Introduction
Melanotic neuroectodermal tumor of infancy (MNTI) is
a mixed mesenchymal-neuroectodermal tumor characterized by the
presence of pigment cells containing melanin, which usually appears
in the first year of life (1). The
tumor is benign, but due to its rapid growth, it can damage the
surrounding structures, which makes it dangerous (2). Most commonly, the tumor is located in
the anterior part of the alveolar process; less frequently in the
skull, brain or mandible (3). The
treatment of choice is surgical excision of the tumor and
chemotherapy (4). Chemotherapy is
one of the primary methods of treatment in cancer therapy, but it
may be associated with specific side effects (5). The most commonly used anti-cancer drug
is cisplatin, which has a nephrotoxic and ototoxic impact (6). Chemotherapy based on platinum compounds
is very useful in the treatment of neuroectodermal neoplasms in
children. Unfortunately, their use can lead to morbid infections
(7,8)
as well as irreversible hearing loss (9). Literature data show that between 40 and
80% of cisplatin-treated patients experience permanent hearing loss
(10,11). Some authors report that
cisplatin-induced ototoxicity has been observed in 7 and 90% of
cases at standard doses (12), as
well as at different doses and in various age groups (13), including children (14). Clinically, ototoxicity manifests
itself as bilateral hearing loss accompanied by tinnitus (15). Hearing loss begins in the
high-frequency range and progresses towards lower frequencies
(16,17).
As a consequence, ototoxicity can lead to delayed
speech development, learning difficulties, and even a deterioration
in psychosocial, emotional and general psychological well-being
(16). Also, ototoxicity has been
shown to have a progressive nature (11,15).
Hearing impairment or delayed hearing loss can appear a few years
after the end of treatment. Therefore, long-term specialist
monitoring of the condition of the auditory system for a minimum of
10 years is recommended. Ototoxicity risk factors include the
cumulative dose, impaired renal function, route of administration,
cranial irradiation, previous sensorineural hearing loss, age under
five years, concomitant use of ototoxic drugs, genetic
susceptibility, and tumor localization (16). The study aimed to evaluate
ototoxicity after MNTI chemotherapy from a long-term
perspective.
Case study
This case study presents a long-term ototoxic
effects after chemotherapy with cisplatin, vincristine,
cyclophosphamide, teniposide and adriamycin in a 10-year-old female
patient, who was administered this combination of drugs before and
after surgical removal of MNTI at the age of 8 months. A female
patient aged three months was admitted to the Department of
Haematology and Paediatric Oncology of the Karol Jonscher Clinical
Hospital in Poznan with a mixed mesenchymal-neuroectodermal MNTI, a
solid tumor within the alveolar ridge. Histopathological
examination confirmed MNTI. General tests were performed:
Morphology, biochemistry, and immunochemistry, which did not show
any abnormalities. Diagnostic imaging examinations, which consisted
of a chest X-ray and abdominal ultrasound, were also standard. A
computed tomography head scan showed lytic and osteogenic bone
lesions on the left side. The lytic lesion was 26×15 mm in size and
was located within the alveolar ridge of the maxilla. The
osteogenic lesions were found in the body of the maxilla near the
nasal wings. ‘Floating teeth’ (incisors) were visible within the
soft tissues of the alveolar ridge. It was decided to administer
chemotherapy before tumor resection. Chemotherapy according to the
CWS protocol for standard risk rhabdomyosarcoma, which consisted of
7 treatments with vincristine and dactinomycin, was distributed.
Before the introduction of chemotherapy, the patient underwent a
hearing examination. Due to the patient's age and her apparent lack
of cooperation, a non-invasive, objective hearing test was
performed, namely a 3/5 otoacoustic emissions (OAEs) screening
test. This test makes it possible to detect hearing loss of
cochlear origin and to assess the function of external hair cells.
It involves the recording of a very quiet acoustic signal that
arises in the cochlea due to the contraction of outer auditory
cells. For both ears, responses for all the frequencies were
recorded, which means that the acoustic cell responded to the
two-tone stimuli (Table I).
 | Table I.Results of otoacoustic emissions tests
for the right and left ear before chemotherapy. |
Table I.
Results of otoacoustic emissions tests
for the right and left ear before chemotherapy.
| l1 (dB) R/L | l2 (dB) R/L | F1 (Hz) R/L | F2 (Hz) R/L | GM (Hz) R/L | DP (dB) R/L | NF (dB) R/L | DP-NF(dB) R/L | Result |
|---|
| 64.8/65.6 | 55.0/53.8 | 4170/4170 | 5014/5014 | 4573/4573 | 13.3/7.1 | 4.4/‒1.5 | 8.9/8.9 | Pass |
| 65.1/66.2 | 55.1/55.6 | 3514/3514 | 4217/4217 | 3850/3850 | 16.7/13.1 | −7.8/3.4 | 24.5/9.8 | Pass |
| 65.3/64.6 | 55.7/55.9 | 2905/2905 | 3514/3514 | 3195/3195 | 15.1/12.9 | −13.5/ 4.7 | 28.6/8.2 | Pass |
| 65.1/67.9 | 55.5/56.0 | 2296/2296 | 2765/2765 | 2519/2519 | 16.1/10.4 | 3.4/1.4 | 12.7/9.0 | Pass |
| 65.7/67.3 | 55.3/56.5 | 1687/1687 | 2015/2015 | 1844/1844 | 16.4/13.4 | 4.7/2.7 | 11.7/10,7 | Pass |
At the age of 8 months, the patient underwent
surgical removal of the tumor in the Department of Oncological
Surgery for Children at the Institute of Mother and Child in
Warsaw. The removed fragment of the maxillary bone was 2.5×1.4×1.5
cm in size, was covered by overlying mucosa, and contained pieces
of tooth structure. Next, multidrug chemotherapy was introduced
with 23.5 mg cisplatin, 95 mg cyclophosphamide, 9.5 mg adriamycin,
and 23.5 mg teniposide injected intravenously. One year after the
completion of chemotherapy, another 3/5 otoacoustic emissions
(OAEs) screening test was performed. The results for the left and
right ears were normal (Table
II).
 | Table II.Results of otoacoustic emissions tests
for the right and left ear one year after chemotherapy. |
Table II.
Results of otoacoustic emissions tests
for the right and left ear one year after chemotherapy.
| l1 (dB) R/L | l2 (dB) R/L | F1 (Hz) R/L | F2 (Hz) R/L | GM (Hz) R/L | DP (dB) R/L | NF (dB) R/L | DP-NF(dB) R/L | Result |
|---|
| 67.3/64.4 | 56.3/53.2 | 4077/4170 | 4873/5014 | 4457/4573 | 6.7/‒3.9 | −3.9/‒12.4 | 10.6/8.5 | Pass |
| 67.1/66.3 | 57.4/54.9 | 3514/3514 | 4217/4217 | 3850/3850 | 9.1/6.1 | −6.7/‒4.5 | 15.8/10.6 | Pass |
| 64.0/64.8 | 55.6/54.4 | 2905/2905 | 3514/3514 | 3195/3195 | 8.8/‒0.7 | −7.5/‒17.3 | 16.3/16.6 | Pass |
| 64.4/65.3 | 52.2/54.8 | 2296/2296 | 2765/2765 | 2519/2519 | 14.9/7.6 | −16.8/‒6.1 | 31.7/13.7 | Pass |
| 64.2/64.8 | 54.1/55.3 | 1687/1687 | 2015/2015 | 1844/1844 | 13.7/15.5 | −3.7/‒10.4 | 17.4/25.9 | Pass |
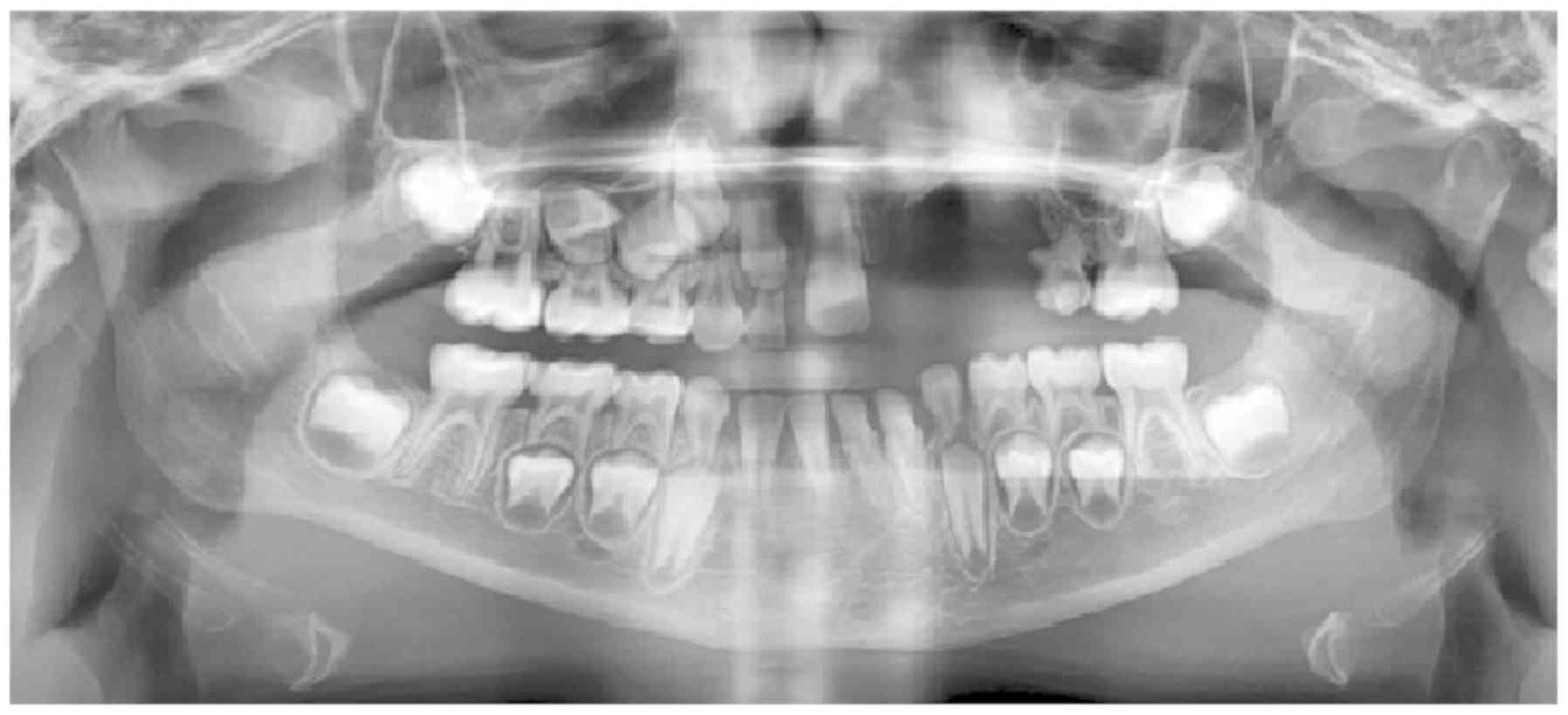
At the age of 8 years (Fig. 1) the girl came for consultation to
the Clinic of Maxillofacial Orthopaedics and Orthodontics at the
University of Medical Sciences in Poznań, of which she has been a
patient ever since. To improve the aesthetics and function of the
masticatory apparatus after the resection procedure, orthodontic
treatment was planned and implemented. There were no changes in the
structures of soft and bone tissues other than those connected with
post-operative healing. After two years, as part of the orthodontic
treatment, the patient was referred to the Department of Hearing
Healthcare Profession, Chair of Biophysics Poznan University of
Medical Sciences, Poland for a hearing test. Otoscopic examination
revealed no contraindications for performing the audiological
evaluation. Subjective tests were conducted using a Madsen Itera II
diagnostic audiometer and included pure-tone audiometry for the
extended frequency range from 125 Hz to 16 kHz and speech
audiometry. In accordance with the cross-check principle, objective
tests were also performed: Classic tympanometry for the 226 Hz
frequency; wideband tympanometry for the frequency range 226–8,000
Hz; stapedial reflex assessment with a Titan tympanometer
(Interacoustic); and a Distortion Product Otoacoustic Emissions
(DPOAE) test using a Madsen Capella 2 device (Medicus). Otoacoustic
emissions evaluation makes it possible to measure the activity of
external auditory cells; in particular, the DPOAE test indicates
the frequency ranges in which external auditory cells are affected
by platinum compounds.
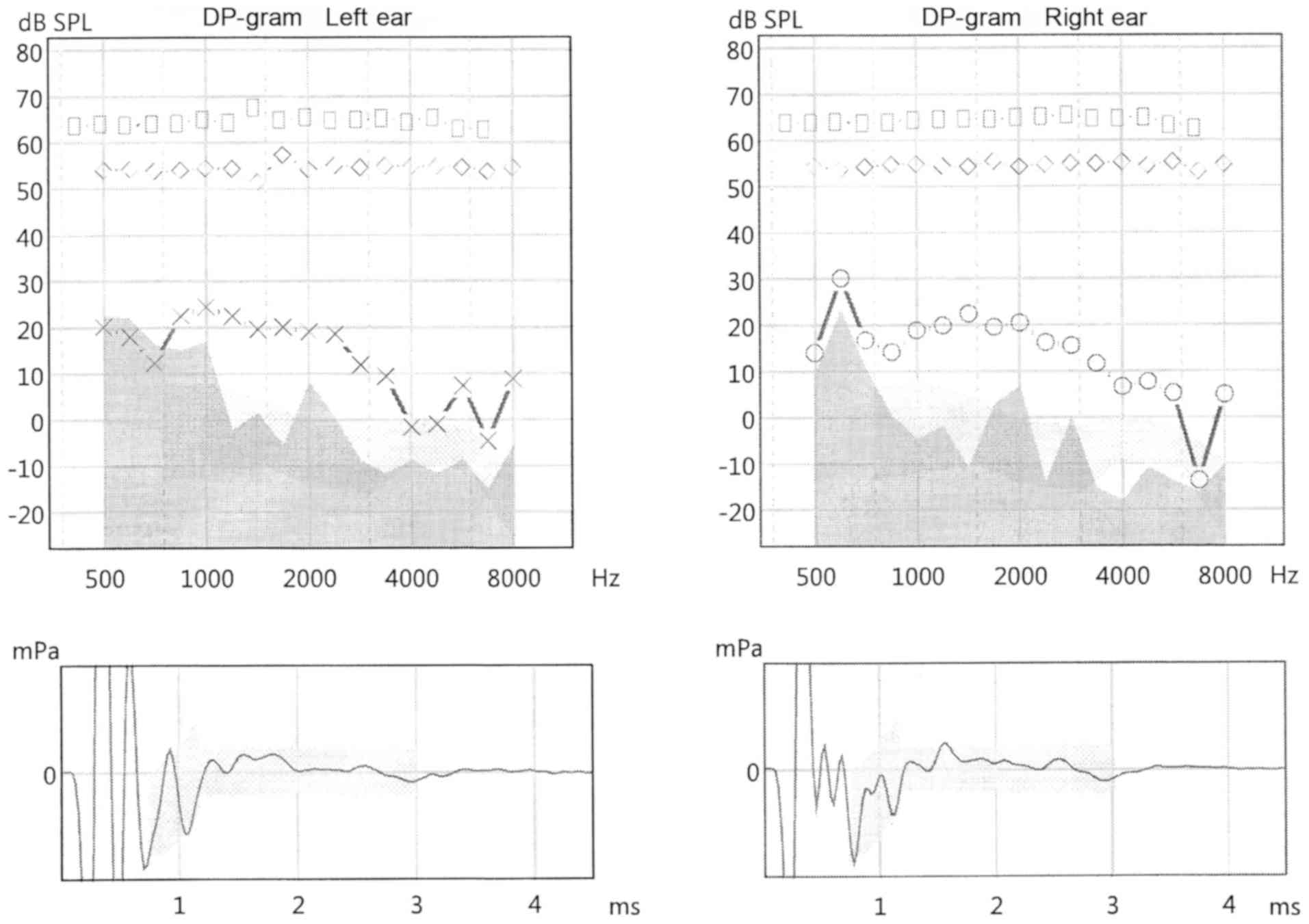
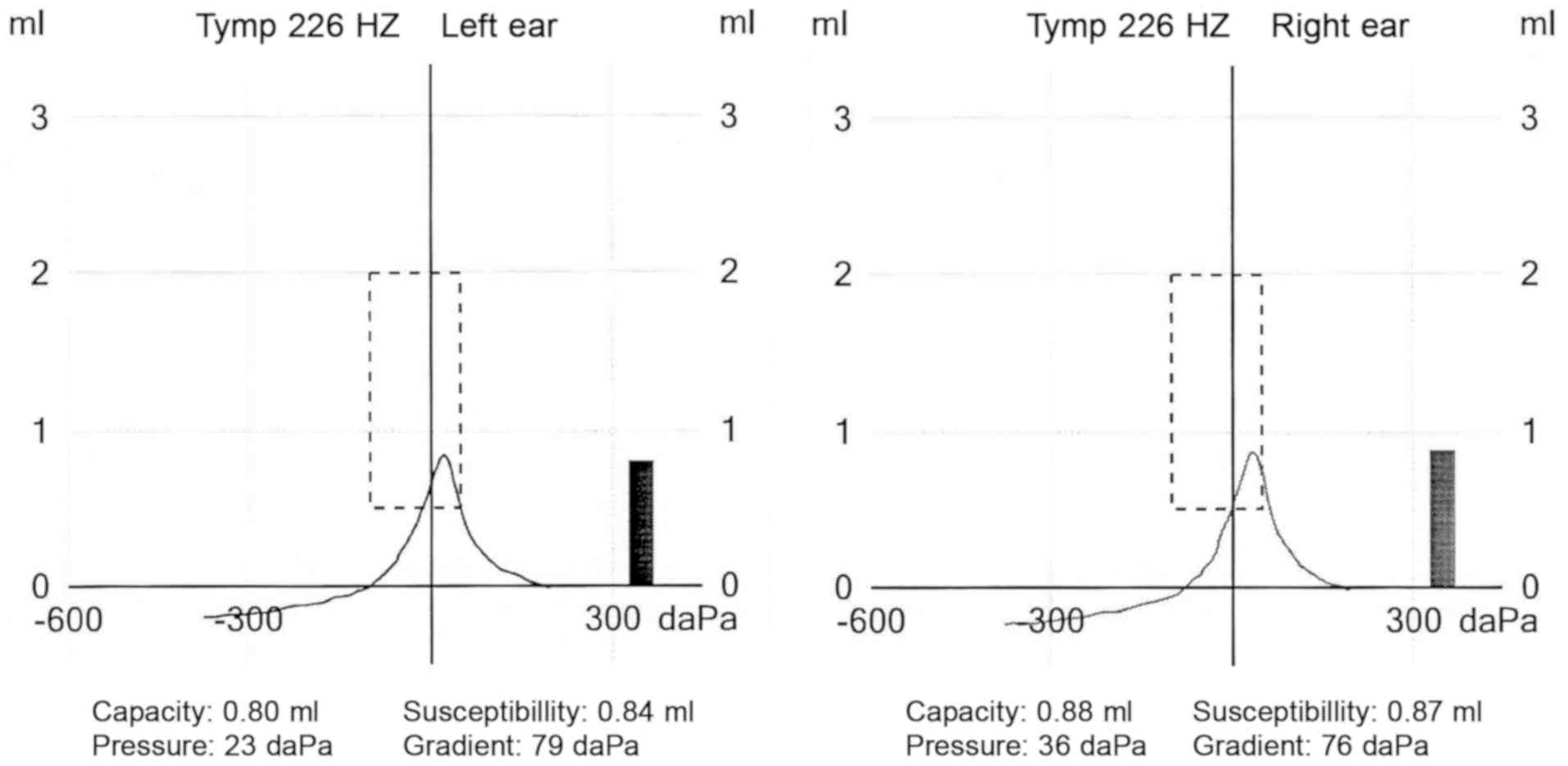
The tests yielded the following results: Otoacoustic
emissions were correct in both ears, normal tympanograms were
obtained for both the right and left ear (type A), with correct
stapedial muscle reflexes for all frequencies (Figs. 2 and 3; Table
III). Absorbance measurements for both ears revealed
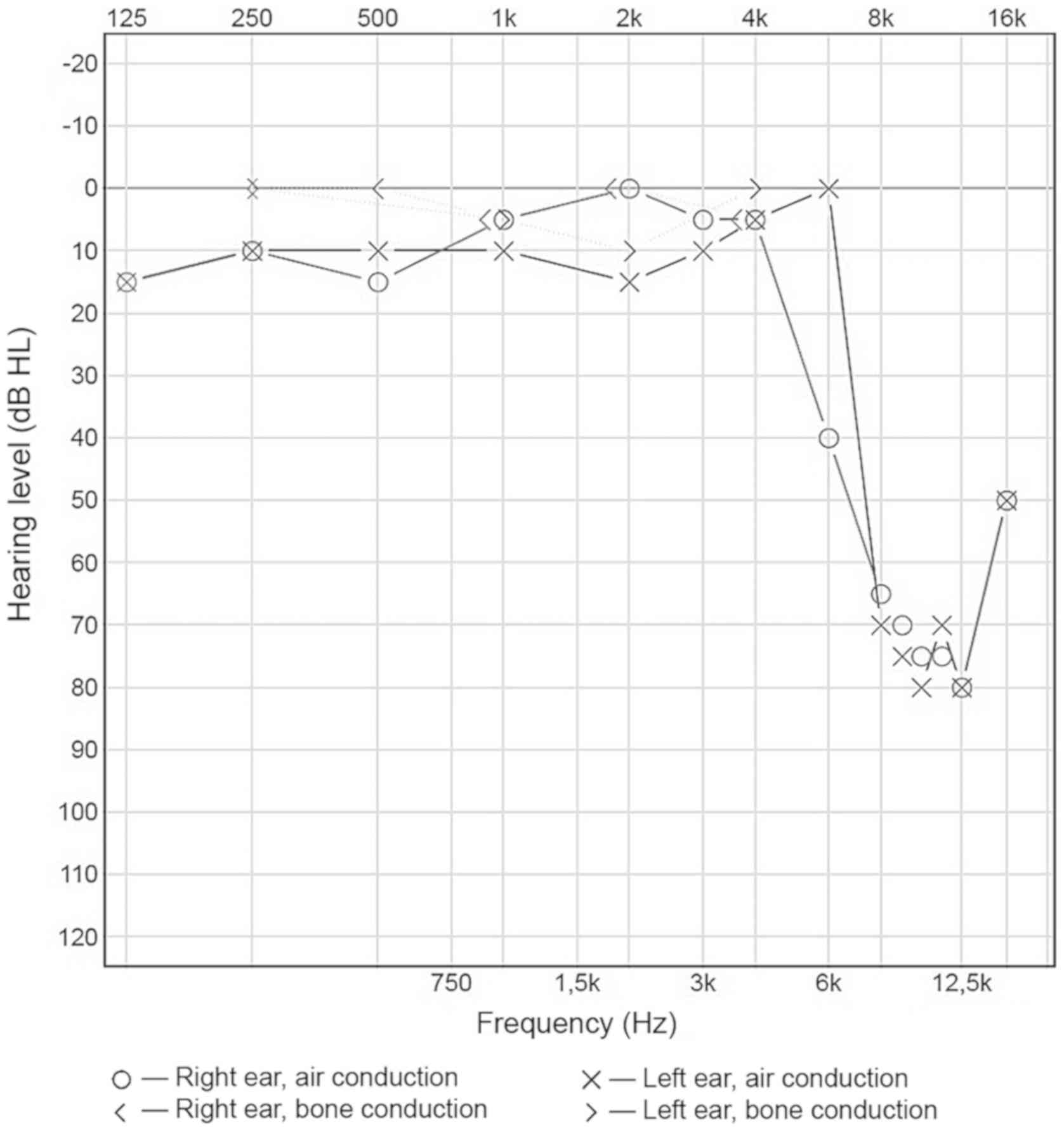
characteristic peaks at around 1,000 and 3,000 Hz. The hearing
threshold determined for the frequencies of 500, 1,000, 2,000, and
4,000 Hz was five dBHL for the right ear, and ten dBHL for the left
ear (Fig. 4). Speech audiometry
results were consistent with the results of pure-tone audiometry:
The Speech Reception Threshold (SRT) was 35 dBSPL for both ears.
However, a significant increase in the hearing threshold of both
ears was recorded for the frequency range between 6,000 and 16,000
Hz. The results obtained reveal substantial abnormalities.
 | Table III.Results of distortion product
otoacoustic emissions tests for the right and left ear, 10 years
after chemotherapy. |
Table III.
Results of distortion product
otoacoustic emissions tests for the right and left ear, 10 years
after chemotherapy.
| F2 (Hz) R/L | GM (dB) R/L | l1/l2 R/L | DP1 (dB) R/L | NF (dB) R/L | SNR (dB) R/L | Result R/L |
|---|
| 498/498 | 452/452 | 64/55/64/55 | 14/20 | 9/23 | 5/−2 |
Rejected/Rejected |
| 596/596 | 539/539 | 64/54/64/54 | 30/18 | 24/22 | 7/−4 | Pass/Rejected |
| 703/703 | 636/636 | 64/54/64/54 | 17/12 | 11/16 | 6/−4 |
Rejected/Rejected |
| 840/840 | 763/763 | 64/55/64/54 | 14/23 | 0/15 | 14/7 | Pass/Pass |
| 996/996 | 904/904 | 64/55/64/54 | 19/23 | −5/17 | 23/7 | Pass/Pass |
| 1191/1191 | 1079/1079 | 64/54/64/54 | 20/22 | −2/−2 | 22/25 | Pass/Pass |
| 1416/1416 | 1283/1283 | 65/55/64/51 | 22/20 | −11/2 | 33/18 | Pass/Pass |
| 1680/1680 | 1521/1521 | 65/55/68/57 | 20/20 | 7/−5 | 14/26 | Pass/Pass |
| 2002/2002 | 1812/1812 | 65/54/65/54 | 20/19 | 7/8 | 14/11 | Pass/Pass |
| 2383/2383 | 2157/2157 | 65/55/65/55 | 16/19 | −14/1 | 30/18 | Pass/Pass |
| 2832/2832 | 2560/2560 | 65/55/65/55 | 16/12 | 0/−9 | 15/21 | Pass/Pass |
| 3359/3359 | 3042/3042 | 65/55/65/55 | 12/9 | −15/−12 | 27/21 | Pass/Pass |
| 4004/4004 | 3625/3625 | 65/55/65/55 | 7/−2 | −18/−9 | 25/7 | Pass/Pass |
| 4756/4756 | 4305/4305 | 65/5464/55 | 8/−1 | −11/−12 | 18/11 | Pass/Pass |
| 5654/5654 | 5121/5121 | 65/55/65/55 | 5/7 | −14/−9 | 19/16 | Pass/Pass |
| 6729/6729 | 6093/6093 | 63/53/63/54 | −14/−5 | −16/−15 | 3/10 | Rejected/Pass |
| 7998/7998 | 7239/7239 | 63/55/63/55 | 5/9 | −10/−5 | 15/15 | Pass/Pass |
The present study was reviewed and approved by the
institutional ethics committee of Poznan University of Medical
Sciences. All the procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards (no. 645/16). Written informed consent was obtained from
all parents prior to enrollment.
Discussion
Platinum compounds are used in the standard
treatment of mesenchymal-neuroectodermal tumors in pediatric
oncology. The use of cisplatin is one of the most common causes of
drug-induced hearing loss because its ototoxicity has a destructive
effect on external auditory cells, which are not capable of
regeneration (18). The means that
the hearing loss after cisplatin-based treatment is irreversible
(19). In this study, a patient with
a mesenchymal-neuroectodermal tumor who had been treated with
cisplatin was diagnosed with bilateral high-frequency hearing loss,
which is consistent with literature reports (10–13). The
damage associated with chemotherapy begins in the first row of the
external auditory cells, at the base of the cochlea, where
high-frequency sounds are processed. As a result, chemotherapy
using cisplatin causes bilateral high-frequency sensorineural
hearing loss, which is consistent with our findings (20). High frequencies are not crucial for
the understanding of speech; however, with higher doses and the
passage of time after the completion of the treatment, hearing loss
may in some cases also affect lower frequencies (21).
As a consequence, cisplatin-induced ototoxicity can
impair a child's development, learning, and behavior (12,22).
Unfortunately, in our case no previous pure-tone audiometry tests
were performed, which makes it impossible to determine whether the
hearing loss is progressive or whether it has remained at the same
level since the end of chemotherapy. In the literature, reports are
stating that after the completion of treating hearing loss is
permanent and stable (19,23). However, many authors have observed
progressive hearing loss following chemotherapy with platinum
compounds in children treated for solid tumors (15,21,22).
It is worth noting the research of Liberman et
al, conducted on a group of 200 patients to assess hearing loss
caused by cancer treatment in childhood. The types of cancer from
which the studied patients suffered included solid tumors. All the
patients were seen at least eight years after the cancer treatment,
which consisted of a combination of radiotherapy and chemotherapy
with or without the use of cisplatin (CDDP). The audiological
evaluation included pure-tone audiometry, speech audiometry, and
impedance audiometry. The assessment of hearing loss was made
according to the criteria adopted by the International Office for
Audiophonology, where a hearing loss means the presence of pure
tones >20 dBHL for the frequency range 500–4,000 Hz. The authors
found symmetric, bilateral hearing loss at the 4, 6 and 8 kHz
frequencies in patients who had undergone chemotherapy with CDDP,
and in those after radiotherapy combined with chemotherapy using
CDDP. Hearing loss was not observed in patients who had experienced
only radiotherapy or chemotherapy without CDDP. It was found that
the risk factors for hearing loss are the use of CDDP in cancer
therapy and the patient's age at the time of cancer diagnosis
(24). Evaluation of the patient
discussed in this paper conducted ten years after the completion of
chemotherapy clearly shows a high-frequency hearing loss, which is
consistent with the foregoing study. Cooperation with the child's
parents/guardians is essential. Their consent and help in the
multi-faceted therapy of the child (regardless of the disease
entity) is a prerequisite for the implementation of treatment and
rehabilitation procedures, which was emphasized in many items
cited, including the study, references.
Most of the available literature does not contain
reports on the possibility of complications resulting from the
administration of cisplatin-based chemotherapy in the treatment of
MNTI. One of the possible side effects of cisplatin is ototoxicity,
which developed in the patient discussed in this paper, an
occurrence which is confirmed by literature reports.
Cisplatin-induced hearing loss develops in patients in the
long-term and initially affects only the high-frequency range. In
the presented case, hearing loss was observed ten years after the
completion of chemotherapy, and it concerned high frequencies in
the 6,000 to 16,000 Hz range for both ears. Thus, it is essential
to inform the parents or legal guardians of a child patient in
advance about the possibility of ototoxicity and to acquaint them
with the possible consequences of hearing the loss in children. It
is also crucial to ensure multidisciplinary cooperation between
doctors and hearing care professionals monitor the auditory system
during and after chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DHJ conceived the current study, developed the
methodology, supervised the experiments and critically revised the
manuscript. ACh and ACz analyzed and interpreted orthodontic
treatment data, and analyzed orthodontics literature. ACh edited
the figures and wrote the manuscript. MMK carried out orthodontic
treatment, collected data, was responsible for patient approval and
secured intellectual property from the patient and parents. AM and
MUO analyzed and interpreted hearing system data. AM analyzed
ototoxity literature. MUO carried out audiological examination and
wrote the manuscript. TMB conceived and scheduled the experiments,
interpreted results, edited and revised the manuscript, and
approved the final version of the manuscript for publication. All
authors read and approved the final manuscript.
Ethics and consent to participate
The present study was reviewed and approved by the
Institutional Ethics Committee of Poznan University of Medical
Sciences. All the procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards (no. 645/16). Written informed consent was obtained from
all parents prior to enrollment.
Patient consent for publication
Parents provided written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chaudhary S, Manuja N, Ravishankar CT,
Sinha A, Vijayran M and Singh M: Oral melanotic neuroectodermal
tumor of infancy. J Indian Soc Pedod Prev Dent. 32:71–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrade NN, Mathai PC, Sahu V, Aggarwal N
and Andrade T: Melanotic neuroectodermal tumour of infancy-A rare
entity. J Oral Biol Craniofac Res. 6:237–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neven J, Hulsbergen-van der Kaa C,
Groot-Loonen J, de Wilde PC and Merkx MA: Rucurrent melanotic
neuroectodermal tumor of infancy: A proposal for treatment protocol
with surgery and adjuvant chemotherapy. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 106:493–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ubale P, Baldwa N and Gujjar P:
Anaesthetic management of a neuroectodermal tumor of infancy: A
rare case report. Anaesth Essays Res. 11:251–253. 2017. View Article : Google Scholar
|
|
5
|
Łyskawa W: Chemiotherapy in the
neoplasmatic diseases treatment and its neurotoxicity. Anestezjol i
Ratow. 3:80–87. 2009.
|
|
6
|
Karasawa T and Steyger PS: An integrated
view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol
Lett. 237:219–227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Işık A, Grassi A and Soran A: Positive
axilla in breast cancer; clinical practice in 2018. Eur J Breast
Health. 14:134–135. 2018.PubMed/NCBI
|
|
8
|
Isik A, Karavas E and Firat D: Spontaneous
milk fistula from an axillary accessory breast. Breast J.
25:1542019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Olgun Y, Aktaş S, Altun Z, Kırkım G,
Kızmazoğlu DÇ, Erçetin AP, Demir B, İnce D, Mutafoğlu K, Demirağ B,
et al: Analysis of genetic and non genetic risk factors for
cisplatin ototoxicity in pediatric patients. Int J Pediatr
Otorhinolaryngol. 90:64–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Breglio AM, Rusheen AE, Shide ED,
Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L and
Cunningham LL: Cisplatin is retained in the cochlea indefinitely
following chemotherapy. Nat Commun. 8:16542017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Einarsson EJ, Petersen H, Wiebe T,
Fransson PA, Grenner J, Magnusson M and Moëll C: Long term hearing
degeneration after platinum-based chemotherapy in childhood. Int J
Audiol. 49:765–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dean JB, Hayashi SS, Albert CM, King AA,
Karzon R and Hayashi RJ: Hearing loss in pediatric oncology
patients receiving carboplatin-containing regimens. J Pediatr
Hematol Oncol. 30:130–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheth S, Mukherjea D, Rybak LP and
Ramkumar V: Mechanisms of cisplatin-induced ototoxicity and
otoprotection. Front Cell Neurosci. 11:3382017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Clemens E, de Vries AC, Pluijm SF, Am
Zehnhoff-Dinnesen A, Tissing WJ, Loonen JJ, van Dulmen-den Broeder
E, Bresters D, Versluys B, Kremer LC, et al: Determinants of
ototoxicity in 451 platinum-treated Dutch survivors of childhood
cancer: A DCOG late-effects study. Eur J Cancer. 69:77–85. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bertolini P, Lassalle M, Mercier G, Raquin
MA, Izzi G, Corradini N and Hartmann O: Platinum compound-related
ototoxicity in children: Long-term follow-up reveals continuous
worsening of hearing loss. J Pediatr Hematol Oncol. 26:649–655.
2004. View Article : Google Scholar
|
|
16
|
Bess FH, Dodd-Murphy J and Parker RA:
Children with minimal sensorineural hearing loss: Prevalence,
educational performance, and functional status. Ear Hear.
19:339–354. 1998. View Article : Google Scholar
|
|
17
|
Davis JM, Elfenbein J, Schum R and Bentler
RA: Effects of mild and moderate hearing impairments on language,
educational, and psychosocial behavior of children. J Speech Hear
Disord. 51:53–62. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sergi B, Ferraresi A, Troiani D, Paludetti
G and Fetoni AR: Cisplatin ototoxicity in the guinea pig:
Vestibular and cochlear damage. Hear Res. 182:56–64. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clemens E, de Vries AC, Am
Zehnhoff-Dinnesen A, Tissing WJ, Loonen JJ, Pluijm SF, van
Dulmen-den Broeder E, Bresters D, Versluys B, Kremer LC, et al:
Hearing loss after platinum treatment is irreversible in noncranial
irradiated childhood cancer survivors. Pediatr Hematol Oncol.
34:120–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CP, Liu JD, Chow JM, Liu CR and Liu
HE: Small-molecule c-Myc inhibitor, 10058-F4, inhibits
proliferation, downregulates human telomerase reverse transcriptase
and enhances chemosensitivity in human hepatocellular carcinoma
cells. Anticancer Drugs. 18:161–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fetoni AR, Ruggiero A, Lucidi D, De Corso
E, Sergi B, Conti G and Paludetti G: Audiological monitoring in
children treated with platinum chemotherapy. Audiol Neurootol.
21:203–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waissbluth S, Chuang A, Del Valle Á and
Cordova M: Long term platinum-induced ototoxicity in pediatric
patients. Int J Pediatr Otorhinolaryngol. 107:75–79. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kushner BH, Budnick A, Kramer K, Modak S
and Cheung NK: Ototoxicity from high-dose use of platinum compounds
in patients with neuroblastoma. Cancer. 107:417–422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liberman PH, Goffi-Gomez MV, Schultz C,
Novaes PE and Lopes LF: Audiological profile of patients treated
for childhood cancer. Braz J Otorhinolaryngol. 82:623–629. 2016.
View Article : Google Scholar : PubMed/NCBI
|