Oral microbiome: A new biomarker reservoir for oral
and oropharyngeal cancers. Theranostics. 7:4313–4321. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cali F, Cantone M, Cosentino FII, Lanza G,
Ruggeri G, Chiavetta V, Salluzzo R, Ragalmuto A, Vinci M and Ferri
R: Interpreting genetic variants: hints from a family cluster of
Parkinson's disease. J Parkinsons Dis. 9:203–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pennisi M, Lanza G, Cantone M, Schepis C,
Ferri R, Barone R and Bella R: Unusual neurological presentation of
nevoid basal cell carcinoma syndrome (Gorlin-Goltz Syndrome). J
Clin Neurol. 13:439–441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhang Y, Wang X, Li H, Ni C, Du Z and Yan
F: Human oral microbiota and its modulation for oral health. Biomed
Pharmacother. 99:883–893. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Guarneri C, Bevelacqua V, Polesel J,
Falzone L, Cannavò PS, Spandidos DA, Malaponte G and Libra M: NF-κB
inhibition is associated with OPN/MMP 9 downregulation in cutaneous
melanoma. Oncol Rep. 37:737–746. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.(Review). PubMed/NCBI
|
|
75
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pang X, Tang YJ, Ren XH, Chen QM, Tang YL
and Liang XH: Microbiota, epithelium, inflammation, and TGF-β
signaling: An intricate interaction in oncogenesis. Front
Microbiol. 9:13532018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Couturier-Maillard A, Secher T, Rehman A,
Normand S, De Arcangelis A, Haesler R, Huot L, Grandjean T,
Bressenot A, Delanoye-Crespin A, et al: NOD2-mediated dysbiosis
predisposes mice to transmissible colitis and colorectal cancer. J
Clin Invest. 123:700–711. 2013.PubMed/NCBI
|
|
78
|
Hu B, Elinav E, Huber S, Strowig T, Hao L,
Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC, et
al: Microbiota-induced activation of epithelial IL-6 signaling
links inflammasome-driven inflammation with transmissible cancer.
Proc Natl Acad Sci USA. 110:9862–9867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Francescone R, Hou V and Grivennikov SI:
Microbiome, inflammation, and cancer. Cancer J. 20:181–189. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ojesina AI, Lichtenstein L, Freeman SS,
Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, Cherniack AD, Ambrogio
L, Cibulskis K, Bertelsen B, et al: Landscape of genomic
alterations in cervical carcinomas. Nature. 506:371–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Allen J and Sears CL: Impact of the gut
microbiome on the genome and epigenome of colon epithelial cells:
Contributions to colorectal cancer development. Genome Med.
11:112019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Multhoff G, Molls M and Radons J: Chronic
inflammation in cancer development. Front Immunol. 2:982012.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Szkaradkiewicz AK: Karpiński TM:
Microbiology of chronic periodontitis. J Biol Earth Sci. 3:M14–M20.
2013.
|
|
84
|
Leonardi R, Talic NF and Loreto C: MMP-13
(collagenase 3) immunolocalisation during initial orthodontic tooth
movement in rats. Acta Histochem. 109:215–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Salemi R, Falzone L, Madonna G, Polesel J,
Cinà D, Mallardo D, Ascierto PA, Libra M and Candido S: MMP-9 as a
candidate marker of response to BRAF inhibitors in melanoma
patients with BRAFV600E mutation detected in circulating-free DNA.
Front Pharmacol. 9:8562018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Silva EM, Mariano VS, Pastrez PRA, Pinto
MC, Castro AG, Syrjanen KJ and Longatto-Filho A: High systemic IL-6
is associated with worse prognosis in patients with non-small cell
lung cancer. PLoS One. 12:e01811252017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Falzone L, Salemi R, Travali S, Scalisi A,
McCubrey JA, Candido S and Libra M: MMP-9 overexpression is
associated with intragenic hypermethylation of MMP9 gene in
melanoma. Aging (Albany NY). 8:933–944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Katz J, Wallet S and Cha S: Periodontal
disease and the oral-systemic connection: ‘is it all the RAGE?’.
Quintessence Int. 41:229–237. 2010.PubMed/NCBI
|
|
89
|
Zhang G and Ghosh S: Molecular mechanisms
of NF-kappaB activation induced by bacterial lipopolysaccharide
through Toll-like receptors. J Endotoxin Res. 6:453–457. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Karpiński TM: Role of oral microbiota in
cancer development. Microorganisms. 7:E202019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hou LT, Liu CM, Liu BY, Lin SJ, Liao CS
and Rossomando EF: Interleukin-1beta, clinical parameters and
matched cellular-histopathologic changes of biopsied gingival
tissue from periodontitis patients. J Periodontal Res. 38:247–254.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Konopka Ł and Brzezinska Błaszczyk E:
Cytokines in gingival crevicular fluidas potential diagnostic and
prognostic markers of periodontitis. Dent Med Probl. 47:206–213.
2010.
|
|
93
|
Carmi Y, Dotan S, Rider P, Kaplanov I,
White MR, Baron R, Abutbul S, Huszar M, Dinarello CA, Apte RN, et
al: The role of IL-1β in the early tumor cell-induced angiogenic
response. J Immunol. 190:3500–3509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A,
Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, et al:
Expression of interleukin-1beta in human breast carcinoma. Cancer.
80:421–434. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Voronov E, Shouval DS, Krelin Y, Cagnano
E, Benharroch D, Iwakura Y, Dinarello CA and Apte RN: IL-1 is
required for tumor invasiveness and angiogenesis. Proc Natl Acad
Sci USA. 100:2645–2650. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Pannone G, Santoro A, Feola A, Bufo P,
Papagerakis P, Lo Muzio L, Staibano S, Ionna F, Longo F, Franco R,
et al: The role of E-cadherin down-regulation in oral cancer: CDH1
gene expression and epigenetic blockage. Curr Cancer Drug Targets.
14:115–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kossakowska AE, Edwards DR, Prusinkiewicz
C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA and
Janowska-Wieczorek A: Interleukin-6 regulation of matrix
metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of
metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's
lymphomas. Blood. 94:2080–2089. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Natali P, Nicotra MR, Cavaliere R, Bigotti
A, Romano G, Temponi M and Ferrone S: Differential expression of
intercellular adhesion molecule 1 in primary and metastatic
melanoma lesions. Cancer Res. 50:1271–1278. 1990.PubMed/NCBI
|
|
100
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Szlosarek P, Charles KA and Balkwill FR:
Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer.
42:745–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rivas MA, Carnevale RP, Proietti CJ,
Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S,
Brouckaert P, et al: TNF alpha acting on TNFR1 promotes breast
cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent
pathways. Exp Cell Res. 314:509–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Karin M: NF-kappaB as a critical link
between inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Agassandian M and Shurin GV: Bacterial
Infections and Cancar Development. In: Shurin MR, Thanavala Y,
Ismail N, editors Infection and Cancaer: Bi-Directorial
Interactions; 1 ed. Springer International Publishing;.
2015:4082015.
|
|
106
|
Hussain SP, Hofseth LJ and Harris CC:
Radical causes of cancer. Nat Rev Cancer. 3:276–285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Piao JY, Lee HG, Kim SJ, Kim DH, Han HJ,
Ngo HK, Park SA, Woo JH, Lee JS, Na HK, et al: Helicobacter
pylori activates IL-6-STAT3 signaling in human gastric cancer
cells: Potential roles for reactive oxygen species. Helicobacter.
21:405–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Brauncajs M and Sakowska D: Krzemiński Z:
Production of hydrogen peroxide by lactobacilli colonising the
human oral cavity. Med Dosw Mikrobiol. 53:331–336. 2001.
|
|
109
|
Abranches J, Zeng L, Kajfasz JK, Palmer
SR, Chakraborty B, Wen ZT, Richards VP, Brady LJ and Lemos JA:
Biology of oral streptococci. Microbiol Spectr. 6:62018. View Article : Google Scholar
|
|
110
|
Carbonero F, Benefiel AC,
Alizadeh-Ghamsari AH and Gaskins HR: Microbial pathways in colonic
sulfur metabolism and links with health and disease. Front Physiol.
3:4482012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bhatt AP, Redinbo MR and Bultman SJ: The
role of the microbiome in cancer development and therapy. CA Cancer
J Clin. 67:326–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Karpiński TM and Szkaradkiewicz AK:
Karpiński TM, Szkaradkiewicz AK: Characteristic of bacteriocines
and their application. Pol J Microbiol. 62:223–235. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Senneby A, Davies JR, Svensäter G and
Neilands J: Acid tolerance properties of dental biofilms in vivo.
BMC Microbiol. 17:1652017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Downes J and Wade WG:
Peptostreptococcus stomatis sp. nov., isolated from the
human oral cavity. Int J Syst Evol Microbiol. 56:751–754. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lunt SJ, Chaudary N and Hill RP: The tumor
microenvironment and metastatic disease. Clin Exp Metastasis.
26:19–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mazzio EA, Smith B and Soliman KF:
Evaluation of endogenous acidic metabolic products associated with
carbohydrate metabolism in tumor cells. Cell Biol Toxicol.
26:177–188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yost S, Stashenko P, Choi Y, Kukuruzinska
M, Genco CA, Salama A, Weinberg EO, Kramer CD and Frias-Lopez J:
Increased virulence of the oral microbiome in oral squamous cell
carcinoma revealed by metatranscriptome analyses. Int J Oral Sci.
10:322018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Franco R, Schoneveld O, Georgakilas AG and
Panayiotidis MI: Oxidative stress, DNA methylation and
carcinogenesis. Cancer Lett. 266:6–11. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Murray IA, Patterson AD and Perdew GH:
Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat
Rev Cancer. 14:801–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu
Z, Boggon TJ, Jin P, Yi H, Wright ER, et al: Glutamate
dehydrogenase 1 signals through antioxidant glutathione peroxidase
1 to regulate redox homeostasis and tumor growth. Cancer Cell.
27:257–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Liu G, Zhu J, Yu M, Cai C, Zhou Y, Yu M,
Fu Z, Gong Y, Yang B, Li Y, et al: Glutamate dehydrogenase is a
novel prognostic marker and predicts metastases in colorectal
cancer patients. J Transl Med. 13:1442015. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Pavlova SI, Jin L, Gasparovich SR and Tao
L: Multiple alcohol dehydrogenases but no functional acetaldehyde
dehydrogenase causing excessive acetaldehyde production from
ethanol by oral streptococci. Microbiology. 159((159Pt 7)):
1437–1446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Marttila E, Bowyer P, Sanglard D, Uittamo
J, Kaihovaara P, Salaspuro M, Richardson M and Rautemaa R:
Fermentative 2-carbon metabolism produces carcinogenic levels of
acetaldehyde in Candida albicans. Mol Oral Microbiol. 28:281–291.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Salaspuro M: Microbial metabolism of
ethanol and acetaldehyde and clinical consequences. Addict Biol.
2:35–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Meurman JH and Uittamo J: Oral
micro-organisms in the etiology of cancer. Acta Odontol Scand.
66:321–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Schwabe RF and Jobin C: The microbiome and
cancer. Nat Rev Cancer. 13:800–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Sellers RS and Morton D: The colon: From
banal to brilliant. Toxicol Pathol. 42:67–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Taur Y and Pamer EG: Microbiome mediation
of infections in the cancer setting. Genome Med. 8:402016.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Arthur JC, Perez-Chanona E, Mühlbauer M,
Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B,
Rogers AB, et al: Intestinal inflammation targets cancer-inducing
activity of the microbiota. Science. 338:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
White RA, Malkoski SP and Wang XJ: TGFβ
signaling in head and neck squamous cell carcinoma. Oncogene.
29:5437–5446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yang L: TGFbeta and cancer metastasis: An
inflammation link. Cancer Metastasis Rev. 29:263–271. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lanza G, Centonze SS, Destro G, Vella V,
Bellomo M, Pennisi M, Bella R and Ciavardelli D: Shiatsu as an
adjuvant therapy for depression in patients with Alzheimer's
disease: A pilot study. Complement Ther Med. 38:74–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Lanza G, Bella R, Cantone M, Pennisi G,
Ferri R and Pennisi M: Cognitive impairment and celiac disease: Is
transcranial magnetic stimulation a trait d'union between gut and
brain? Int J Mol Sci. 19:E22432018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Pennisi M, Bramanti A, Cantone M, Pennisi
G, Bella R and Lanza G: Neurophysiology of the ‘Celiac Brain’:
Disentangling Gut-Brain Connections. Front Neurosci. 11:4982017.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Bella R, Lanza G, Cantone M, Giuffrida S,
Puglisi V, Vinciguerra L, Pennisi M, Ricceri R, D'Agate CC,
Malaguarnera G, et al: Effect of a gluten-free diet on cortical
excitability in adults with celiac disease. PLoS One.
10:e01292182015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Pennisi G, Lanza G, Giuffrida S,
Vinciguerra L, Puglisi V, Cantone M, Pennisi M, D'Agate CC, Naso P,
Aprile G, et al: Excitability of the motor cortex in de novo
patients with celiac disease. PLoS One. 9:e1027902014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Pennisi M, Lanza G, Cantone M, Ricceri R,
Ferri R, D'Agate CC, Pennisi G, Di Lazzaro V and Bella R: Cortical
involvement in celiac disease before and after long-term
gluten-free diet: A transcranial magnetic stimulation study. PLoS
One. 12:e01775602017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Tiffon C: The impact of nutrition and
environmental epigenetics on human health and disease. Int J Mol
Sci. 19:E34252018. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Acquaviva R, Sorrenti V, Santangelo R,
Cardile V, Tomasello B, Malfa G, Vanella L, Amodeo A, Genovese C,
Mastrojeni S, et al: Effects of an extract of Celtis aetnensis
(Tornab.) Strobl twigs on human colon cancer cell cultures. Oncol
Rep. 36:2298–2304. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Malfa GA, Tomasello B, Acquaviva R,
Genovese C, La Mantia A, Cammarata FP, Ragusa M, Renis M and Di
Giacomo C: Betula etnensis Raf. (Betulaceae) extract induced
HO-1 expression and ferroptosis cell death in human colon cancer
cells. Int J Mol Sci. 20:E27232019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Soldati L, Di Renzo L, Jirillo E, Ascierto
PA, Marincola FM and De Lorenzo A: The influence of diet on
anti-cancer immune responsiveness. J Transl Med. 16:752018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Farhud D and Zarif Yeganeh M and Zarif
Yeganeh M: Nutrigenomics and nutrigenetics. Iran J Public Health.
39:1–14. 2010.PubMed/NCBI
|
|
144
|
Martucci M, Ostan R, Biondi F, Bellavista
E, Fabbri C, Bertarelli C, Salvioli S, Capri M, Franceschi C and
Santoro A: Mediterranean diet and inflammaging within the hormesis
paradigm. Nutr Rev. 75:442–455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Minihane AM, Vinoy S, Russell WR, Baka A,
Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, et
al: Low-grade inflammation, diet composition and health: Current
research evidence and its translation. Br J Nutr. 114:999–1012.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Murtaza N, Burke LM, Vlahovich N,
Charlesson B, O'Neill HM, Ross ML, Campbell KL, Krause L and
Morrison M: Analysis of the effects of dietary pattern on the oral
microbiome of elite endurance athletes. Nutrients. 11:E6142019.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kadayifci FZ, Zheng S and Pan YX:
Molecular mechanisms underlying the link between diet and DNA
methylation. Int J Mol Sci. 19:E40552018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Guillemin GJ, Essa MM, Song BJ and
Manivasagam T: Dietary supplements/antioxidants: impact on redox
status in brain diseases. Oxid Med Cell Longev. 2017:50484322017.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Quintanilha BJ, Reis BZ, Duarte GBS,
Cozzolino SMF and Rogero MM: Nutrimiromics: Role of microRNAs and
nutrition in modulating inflammation and chronic diseases.
Nutrients. 9:E11682017. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Battaglia R, Palini S, Vento ME, La
Ferlita A, Lo Faro MJ, Caroppo E, Borzì P, Falzone L, Barbagallo D,
Ragusa M, et al: Identification of extracellular vesicles and
characterization of miRNA expression profiles in human blastocoel
fluid. Sci Rep. 9:842019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Candido S, Lupo G, Pennisi M, Basile MS,
Anfuso CD, Petralia MC, Gattuso G, Vivarelli S, Spandidos DA, Libra
M, et al: The analysis of miRNA expression profiling datasets
reveals inverse microRNA patterns in glioblastoma and Alzheimer's
disease. Oncol Rep. 42:911–922. 2019.PubMed/NCBI
|
|
152
|
Falzone L, Romano GL, Salemi R, Bucolo C,
Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M and Candido
S: Prognostic significance of deregulated microRNAs in uveal
melanomas. Mol Med Rep. 19:2599–2610. 2019.PubMed/NCBI
|
|
153
|
Falzone L, Scola L, Zanghì A, Biondi A, Di
Cataldo A, Libra M and Candido S: Integrated analysis of colorectal
cancer microRNA datasets: Identification of microRNAs associated
with tumor development. Aging (Albany NY). 10:1000–1014. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon
A, et al: Environment and bladder cancer: Molecular analysis by
interaction networks. Oncotarget. 8:65240–65252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
McCubrey JA, Fitzgerald TL, Yang LV,
Lertpiriyapong K, Steelman LS, Abrams SL, Montalto G, Cervello M,
Neri LM, Cocco L, et al: Roles of GSK-3 and microRNAs on epithelial
mesenchymal transition and cancer stem cells. Oncotarget.
8:14221–14250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hafsi S, Candido S, Maestro R, Falzone L,
Soua Z, Bonavida B, Spandidos DA and Libra M: Correlation between
the overexpression of Yin Yang 1 and the expression levels of
miRNAs in Burkitt's lymphoma: A computational study. Oncol Lett.
11:1021–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Dalmasso G, Nguyen HTT, Yan Y, Laroui H,
Charania MA, Ayyadurai S, Sitaraman SV and Merlin D: Microbiota
modulate host gene expression via microRNAs. PLoS One.
6:e192932011. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Peck BCE, Mah AT, Pitman WA, Ding S, Lund
PK and Sethupathy P: Functional transcriptomics in diverse
intestinal epithelial cell types reveals robust microrna
sensitivity in intestinal stem cells to microbial status. J Biol
Chem. 292:2586–2600. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Yuan C and Subramanian S: MicroRNA
mediated tumor-microbiota metabolic interactions in colorectal
cancer. DNA Cell Biol. 38:281–285. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Liu S, da Cunha AP, Rezende RM, Cialic R,
Wei Z, Bry L, Comstock LE, Gandhi R and Weiner HL: The host shapes
the gut microbiota via fecal microrna. Cell Host Microbe. 19:32–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhang L, Chen T, Yin Y, Zhang CY and Zhang
YL: Dietary microRNA-A Novel Functional Component of Food. Adv
Nutr. 10:711–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Teodori L, Petrignani I, Giuliani A,
Prattichizzo F, Gurău F, Matacchione G, Olivieri F, Coppari S and
Albertini MC: Inflamm-aging microRNAs may integrate signals from
food and gut microbiota by modulating common signalling pathways.
Mech Ageing Dev. 182:1111272019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lang C, Karunairetnam S, Lo KR, Kralicek
AV, Crowhurst RN, Gleave AP, MacDiarmid RM and Ingram JR: Common
Variants of the Plant microRNA-168a Exhibit Differing Silencing
Efficacy for Human Low-Density Lipoprotein Receptor Adaptor Protein
1 (LDLRAP1). MicroRNA. 8:166–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Golan-Gerstl R, Elbaum Shiff Y, Moshayoff
V, Schecter D, Leshkowitz D and Reif S: Characterization and
biological function of milk-derived miRNAs. Mol Nutr Food Res.
61:17000092017. View Article : Google Scholar
|
|
166
|
Wagner AE, Piegholdt S, Ferraro M, Pallauf
K and Rimbach G: Food derived microRNAs. Food Funct. 6:714–718.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar
A, Hutchins E, Mu J, Deng Z, Luo C, et al: Plant-derived exosomal
micrornas shape the gut microbiota. Cell Host Microbe.
24:637–652.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Adami GR, Tangney CC, Tang JL, Zhou Y,
Ghaffari S, Naqib A, Sinha S, Green SJ and Schwartz JL: Effects of
green tea on miRNA and microbiome of oral epithelium. Sci Rep.
8:58732018. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Felden B and Gilot D: Modulation of
bacterial srnas activity by epigenetic modifications: Inputs from
the eukaryotic miRNAs. Genes (Basel). 10:E222018. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Bloch S, Węgrzyn A, Węgrzyn G and
Nejman-Faleńczyk B: Small and smaller-sRNAs and microRNAs in the
regulation of toxin gene expression in prokaryotic cells: A
mini-review. Toxins (Basel). 9:E1812017. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Yuan C, Steer CJ and Subramanian S:
Host-microRNA-microbiota interactions in colorectal cancer. Genes
(Basel). 10:E2702019. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Miro-Blanch J and Yanes O: Epigenetic
regulation at the interplay between gut microbiota and host
metabolism. Front Genet. 10:6382019. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Patrignani P, Tacconelli S and Bruno A:
Gut microbiota, host gene expression, and aging. J Clin
Gastroenterol. 48 (Suppl 1):S28–S31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Masotti A: Interplays between gut
microbiota and gene expression regulation by miRNAs. Front Cell
Infect Microbiol. 2:1372012. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zhao H, Chu M, Huang Z, Yang X, Ran S, Hu
B, Zhang C and Liang J: Variations in oral microbiota associated
with oral cancer. Sci Rep. 7:117732017. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Al-Hebshi NN, Nasher AT, Maryoud MY,
Homeida HE, Chen T, Idris AM and Johnson NW: Inflammatory
bacteriome featuring Fusobacterium nucleatum and
Pseudomonas aeruginosa identified in association with oral
squamous cell carcinoma. Sci Rep. 7:18342017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Audirac-Chalifour A, Torres-Poveda K,
Bahena-Román M, Téllez-Sosa J, Martínez-Barnetche J,
Cortina-Ceballos B, López-Estrada G, Delgado-Romero K,
Burguete-García AI, Cantú D, et al: Cervical microbiome and
cytokine profile at various stages of cervical cancer: A pilot
study. PLoS One. 11:e01532742016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW,
Lin YJ, Deng YF, Hsu WT, Wu CS and Li C: Increased abundance of
Clostridium and Fusobacterium in gastric microbiota
of patients with gastric cancer in Taiwan. Sci Rep. 8:1582018.
View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Yamamura K, Baba Y, Nakagawa S, Mima K,
Miyake K, Nakamura K, Sawayama H, Kinoshita K, Ishimoto T, Iwatsuki
M, et al: Human microbiome Fusobacterium nucleatum in esophageal
cancer tissue is associated with prognosis. Clin Cancer Res.
22:5574–5581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yang CY, Yeh YM, Yu HY, Chin CY, Hsu CW,
Liu H, Huang PJ, Hu SN, Liao CT, Chang KP, et al: Oral microbiota
community dynamics associated with oral squamous cell carcinoma
staging. Front Microbiol. 9:8622018. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Lim Y, Fukuma N, Totsika M, Kenny L,
Morrison M and Punyadeera C: The performance of an oral microbiome
biomarker panel in predicting oral cavity and oropharyngeal
cancers. Front Cell Infect Microbiol. 8:2672018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sasaki M, Yamaura C, Ohara-Nemoto Y,
Tajika S, Kodama Y, Ohya T, Harada R and Kimura S: Streptococcus
anginosus infection in oral cancer and its infection route.
Oral Dis. 11:151–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Pushalkar S, Ji X, Li Y, Estilo C,
Yegnanarayana R, Singh B, Li X and Saxena D: Comparison of oral
microbiota in tumor and non-tumor tissues of patients with oral
squamous cell carcinoma. BMC Microbiol. 12:1442012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Galvão-Moreira LV and da Cruz MC: Oral
microbiome, periodontitis and risk of head and neck cancer. Oral
Oncol. 53:17–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Shiga K, Tateda M, Saijo S, Hori T, Sato
I, Tateno H, Matsuura K, Takasaka T and Miyagi T: Presence of
Streptococcus infection in extra-oropharyngeal head and neck
squamous cell carcinoma and its implication in carcinogenesis.
Oncol Rep. 8:245–248. 2001.PubMed/NCBI
|
|
186
|
Narikiyo M, Tanabe C, Yamada Y, Igaki H,
Tachimori Y, Kato H, Muto M, Montesano R, Sakamoto H, Nakajima Y,
et al: Frequent and preferential infection of Treponema
denticola, Streptococcus mitis, and Streptococcus
anginosus in esophageal cancers. Cancer Sci. 95:569–574. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yang SF, Huang HD, Fan WL, Jong YJ, Chen
MK, Huang CN, Chuang CY, Kuo YL, Chung WH and Su SC: Compositional
and functional variations of oral microbiota associated with the
mutational changes in oral cancer. Oral Oncol. 77:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Kumar M, Kumar A, Nagpal R, Mohania D,
Behare P, Verma V, Kumar P, Poddar D, Aggarwal PK, Henry CJ, et al:
Cancer-preventing attributes of probiotics: An update. Int J Food
Sci Nutr. 61:473–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Yu AQ and Li L: The potential role of
probiotics in cancer prevention and treatment. Nutr Cancer.
68:535–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Lee JW, Shin JG, Kim EH, Kang HE, Yim IB,
Kim JY, Joo HG and Woo HJ: Immunomodulatory and antitumor effects
in vivo by the cytoplasmic fraction of Lactobacillus casei
and Bifidobacterium longum. J Vet Sci. 5:41–48. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Ma EL, Choi YJ, Choi J, Pothoulakis C,
Rhee SH and Im E: The anticancer effect of probiotic Bacillus
polyfermenticus on human colon cancer cells is mediated through
ErbB2 and ErbB3 inhibition. Int J Cancer. 127:780–790.
2010.PubMed/NCBI
|
|
192
|
Kim Y, Oh S, Yun HS, Oh S and Kim SH:
Cell-bound exopolysaccharide from probiotic bacteria induces
autophagic cell death of tumour cells. Lett Appl Microbiol.
51:123–130. 2010.PubMed/NCBI
|
|
193
|
Borowicki A, Michelmann A, Stein K,
Scharlau D, Scheu K, Obst U and Glei M: Fermented wheat aleurone
enriched with probiotic strains LGG and Bb12 modulates markers of
tumor progression in human colon cells. Nutr Cancer. 63:151–160.
2011.PubMed/NCBI
|
|
194
|
Stein K, Borowicki A, Scharlau D,
Schettler A, Scheu K, Obst U and Glei M: Effects of synbiotic
fermentation products on primary chemoprevention in human colon
cells. J Nutr Biochem. 23:777–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Wan LH, Yao Z, Ni F, Wei M, Zhou Z, Wang
HQ, Sun Y and Zhong ZX: Biosynthesis of genipin from gardenoside
catalyzed by β-glucosidase in two-phase medium. CIESC J.
65:3583–3591. 2014.
|
|
196
|
Asoudeh-Fard A, Barzegari A, Dehnad A,
Bastani S, Golchin A and Omidi Y: Lactobacillus plantarum
induces apoptosis in oral cancer KB cells through upregulation of
PTEN and downregulation of MAPK signalling pathways. Bioimpacts.
7:193–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Aghazadeh Z, Pouralibaba F and Yari
Khosroushahi A: The prophylactic effect of Acetobacter
syzygii probiotic species against squamous cell carcinoma. J
Dent Res Dent Clin Dent Prospects. 11:208–214. 2017.PubMed/NCBI
|
|
198
|
Orlandi E, Iacovelli NA, Tombolini V,
Rancati T, Polimeni A, De Cecco L, Valdagni R and De Felice F:
Potential role of microbiome in oncogenesis, outcome prediction and
therapeutic targeting for head and neck cancer. Oral Oncol.
99:1044532019. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Christofi T, Baritaki S, Falzone L, Libra
M and Zaravinos A: Current perspectives in cancer immunotherapy.
Cancers (Basel). 11:E14722019. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Guerrero-Preston R, Godoy-Vitorino F,
Jedlicka A, Rodríguez-Hilario A, González H, Bondy J, Lawson F,
Folawiyo O, Michailidi C, Dziedzic A, et al: 16S rRNA amplicon
sequencing identifies microbiota associated with oral cancer, human
papilloma virus infection and surgical treatment. Oncotarget.
7:51320–51334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Tuaeva NO, Falzone L, Porozov YB, Nosyrev
AE, Trukhan VM, Kovatsi L, Spandidos DA, Drakoulis N, Kalogeraki A,
Mamoulakis C, et al: Translational application of circulating DNA
in oncology: Review of the last decades achievements. Cells.
8:E12512019. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Silantyev AS, Falzone L, Libra M, Gurina
OI, Kardashova KS, Nikolouzakis TK, Nosyrev AE, Sutton CW, Mitsias
PD and Tsatsakis A: Current and future trends on diagnosis and
prognosis of glioblastoma: From molecular biology to proteomics.
Cells. 8:E8632019. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Ribeiro IP, de Melo JB and Carreira IM:
Head and neck cancer: Searching for genomic and epigenetic
biomarkers in body fluids - the state of art. Mol Cytogenet.
12:332019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Falzone L, Salomone S and Libra M:
Evolution of cancer pharmacological treatments at the turn of the
third millennium. Front Pharmacol. 9:13002018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Aas JA, Paster BJ, Stokes LN, Olsen I and
Dewhirst FE: Defining the normal bacterial flora of the oral
cavity. J Clin Microbiol. 43:5721–5732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Chattopadhyay I, Verma M and Panda M: Role
of oral microbiome signatures in diagnosis and prognosis of oral
cancer. Technol Cancer Res Treat. 18:15330338198673542019.
View Article : Google Scholar : PubMed/NCBI
|