Introduction
In July 2015, the European Medicines Agency approved
the use of immunotherapy (IT) targeting the programmed cell death 1
(PDCD-1)/programmed death-ligand 1 (PD-L1) (also known as CD274
molecule) interaction in patients with locally advanced,
non-resectable or metastatic non-small cell lung cancers (NSCLCs)
(1,2). However, since the response rate of
NSCLC to IT varies from 12 to 49%, it is crucial to determine
biomarkers to identify responders and non-responders (1).
Numerous studies have reported the use of PD-L1
expression evaluation by immunohistochemistry (IHC) as a biomarker
that could predict the effectiveness of anti-PDCD-1/PD-L1 IT, in
order to select patients who would benefit from this type of
therapy (3–5). The Checkmate 057 (6), Keynote 001 (7) and POPLAR (8) clinical studies demonstrated that, in
NSCLC, high expression of PD-L1 in tumor cells is associated with a
better response to IT; however the Checkmate 017 (9) study reported that some patients
responded positively to nivolumab, another IT drug, although their
lung tumor did not express PD-L1. At present, only the use of
pembrolizumab is based on PD-L1 expression (1,10,11).
The contradictory results regarding the theranostic
role of PD-L1 expression can be explained by several parameters.
Firstly, PD-L1 expression is heterogeneous and dynamic. It varies
between the primary tumor and metastasis, and within the tumor
itself (4,12). Hendry et al (13) analyzed PD-L1 expression in numerous
samples from the same tumor with a low to moderate agreement
(13). This heterogeneity partly
explains the differences in PD-L1 expression observed by Ilie et
al (14) in biopsies compared
with that in surgical resections, with a lower PD-L1 expression in
biopsies. Conversely, previous studies reported a good agreement
between histological and cytological samples, which are widely used
in clinical routines (10,15,16).
Cytological sample analysis could therefore be recommended
routinely, although its use has not yet been validated in clinical
trials (11). Secondly, it should be
noted that for each IT molecule, a specific test for the evaluation
of PD-L1 expression is developed (1). These tests involve different antibodies
and platforms that make cross-comparisons difficult. However,
numerous studies have reported encouraging results (10,17–19). For
example, the Blueprint Phase 1 (20)
and the French Harmonization studies (21) reported a good agreement for 28–8,
22C3 and SP263 antibodies, suggesting they can be potentially
interchangeable, whereas the SP142 assay exhibited fewer stained
tumor cells (7,13). The Blueprint Phase 2 study which used
clinical routine samples confirmed the lower sensitivity of the
SP142 assay and reported a higher sensitivity with the 73–10
antibody (16). Thirdly,
interpretation of PD-L1 expression by IHC can be difficult. PD-L1
is expressed by immune, tumor and necrotic cells in the membrane or
cytoplasm, whereas the theranostic criterion only considers the
membrane staining of viable tumor cells (1). These difficulties can negatively impact
the intra- and inter-observer concordance regarding the PD-L1
assessment, which are the levels of agreement respectively when the
same pathologist assesses the slides twice and when two different
pathologists assess the same slides double-blinded (10,17,22–24). In
all cases, the intra-observer agreement increases with the PD-L1
expression (10).
The aforementioned data were obtained from
retrospective and prospective clinical studies conducted on
homogeneous series, according to numerous selection criteria. Apart
from the Blueprint Phase 2, only a few studies were conducted on
series extracted from clinical routine samples (25). Therefore the influence of the
complexity (in regards to the biology, technicality or
interpretation) of PD-L1 analysis on the daily management of
patients was evaluated. The present retrospective study analyzed
the impact of pathological and technological factors on the daily
evaluation of PD-L1 in patients with NSCLC. Nowadays molecular
tumor profiling represents an integral part of pathologist's daily
practice for patients with NSCLC and allows personalized medicine.
At Erasme Hospital (Brussels, Belgium), NSCLCs are daily
characterized using a next generation sequencing (NGS)-based gene
panel targeting 22 genes (AKT1, ALK, BRAF, CTNNB1, DDR2, EGFR,
ERBB2, ERBB4, FBXW7, FGFR1, FGFR2, FGFR3, KRAS, MAP2K1, MET,
NOTCH1, NRAS, PIK3CA, PTEN, SMAD4, STK11 and TP53). These data were
therefore included in the present study, and variations in PD-L1
expression according to molecular (NGS) data were analyzed.
Materials and methods
Clinical series
The present retrospective study included 454
patients with NSCLC for whom the PD-L1 status was requested by
clinicians, and was approved by the Ethics Committee of Erasme
Hospital (Brussels, Belgium; approval no. P2017/581). The Ethics
Committee waived the need for written informed consent from all
participants. Between November 2016 and January 2018, a total of
454 formalin-fixed paraffin-embedded (FFPE) samples obtained from
patients with NSCLC following biopsy and/or surgical resection were
received from 12 different institutions and used for the analysis
of PD-L1 expression by IHC (on 4-µm thick tissue sections). The
FFPE blocks were provided by the Pathology Department of Erasme
Hospital (Brussels, Belgium), the Centre Universitaire Inter
Regional d'Expertise en Anatomie Pathologique Hospitalière
(CurePath, Charleroi, Belgium), the Centre Hospitalier
Universitaire (CHU) Université Catholique de Louvain (UCL) Namur
(Godinne Site, Belgium), the Centre de Morphologie Pathologique
(CMP; Brussels, Belgium), the Institut Jules Bordet (Brussels,
Belgium), the CHU Charleroi (Belgium), the CHU Brugmann (Brussels,
Belgium), the Centre Hospitalier de Mouscron (Belgium), the
Cliniques du Sud Luxembourg (Edmond-Jacques Site, Belgium), the CHR
Verviers (Belgium), the Centre Hospitalier EpiCURA (Frameries Site,
Belgium), and the Centre Hospitalier de Wallonie picarde (CHwapi,
Union Site, Belgium). PD-L1 expression analysis by IHC was
performed at the Pathology Department of Erasme Hospital. To
respond to recurrent clinician requests, among the 454 patients we
included 87 patients for whom only cytological samples were
available (including endobronchial ultrasound-guided transbronchial
needle aspiration, pleural effusion and bronchial aspiration). In
the present study, the sampling type (cytology, biopsy or surgery)
and the sample location (primary tumor, LN or distant metastasis)
were distinguished. The pathological tumor (pT), node (pN),
metastasis (pM) and stage were revised according to the 8th UICC
edition (26). All samples included
in the present study met the acceptability criterion of ≥100 viable
(non-necrotic) tumor cells that was required for the companion test
used (Dako PharmDx 22C3; Dako; Agilent Technologies, Inc.).
Test requests from external centres were reviewed
and, when provided, clinical, histopathological, IHC and molecular
data were extracted. Moreover, for cases from Erasme Hospital,
clinical, histopathological, IHC and molecular data were extracted
from the medical records. Tables I
and II present the clinical,
histopathological, immunohistochemical and molecular data so
collected for the patients included in the present study. As some
information was missing, the total number of data available varies
between the different features. As mentioned above, the molecular
data were already available in the medical records and were the
result of targeted NGS using the Colon and Lung AmpliSeq panel
(Thermo Fisher Scientific, Inc.), as previously described (27).
 | Table I.Clinicopathological characteristics
of the patients with NSCLC included in the present study. |
Table I.
Clinicopathological characteristics
of the patients with NSCLC included in the present study.
|
Characteristics | Number |
|---|
| Median age (n=454),
(range) | 66 (35–91) |
| Sex (%)
(n=454) |
|
| Female,
n | 172 (37.9) |
| Male,
n | 282 (62.1) |
| Smoking history (%)
(n=145) |
|
| Yes,
n | 132 (91.0) |
| No,
n | 13 (9.0) |
| Histology (%)
(n=432) |
|
| SCC,
n | 112 (25.9) |
| ADC,
n | 290 (67.1) |
| NSCLC
NOS, n | 24 (5.6) |
|
Othera, n | 6 (1.4) |
| Sampling type (%)
(n=416) |
|
|
Cytology, n | 87 (20.9) |
| Biopsy,
n | 235 (56.5) |
|
Surgical specimen, n | 94 (22.6) |
| Sample location (%)
(n=414) |
|
| Primary
tumor | 260 (62.8) |
| LN | 88 (21.3) |
| Distant
metastasis | 66 (15.9) |
| pT (%) (n=74) |
|
| T1 | 43 (58.1) |
| T2 | 12 (16.2) |
| T3 | 14 (18.9) |
| T4 | 5 (6.8) |
| pN (%) (n=153) |
|
| N0 | 50 (32.7) |
|
N1–2 | 103 (67.3) |
| pM (%) (n=112) |
|
| M0 | 42 (37.5) |
| M1 | 70 (62.5) |
| Stage (%)
(n=112) |
|
| 1 | 19 (17.0) |
| 2 | 16 (14.3) |
| 3 | 7 (6.2) |
| 4 | 70 (62.5) |
| FFPE block age (%)
(n=453) |
|
| <3
years | 446 (98.5) |
| ≥3
years | 7 (1.5) |
| EGFR
mutation (%) (n=294) |
|
|
Yes | 26 (8.8) |
| No | 268 (91.2) |
| KRAS
mutation (%) (n=289) |
|
|
Yes | 108 (37.4) |
| No | 181 (62.6) |
| TP53
mutation (%) (n=287) |
|
|
Yes | 108 (37.6) |
| No | 179 (62.4) |
| Other mutations (%)
(n=289) |
|
|
Yes | 54 (18.7) |
| No | 235 (81.3) |
| Total number of
mutations (%) (n=287) |
|
| 0 | 61 (21.3) |
| 1 | 162 (56.4) |
| 2 | 53 (18.5) |
| 3 | 10 (3.5) |
| 4 | 1 (0.3) |
 | Table II.Localization of distant
metastasis. |
Table II.
Localization of distant
metastasis.
| Tissue/organ | Number (n=66) |
|---|
| Pleura | 13 |
| Brain | 10 |
| Liver | 9 |
| Pleural fluid | 5 |
| Bone | 5 |
| Adrenal glands | 5 |
| Cerebellum | 3 |
| Vertebra | 3 |
| Muscle | 2 |
| Diaphragm | 2 |
| Skin | 2 |
| Controlateral
lung | 1 |
| Pericardial
fluid | 1 |
| Mesentery | 1 |
| Pericardium | 1 |
| Pancreas | 1 |
| Subcutaneous | 1 |
| Paravertebra | 1 |
IHC
IHC staining for PD-L1 was performed using PD-L1 IHC
22C3 pharmDx kit (cat. no. SK006; Dako; Agilent Technologies, Inc.)
on the Autostainer Link 48 system (Dako; Agilent Technologies,
Inc.) according to the manufacturer's instructions. Briefly, FFPE
samples were cut into 4-µm thick sections. Deparaffinization,
Rehydration and Target Retrieval (3-in-1) Procedure (reagents
included in the PD-L1 IHC 22C3 pharmDx kit) was performed using PT
Link Pre-treatment Module (Dako; Agilent Technologies, Inc.)
according to the manufacturer's instructions. PD-L1 IHC was
performed on the Autostainer Link 48 system (Dako; Agilent
Technologies, Inc.) using the 22C3 antibody (monoclonal mouse
anti-PD-L1 antibody; clone 22C3; ready-to-use; provided in the IHC
22C3 pharmDx kit). The sections were counterstained for 5 min with
haematoxylin at room temperature. Quality controls were included in
each staining run. Controls used FFPE tonsil samples in addition to
PD-L1 IHC 22C3 pharmDx Slide of Control Cell Lines (provided in the
IHC 22C3 pharmDx kit). Moreover, Negative Control Reagent
(monoclonal mouse control IgG antibody; ready to use; provided in
the IHC 22C3 pharmDx kit) was also used for each sample. IHC slides
were analyzed using a bright field microscope (magnifications, ×2
to ×40; Olympus).
PD-L1 tumor proportion score (TPS)
evaluation and heterogeneity analysis
The PD-L1 TPS was evaluated as the percentage of
viable tumor cells presenting partial or complete PD-L1 expression
at the cell membrane, as previously described (1). TPS was classified into three large
categories, corresponding to <1, 1–49 or ≥50% of positively
stained cells and labelled as such to facilitate understanding of
the results. Depending of the type of statistical analysis, the
ordinal property of these 3 categories was taken into account or
not. In addition, TPS was also evaluated in 13 ordered and more
specific categories, corresponding to 0, 1, 5, 10, 20, 30, 40, 50,
60, 70, 80, 90 and 100% of positively stained cells and labelled as
such to facilitate understanding of the results. Subsequently,
these 13 categories are referred to as ‘the precise TPS
values’.
The heterogeneity of PD-L1 expression was evaluated
in patients with multiple analysable samples. The PD-L1 TPS of
these cases (n=80) was calculated by two pathologists [Termed
pathologist 1 (junior) and pathologist 2 (senior)] who were
double-blinded, allowing the assessment of intra- and
inter-observer agreements. The first assessment for pathologist 2
was conducted prospectively (during the clinical routine) and the
second retrospectively (i.e., all slides were reassessed together
in a single evaluation session), whereas pathologist 1 performed
each assessment retrospectively, with a wash-out period of two
weeks between the two sessions. Regarding the analysis of
inter-observer concordance, for each pathologist we used the
average of their two precise TPS evaluations performed per case.
Retrospective evaluation by a third pathologist (3, junior) was
added to complete the inter-observer concordance analysis. For the
analysis of the whole series, the PD-L1 values used correspond to
the value retrieved from the pathological report and were
considered as the first assessment by pathologist 2 in the
concordance analysis.
For some patients (n=28), multiple samples from
different locations were available and analysable (28 pairs,
including 16 patients with primary tumor and LN samples and 12
patients with primary tumor and distant metastasis samples). The
paired PD-L1 expression levels obtained were compared. Comparative
analysis were also performed in 14 other patients, for whom two
types of samples (surgical resection and either cytology or biopsy)
were available.
Statistical analysis
The statistical analyses were performed using
Statistica 7.1 software (StatSoft, Inc.). χ2 tests were
used to analyze the associations between the PD-L1 TPS assessed in
3 categories (<1, 1–49 or ≥50%) and all the categorical
clinicopathological variables described in Table I. In these analyses the ordinal
property of the 3 TPS categories was not considered. Before
applying the χ2 it was checked whether the expected
frequencies (computed under the null hypothesis of independence)
were non-zero and that the percentage of expected frequencies <5
was below 20%. In some case, categories were merged resulting in
2×2 contingency tables, on which we applied the Fisher's exact
test. The variations of the PD-L1 TPS assessed in 13 ordered
categories were also analyzed and considered as ranked data between
two or more independent groups determined by clinicopathological
variables, using Mann-Whitney tests or Kruskal-Wallis tests (with
Dunn's multiple comparisons post hoc test using rank sums)
respectively (see details in the results). The intra- and
inter-observer concordance analyses were based on either the
weighted κ coefficient (computed with an online calculator,
©Richard Lowry 2001–2019, http://vassarstats.net/kappa.html) to take into
account the ordinal property of the TPS assessed in the three large
categories, or Lin's concordance correlation coefficient (CCC) for
TPS assessed by the means of the 13 more precise percentages that
were considered as quantitative data (28).
Results
Factors impacting PD-L1 expression and
its TPS value
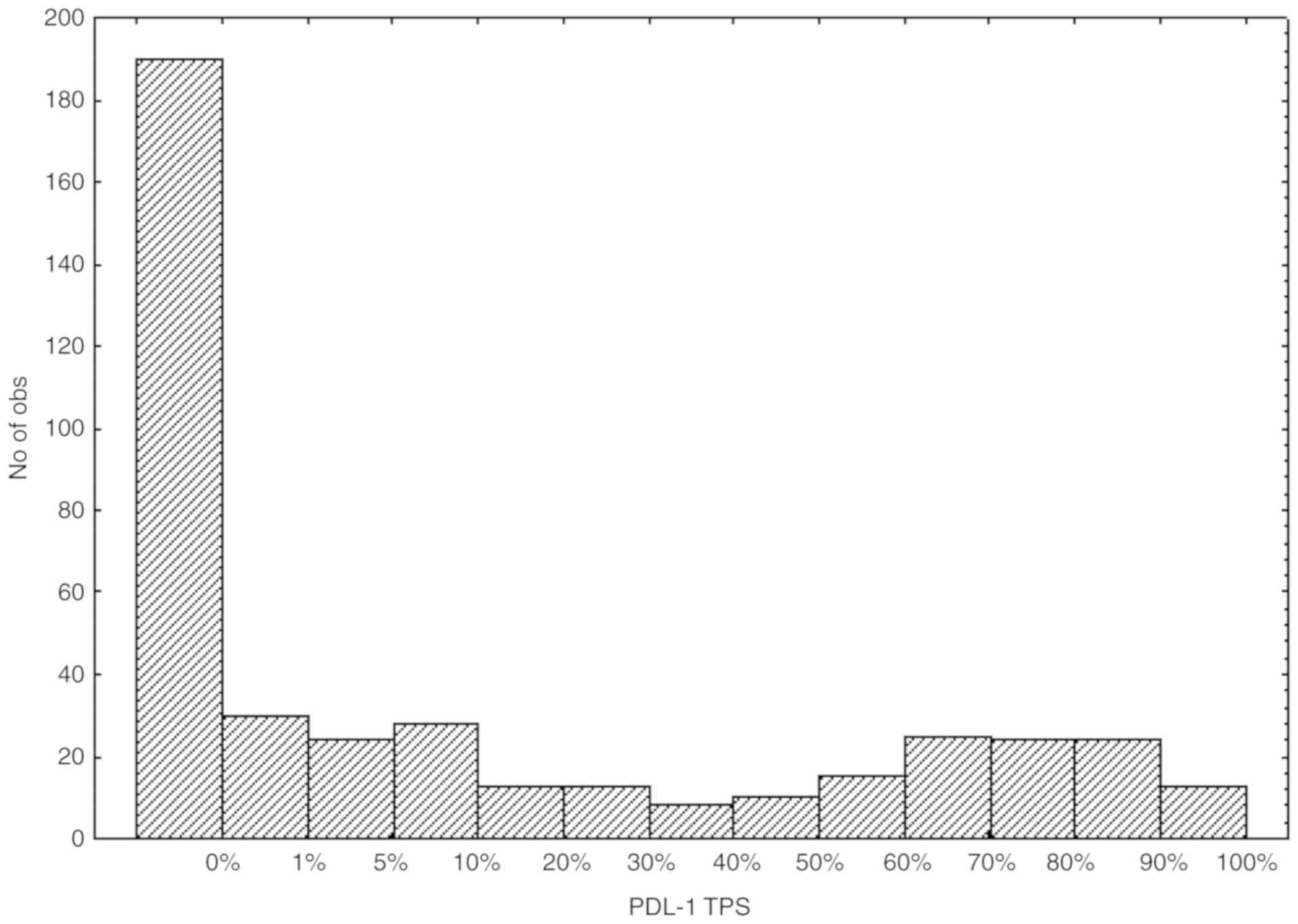
The distribution of the PD-L1 TPS from the NSCLC
samples according to the 13 categories is presented in Fig. 1. Following grouping of the TPS values
in three large categories, 190 cases (41.85%), 133 cases (29.30%)
and 131 cases (28.85%) had a TPS score corresponding to <1, 1–49
and ≥50% of positively stained cells, respectively.
Subsequently, whether and how the
clinicopathological characteristics listed in Tables I and II affect the PD-L1 TPS evaluation was
analyzed. The different methods used to score PD-L1 expression
(using either three or 13 categories) were considered in the
analysis. Only significant variations are summarized in Table III, including a refined analysis
carried out on the positive cases (precisely TPS values ≥1%)
only.
 | Table III.Significant variations observed in
PD-L1 TPS in relation to the clinicopathological characteristics
described in Table I. |
Table III.
Significant variations observed in
PD-L1 TPS in relation to the clinicopathological characteristics
described in Table I.
|
| PD-L1 TPS (3
categories) | Precise TPS
valuea | Precise TPS value
≥1%a |
|---|
|
|
|
|
|
|---|
| Variable | <1%, n (%) | 1–49%, n (%) | ≥50%, n (%) |
P-valueb |
P-valuec,d |
P-valuec,d |
|---|
| Sample location
(n=414) |
|
|
|
|
| (n=241) |
|
Primary | 107 (41) | 90 (35) | 63 (24) | 0.005 | NSd | 0.020d |
| LN | 42
(48) | 13 (15) | 33 (38) |
|
|
|
|
Metastasis | 24
(36) | 19 (29) | 23 (35) |
|
|
|
| Stagee (n=42) |
|
|
|
|
| (n=27) |
|
1–2 | 9
(29) | 13 (42) | 9
(29) | NA | 0.049c | NAc |
|
3–4 | 6
(55) | 4
(36) | 1 (9) |
|
|
|
| Histology
(n=426)f |
|
|
|
|
| (n=247) |
|
SCC | 37
(33) | 47 (42) | 28 (25) | 0.026 | NSe | 0.036e |
|
ADC | 129 (44) | 79 (27) | 82 (28) |
|
|
|
| NSCLC
NOS | 13
(54) | 4
(17) | 7
(29) |
|
|
|
| Total number of
mutations (n=287) |
|
|
|
|
| (n=158) |
| 0 | 37
(61) | 10 (16) | 14 (23) | 0.004 | 0.002d | NSd |
| 1 | 70
(43) | 50 (31) | 42 (26) |
|
|
|
|
>1 | 22
(34) | 14 (22) | 28 (44) |
|
|
|
| KRAS
mutations (n=256)g |
|
|
|
|
| (n=138) |
|
Yes | 35
(36) | 28 (29) | 35 (36) | 0.024 | 0.0006c | NSc |
| No | 83
(53) | 38 (24) | 37 (23) |
|
|
|
First, the sampling type (surgical resection, biopsy
or cytology) and the age of the FFPE block had no significant
impact on PD-L1 expression. The absence of variation between the
different sampling types was confirmed in a small series of 14
patients for whom two types of samples were available (surgical
resection and either cytology or biopsy). For these 14 sample
pairs, the TPS evaluation in three categories perfectly matched,
and the precise TPS evaluation agreed with a CCC of 0.965 and a 95%
confidence interval (CI) of (0.895–0.989).
Conversely, as detailed in the top of Table III, the sample location is
significantly associated with the TPS assessed in 3 categories
(P=0.005). TPS was >50% in 35% (23/66) of distant metastases and
in 38% (33/88) of LN, whereas it was only observed in 24% (63/260)
of primary tumors. In addition, LN exhibited the highest rate of
PD-L1-negative cases (48% vs. 41 and 36% for primary tumor and
distant metastases, respectively). However, in the positive cases
(TPS ≥1%), a refined analysis on the precise TPS values
demonstrated a significant TPS increase in LN compared with primary
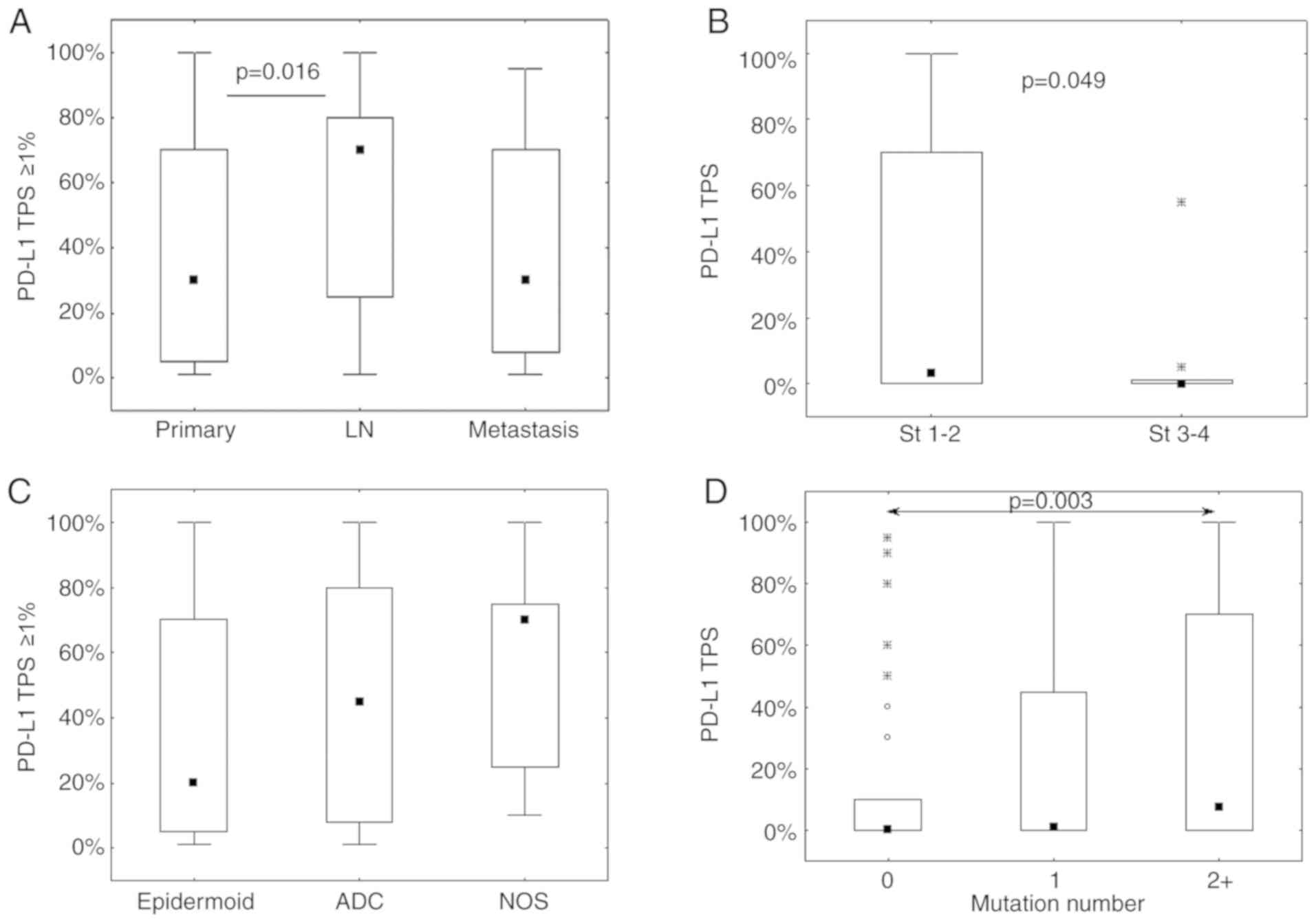
tumors (Fig. 2A). The analysis was
completed by comparing pairs of samples from the same patient (from
primary tumor and either LN or distant metastasis; Table IV and Fig. 3). The data was fairly heterogeneous
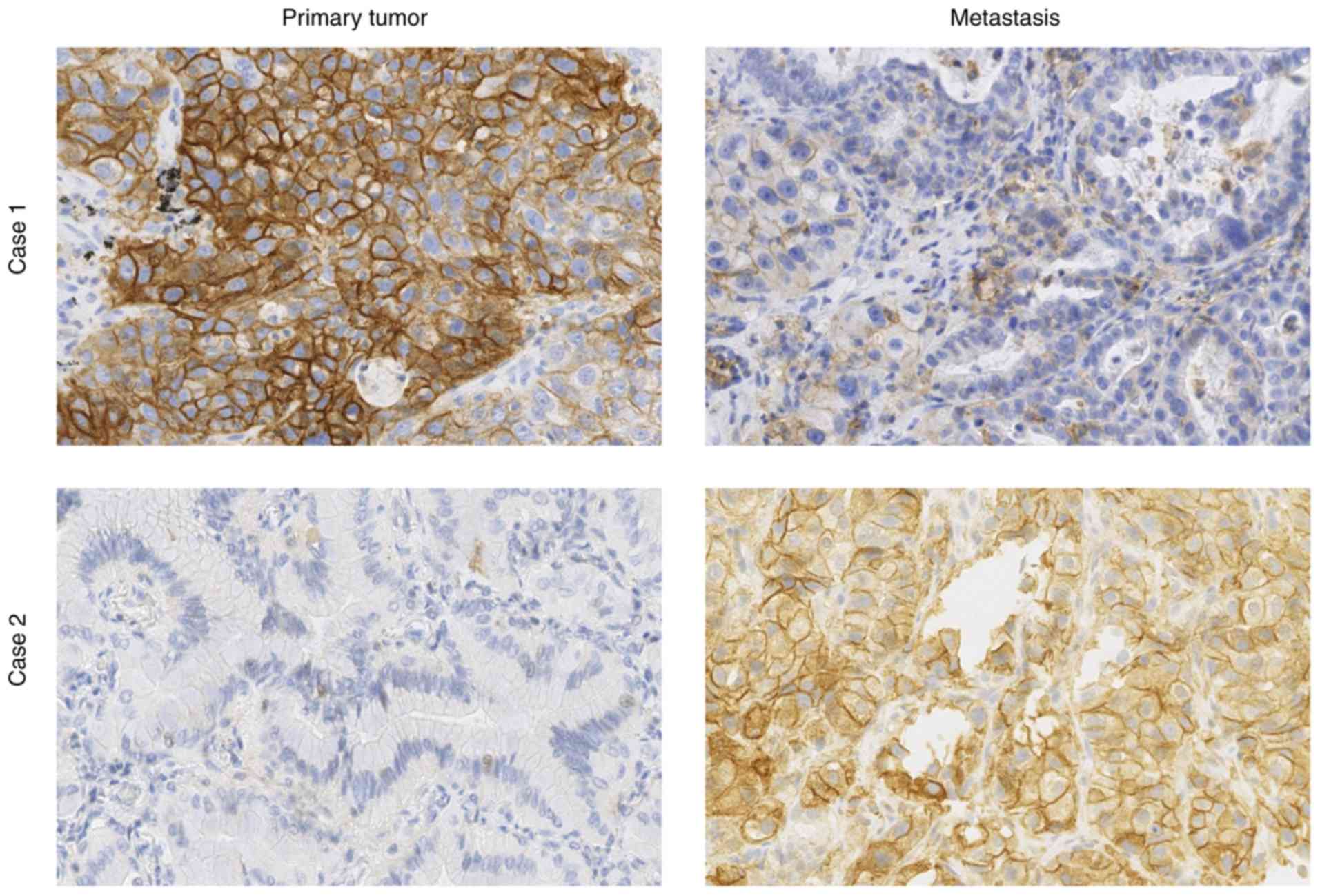
from one patient to another, as shown in Fig. 3. Two very different PD-L1 staining
profiles in primary tumor and metastasis from the same patient are
presented (Fig. 3). In case 1 the
expression was strongly higher in the primary tumor than in the
metastasis, whereas the converse was true in case 2. It was
observed that 43% of primary tumors not expressing PD-L1 had
PD-L1-positive LN or metastasis, whereas 29% of PD-L1-positive
primary tumors had PD-L1-negative LN or metastasis. The PD-L1 TPS
categories were the same for only 11 of 28 pairs (see diagonal in
Table IV), and precise TPS values
were concordant for eight pairs only, with no significant
concordance correlation (CCC, 0.189; CI, −0.166–0.501; data not
shown). The observations from these paired samples demonstrated
that most of the positive cases exhibited intermediate PD-L1 TPS
(i.e. 1–49%) in LN/metastases vs. high TPS (i.e. ≥50%) in primary
tumors, contrasting with the results obtained in the complete
series (Table III).
 | Table IV.Breakdown of matched data
characterizing PD-L1 TPS into three categories in primary tumor, LN
or distant metastasis samples from the same patient. |
Table IV.
Breakdown of matched data
characterizing PD-L1 TPS into three categories in primary tumor, LN
or distant metastasis samples from the same patient.
|
| LN/Metastasis TPS,
n |
|
|---|
|
|
|
|
|---|
| Primary tumor
TPS | <1% | 1–49% | ≥50% | Total, n |
|---|
| <1% | 8 | 3 | 3 | 14 |
| 1–49% | 3 | 1 | 0 | 4 |
| ≥50% | 1 | 7 | 2 | 10 |
| Total, n | 12 | 11 | 5 | 28 |
Considering the variations observed with the sample
location, the impact of stage and pT, pN, pM on PD-L1 expression
were evaluated for the primary tumors only. Negative PD-L1
expression (TPS <1%) was observed in 55% of stages 3–4 and 29%
of stages 1–2 (see Table III).
Significant variations were evidenced between stages when
considering precise TPS values (see Fig.
2B). No significant variations were observed with respect to
the pTNM variables (data not shown).
Histology is also significantly associated with the
TPS assessed in 3 categories (P=0.026, see Table III). PD-L1 expression was less
often negative in SCC (37/112; 33%) than in ADC (129/290; 44%) and
NOS histological subtypes (13/24; 54%). However, the positive PD-L1
TPS in SCC was more frequently between 1 and 49% compared with 50%
or more, whereas the opposite trend was observed for the positive
TPS values in ADC and NOS (Table
III). These data were confirmed by the analysis of the precise
positive PD-L1 scores (TPS ≥1%) that demonstrated a significant
variation (with the same trend) among the three histological
subgroups (P=0.036; Fig. 2C).
The results from targeted NGS demonstrated a
significant association between the number of mutations and PD-L1
expression (Table III). It was
observed that the absence of any mutations was associated with an
absence of PD-L1 expression, whereas PD-L1 TPS increased with the
mutation number (Table III and
Fig. 2D). Regarding the specific
gene mutations, no significant association was found between PD-L1
expression and epidermal growth factor receptor (EGFR) or
tumor protein p53, with or without considering histological subtype
stratification (data not shown). Conversely, we observed that the
patients with NSCLC and presenting with KRAS mutations
expressed PD-L1 more frequently (65% of TPS values ≥1%) compared
with patients with NSCLC without KRAS mutations (47% of TPS
values ≥1%), resulting in a significant association between the two
categorical variables (P=0.024; Table
III). A significant difference between the two KRAS
mutation-related groups was also observed in terms of the precise
evaluation of the PD-L1 TPS, which was higher in the mutated group
(P=0.0006, data not shown). Furthermore, the available data showed
that at least 80% of SCC and NSCLC NOS samples (i.e. 12 of 14 and
16 of 20 cases respectively) presented no KRAS mutations
compared with only 60% (i.e. 142 of 236) for ADC samples (P=0.041).
A specific analysis on patients with ADC only provided similar
results to all patients, and demonstrated 62% (i.e. 55 of 89 cases)
of positive PD-L1 expression in the presence of KRAS
mutation vs. 45% (i.e. 59 of 132) in the absence of KRAS
mutation (Fisher's exact test: P=0.014). The analysis of the three
most frequent KRAS mutations (G12C, G12V and
G12D) did not provide significant association with PD-L1
expression.
Intra- and inter-observer concordance
analysis
The weighted κ index characterizing the
intra-observer agreement of the PD-L1 TPS assessment in three
categories was 0.779 (CI, 0.665–0.893) for pathologist 1 and 0.804
(CI, 0.703–0.905) for pathologist 2, with 17.5 and 16% of cases
with discordances, respectively (Table
V). For both observers, these discordances concerned the three
sampling types (surgical resection, biopsy and cytology). Most
discordances were found in the intermediate 1–49% category, where
~1/3 of the cases classified as intermediate (1–49%) at the first
assessment were reclassified as negative (<1%) at the second
assessment (Table V). The results
also demonstrated that only four intra-observer discordances were
common to both pathologists and concerned different types of
samples (one surgical resection, one biopsy and two cytology
samples). The CCC characterizing the intra-observer agreement of
the PD-L1 TPS assessment in 13 precise values were 0.978 (CI,
0.959–0.988) for pathologist 1 and 0.946 (CI, 0.888–0.975) for
pathologist 2.
 | Table V.Intra-observer agreement of the PD-L1
molecule TPS assessments into three categories by pathologist 1
(junior) and pathologist 2 (senior). |
Table V.
Intra-observer agreement of the PD-L1
molecule TPS assessments into three categories by pathologist 1
(junior) and pathologist 2 (senior).
| A, Pathologist
1-WKI: 0.779 (CI, 0.665–0.893) |
|---|
|
|---|
|
| 2nd TPS assessment,
n |
|
|---|
|
|
|
|
|---|
| 1st TPS assessment,
n | <1% | 1–49% | ≥50% | Total, n |
|---|
| <1% | 38 | 4 | 0 | 42 |
| 1–49% | 9 | 14 | 1 | 24 |
| ≥50% | 0 | 0 | 14 | 14 |
| Total, n | 47 | 18 | 15 | 80 |
|
| B, Pathologist
2-WKI: 0.804 (CI, 0.703–0.905) |
|
|
| 2nd TPS
assessment, n |
|
|
|
|
|
| 1st TPS
assessment, n | <1% | 1–49% | ≥50% | Total |
|
| <1% | 40 | 2 | 0 | 42 |
| 1–49% | 7 | 12 | 2 | 21 |
| ≥50% | 0 | 2 | 15 | 17 |
| Total, n | 47 | 16 | 17 | 80 |
In order to evaluate the inter-observer agreement,
the average of the precise TPS values obtained after the two
assessments of each pathologist were computed and reclassified into
the three large categories (<1, 1–49 and ≥50%) for each
pathologist. We observed 15% of discordant cases between
pathologists 1 and 2 for which the average TPS values were in the
1–49% category for one pathologist and <1% for the other (data
not shown). The weighted κ index characterizing these data was
0.814 (CI, 0.709–0.918). Fig. 4
shows the scatterplot of the precise TPS averages obtained for the
two pathologists, including numerous overlaps in the case of small
values (see figure legend for details). The CCC computed on these
TPS averages was 0.967 (CI, 0.950–0.978). These findings revealed
that the 1–49% category was the least reproducible from one
assessment to another, without evidence of an impact from sampling
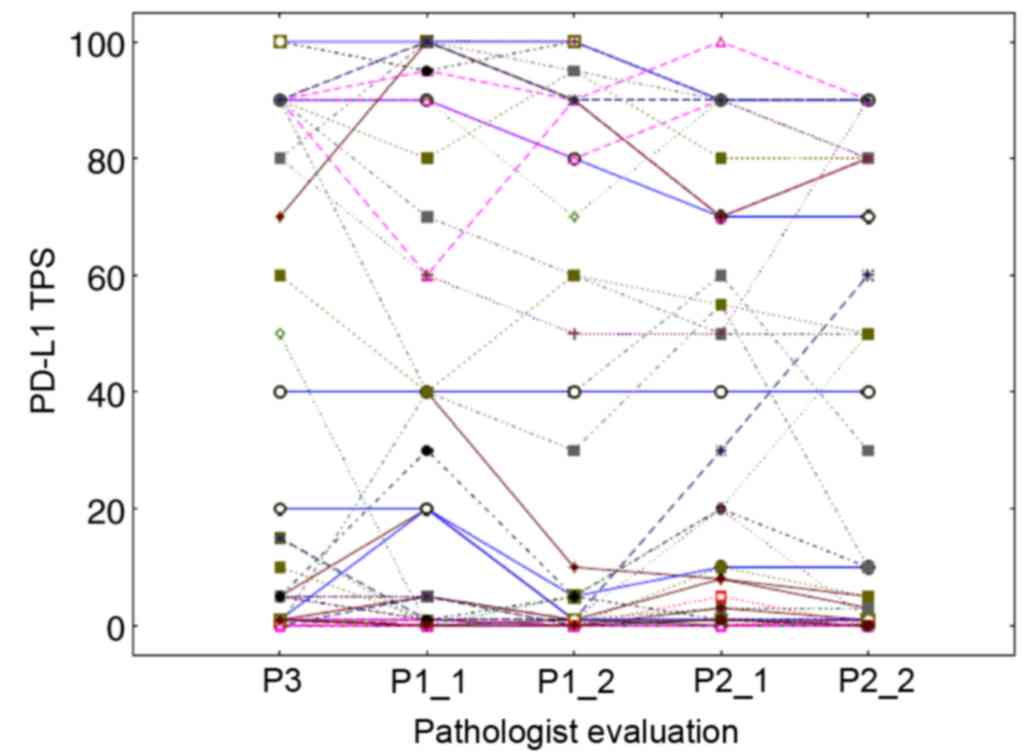
type (Fig. 4). Evaluation from a
third pathologist allowed the completion of these inter-observer
data (Fig. 5). For the three TPS
categories, the weighted κ index was 0.722 (CI, 0.599–0.844)
between pathologist 3 and 2 and 0.693 (CI, 0.566–0.820) between
pathologist 3 and 1. The CCC computed on the precise TPS (Fig. 5) between pathologist 3 and 1 or 2 was
0.942 (CI, 0.912–0.963) and 0.943 (CI, 0.914–0.962), respectively.
For pathologist 1 and 2, the latter results were obtained based on
the TPS averages calculated from their two assessments (possibly
smoothing out some variations). Fig.
5 details all the intra- and inter-observer variations by
showing the precise TPS values (linked per case) provided by the
three pathologists with two assessments for two of them. It should
be noted that 24 perfect agreements of the 5 assessments on the TPS
value of 0 were observed, resulting in overlapping horizontal lines
at level 0.
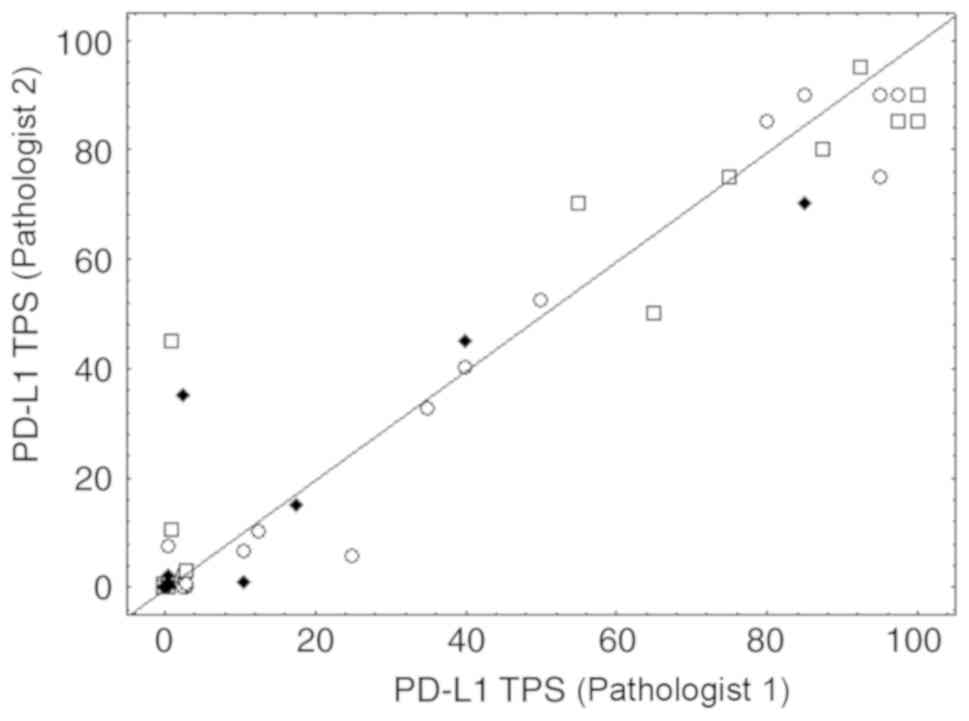 | Figure 4.Inter-observer concordance.
Inter-observer concordance for the precise PD-L1 TPS assessments
between pathologist 1 (junior) and pathologist 2 (senior). Dots,
squares and diamonds represent surgery, biopsy and cytological
samples, respectively. Some pairs with small values resulted in
many overlaps. The value pair (0,0) was observed 33 times, the pair
(0,0.5) 4 times, (0.5,0) 5 times, the pair (0.5,0.5) for 2 times
and the pair (3,0.5) for 2 times. PD-L1, PD-L1 molecule; TPS, tumor
proportion score. |
Discussion
Since PD-L1 expression is heterogeneous, dynamic and
difficult to interpret, the present study aimed to identify factors
that may affect the daily routine evaluation of PD-L1 expression
from patients with NSCLC.
Regarding the pathological factors, the current
study demonstrated that the sample location was significantly
associated with PD-L1 expression. In particular, analysis of
matched specimens from the same patient demonstrated a poor
agreement between primary tumors and LN or distant metastasis. The
results demonstrated that 43% patients with primary tumors not
expressing PD-L1 had PD-L1-positive LN or metastasis, whereas 29%
patients with PD-L1-positive primary tumors had PD-L1-negative LN
or distant metastasis. This lack of agreement between the
negative/positive PD-L1 status would have an impact on the
possibility for patients to benefit from pembrolizumab. Only a few
patients from the present study were treated with immunotherapy,
which was a potential limitation of the present study. The results
from the current study were similar to findings from Cho et
al (25), who reported a 67%
agreement between negative and positive PD-L1 expression on 91
matched samples of primary NSCLC and metastasis. In addition it was
reported that 28% of the samples did not express PD-L1 after the
first assessment but expressed PD-L1 after the second one (25). Conversely, 37% of PD-L1-positive
samples became negative after the second assessment (25). In contrast, the ATLANTIC study
reported an 89% agreement on 88 samples matched between primary
tumors and metastasis (commercially available tissue samples);
however, the result concerned PD-L1 expression rates of 25% and
more (29). The data from Cho et
al (25) and the present study
suggested that these investigations should be performed on a larger
sample size of matched samples from patients with clinical
information regarding IT response in order to obtain more reliable
data.
The present study also demonstrated that PD-L1 was
more frequently expressed in SCC tissues compared with that in
other histological types, and that its expression rate was mostly
between 1 and 49%. Conversely, when ADCs and NSCLC NOS cases
expressed PD-L1, the TPS score was higher. The SCC type has been
proposed as a response factor to IT, as well as the smoking habit
and the mutation number (3,30). These three variables may therefore be
interconnected. SCC is more frequent in smokers, since tobacco, by
increasing the mutation rate, produces neo-antigens that stimulate
the immune response (3,5,31,32). SCC
tumors may therefore be more sensitive to IT.
Regarding the specific mutations, a previous study
on patients with EGFR-mutant NSCLC reported a significant
association between PD-L1 positivity and EGFR mutations
other than the L858R mutation or exon 19 deletion (33). The present study did not detect this
association, as only 26 patients presented with EGFR
mutations, including seven mutations other than the L858R mutation
or exon 19 deletion. However, the present study demonstrated that
KRAS mutations were associated with PD-L1 expression score.
Patients with NSCLC and presenting with KRAS mutations
express PD-L1 more frequently (64% (63/98) of KRAS mutated samples
and 47% of KRAS non-mutated samples showed a TPS values ≥1%), as
similarly observed by Li et al (34).
As reported by previous studies (10,15,35), the
present study reported no significant difference in the PD-L1
expression rate between the different sampling types (cytology,
biopsy or surgical specimen) in all patients combined. This result
was confirmed by very good agreements between sample pairs from the
same patient, as similarly described by Cho et al (25). Comparison of the present results with
results from the Blueprint Phase 2B study, which compares the PD-L1
status between various samples types from the same lung tumor
(16), is not yet possible.
The difficulty in correctly evaluating PD-L1
expression by IHC induces post-analytical heterogeneity, leading to
intra- and inter-observer discrepancies. The concordance data from
the present study were similar to those from the DREAM study
(11,22). Regarding PD-L1 TPS assessment in
three categories, the present study reported intra-observer
discrepancies between 16 and 17.5%, suggesting a hypothesis that,
in daily practice, 1–2 out of 10 patients were potentially
misdiagnosed. Inter-observer discrepancies were not correlated with
one particular sampling type. However, the most discordant cases
were for patients with a pleural effusion sample, which is the most
difficult type of sample to assess.
One current marker of interest is the Tumor Mutation
Burden (TMB), which is defined as the total number of nonsynonymous
mutations per coding area of a tumor genome. Although TMB is
associated with IT effectiveness, it is not associated with PD-L1
expression (2,10,30–32). At
present in our daily clinical practice, a NGS panel of 22 genes is
used to search for ‘actionable’ mutations. The present study shows
that this panel, which is smaller than the panels used for TMB,
established a significant association between mutation number and
PD-L1 expression, with a major difference between non-mutated NSCLC
cases and NSCLC cases with >2 mutations.
In conclusion, to the best of our knowledge, the
present study was one of the first investigating the impact of
pathological and technological factors (such as tumor location and
sampling type, respectively) in the daily clinical assessment of
PD-L1 expression. The current study confirmed that cytological
samples, which are often used routinely, can be used for the
evaluation of PD-L1. The results from the present study also
demonstrated significant associations between PD-L1 expression and
sample location, histology, total number of mutations and KRAS
mutations. These data will require further confirmation using a
larger sample size. The results from the present study suggested
that PD-L1 may be considered as a useful, although dynamic and
heterogeneous, marker that may be associated with other
clinicopathological characteristics of patients with NSCLC. Further
investigation would improve its theranostic value.
Acknowledgements
The authors would like to thank Fonds National de la
Recherche Scientifique (Brussels, Belgium) for supporting the work
of research associate, Professor Christine Decaestecker,
financially. The authors would like to thank the Institut Jules
Bordet (Brussels, Belgium), CHU Brugmann (Brussels, Belgium), CHU
Charleroi (Charleroi, Belgium), CHR Verviers (Verviers, Belgium),
Centre Hospitalier de Wallonie Picarde (Union Site, Tournai,
Belgium), Cliniques du Sud Luxembourg (Virton, Belgium), CMP
(Brussels, Belgium), Centre Hospitalier EpiCURA (Frameries Site,
Frameries, Belgium), Centre Hospitalier de Mouscron (Mouscron,
Belgium) for providing the samples.
Funding
This study was supported by the Fonds Yvonne Boël
(SecundOS project) (Brussels, Belgium). The Center for Microscopy
and Molecular Imaging (CMMI) is supported by the European Regional
Development Fund and the Walloon Region (Wallonia-biomed; grant no.
411132-957270; project ‘CMMI-ULB’).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the first author on reasonable
request.
Authors' contributions
ND, IS and CD designed and supervised the study. CV,
LR and ND performed the PD-L1 assessments. SD and AV performed the
immunohistochemistry. CSP and MR performed the pathological
diagnosis. CV, ND and CD analyzed the data and wrote the
manuscript. ZM, SO and CC contributed to the acquisition,
interpretation and analysis of clinicopathological data and
reviewed the manuscript. All authors approved the final version of
the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Erasme Hospital (approval no. P2017/581).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ADC
|
adenocarcinoma
|
|
IHC
|
immunohistochemistry
|
|
IT
|
immunotherapy
|
|
KRAS
|
KRAS proto-oncogene
|
|
LN
|
lymph node metastasis
|
|
NGS
|
next generation sequencing
|
|
NSCLC
|
non-small cell lung cancer
|
|
PD-L1
|
programmed death-ligand 1
|
|
PDCD-1
|
programmed cell death 1
|
|
SCC
|
squamous cell carcinoma
|
|
TPS
|
tumor proportion score
|
References
|
1
|
IASCL: Atlas of PD-L1 immunohistochemistry
testing in lung cancer. Editorial Rx Press; USA: 2017
|
|
2
|
Hersom M and Jørgensen JT: Companion and
complementary diagnostics-focus on PD-L1 expression assays for
PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer.
Ther Drug Monit. 40:9–16. 2018.PubMed/NCBI
|
|
3
|
Melosky B, Chu Q, Juergens R, Leighl N,
McLeod D and Hirsh V: Pointed progress in Second-line advanced
Non-small-cell lung cancer: The rapidly evolving field of
checkpoint inhibition. J Clin Oncol. 34:1676–1688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gibney GT, Weiner LM and Atkins MB:
Predictive biomarkers for checkpoint inhibitor-based immunotherapy.
Lancet Oncol. 17:e542–e551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khunger M, Hernandez AV, Pasupuleti V,
Rakshit S, Pennell NA, Stevenson J, Mukhopadhyay S, Schalper K and
Velcheti V: Programmed cell death 1 (PD-1) ligand (PD-L1)
expression in solid tumors as a predictive biomarker of benefit
from PD-1/PD-L1 axis inhibitors: A systematic review and
meta-analysis. JCO Precis Oncol. 1:1–15. 2017. View Article : Google Scholar
|
|
6
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado H, et al:
Nivolumab versus docetaxel in advanced nonsquamous Non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the Treatment of Non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt W, Poddubskaya E, Antonia S, Pluzanski A, Vokes E,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell Non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Büttner R, Gosney JR, Skov BG, Adam J,
Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F,
Penault-Llorca F, et al: Programmed Death-ligand 1
Immunohistochemistry testing: A review of analytical assays and
clinical implementation in Non-small-cell lung cancer. J Clin
Oncol. 35:3867–3876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanteujoul S, Adam J, Girard N,
Duruisseaux M, Mansuet-Lupo A, Cazes A, Rouquette I, Gibault L,
Garcia S, Antoine M, et al: Tests immunohistochimiques PD-L1 dans
les cancers du poumon non à petites cellules: Recommandations par
le groupe PATTERN de pathologistes thoraciques PD-L1 testing in
non-small cell lung carcinoma: Guidelines from the PATTERN group of
thoracic pathologists. Ann Pathol. 38:110–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McLaughlin J, Han G, Schalper KA,
Carvajal-Hausdorf D, Pelakanou V, Rehman J, Velcheti V, Herbst R,
LoRusso P and Rimm DL: Quantitative assessment of the heterogeneity
of PD-L1 Expression in Non-small cell lung cancer. JAMA Oncol.
2:46–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendry S, Byrne DJ, Wright GM, Young RJ,
Sturrock S, Cooper WA and Fox SB: Comparison of four PD-L1
immunohistochemical assays in lung cancer. J Thorac Oncol.
13:367–376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ilie M, Long-Mira E, Bence C, Butori C,
Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K,
et al: Comparative study of the PD-L1 status between surgically
resected specimens and matched biopsies of NSCLC patients reveal
major discordances: A potential issue for anti-PD-L1 therapeutic
strategies. Ann Oncol. 27:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skov BG and Skov T: Paired comparison of
PD-L1 expression on cytologic and histologic specimens from
malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and
PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol.
25:453–459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsao MS, Kerr KM, Kockx M, Beasley MB,
Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, et
al: PD-L1 immunohistochemistry comparability study in real-life
clinical samples: Results of blueprint phase 2 project. J Thorac
Oncol. 13:1302–1311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thunnissen E, Allen TC, Adam J, Aisner DL,
Beasley MB, Borczuk AC, Cagle PT, Capelorri VL, Cooper W, Hariri
LP, et al: Immunohistochemistry of pulmonary biomarkers: A
perspective from members of the pulmonary pathology society. Arch
Pathol Lab Med. 142:408–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rimm DL, Han G, Taube JM, Yi ES, Bridge
JA, Flieder DB, Homer R, West WW, Wu H, Roden AC, et al: A
prospective, Multi-institutional, Pathologist-based assessment of 4
immunohistochemistry assays for PD-L1 expression in Non-small cell
lung cancer. JAMA Oncol. 3:1051–1058. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scheel AH, Baenfer G, Baretton G, Dietel
M, Diezko R, Henkel T, Heukamp LC, Jasani B, Jöhrens K, Kirchner T,
et al: Interlaboratory concordance of PD-L1 immunohistochemistry
for non-small-cell lung cancer. Histopathology. 72:449–459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirsch FR, McElhinny A, Stanford D,
Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P,
Hanks D, Vannapusa B, et al: PD-L1 immunohistochemistry assays for
lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay
comparison project. J Thorac Oncol. 12:208–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adam J, Rouquette I, Damotte D, Badoual C,
Danel C, Damiola F, Penault-Llorca F and Lantuejoul S: PL04a.04:
Multicentric French harmonization study for PD-L1 IHC testing in
NSCLC. J Thorac Oncol. 12 (Suppl):S11–S12. 2017. View Article : Google Scholar
|
|
22
|
Cooper WA, Russell PA, Huot-Marchand P,
Cherian M, Duhig EE, Godbolt D, Jessup PJ, Khoo C, Leslie C, Mahar
A, et al: Intra-and Inter-observer reproducibility study of PD-L1
biomarker in Non-small cell lung cancer (NSCLC)-the DREAM STUDY. J
Thorac Oncol. 12 (Suppl):S814–S815. 2017. View Article : Google Scholar
|
|
23
|
Ratcliffe MJ, Sharpe A, Midha A, Barker C,
Scott M, Scorer P, Al-Masri H, Rebelatto MC and Walker J: Agreement
between programmed cell death ligand-1 diagnostic assays across
multiple protein expression cutoffs in non-small cell lung cancer.
Clin Cancer Res. 23:3585–3591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roach C, Zhang N, Corigliano E, Jansson M,
Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D and
Kulangara K: Development of a companion diagnostic PD-L1
immunohistochemistry assay for pembrolizumab therapy in
Non-small-cell lung cancer. Appl Immunohistochem Mol Morphol.
24:392–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho JH, Sorensen SF, Choi YL, Feng Y, Kim
TE, Choi H, Georgsen JB, Dolled-Filhart M, Emancipator K, Meldgaard
P, et al: Programmed death ligand 1 expression in paired non-small
cell lung cancer tumor samples. Clin Lung Cancer. 18:e473–e479.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Union for International Cancer Control
UICC, . TNM Classification of Malignant Tumours Eight Edition. John
Wiley & Sons; UK: 2017
|
|
27
|
D'Haene N, Le Mercier M, De Nève N,
Blanchard O, Delaunoy M, El Housni H, Dessars B, Heimann P,
Remmelink M, Demetter P, et al: Clinical validation of targeted
next generation sequencing for colon and lung cancers. PLoS One.
10:e01382452015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin LI: A concordance correlation
coefficient to evaluate reproducibility. Biometrics. 45:255–268.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Midha A, Sharpe A, Scott M, Walker J, Shi
K, Ballas M, Garassino MC and Rizvi NA: PD-L1 expression in
advanced NSCLC: Primary lesions versus metastatic sites and impact
of sample age. J Clin Oncol. 34 (15 Suppl):S30252016. View Article : Google Scholar
|
|
30
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to Anti-programmed cell
death (PD)-1 and Anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line Nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho J, Zhou W, Choi Y, Sun JM, Choi H, Kim
TE, Dolled-Filhart M, Emancipator K, Rutkowski MA and Kim J:
Retrospective molecular epidemiology study of PD-L1 expression in
patients with EGFR-mutant non-small cell lung cancer. Cancer Res
Treat. 50:95–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li D, Zhu X, Wang H and Li N: Association
between PD-L1 expression and driven gene status in NSCLC: A
meta-analysis. Eur J Surg Oncol. 43:1372–1379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heymann JJ, Bulman WA, Swinarski D, Pagan
CA, Crapanzano JP, Haghighi M, Fazlollahi L, Stoopler MB, Sonett
JR, Sacher AG, et al: PD-L1 expression in non-small cell lung
carcinoma: Comparison among cytology, small biopsy, and surgical
resection specimens. Cancer Cytopathol. 125:896–907. 2017.
View Article : Google Scholar : PubMed/NCBI
|