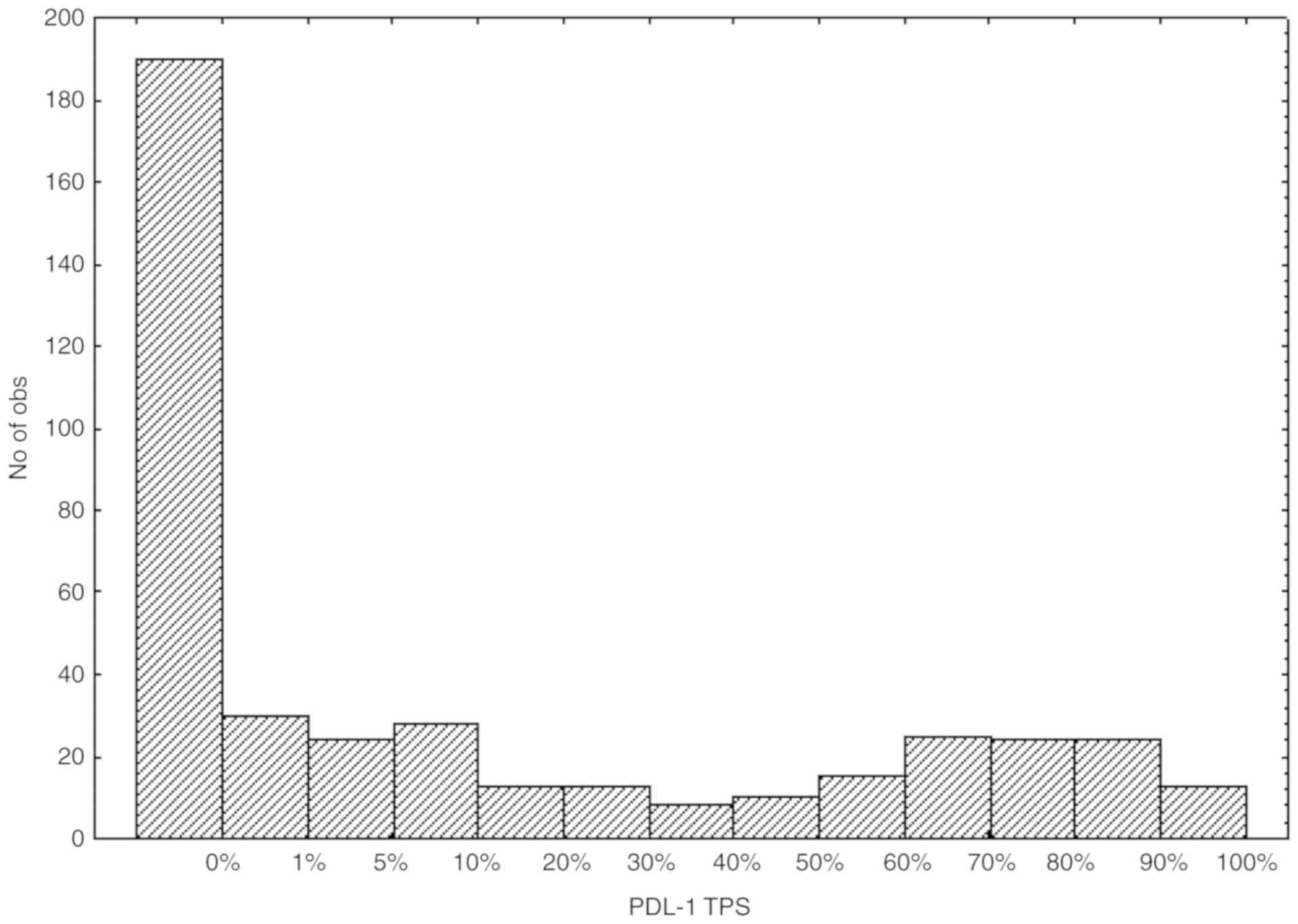
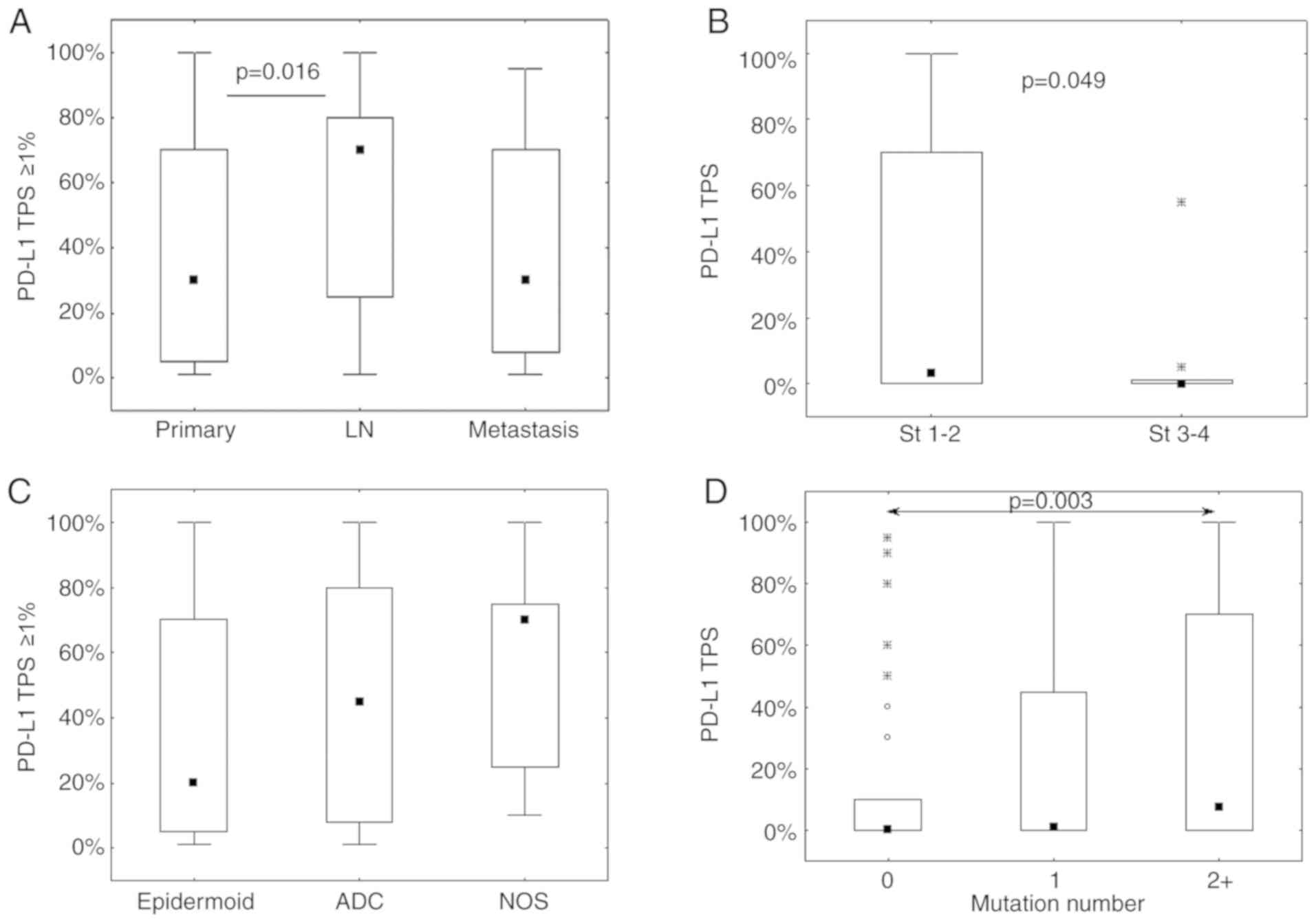
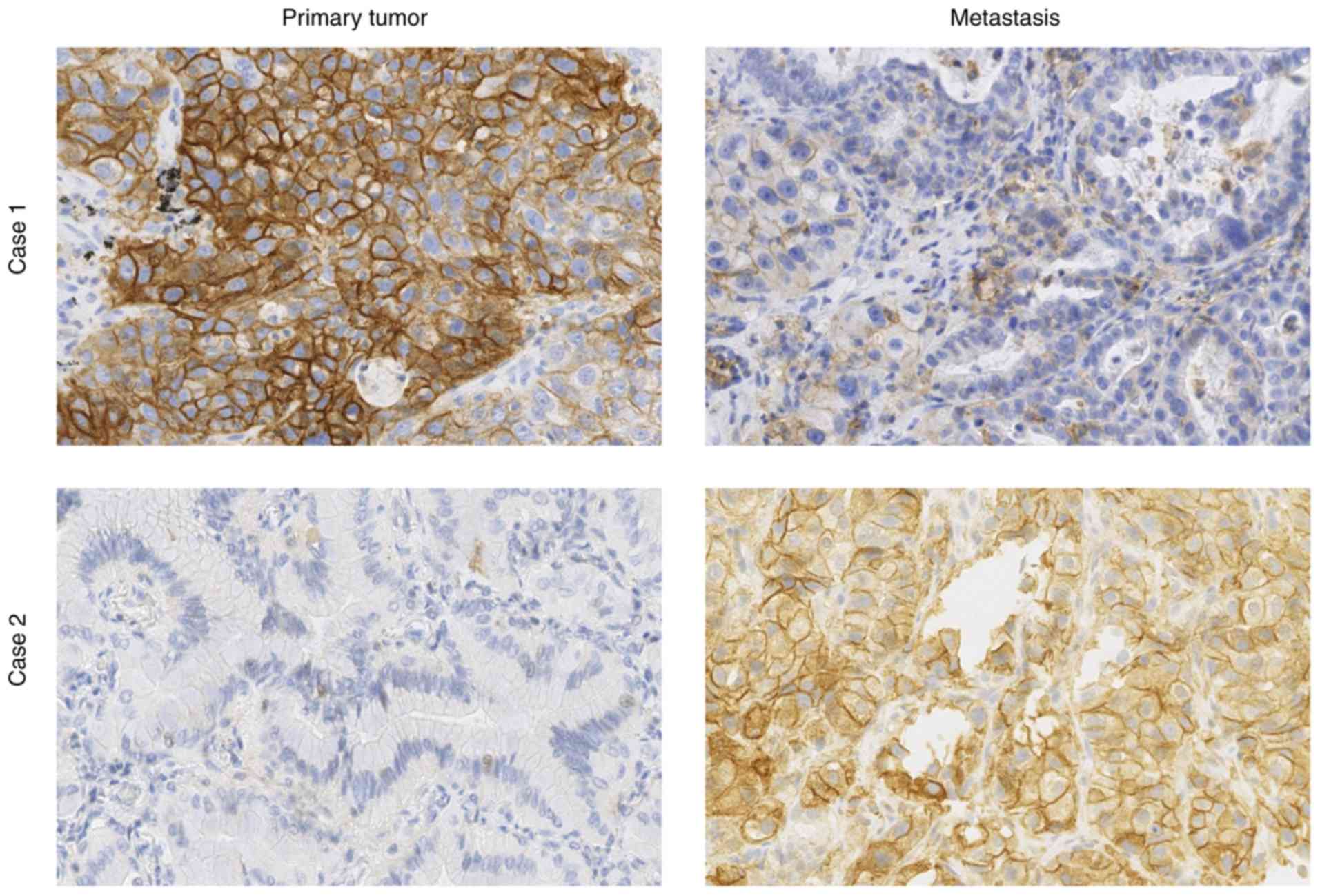
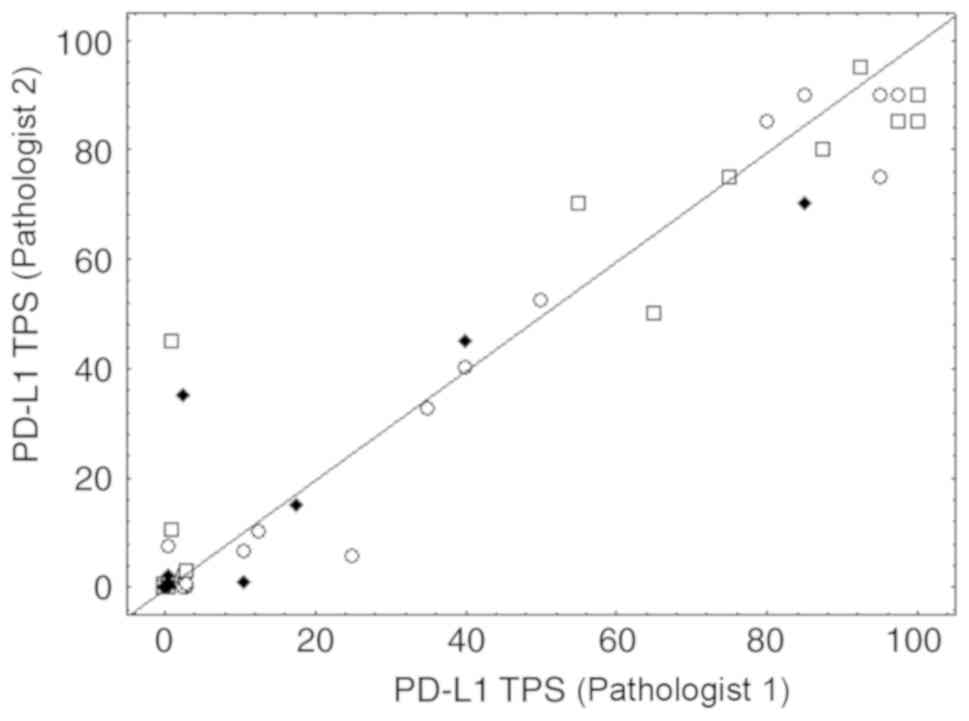
|
1
|
IASCL: Atlas of PD-L1 immunohistochemistry
testing in lung cancer. Editorial Rx Press; USA: 2017
|
|
2
|
Hersom M and Jørgensen JT: Companion and
complementary diagnostics-focus on PD-L1 expression assays for
PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer.
Ther Drug Monit. 40:9–16. 2018.PubMed/NCBI
|
|
3
|
Melosky B, Chu Q, Juergens R, Leighl N,
McLeod D and Hirsh V: Pointed progress in Second-line advanced
Non-small-cell lung cancer: The rapidly evolving field of
checkpoint inhibition. J Clin Oncol. 34:1676–1688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khunger M, Hernandez AV, Pasupuleti V,
Rakshit S, Pennell NA, Stevenson J, Mukhopadhyay S, Schalper K and
Velcheti V: Programmed cell death 1 (PD-1) ligand (PD-L1)
expression in solid tumors as a predictive biomarker of benefit
from PD-1/PD-L1 axis inhibitors: A systematic review and
meta-analysis. JCO Precis Oncol. 1:1–15. 2017. View Article : Google Scholar
|
|
6
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado H, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the Treatment of Non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt W, Poddubskaya E, Antonia S, Pluzanski A, Vokes E,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell Non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Büttner R, Gosney JR, Skov BG, Adam J,
Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F,
Penault-Llorca F, et al: Programmed Death-ligand 1
Immunohistochemistry testing: A review of analytical assays and
clinical implementation in Non-small-cell lung cancer. J Clin
Oncol. 35:3867–3876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanteujoul S, Adam J, Girard N,
Duruisseaux M, Mansuet-Lupo A, Cazes A, Rouquette I, Gibault L,
Garcia S, Antoine M, et al: Tests immunohistochimiques PD-L1 dans
les cancers du poumon non à petites cellules: Recommandations par
le groupe PATTERN de pathologistes thoraciques PD-L1 testing in
non-small cell lung carcinoma: Guidelines from the PATTERN group of
thoracic pathologists. Ann Pathol. 38:110–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelakanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 Expression in Non-small cell lung cancer. JAMA Oncol.
2:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendry S, Byrne DJ, Wright GM, Young RJ,
Sturrock S, Cooper WA and Fox SB: Comparison of four PD-L1
immunohistochemical assays in lung cancer. J Thorac Oncol.
13:367–376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ilie M, Long-Mira E, Bence C, Butori C,
Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K,
et al: Comparative study of the PD-L1 status between surgically
resected specimens and matched biopsies of NSCLC patients reveal
major discordances: A potential issue for anti-PD-L1 therapeutic
strategies. Ann Oncol. 27:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skov BG and Skov T: Paired comparison of
PD-L1 expression on cytologic and histologic specimens from
malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and
PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol.
25:453–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsao MS, Kerr KM, Kockx M, Beasley MB,
Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et
al: PD-L1 immunohistochemistry comparability study in real-life
clinical samples: Results of blueprint phase 2 project. J Thorac
Oncol. 13:1302–1311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thunnissen E, Allen TC, Adam J, Aisner DL,
Beasley MB, Borczuk AC, Cagle PT, Capelorri VL, Cooper W, Hariri
LP, et al: Immunohistochemistry of pulmonary biomarkers: A
perspective from members of the pulmonary pathology society. Arch
Pathol Lab Med. 142:408–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge
JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A
prospective, Multi-institutional, Pathologist-based assessment of 4
immunohistochemistry assays for PD-L1 expression in Non-small cell
lung cancer. JAMA Oncol. 3:1051–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheel AH, Baenfer G, Baretton G, Dietel
M, Diezko R, Henkel T, Heukamp LC, Jasani B, Jöhrens K, Kirchner T,
et al: Interlaboratory concordance of PD-L1 immunohistochemistry
for non-small-cell lung cancer. Histopathology. 72:449–459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch FR, McElhinny A, Stanford D,
Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P,
Hanks D, Vannapusa B, et al: PD-L1 immunohistochemistry assays for
lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay
comparison project. J Thorac Oncol. 12:208–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adam J, Rouquette I, Damotte D, Badoual C,
Danel C, Damiola F, Penault-Llorca F and Lantuejoul S: PL04a.04:
Multicentric French harmonization study for PD-L1 IHC testing in
NSCLC. J Thorac Oncol. 12 (Suppl):S11–S12. 2017. View Article : Google Scholar
|
|
22
|
Cooper WA, Russell PA, Huot-Marchand P,
Cherian M, Duhig EE, Godbolt D, Jessup PJ, Khoo C, Leslie C, Mahar
A, et al: Intra-and Inter-observer reproducibility study of PD-L1
biomarker in Non-small cell lung cancer (NSCLC)-the DREAM STUDY. J
Thorac Oncol. 12 (Suppl):S814–S815. 2017. View Article : Google Scholar
|
|
23
|
Ratcliffe MJ, Sharpe A, Midha A, Barker C,
Scott M, Scorer P, Al-Masri H, Rebelatto MC and Walker J: Agreement
between programmed cell death ligand-1 diagnostic assays across
multiple protein expression cutoffs in non-small cell lung cancer.
Clin Cancer Res. 23:3585–3591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roach C, Zhang N, Corigliano E, Jansson M,
Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D and
Kulangara K: Development of a companion diagnostic PD-L1
immunohistochemistry assay for pembrolizumab therapy in
Non-small-cell lung cancer. Appl Immunohistochem Mol Morphol.
24:392–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho JH, Sorensen SF, Choi YL, Feng Y, Kim
TE, Choi H, Georgsen JB, Dolled-Filhart M, Emancipator K, Meldgaard
P, et al: Programmed death ligand 1 expression in paired non-small
cell lung cancer tumor samples. Clin Lung Cancer. 18:e473–e479.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Union for International Cancer Control
UICC, . TNM Classification of Malignant Tumours Eight Edition. John
Wiley & Sons; UK: 2017
|
|
27
|
D'Haene N, Le Mercier M, De Nève N,
Blanchard O, Delaunoy M, El Housni H, Dessars B, Heimann P,
Remmelink M, Demetter P, et al: Clinical validation of targeted
next generation sequencing for colon and lung cancers. PLoS One.
10:e01382452015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin LI: A concordance correlation
coefficient to evaluate reproducibility. Biometrics. 45:255–268.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Midha A, Sharpe A, Scott M, Walker J, Shi
K, Ballas M, Garassino MC and Rizvi NA: PD-L1 expression in
advanced NSCLC: Primary lesions versus metastatic sites and impact
of sample age. J Clin Oncol. 34 (15 Suppl):S30252016. View Article : Google Scholar
|
|
30
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to Anti-programmed cell
death (PD)-1 and Anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line Nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho J, Zhou W, Choi Y, Sun JM, Choi H, Kim
TE, Dolled-Filhart M, Emancipator K, Rutkowski MA and Kim J:
Retrospective molecular epidemiology study of PD-L1 expression in
patients with EGFR-mutant non-small cell lung cancer. Cancer Res
Treat. 50:95–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li D, Zhu X, Wang H and Li N: Association
between PD-L1 expression and driven gene status in NSCLC: A
meta-analysis. Eur J Surg Oncol. 43:1372–1379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heymann JJ, Bulman WA, Swinarski D, Pagan
CA, Crapanzano JP, Haghighi M, Fazlollahi L, Stoopler MB, Sonett
JR, Sacher AG, et al: PD-L1 expression in non-small cell lung
carcinoma: Comparison among cytology, small biopsy, and surgical
resection specimens. Cancer Cytopathol. 125:896–907. 2017.
View Article : Google Scholar : PubMed/NCBI
|