Introduction
Endometrial carcinoma (EC) is one of the most common
gynecological malignancies in developed countries, accounting for
20–30% of female malignancies, with a prevalence that is increasing
annually (1,2). Early diagnosis and treatment has
allowed a possible 5-year survival rate of 90% (3,4).
Mucinous endometrial carcinoma (MEC) is an independent and
infrequent pathological pattern of EC and accounts for 1–9% of all
uterine carcinomas (5). Previous
studies describing its pathomorphism and diagnostic criteria have
suggested that MEC and endometroid adenocarcinoma (EA) differ with
respect to patient age and lymphatic metastasis (6–9). MEC is
more frequent in older women, and is more likely to involve
lymphatic metastasis (6–9). Compared with EA, MEC samples exhibit
decreased paired box 2 expression levels, low TP53 mutation rates
and increased CD10, estrogen receptor (ER), progesterone receptor
(PR), K-ras, p16, c-MET, epidermal growth factor receptor (EGFR),
PTEN and PD-L1 expression levels and sporadic promoter
hypermethylation of the mutL homolog 1 gene (10–15).
Like many malignancies, endometrial cancer is a
complex disease driven by genetic, epigenetic and environmental
factors (16–18). DNA methylation at the 5-C of a
cytosine ring of a CpG island is one of the most important
epigenetic alterations in cancer initiation (19). Studies have demonstrated that
aberrant DNA methylation is associated with malignant formation
(20–23). Tumor suppressor genes (TSG) may be
hypermethylated, which leads to decreased expression levels and
alteration of cell growth, differentiation, proliferation and
apoptosis, resulting in the development of tumors (20–23).
Therefore, assaying DNA methylation status may be a method for
early diagnosis (24,25).
Hypermethylation of promoters of a number of TSGs,
such as EGF-containing fibulin extracellular matrix protein 1,
glutathione S-transferase P1, suppressor of cytokine signaling 3,
3OST2, basic helix-loop-helix family member E22/cysteine
deoxygenase 1/CUGBP Elva-like family member 4, SHP1 and
transmembrane protein with EGF-like and two follistatin-like
domains 2, have been studied in certain EC tissues and cell lines
(such as Ishikawa and KLE) and the methylation frequency is high
(26–31). The present study investigated five
TSGs (HOXD10, SHH, ZNF545, PCDH17 and MEIS1) whose promoters are
reported to be hypermethylated in other malignancies but have not
been evaluated for methylation in EC (32–40). For
example, HOXD10 is hypermethylated and lowly expressed in colon
adenocarcinoma cells, which downregulates the RHOC/AKT/MAPK pathway
to enhance apoptosis and restrict the proliferation, migration and
invasion of colon adenocarcinoma (32,33).
Methylation of the Shh promoter and reduced expression of SHH is
frequent in basal cell carcinoma (34,35).
ZNF545 functions as a tumor suppressor in colorectal cancer and is
frequently inactivated by promoter methylation (36,37).
Hypermethylation of PCDH17 is correlated to poor prognosis in acute
lymphoblastic leukemia (38,39). MEIS1 genes are frequently
hypermethylated in different types of leukemia (40). The present study measured the
methylation statuses of these genes, and then selected the most
hypermethylated genes and measured protein expression levels in
tumor and control samples. The present study aimed to analyze the
methylation status of the TSG in EC and to elucidate the
association between methylation, expression levels and
clinicopathological characteristics. In order to further validate
the association between the methylation status of a gene and its
expression level, the present study detected the expression level
of HOXD10 before and after DNA methylation transferase inhibitor
treatment.
Materials and methods
Patients and samples
A total of 132 well-conserved paraffin-embedded
tissue blocks were obtained from the Department of Pathology,
Shandong University Qilu Hospital (Shandong, China), which were
initially taken between 2006 and 2015, including endometrial
adenocarcinoma (n=50), mucinous endometrial carcinoma (n=12),
simple hyperplasia (n=22) and complex hyperplasia (n=48). Patient
data, such as age, tumor differentiation, depth of myometrial
invasion and lymph node metastasis, were collected. Case diagnoses
were made according to World Health Organization Classification of
Tumors of Female Reproductive Organs (4th edition) (41). The present study was approved by the
Ethical Research Committee at Shandong University (approval no.
mecsdums 2012032).
Cell lines and culture
The human endometrial carcinoma cell line Ishikawa
was obtained from the European Collection of Cell Cultures
(Sigma-Aldrich; Merck KGaA) and maintained at 37°C in Dulbecco's
modified Eagle's medium/Ham's F-12 medium (DMEM/F12; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). Cell lines were
treated at 37°C for 72 h with 5-Aza-2′-deoxycytidine (5-Aza-CdR;
Sigma-Aldrich; Merck KGaA) at a concentration of 1, 2, 5 µM as a
demethylation treatment. Media and 5-Aza-CdR were replaced every 24
h.
Methylation-specific PCR (MSP)
DNA samples were extracted from paraffin-embedded
tissue blocks using a genomic DNA purification kit (Qiagen GmbH),
and treated to convert unmethylated cytosine to uracil using a
cpGenome DNA modification kit (InterGen Co.). Primer sequences,
conditions and product length for MSP are presented in Table SI. MSP reactions were performed in a
10 µl system, using 1 µl (20 ng) template DNA for each reaction.
H2O was used as a negative control.
Immunohistochemistry
A two-step method was used according to the
manufacturer's instructions regarding primary antibody and
secondary antibody described below. Sections (5-µm thick) were
pre-treated using sodium citrate buffer (pH 6.0; 0.01 mol/l;
Beijing Solarbio Science & Technology Co., Ltd.) at 98°C for 5
min to retrieve cell antigens and blocked with goat serum (1:10;
cat. no. ZLI-9021; OriGene Technologies, Inc.) at room temperature
for 20 min. Sections were incubated overnight at 4°C with primary
antibody (anti-HOXD10; 1:150; cat. no. ab172865; Abcam). Colonic
carcinoma tissue sections (5-µm thick) were used as the positive
control. The primary antibody was replaced with phosphate-buffered
saline and was used as a negative control. The sections were
incubated with horseradish peroxidase-labeled secondary antibody
(1:100; Universal PV9000 kit; OriGene Technologies) for 30 min at
37°C and visualized using DAB (1:20; Beijing Solarbio Science &
Technology Co., Ltd.). The sections were stained with hematoxylin
for 2 min at room temperature. Optical microscope (magnification,
×100 and ×400; Carl Zeiss AG) was used for visualization.
A semi-quantitative scoring system was used to
obtain a staining score for HOXD10 expression levels. A total of
five high power fields from each section were randomly selected,
and scores were assigned according to intensity and percentage of
stained cells. Scores were averaged to yield a final score.
Intensity scores were classified according to staining intensity:
Negative staining (staining intensity score, 0); positive yellow
staining (staining intensity score, 1); positive brown staining
(staining intensity score ≤2); and positive dark brown staining
(staining intensity score, 3). Percentage score was classified
according to percentage of stained cells: No staining (score =0);
positive staining 0–10% (score, 1); positive staining 11–50%
(score, 2); positive staining 50–80% (score, 3); 80–100% (score,
4). A two-score average was used as the score of the fields. Final
scores of 0–3 were considered negative, while scores >4 were
considered positive.
Western blot analysis
Ishikawa cells were harvested and subjected to
protein extraction with RIPA lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd.). The concentration of protein was
measured using the BCA method (BCA Protein Assay kit; cat. no.
PC0020; Beijing Solarbio Science & Technology Co., Ltd.). Equal
amounts of protein (50 µg) were separated by 10% SDS-PAGE gel and
transferred to PVDF membranes (EMD Millipore). After blocking with
5% skim milk (Beijing Solarbio Science & Technology Co., Ltd.)
at room temperature for 1 h, the membranes were incubated overnight
at 4°C with primary antibodies as follows: Anti-HOXD10 (1:100; cat.
no. ab172865; Abcam) and anti-β-actin (1:1,000; cat. no. 4970T;
Cell Signaling Technology, Inc.). The immune complexes were
incubated with horseradish peroxidase-conjugated secondary
antibody. The blots were developed using chemiluminescence (EMD
Millipore) with the Las-4000 Imaging system (Fujifilm).
Statistical analysis
SPSS software (version 22.0; IBM, Corp.) was used
for statistical analysis. A Pearson χ2 test was used to
compare gene methylation status with clinical data among patients
with cancer. P<0.05 was considered to indicate a statistically
significant difference.
Results
HOXD10 promoter hypermethylation in
EC
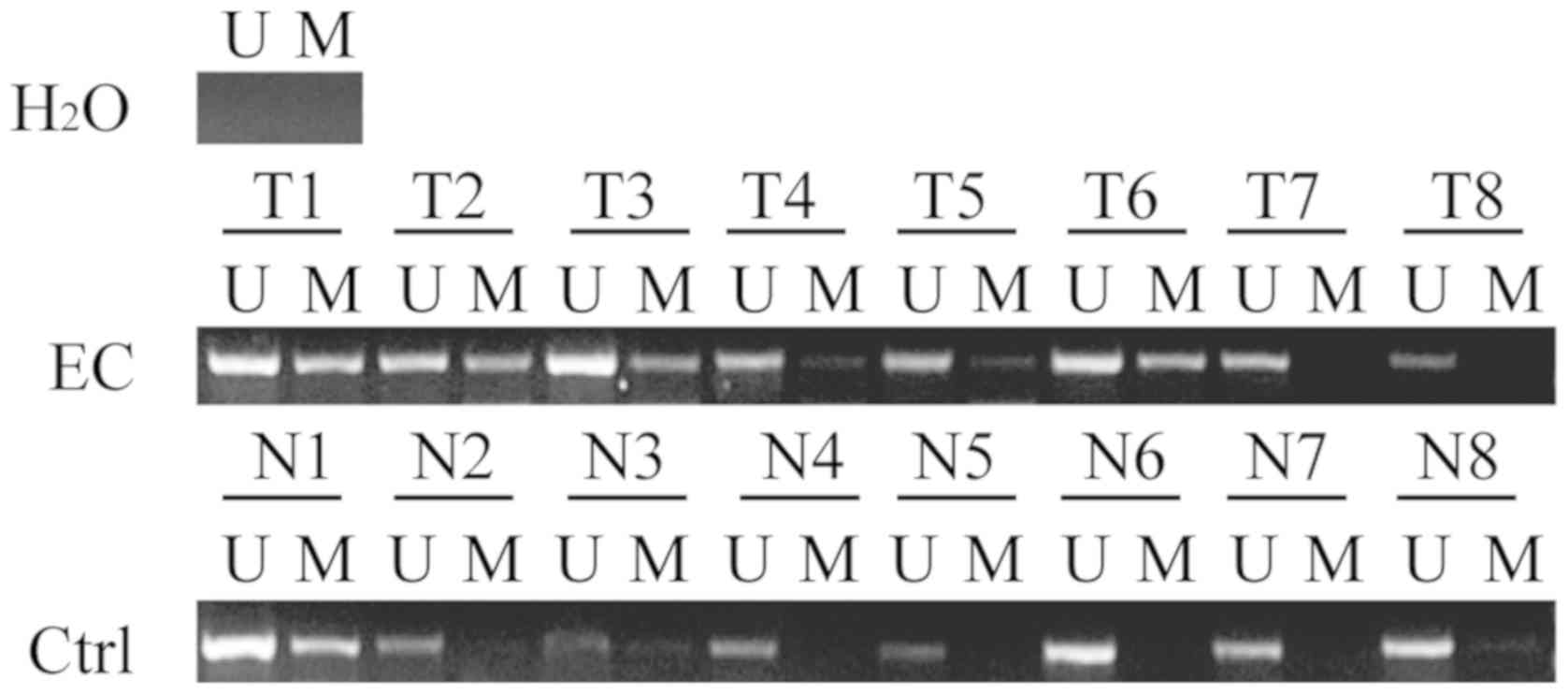
HOXD10 promoter methylation measured by MSP was
greater in cancer samples compared with non-cancerous samples
(Fig. 1 and Table I). Hypermethylation of SHH, ZNF545,
PCDH17 and MEIS1 in EA and MEC occurred, but a similar methylation
frequency was observed in non-cancerous lesions (Fig. S1).
 | Table I.MSP data for HOXD10 in primary
endometrial lesions and clinical pathological correlations. |
Table I.
MSP data for HOXD10 in primary
endometrial lesions and clinical pathological correlations.
|
| HOXD10 |
|
|---|
|
|
|
|
|---|
| Category | Methylated | Unmethylated | P-value |
|---|
| Simple
hyperplasia | 4 | 18 | 0.4750 |
| Complex and
atypical hyperplasia | 7 | 41 |
|
| Endometroid
adenocarcinoma | 35 | 15 | 0.5380 |
| Mucinous
endometrial | 8 | 4 |
|
| Total endometrial
carcinoma | 43 | 19 | <0.0001 |
| Total control
group | 11 | 59 |
|
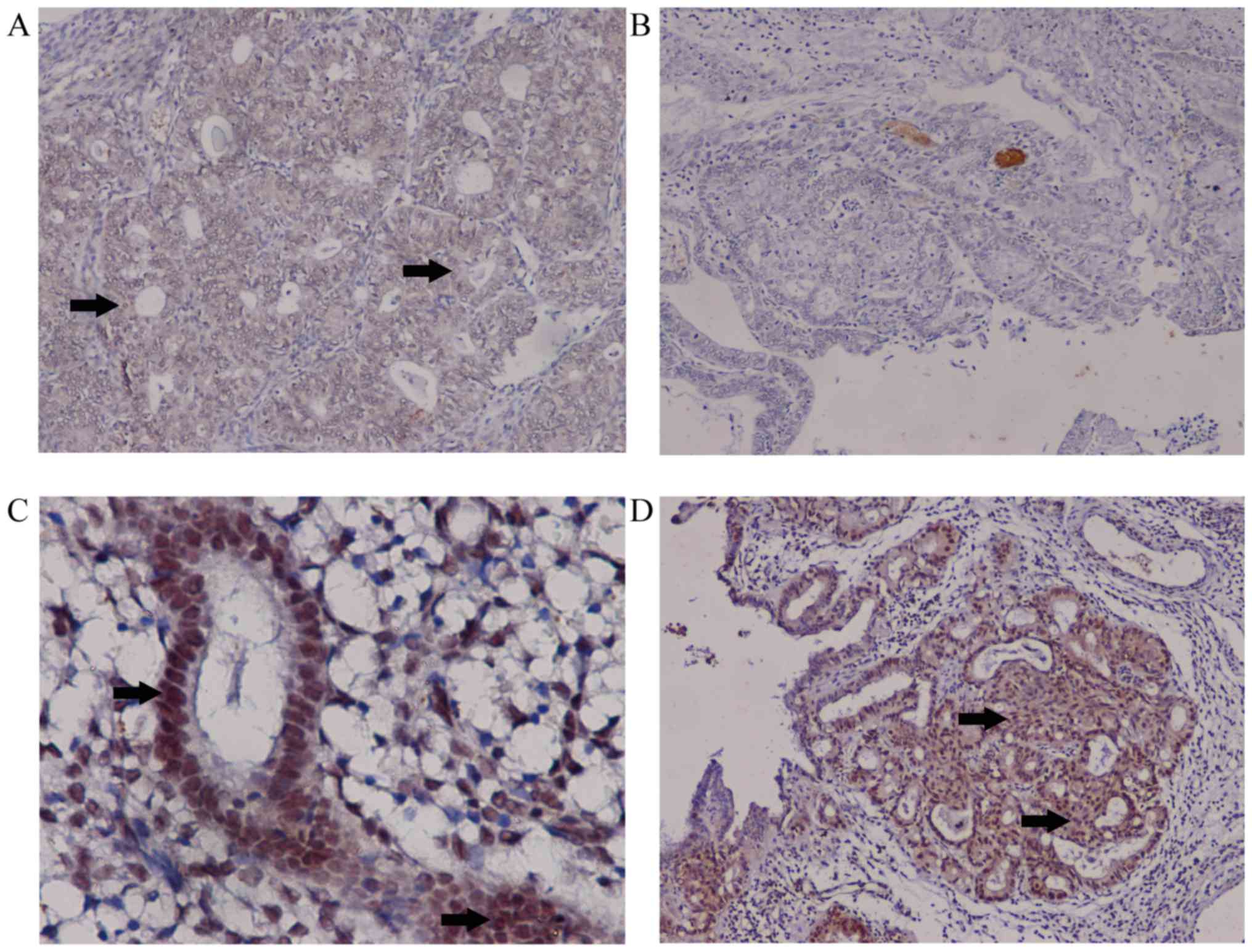
Decreased expression levels of HOXD10
in EC
HOXD10 expression levels were measured in cancer and
control samples. Downregulation of HOXD10 in cancer samples was
confirmed at the protein level using immunohistochemistry. HOXD10
expression levels were decreased in cancer cases (EA and MEC)
compared with the controls (37/62 vs. 14/70; P<0.01). Compared
with EA, MEC samples did not express HOXD10 (P<0.001; Table II). In EC, positive staining for
HOXD10 occurred in the cytoplasm. For simple and complex
hyperplasia, positive staining was evident in the nucleus and
cytoplasm (Fig. 2). Staining of
colonic carcinoma tissues was provided as positive control
(Fig. S2).
 | Table II.Immunohistochemistry data of HOXD10
in primary endometrial lesions. |
Table II.
Immunohistochemistry data of HOXD10
in primary endometrial lesions.
|
| HOXD10 |
|
|---|
|
|
|
|
|---|
| Category | Positive | Negative | P-value |
|---|
| EA | 25 | 25 |
0.0010 |
| MEC | 0 | 12 |
|
| Cancer samples | 25 | 37 | <0.0001 |
| Control
samples | 56 | 14 |
|
Association between HOXD10
methylation, gene expression levels and clinicopathological
features in EC
The present study compared methylation of the HOXD10
gene and its expression levels in cancer and control samples.
Staining intensity of HOXD10 was negatively associated with
promoter methylation (P<0.05). The majority of samples with
promoter methylation lacked HOXD10 protein expression levels,
indicating a significant association between promoter
hypermethylation and transcriptional silencing (P<0.05).
There was no significant difference in HOXD10
methylation between EA and MEC samples (P>0.05). No association
between HOXD10 methylation and clinical characteristics (patient
age, tumor differentiation, tumor size, depth of myometrial
invasion and lymph node metastases) was observed in EA or MEC cases
(Table III).
 | Table III.Promoter methylation status of HOXD10
in primary endometrial lesions and associations with clinical
features. |
Table III.
Promoter methylation status of HOXD10
in primary endometrial lesions and associations with clinical
features.
|
| HODX10 |
|
|---|
|
|
|
|
|---|
| Clinical
feature | Methylated | Unmethylated | P-value |
|---|
| Age, years | 50.56±16.17 | 53.32±16.63 | 0.700 |
| Differentiation,
n |
|
|
|
|
Well | 22 | 13 |
|
|
Moderate | 12 | 3 | 0.630 |
|
Poor | 9 | 3 |
|
| Depth of myometrial
invasion, n |
|
|
|
|
<1/2 | 38 | 16 | 0.652 |
|
≥1/2 | 5 | 3 |
|
| Lymph node
metastasis, n |
|
|
|
|
Yes | 38 | 19 | 0.149 |
| No | 5 | 0 |
|
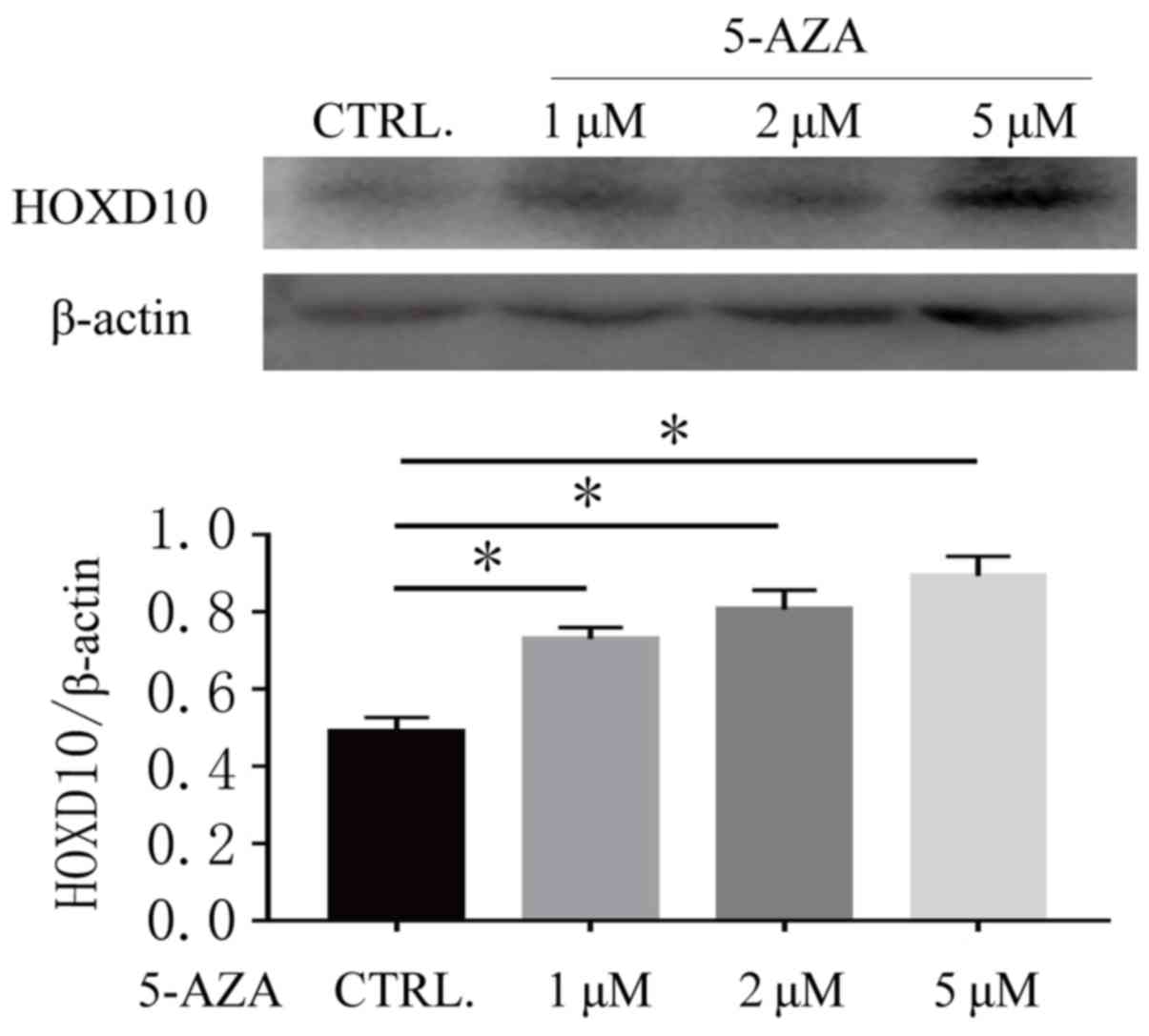
5-Aza-CdR treatment reverses the
expression levels of HOXD10 in Ishikawa cells
In order to further validate the association between
the methylation status of the HOXD10 gene and its expression level,
the present study detected the expression level of HOXD10 in the
Ishikawa cell line both before and after DNA methylation
transferase inhibitor treatment. As presented in Fig. 3, HOXD10 was weakly expressed in
Ishikawa cell lines and the expression level of HOXD10 was
increased after 72 h treatment with 5-Aza-CdR (P<0.01). These
results suggested that DNA methylation may be associated with the
promoter methylation status of HOXD10.
Discussion
DNA promoter methylation is considered to be a
promising diagnostic biomarker for cancer. For certain
malignancies, aberrant methylation occurs at the promoter of TSG,
which plays a crucial role in the regulation of tumor growth and
cell differentiation, proliferation and apoptosis. This causes low
gene expression levels, leading to alterations in tumor growth and
cell differentiation, proliferation and apoptosis, finally
resulting in malignancies that may occur prior to tumor formation.
Therefore, DNA methylation may be used as a marker in early cancer
screening (24,42–44).
Hypermethylation of specific TSG, as well as low mRNA and protein
expression levels, has been reported in a number of human
malignancies, including EC (28,45,46).
HOXD10 is one of the ANTP homeobox genes located on
chromosome 2 (2p31). This gene cluster consists of 39 genes and
multiple transcripts and can be divided into four groups (HOX A, B,
C and D) (47). HOX regulates stem
cell differentiation and embryonic development (48). Abnormal HOX gene expression levels
are responsible for certain malignancies, such as breast, thyroid
and ovarian cancer (49). The
potential use of homeobox genes as diagnostic and prognostic
biomarkers has been described in the literature (50,51).
HOXD10 acts as a TSG in a number of malignancies,
such as breast cancer (52), gastric
carcinogenesis (53) and
cholangiocellular carcinoma (54).
It inhibits tumorigenesis (55) and
angiogenesis and inhibits vascular EGFR, matrix metalloproteinase
14 and uPAR (56). In certain tumor
cell lines, upregulation of HOXD10 decreases invasiveness,
migration and survival of cancer cells (40,34,44). For
example, HOXD10 acts as a tumor-suppressive factor that suppresses
proliferation and invasion, promotes G1/S progression arrest and
apoptosis via inhibition of the RHOC/AKT/MAPK pathway in human
cholangiocellular carcinoma (40).
Low or absent HOXD10 expression levels promoted invasion and
migration in colorectal cancer (54,57) and
inhibited benign transformation of breast tumors (58).
The present study demonstrated that hypermethylation
of the HOXD10 promoter occurred more frequently in EC and thus may
be a diagnostic tool for EC compared with non-cancerous tissue. In
EA and MEC samples, HOXD10 expression levels were negatively
associated with methylation status. Aberrant methylation of the
HOXD10 promoter may decrease HOXD10 protein expression levels.
DNA methylation transferase inhibitor 5-Aza-CdR
treatment partly reverted the expression level of HOXD10 in the
Ishikawa cell line. These findings suggest that aberrant DNA
methylation may be one of the mechanisms underlying HOXD10
downregulation in EC.
In pathological and clinical diagnostic practice,
MEC is typically mucinous (5), but
better biomarkers to clearly identify MEC are lacking. MEC often
co-occurs with EA and lacks clear boundaries, which complicates
diagnosis (7). Previous studies have
suggested that in MEC, immunohistochemistry has demonstrated
diffuse positivity for ER/PR and vimentin, high p16 and low Ki-67
labeling index, has proven useful for the differential diagnosis of
EA (12,51). In the present study,
immunohistochemistry data revealed a lack of HOXD10 expression
levels in MEC, so this may be a reliable diagnostic biomarker.
In the present study, MSP data indicated no
differences in methylation of the HOXD10 promoter between EA and
MEC (EA samples vs. MEC; 70 vs. 66.6%; P>0.05). Dysregulation of
HOXD10 may have mechanisms other than promoter methylation. The
precise mechanism of dysregulation of HOXD10 in cancer is not
clear. In hepatocellular carcinoma, and colon, gastric and
papillary thyroid cancer, it has been demonstrated that promoter
methylation of HOXD10 can lead to loss of HOXD10 expression levels
(32,33,53,59).
MicroRNA (miR) can affect HOXD10 expression levels at the
post-transcriptional level in breast, colorectal, ovarian, gastric
and non-small cell lung cancer, as well as hepatocellular
carcinoma, glioma and hemangioma. miR-10b, miR-224, miR-23a,
miR-501, miR-92b-3p and miR-376b reportedly accelerate cancer
progression by directly targeting HOXD10 within the 3′UTR (60–65).
Other suggestions of mechanisms of HOXD10 dysregulation include the
possible role of long non-coding RNAs (lncRNA), such as HOX
transcript antisense RNA (HOTAIR). lncRNA HOTAIR expression is
dysregulated in breast cancer and can inhibit HOXD10 expression
levels (66). No evidence of HOXD10
mutations has been identified in cancer. Further studies are
required to understand the pathology underlying MEC.
In summary, as decreased protein expression levels
caused by hypermethylation of TSG may result in tumor progression,
the present study demonstrated that promoter
hypermethylation-mediated silencing of HOXD10 is a frequent event
in EC and thus this may be used to diagnose cancers and guide
epigenetic treatment for EA and MEC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant nos. 81272277 and 81372810).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ and CZ designed and supervised experiments. SM,
FY and YD wrote the manuscript. SG, JW and HL collected research
data. FY and DL performed the MSP and immunohistochemistry. YD and
SM conducted the cell line experiments. DL performed clinical
evaluations and statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the Committee of
Ethical Research at Shandong University (approval no.
mecsdums2012032). Written informed consent was obtained from
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EA
|
endometroid adenocarcinoma
|
|
EC
|
endometrial carcinoma
|
|
MEC
|
mucinous endometrial carcinoma
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
PCDHs
|
protocadherins
|
|
ZNF
|
zinc finger protein
|
|
TSG
|
tumor suppressor gene
|
|
MSP
|
methylation-specific PCR
|
|
5-aza-CdR
|
5-aza-2′-deoxycytidine
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eiriksson L, Cuartero J, Steed H, Pearcey
R, Capstick V, Schepansky A, Faught W and Dundas G: Assessment of
outcomes in surgically staged I/II endometrial adenocarcinoma
patients treated with postoperative vaginal vault radiotherapy
only. Int J Gynecol Cancer. 20:1356–1362. 2010.PubMed/NCBI
|
|
5
|
Gungorduk K, Ozdemir A, Ertas IE, Selcuk
I, Solmaz U, Ozgu E, Mat E, Gokcu M, Karadeniz T, Akbay S, et al:
Is mucinous adenocarcinoma of the endometrium a risk factor for
lymph node involvement? A multicenter case-control study. Int J
Clin Oncol. 20:782–789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jalloul RJ, Elshaikh MA, Ali-Fehmi R,
Haley MM, Yoon J, Mahan M and Munkarah AR: Mucinous adenocarcinoma
of the endometrium: Case series and review of the literature. Int J
Gynecol Cancer. 22:812–818. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rauh-Hain JA, Vargas RJ, Clemmer J, Clark
RM, Bradford LS, Growdon WB, Goodman A, Boruta DM II, Schorge JO
and del Carmen MG: Mucinous adenocarcinoma of the endometrium
compared with endometrioid endometrial cancer: A SEER analysis. Am
J Clin Oncol. 39:43–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Musa F, Huang M, Adams B, Pirog E and
Holcomb K: Mucinous histology is a risk factor for nodal metastases
in endometrial cancer. Gynecol Oncol. 125:541–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galic V, Schiavone MB, Herzog TJ, Holcomb
K, Lewin SN, Lu YS, Neugut AI, Hershman DL and Wright JD:
Prognostic significance of mucinous differentiation of endometrioid
adenocarcinoma of the endometrium. Cancer Invest. 31:500–504. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jackson CL, Hang S, Hansen K, He M, Sung
CJ, Quddus MR, Xiong M, Wang Y, Patel NR, Lawrence WD and Xiong J:
Endometrial adenocarcinomas with significant mucinous
differentiation: A characterization of intratumoral heterogeneity
of KRAS mutations in mucinous and endometrioid histologic
components. Int J Gynecol Cancer. 28:241–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sloan EA, Moskaluk CA and Mills AM:
Mucinous differentiation with tumor infiltrating lymphocytes is a
feature of sporadically methylated endometrial carcinomas. Int J
Gynecol Pathol. 36:205–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lax SF: Pathology of endometrial
carcinoma. Adv Exp Med Biol. 943:75–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fadare O, Roma AA, Mhawech-Fauceglia P,
Parkash V and Rabban JT: The diagnosis of mucinous lesions in
endometrial samplings by gynaecological pathologists: An analysis
of diagnostic reproducibility. Pathology. 50:276–285. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones NL, Xiu J, Chatterjee-Paer S,
Buckley de Meritens A, Burke WM, Tergas AI, Wright JD and Hou JY:
Distinct molecular landscapes between endometrioid and
nonendometrioid uterine carcinomas. Int J Cancer. 140:1396–1404.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee S, Sahasrabuddhe VV, Mendoza-Cervantes
D, Zhao R and Duggan MA: Tissue-based immunohistochemical biomarker
expression in malignant glandular lesions of the uterine cervix: A
systematic review. Int J Gynecol Pathol. 37:128–140. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stampoliou A, Arapantoni-Dadioti P and
Pavlakis K: Epigenetic mechanisms in endometrial cancer. J BUON.
21:301–306. 2016.PubMed/NCBI
|
|
17
|
Zhou JY, Zhang L, Wei LH and Wang JL:
Endometrial carcinoma-related genetic factors: Application to
research and clinical practice in China. BJOG. 123 (Suppl
3):S90–S96. 2016. View Article : Google Scholar
|
|
18
|
Banno K, Yanokura M, Iida M, Masuda K and
Aoki D: Carcinogenic mechanisms of endometrial cancer: Involvement
of genetics and epigenetics. J Obstet Gynaecol Res. 40:1957–1967.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Men C, Chai H, Song X, Li Y, Du H and Ren
Q: Identification of DNA methylation associated gene signatures in
endometrial cancer via integrated analysis of DNA methylation and
gene expression systematically. J Gynecol Oncol. 28:e832017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caplakova V, Babusikova E, Blahovcova E,
Balharek T, Zelieskova M and Hatok J: DNA methylation machinery in
the endometrium and endometrial cancer. Anticancer Res.
36:4407–4420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romanek-Piva K, Gałczyński K,
Adamiak-Godlewska A, Rechberger T and Postawski K: DNA methylation
and DNA methyltransferase (DNMT1) activity pattern in endometrial
carcinoma. Ginekol Pol. 87:6–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fialkova V, Vidomanova E, Balharek T,
Marcinek J, Kudela E, Hanysova S, Visnovsky J, Dobrota D and Hatok
J: DNA methylation as mechanism of apoptotic resistance development
in endometrial cancer patients. Gen Physiol Biophys. 36:521–529.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bartosch C, Lopes JM and Jeronimo C:
Epigenetics in endometrial carcinogenesis-part 1: DNA methylation.
Epigenomics. 9:737–755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai HC, Wang YC, Yu MH, Huang RL, Yuan CC,
Chen KJ, Wu CC, Chiang KJ and Chao TK: DNA methylation as a
biomarker for the detection of hidden carcinoma in endometrial
atypical hyperplasia. Gynecol Oncol. 135:552–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan Y, Liu G, Zhou F, Su B and Li Y: DNA
methylation profiles in cancer diagnosis and therapeutics. Clin Exp
Med. 18:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang T, Qiu H, Bao W, Li B, Lu C, Du G,
Luo X, Wang L and Wan X: Epigenetic inactivation of EFEMP1 is
associated with tumor suppressive function in endometrial
carcinoma. PLoS One. 8:e674582013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fiolka R, Zubor P, Janusicova V, Visnovsky
J, Mendelova A, Kajo K, Lasabova Z, Plank L and Danko J: Promoter
hypermethylation of the tumor-suppressor genes RASSF1A, GSTP1 and
CDH1 in endometrial cancer. Oncol Rep. 30:2878–2886. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sheng Y, Wang H, Liu D and Zhang C, Deng
Y, Yang F, Zhang T and Zhang C: Methylation of tumor suppressor
gene CDH13 and SHP1 promoters and their epigenetic regulation by
the UHRF1/PRMT5 complex in endometrial carcinoma. Gynecol Oncol.
140:145–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YC, Tsao CM, Kuo CC, Yu MH, Lin YW,
Yang CY, Li HJ, Yan MD, Wang TJ, Chou YC and Su HY: Quantitative
DNA methylation analysis of selected genes in endometrial
carcinogenesis. Taiwan J Obstet Gynecol. 54:572–579. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang RL, Su PH, Liao YP, Wu TI, Hsu YT,
Lin WY, Wang HC, Weng YC, Ou YC, Huang TH and Lai HC: Integrated
epigenomics analysis reveals a DNA methylation panel for
endometrial cancer detection using cervical scrapings. Clin Cancer
Res. 23:263–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Zhang C, Sheng Y, Yao S, Liu Z,
Zhang C and Zhang T: Frequent SOCS3 and 3OST2 promoter methylation
and their epigenetic regulation in endometrial carcinoma. Am J
Cancer Res. 5:180–190. 2015.PubMed/NCBI
|
|
32
|
Guo Y, Peng Y, Gao D, Zhang M, Yang W,
Linghu E, Herman JG, Fuks F, Dong G and Guo M: Silencing HOXD10 by
promoter region hypermethylation activates ERK signaling in
hepatocellular carcinoma. Clin Epigenetics. 9:1162017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan YH, Wang HY, Lai Y, Zhong W, Liang
WL, Yan FD, Yu Z, Chen JK and Lin Y: Epigenetic inactivation of
HOXD10 is associated with human colon cancer via inhibiting the
RHOC/AKT/MAPK signaling pathway. Cell Commun Signal. 17:92019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang LH, Choi YL, Hua XY, Shin YK, Song
YJ, Youn SJ, Yun HY, Park SM, Kim WJ, Kim HJ, et al: Increased
expression of sonic hedgehog and altered methylation of its
promoter region in gastric cancer and its related lesions. Mod
Pathol. 19:675–683. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brinkhuizen T, van den Hurk K,
Winnepenninckx VJ, de Hoon JP, van Marion AM, Veeck J, van Engeland
M and van Steensel MA: Epigenetic changes in basal cell carcinoma
affect SHH and WNT signaling components. PLoS One. 7:e517102012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan Y, Wang Y, Fu S, Liu D and Lin S:
Methylation-regulated ZNF545 inhibits growth of the p53-mutant
KYSE150 cell line by inducing p21 and Bax. Exp Ther Med.
18:1563–1570. 2019.PubMed/NCBI
|
|
37
|
Xiang S, Xiang T, Xiao Q, Li Y, Shao B and
Luo T: Zinc-finger protein 545 is inactivated due to promoter
methylation and functions as a tumor suppressor through the
Wnt/β-catenin, PI3K/AKT and MAPK/ERK signaling pathways in
colorectal cancer. Int J Oncol. 51:801–811. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin YL, Wang YP, Li HZ and Zhang X:
Aberrant promoter methylation of PCDH17 (Protocadherin 17) in serum
and its clinical significance in renal cell carcinoma. Med Sci
Monit. 23:3318–3323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uyen TN, Sakashita K, Al-Kzayer LF,
Nakazawa Y, Kurata T and Koike K: Aberrant methylation of
protocadherin 17 and its prognostic value in pediatric acute
lymphoblastic leukemia. Pediatr Blood Cancer. 64:2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Musialik E, Bujko M, Kober P, Grygorowicz
MA, Libura M, Przestrzelska M, Juszczyński P, Borg K, Florek I,
Jakóbczyk M and Siedlecki JA: Promoter DNA methylation and
expression levels of HOXA4, HOXA5 and MEIS1 in acute myeloid
leukemia. Mol Med Rep. 11:3948–3954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO classification of tumours of female reproductive
organs. International Agency for Research on Cancer. 307:2014.
|
|
42
|
Akhavan-Niaki H and Samadani AA: DNA
methylation and cancer development: Molecular mechanism. Cell
Biochem Biophys. 67:501–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding WJ, Yang Y, Chen ZX, Wang YY, Dong
WL, Cen JN, Qi XF, Jiang F and Chen SN: Methylation level of
Rap1GAP and the clinical significance in MDS. Oncol Lett.
16:7287–7294. 2018.PubMed/NCBI
|
|
44
|
Kim DS, Lee WK and Park JY: Promoter
methylation of Wrap53α, an antisense transcript of p53, is
associated with the poor prognosis of patients with non-small cell
lung cancer. Oncol Lett. 16:5823–5828. 2018.PubMed/NCBI
|
|
45
|
Jones A, Teschendorff AE, Li Q, Hayward
JD, Kannan A, Mould T, West J, Zikan M, Cibula D, Fiegl H, et al:
Role of DNA methylation and epigenetic silencing of HAND2 in
endometrial cancer development. PLoS Med. 10:e10015512013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Meršaková S, Holubeková V, Grendár M,
Višňovský J, Ňachajová M, Kalman M, Kúdela E, Žúbor P, Bielik T,
Lasabová Z and Danko J: Methylation of CADM1 and MAL together with
HPV status in cytological cervical specimens serves an important
role in the progression of cervical intraepithelial neoplasia.
Oncol Lett. 16:7166–7174. 2018.PubMed/NCBI
|
|
47
|
Abbasi AA: Diversification of four human
HOX gene clusters by step-wise evolution rather than ancient
whole-genome duplications. Dev Genes Evol. 225:353–357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Seifert A, Werheid DF, Knapp SM and
Tobiasch E: Role of Hox genes in stem cell differentiation. World J
Stem Cells. 7:583–595. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bhatlekar S, Fields JZ and Boman BM: HOX
genes and their role in the development of human cancers. J Mol Med
(Berl). 92:811–823. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Javed S and Langley SE: Importance of HOX
genes in normal prostate gland formation, prostate cancer
development and its early detection. BJU Int. 113:535–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chekmareva M, Ellenson LH and Pirog EC:
Immunohistochemical differences between mucinous and microglandular
adenocarcinomas of the endometrium and benign endocervical
epithelium. Int J Gynecol Pathol. 27:547–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Vardhini NV, Rao PJ, Murthy PB and
Sudhakar G: HOXD10 expression in human breast cancer. Tumour Biol.
35:10855–10860. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang L, Chen S, Xue M, Zhong J, Wang X,
Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al: Homeobox D10 gene, a
candidate tumor suppressor, is downregulated through promoter
hypermethylation and associated with gastric carcinogenesis. Mol
Med. 18:389–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang H, Zhou J, Mi J, Ma K, Fan Y, Ning J,
Wang C, Wei X, Zhao H and Li E: HOXD10 acts as a tumor-suppressive
factor via inhibition of the RHOC/AKT/MAPK pathway in human
cholangiocellular carcinoma. Oncol Rep. 34:1681–1691. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Joo MK, Park JJ and Chun HJ: Impact of
homeobox genes in gastrointestinal cancer. World J Gastroenterol.
22:8247–8256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Myers C, Charboneau A, Cheung I, Hanks D
and Boudreau N: Sustained expression of homeobox d10 inhibits
angiogenesis. Am J Pathol. 161:2099–2109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Y, Li Z, Zhao X, Zuo X and Peng Z:
miR-10b promotes invasion by targeting HOXD10 in colorectal cancer.
Oncol Lett. 12:488–494. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Carrio M, Arderiu G, Myers C and Boudreau
NJ: Homeobox D10 induces phenotypic reversion of breast tumor cells
in a three-dimensional culture model. Cancer Res. 65:7177–7185.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cao YM, Gu J, Zhang YS, Wei WJ, Qu N, Wen
D, Liao T, Shi RL, Zhang L, Ji QH, et al: Aberrant hypermethylation
of the HOXD10 gene in papillary thyroid cancer with BRAFV600E
mutation. Oncol Rep. 39:338–348. 2018.PubMed/NCBI
|
|
60
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li S, Zhang J, Zhao Y, Wang F, Chen Y and
Fei X: miR-224 enhances invasion and metastasis by targeting HOXD10
in non-small cell lung cancer cells. Oncol Lett. 15:7069–7075.
2018.PubMed/NCBI
|
|
62
|
Yachi K, Tsuda M, Kohsaka S, Wang L, Oda
Y, Tanikawa S, Ohba Y and Tanaka S: miR-23a promotes invasion of
glioblastoma via HOXD10-regulated glial-mesenchymal transition.
Signal Transduct Target Ther. 3:332018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zeng Z, Liu S, Cai J, Li Z, Wu H, Chen H
and Huang Y: miR-501 promotes hemangioma progression by targeting
HOXD10. Am J Transl Res. 11:2439–2446. 2019.PubMed/NCBI
|
|
64
|
Li C, Huo B, Wang Y and Cheng C:
Downregulation of microRNA-92b-3p suppresses proliferation,
migration, and invasion of gastric cancer SGC-7901 cells by
targeting Homeobox D10. J Cell Biochem. 120:17405–17412. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
An N, Luo X, Zhang M and Yu R:
MicroRNA-376b promotes breast cancer metastasis by targeting Hoxd10
directly. Exp Ther Med. 13:79–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|