Introduction
Previous research has shown that lung cancer is the
leading cause of cancer-associated mortality in China, but also
worldwide, with a morbidity rate and mortality rate of 11.6 and
18.4%, respectively (1–3). Due to the high degree of malignancy and
rapid development of lung cancer, lack of clinical symptoms in the
early stage and the lack of an effective screening program, >70%
of patients are diagnosed with advanced stage disease (stage
III/IV) (4). It has been previously
reported that ~60% of patients diagnosed with non-small-cell lung
cancer (NSCLC) die within 1 year, because they are unable to
undergo any surgical resection, and due to the occurrence of local
recurrence and distant metastasis during the treatment process
(5). In recent years, the main
treatments for advanced NSCLC have been chemotherapy,
molecular-targeted therapy and developing immunotherapy (6–8).
Although molecular targeted therapies have resulted important
changes in the treatment of NSCLC, especially for adenocarcinoma,
the odds of patients with lung cancer surviving for at least 5
years after diagnosis remain <15% (9). The pathogenesis behind lung cancer has
not been fully elucidated, and research has shown that factors such
as polygenes may be involved. Research has found that microRNAs
(miRNAs/miR) have an important role in the occurrence and
progression of tumors via the regulation of target genes (10). Therefore, miRNAs may be key for tumor
intervention therapy (11).
miRNA-146a is located in the LOC285628 gene on human chromosome 5.
The LOC285628 gene is composed of two exons separated by ~16 kb.
The sequence encoding miR-146a is located in the second exon. In
2008, Jazdzewski et al (12)
proposed that the pre-miR-146a gene polymorphism may serve a role
in thyroid papillary carcinoma. miR-146a was subsequently examined
in gastric cancer, esophageal cancer, liver cancer, breast cancer,
ovarian cancer and other tumors. In 2011, Vinci et al
(13), studied the genetic
polymorphisms and expression of miRNAs in NSCLC, and later found
that miR-146a may increase the risk of NSCLC. In the past five
years, the number of studies on the effects of miR-146a on NSCLC
has increased greatly. Only 20% (5/20) of the manuscripts regarding
miR-146a in NSCLC could be retrieved before 2014, and the remaining
manuscripts were retrieved from 2015 to 2019.
Research has indicated that miR-146a has great
impact on proliferation, apoptosis, invasion and metastasis of lung
cancer (14). Although the specific
mechanism, to the best of our knowledge, has not been fully
explored, miR-146a may be important for developing new and
effective therapeutic targets, becoming one of the hotspots in
cancer research. In the present study, reverse
transcription-quantitative PCR (RT-qPCR) was used to detect the
miR-146a level in three lung adenocarcinoma cell lines.
Furthermore, the effects of miR-146a on the malignant biology of
lung adenocarcinoma cells were examined. Finally, the molecular
mechanism of the effects of miR-146a was explored.
Materials and methods
Cell lines and cell culture
Human lung epithelial cells, BEAS-2B, were obtained
from China Center for Type Culture Collection and human lung
adenocarcinoma cells, A549, H1299 and PC-9, were obtained from the
Pharmacology Department of Fujian Medical University (Fujian,
China). All cells were cultured in RPMI-1640 medium with 10% FBS
(both HyClone; GE Healthcare Life Sciences) and 100 U/ml penicillin
and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
All cells were incubated at 37°C with 5% CO2.
RT-qPCR and the expression levels of
miR-146a in human lung adenocarcinoma cells
The expression of miR-146a in human lung
adenocarcinoma cells, A549, H1299 and PC-9, and human lung
epithelial cells, BEAS-2B, were measured using RT-qPCR. Total RNA
was extracted from cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). The purity and concentration of RNA were
assessed using NanoDrop 2000 (Thermo Fisher Scientific, Inc.). cDNA
was synthesized according to the instructions included with the
miRNA reverse transcription kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and performed using the following conditions:
Incubation at 42°C for 60 min, 95°C for 5 min, and held at 4°C,
until subsequent experimentation. qPCR reaction system was prepared
with the SYBR Green qPCR Supermix (Promega Corporation), according
to the manufacturer's protocols. qPCR reaction conditions were as
follows: 95°C for 3 min; followed by 40 cycles of 95°C for 15 sec,
60°C for 30 sec, and 68°C for 1 min. U6 served as an internal
normalized reference. The primers of miR-146a and U6 are shown in
Table I. Quantification of relative
expression was performed using the 2−ΔΔCq method
(15), where
ΔΔCq=[Cq(miRNA)-Cq(U6)]experimental
group-[Cq(miRNA)-Cq(U6)]control group.
 | Table I.Primer sequences used for
amplification. |
Table I.
Primer sequences used for
amplification.
| Primer | Sequence
(5′-3′) |
|---|
| RT |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACCCA |
| miR-146a | Forward
CGGCGGTGAGAACTGAATTCCA |
| miR-146a | Reverse
GTGCAGGGTCCGAGGT |
| U6 | Forward
GCTTCGGCAGCACATATACTAAAAT |
| U6 | Reverse
CGCTTCACGAATTTGCGTGTCAT |
| IRAK1 | Forward
GTGGACACGGACACCTTCAG |
| IRAK1 | Reverse
CTCCTCAGCCTCCTCTTCCA |
| TRAF6 | Forward
GGAACCCTAGCCCATCGTCA |
| TRAF6 | Reverse
GGAACCCTAGCCCATCGTCA |
| GAPDH | Forward
AGAAGGCTGGGGCTCATTTG |
| GAPDH | Reverse
AGGGGCCATCCACAGTCTTC |
Experimental group and
transfection
The experimental cells were divided into 3 groups:
Mimics group, transfected with Cy3-miR-146a mimics
(5′-UGAGAACUGAAUUCCAUGGGUU-3′; Shanghai GenePharma Co., Ltd.) as
the experimental group; negative control (NC;
5′-UUCUCCGAACGUGUCACGUTT-3′) group, transfected with miR-146a
analogue nonsense sequences (Shanghai GenePharma Co., Ltd.) as the
negative control group; and mock group, treated with
Lipofectamine® 2000 only (Invitrogen; Thermo Fisher
Scientific, Inc.). The day before transfection, A549 cells were
seeded on a 24-well-plate and allowed to incubate overnight, after
which their confluency was 30–50%. The cells were transfected using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols.
Transfection efficiency of
miR-146a
After 6 h of transfection, the approximate
transfection efficiency was observed and calculated using
fluorescence microscopy in which five random fields were observed
at ×200 magnification. The cells were continued to be cultured
after the medium was replaced. Total RNA was extracted from the
three groups at 48 h after transfection. The expression of miR-146a
in the three groups was measured using RT-qPCR. U6 was used as an
internal reference. Primers are shown in Table I.
Cell proliferation assay
Cell proliferation was assessed by Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology). Briefly,
the three groups of A549 cells were incubated in 96-well plates at
a density of 2.5×103 cells/well. The cells were cultured
for 12, 24, 36, 48, 60 and 72 h. A total of 10 µl CCK-8 reagent was
subsequently added to each well, and cells were returned to
incubation for 1–2 h. Light absorbance was measured at a wavelength
of 450 nm using a microplate reader. The resulting value was used
to represent cell proliferation activity.
Apoptosis assay
A549 cells were inoculated into 6-well plates with
the density of 1×106 cells/well. At 48 h after
transfection, the Annexin V-FITC and propidium iodine (PI) reagents
(Nanjing KeyGen Biotech Co., Ltd.) were added, according to the
protocols of the Annexin V-FITC apoptosis kit. The rate of
apoptosis was determined by flow cytometry after 15 min of
staining.
Transwell and Matrigel assays
Transwell chambers were placed into a 24-well plate
and Matrigel was diluted at 1:16 using RPMI-1640. 60 µl diluted
Matrigel was added to the upper chamber and incubated at 37°C for 3
h. A549 cells of the three groups were digested and resuspended at
24 h after transfection. The cells were diluted with 200 µl
serum-free medium (1×105/ml) and added into the upper
chamber of Transwell chamber, and 600 µl RPMI-1640 culture medium
containing with 20% FBS was added to the lower chamber. After 24 h
of cell culture, the cells were removed from the upper surface of
the polycarbonate membrane with a wet cotton swab. The filter
membrane was fixed with 4% polyformaldehyde for 10 min at room
temperature, stained with 0.1% crystal violet for 30 min at room
temperature, and rinsed with double distilled water for 5 min.
Subsequently, five fields of view were randomly selected using
light microscopy (Olympus Corporation) at low magnification, the
number of invading cells was counted, and the mean average was
calculated.
Wound-healing assay
The three groups of A549 cells were seeded into
6-well plates at a density of 1×106 cells/well. Cells
were starved with serum-free medium for 12 h. A 10 µl pipette tip
was used create a scratch across the center of each well. Images
were obtained at 0 and 48 h of culture to assess wound-healing
using light microscopy (×100 magnification; Olympus
Corporation).
Target gene prediction and
dual-luciferase assay
Target genes of miR-146a were predicted using the
bioinformatics software TargetScan (http://www.targetscan.org/vert_72/) and miRDB
(http://mirdb.org/). Dual-luciferase assay was used to
validate the association between miR-146a and the predicted
targets. The wild-type 3′-untranslated region (UTR; IRAK1-WT and
TRAF6-WT) and mutant 3′-UTR (IRAK1-MUT and TRAF6-MUT) of target
genes were inserted into the pGL3 promoter vector (Shanghai
GenePharma Co., Ltd.) the vector (0.4 mg) and miR-146a mimic or NC
(10 pmol) were transfected into A549 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were diluted to 1×105/ml
and cultured in a 24-well plate. Firefly and Renilla
luciferase activities were quantified using the dual-luciferase
reporter assay system (Promega Corporation) according to the
manufacturer's instructions 48 h following co-transfections. The
automatic microplate reader (Molecular Devices, LLC) was used in
luciferase assays detection.
mRNA expression of target genes as
determined using RT-qPCR
The extraction of total RNA, synthesis of cDNA, and
fluorescence quantitative PCR were performed in accordance with the
steps described above. The relative mRNA expression of the target
genes was calculated using GAPDH as an internal reference. The
primers required for the experiment are presented in Table I.
Protein expression of target genes as
detected by western blot analysis
The three groups of cells were lysed with RIPA lysis
buffer on ice at 48 h after transfection. The supernatant was
retained after high-speed centrifugation (12,000 × g at 4°C) and
the protein concentrations were measured using a BCA kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (15 µg)were
separated by SDS-PAGE on 10% gels (Beijing Solarbio Science &
Technology Co., Ltd.), and transferred to PVDF membranes. The PVDF
membranes were blocked with 5% skim milk at room temperature for 2
h. They were then incubated with primary antibodies against
interleukin-1 receptor-associated kinase 1 (IRAK1; dilution,
1:1,000; cat. no. ab180747; Abcam), TNF receptor associated factor
6 (TRAF6; dilution, 1:1,000; cat. no. ab33915; Abcam) and GAPDH
(dilution, 1:10,000; cat. no. ab181602; Abcam) at 4°C overnight.
The membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(dilution, 1:5,000; cat. no. BB-2202; BestBio Company; http://bestbio.bioon.com.cn/) at room temperature for
2 h. Proteins were visualized by chemiluminescence using an ECL kit
(Beyotime Institute of Biotechnology), according to the
manufacturer's protocols. The expression of proteins was quantified
using densitometry analysis (ChemiDoc™ XRS+ gel imaging system) and
analysed using image lab™ software (version 3.0; both
Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analyzed by SPSS 24.0 (IBM Corp.). Values
are expressed as the mean ± standard deviation of three independent
experiments. All P-values were calculated using unpaired Student's
t-test or one-way analysis of variance with Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-146a is downregulated in human
lung adenocarcinoma cell lines
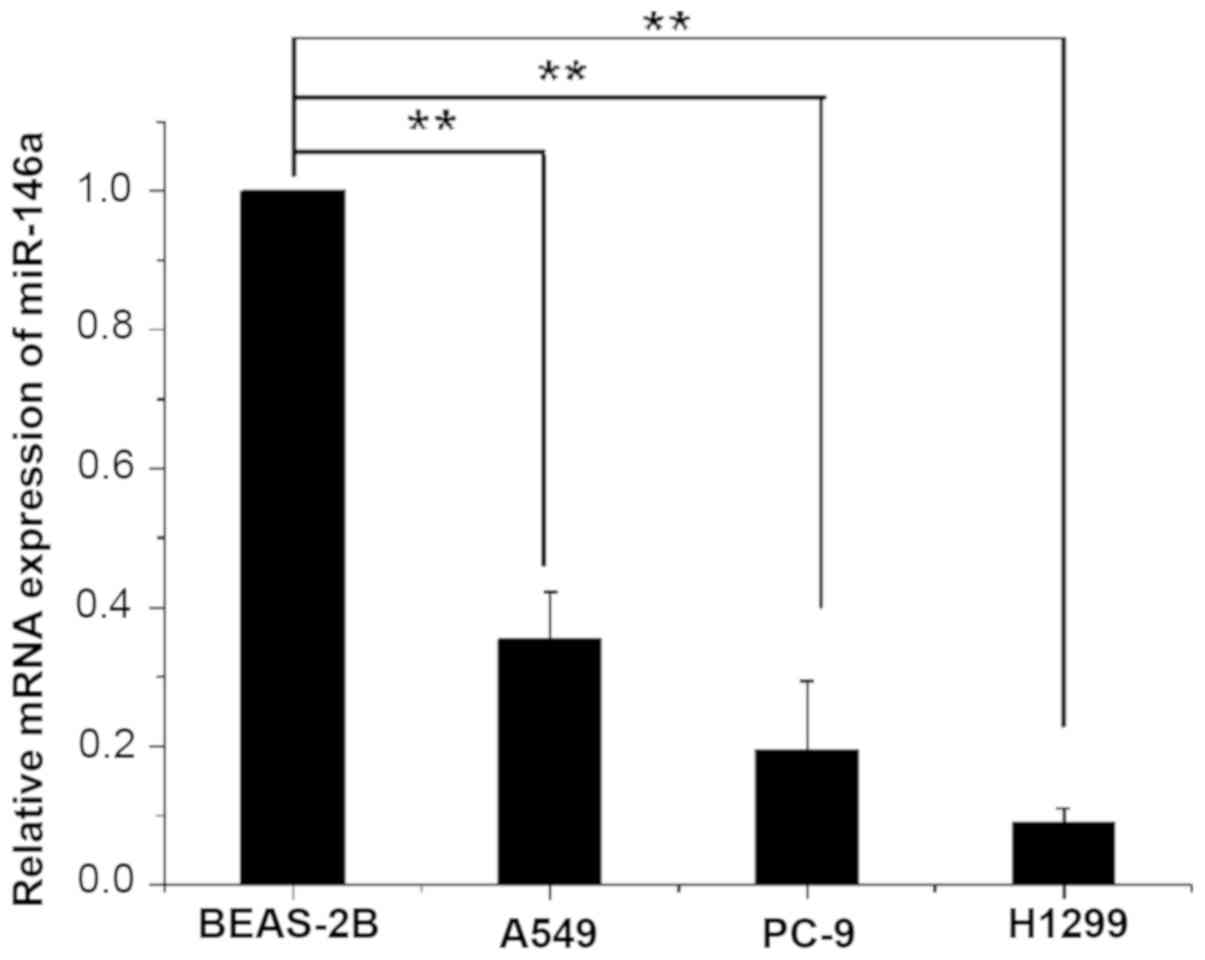
The expression of miR-146a in three human lung
cancer cell lines A549, H1299 and PC-9, and normal human lung
epithelial cells, BEAS-2B, was detected by RT-qPCR. The expression
of miR-146a in BEAS-2B and the relative expression in other cell
lines is presented in Table II. The
results showed that the expression of miR-146a in three human lung
cancer cell lines was significantly lower than in human normal lung
epithelial cells (P<0.01; Fig.
1).
 | Table II.Relative expression level of miR-146a
in the four cell lines. |
Table II.
Relative expression level of miR-146a
in the four cell lines.
| Group | miR-146a (mean ±
standard deviation) |
|---|
| A549 |
0.325±0.069a |
| PC-9 |
0.193±0.100a |
| H1299 |
0.090±0.020a |
| BEAS-2B | 1.000 |
Cell transfection
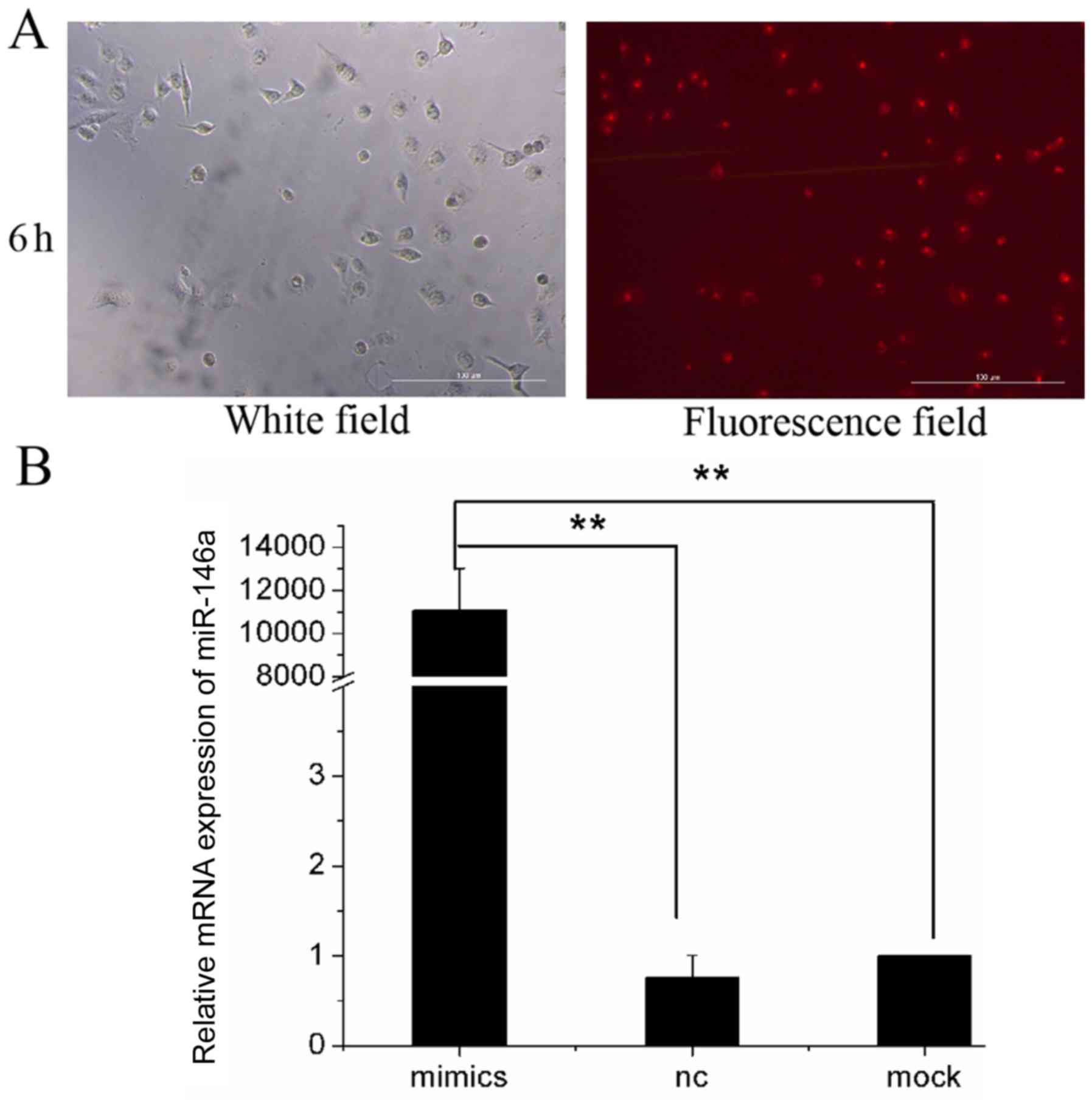
Human lung adenocarcinoma cell line A549 was
transfected with Cy3-miR-146a mimics and the transfected cells were
observed using a fluorescence microscope after 6 h (Fig. 2A). A total of 65±3.5 cells were
counted in light microscopy images, while 62±4.7 cells were counted
in fluorescence microscopy images. The transfection efficiency in
A549 cells reached 95.4%.
At 48 h after transfection, the transfection
efficiency of miR-146a mimics in A549 cells was assessed by
RT-qPCR. The miR-146a expression level in the mock group was set to
1, and the relative expression levels of miR-146a in the NC group
and the mimics group were 0.76±0.24 and 11,097.93±1,926.50,
respectively (Fig. 2B). The results
showed that after the transfection of miR-146a mimics, the
expression of miR-146a was significantly higher in the mimic group
than in the NC and mock groups (P<0.01; Fig. 2B). This indicated that miR-146a was
successfully overexpressed in human lung adenocarcinoma cell line
A549.
Overexpression of miR-146a inhibits
the proliferation and induces cell apoptosis of A549 cells
To determine the role of miR-146a in lung
adenocarcinoma cell lines, miR-146a was overexpressed and the
effect of miR-146a on the proliferation of A459 cells was assessed
by CCK-8 assay. The OD values of A549 cells were measured at 12,
24, 36, 48, 60 and 72 h after transfection (Table III). There were no significant
differences in proliferation among the three groups at 12 h
(P>0.05); however, at 24, 36, 48, 60 and 72 h, the OD values of
the mimics group were significantly lower than those of the nc and
mock groups (P<0.05 and P<0.01; Fig. 3A). The CCK-8 assay indicated that
overexpression of miR-146a reduces the proliferation of A549 cells.
In order to determine whether miR-146a can promote apoptosis of
A549 cells, a flow cytometry assay was performed at 48 h after A549
cells were transfected. The results showed the rates of apoptosis
in the mimics, nc and mock groups to be 19.600±0.990, 9.800±1.131
and 13.701±0.707%, respectively (P<0.05; Fig. 3B and C). These results indicated that
overexpression of miR-146a inhibited the proliferation and promoted
apoptosis of A549 cells.
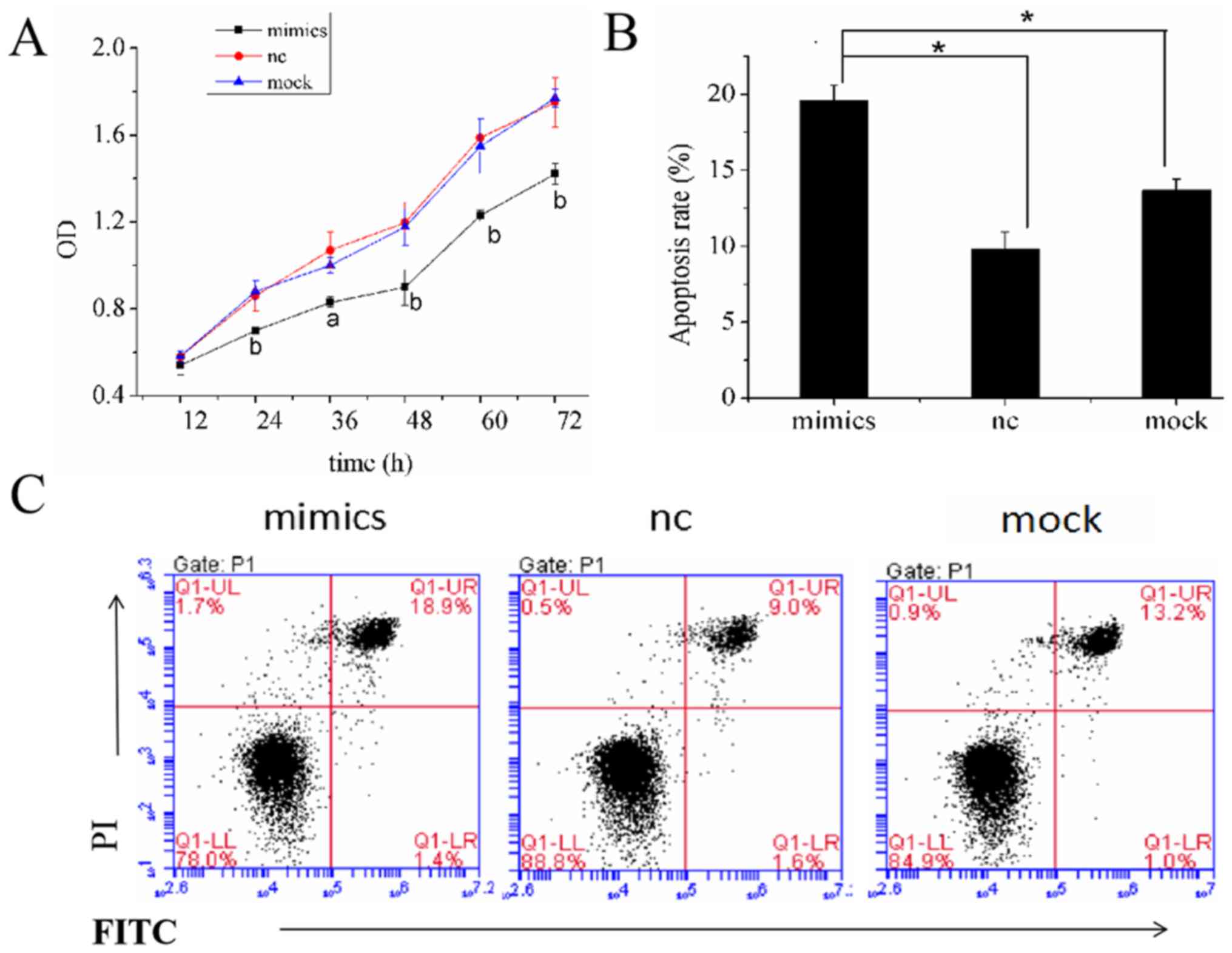 | Figure 3.Effects of miR-146a overexpression on
proliferation and apoptosis in A549 cells. (A) The OD values of
A549 cells were measured at 12, 24, 36, 48, 60 and 72 h after
transfection. The values of the mimics group were smaller than the
nc group and the mock group at 24, 36, 48, 60 and 72 h. (B and C)
At 48 h after transfection, miR-146a overexpression was found to
promote apoptosis of A549 cells. *P<0.05 vs. mock and NC group.
miR-146a, microRNA-146a; NC, negative control; OD, optical density;
PI, propidium iodine. |
 | Table III.Overexpression of miR-146a promotes
proliferation of A549 cells. |
Table III.
Overexpression of miR-146a promotes
proliferation of A549 cells.
|
| Time point (h) |
|---|
|
|
|
|---|
| Group | 12 | 24 | 36 | 48 | 60 | 72 |
|---|
| miR-mimics | 0.539±0.046 |
0.696±0.010a,b |
0.827±0.027c,d |
0.899±0.082a,b |
1.235±0.027a,b |
1.458±0.048a,b |
| miR-NC | 0.582±0.014 | 0.863±0.070 | 1.070±0.085 | 1.197±0.094 | 1.588±0.008 | 1.749±0.114 |
| Mock | 0.575±0.027 | 0.880±0.049 | 1.000±0.035 | 1.176±0.086 | 1.549±0.126 | 1.797±0.040 |
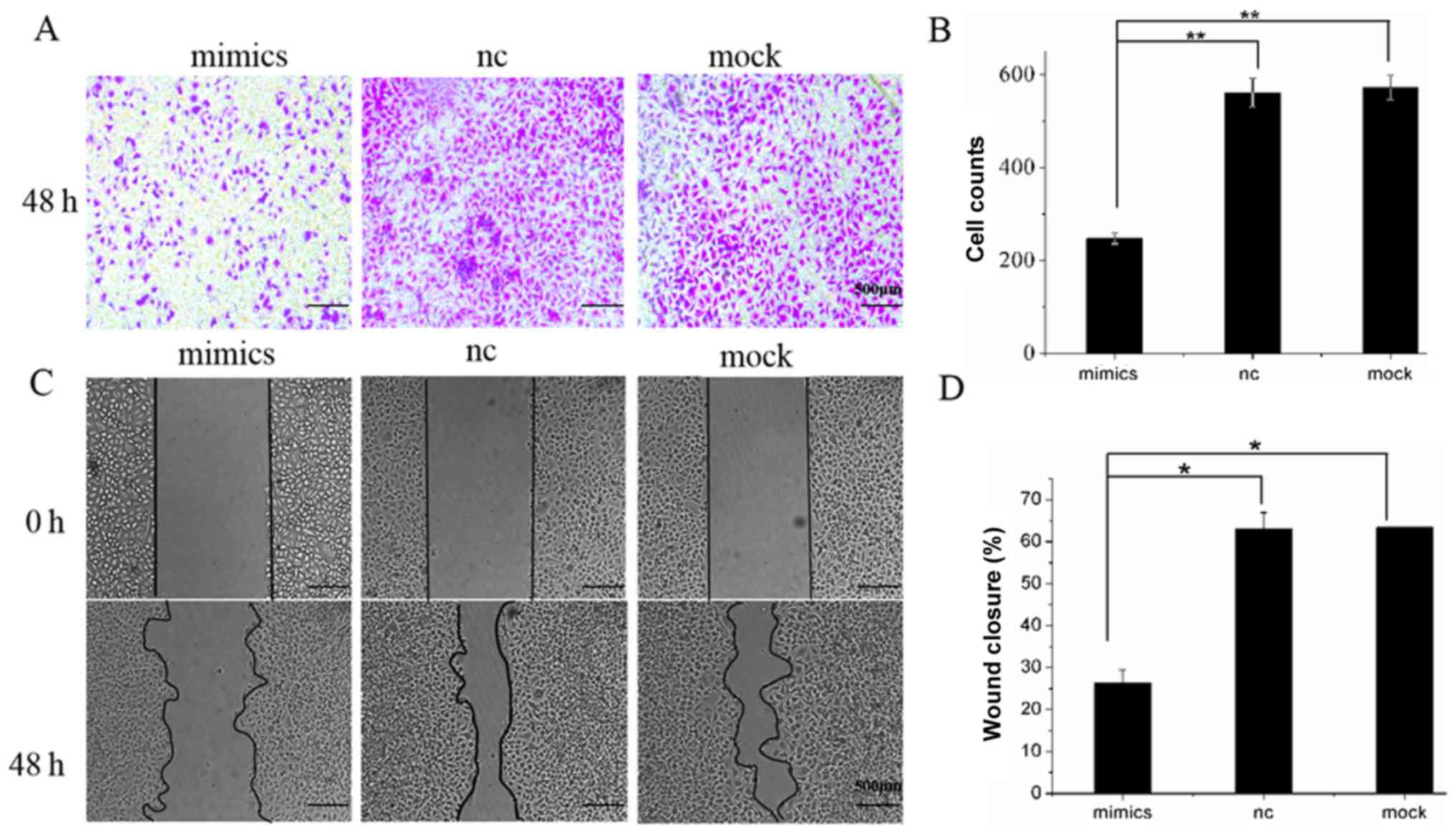
Overexpression of miR-146a inhibits
the migration of A549 cells
Taking into account the low levels of miR-146a
expression in human lung adenocarcinoma cells, and the fact that
miR-146a inhibits proliferation and promotes apoptosis of A549
cells, this study subsequently examined whether miR-146a may affect
cell migration. To address this, Transwell and wound-healing assays
were conducted 48 h after cells were transfected. The results of
the Transwell assay showed that the number of cells passing through
the membrane in the mimics group (212±6.000) was significantly
lower than in the nc group (588±60.357) and mock group (572±25.166;
P<0.01; Fig. 4A). In the
wound-healing assay, images of the scratches in the three groups at
0 and 48 h after transfection, and the sizes of the healing areas
were calculated accordingly. Five randomly selected fields of view
along each wound were marked, and the wound area was measured, and
the average wound-healing area was determined as the wound area of
this wound and calculated as follows: The wound-healing area=wound
area of 0 h-wound area of 48 h. Wound-healing area was first
compared to the wound area at 0 h, and subsequently compared to the
NC and mock groups. The area of cell monolayer healing in the
mimics group was significantly smaller (26.32±3.11%) than that in
the NC group (63.42±0.17%) and in the mock group (63.17±3.73%;
P<0.05; Fig. 4B). Results showed
that overexpression of miR-146a inhibited migration in
vitro.
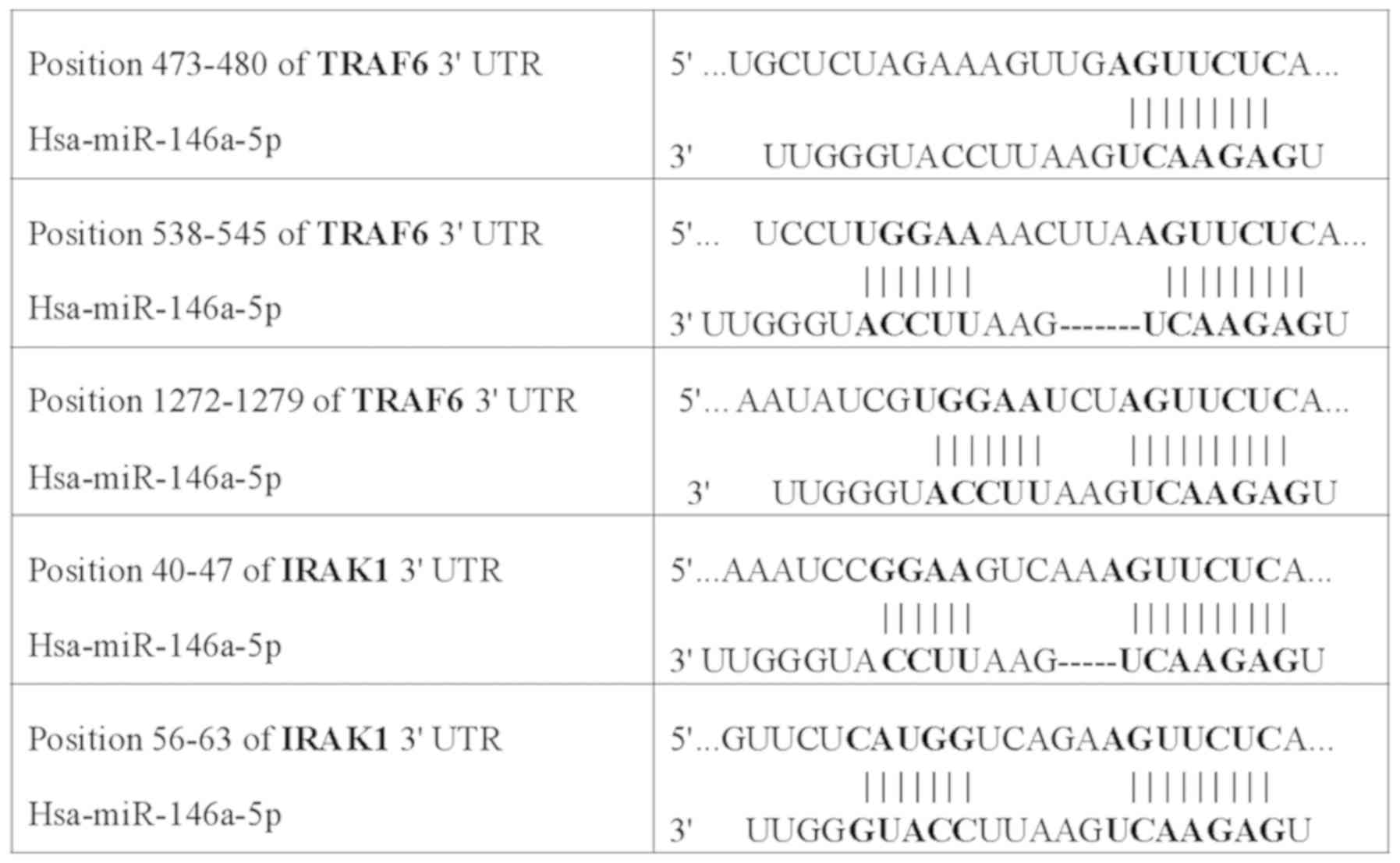
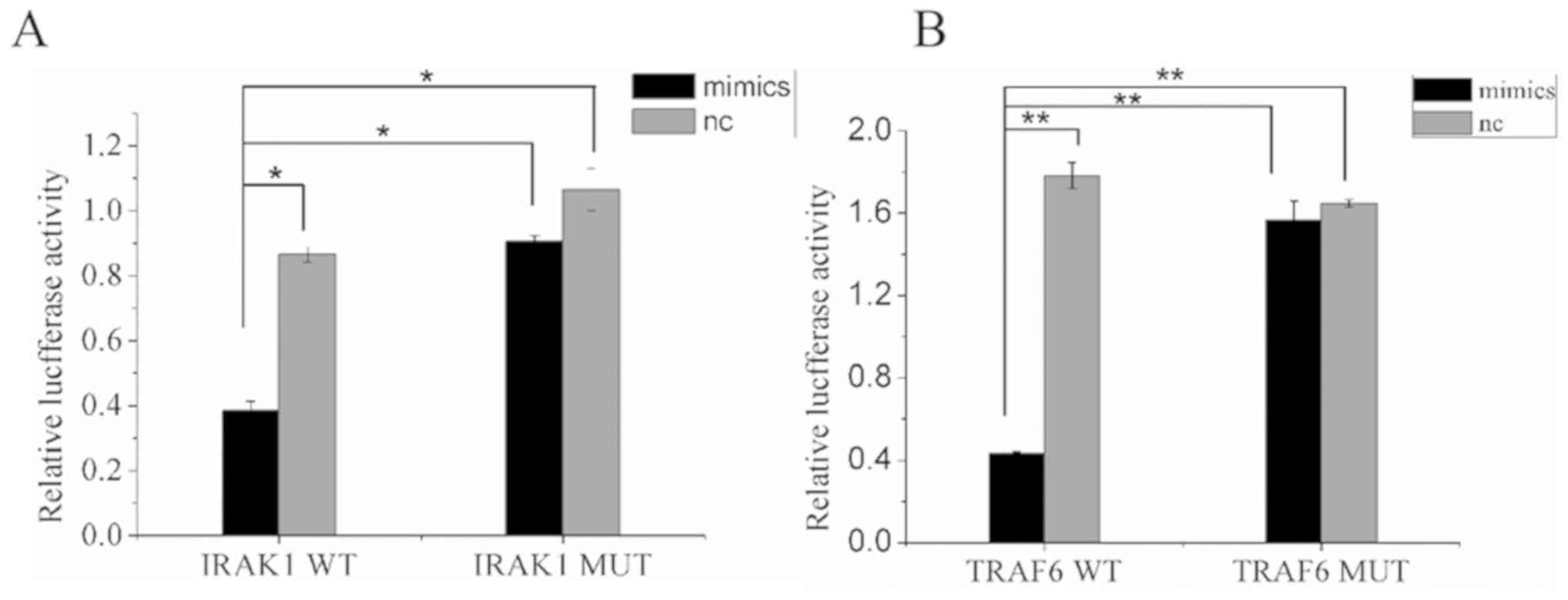
Detection of miR-146a targeted
genes
The target genes of miR-146a were predicted using
bioinformatics tools using the databases TargetScan and miRDB.
After reviewing previous publications (16–18),
IRAK1 and TRAF6 were selected as target genes in lung
cancer and were used for the dual-luciferase assay. Bioinformatics
predicted the presence of miR-146a-binding sites in the 3′-UTRs of
IRAK1 and TRAF6 (Fig.
5). The wild-type and mutant 3′-UTRs of IRAK1 and
TRAF6 were used for the construction of recombinant
luciferase expression vectors IRAK1-WT, IRAK1-MUT, TRAF6-WT, and
TRAF6-MUT, and the vectors were co-transfected with miR-146a mimics
or miR-146a nc to measure luciferase activity. The ratios of
firefly luciferase activity to Renilla luciferase activity
in the co-transfected groups, IRAK1-WT + miR-146a mimics group and
TRAF6-WT + miR-146a mimics group, were significantly lower than in
the WT + NC and the MUT + miR-146a mimics and NC groups (P<0.05
and P<0.01; Fig. 6A and B). The
results indicated that miR-146a could specifically bind to 3′-UTRs
of IRAK1 and TRAF6.
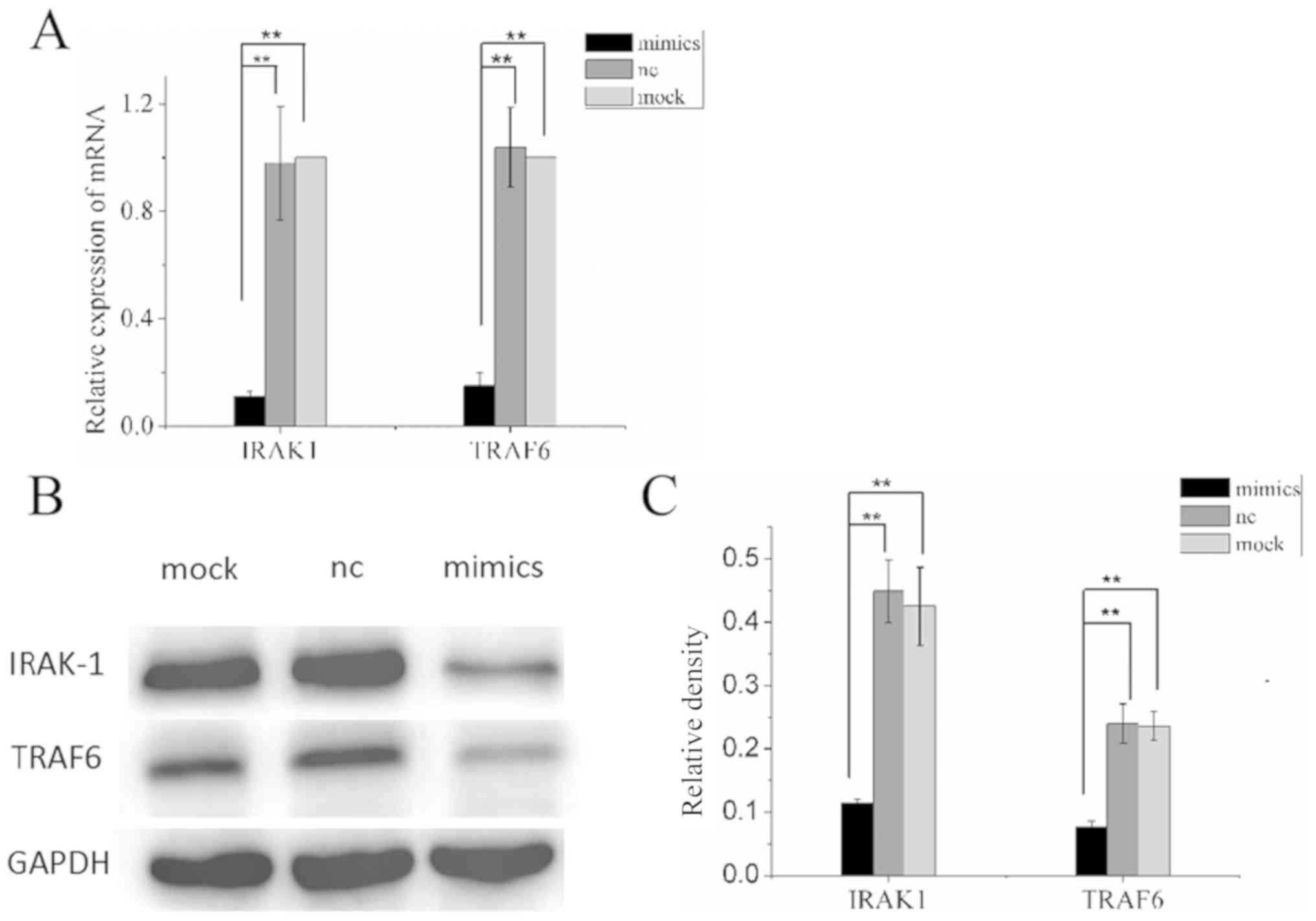
Overexpression of miR-146a inhibits
IRAK1 and TRAF6 in A549 cells
To further examine the signaling pathway that
miR-146a regulates, and prove that miR-146a can regulate
IRAK1 and TRAF6, the mRNA and protein expression
levels of these two genes were measured by RT-qPCR and western blot
analysis. RT-qPCR assay showed that mRNA levels of both target
genes in the experimental group transfected with miR-146a mimics
were significantly lower than in the nc and mock groups (P<0.05
and P<0.01; Fig. 7A). In
addition, the results of the western blot experiment also indicated
that the protein levels of IRAK1 and TRAF6 were
inhibited after miR-146a overexpression in A549 cells (P<0.05
and P<0.01; Fig. 7B and C). This
investigation of the underlying mechanism shows that miR-146a can
regulate the expression of IRAK1 and TRAF6, at the
mRNA and protein level.
Discussion
With the development of modern gene technologies and
the exploration of candidate genes and genomes, evidence has shown
that lung cancer cells accumulate a large number of gene mutations
during cancer development and progression, including inactivation
of tumor suppressor genes and activation of oncogenes, which
promote the growth or survival of tumor cells (19). miRNA molecules have been identified
as a class of novel regulatory molecules that negatively regulate
gene expression. These non-coding small RNAs are generally 18–25
nucleotides long, and they form gene regulation networks through
complex interactions with their target genes, which are cell- and
tissue-specific, and can regulate many physiological and
pathological processes in humans (20). The main mechanism of miRNA regulation
is to induce the degradation of target mRNA and inhibit its
post-transcriptional translation by completely or partially
complementing the 3′-UTR of the target mRNA (21). A number of studies have reported
abnormal expression of miRNA to be associated with the pathogenesis
of various human tumors; miRNA has functions similar to those of
proto-oncogenes or tumor suppressor genes, in addition to playing
important roles in the development and progression of tumors
(22,23).
Lung cancer is the most common clinical primary
malignant tumor, with 80–85% of cases being NSCLC, and lung
adenocarcinoma is the main type of NSCLC (24). Due to the difficulty of early
diagnosis of lung adenocarcinoma, the majority of cases are
diagnosed at the advanced stage and are difficult to radically
resect surgically. Therefore, chemotherapy and targeted therapy are
the main treatments for NSCLC. However, unsatisfactory curative
effect and poor prognosis are observed in a large number of
patients (25). The identification
and assessment of new therapeutic targets has become urgent in the
diagnosis and treatment of lung adenocarcinoma. miR-146a plays
important roles in the development and progression of many types of
tumors, and it has been widely studied and reported in recent
years. The expression of miR-146a is decreased significantly in
breast (26,27), liver (28,29),
prostate (30) and bladder cancer
(31), and upregulation of miR-146a
can inhibit the proliferation, invasion, and metastasis of these
tumor cells and promote apoptosis (26–31);
however, the expression of miR-146a is significantly higher in
thyroid follicular carcinoma (32,33) and
oral carcinoma (34), and the
downregulation of miR-146a expression can inhibit the malignant
biological behaviors of tumor cells. In addition, the negative
feedback mechanism associated with miR-146a is not applicable to
all tumor cells (35). The
underlying mechanism of miR-146a in different types of tumor cells
requires further study.
In 2011, Vinci et al (13) measured the expression of several
miRNAs in NSCLC and found the expression of miR-146a to be
increased, therefore increasing the risk of NSCLC. In 2015, Wang
et al (36) also observed the
upregulation of miR-146a in NSCLC by RT-qPCR. However, in 2013,
Chen et al (37) reported
that the expression of miR-146a was reduced in NSCLC, which
suggested that miR-146a may have a role as a tumor suppressor gene.
Wang et al (38) showed
similar results. These data indicated that, in lung cancer, the
role of miR-146a and whether it acts as an oncogene or a tumor
suppressor gene remains debatable.
In this study, the expression of miR-146a in BEAS-2B
human normal lung epithelial cells and three human lung
adenocarcinoma cell lines (H1299, A549, and PC-9) were measured
using RT-qPCR. The results showed that the expression of miR-146a
in human lung adenocarcinoma cell lines was significantly lower
than that in human normal lung epithelial cells, indicating that
miR-146a plays an important role as a tumor suppressor gene in
NSCLC. The extensively used human lung adenocarcinoma cell line
A549, which has high miR-146a expression was examined. Therefore,
these cells were transfected with miR-146a using the liposome
method to establish miR-146a-overexpressing A549 cells. The results
showed that overexpression of miR-146a not only inhibited the
proliferation of A549 cells, but also promoted apoptosis and
inhibited the migratory ability of A549 cells, suggesting the role
of miR-146a as a tumor suppressor gene in lung cancer.
One miRNA can target several mRNAs, and one target
gene can be co-regulated by multiple miRNAs (39); the biological functions of miRNAs
mainly depend on the roles of the downstream target genes. In 2010,
Li et al (40) reported that,
in pancreatic cancer, the inhibitory effect of miR-146 on the
invasion of pancreatic cancer cells was associated with the
downregulation of metastasis associated 1 family member 2, IRAK1,
epidermal growth factor receptor (EGFR), and NF-κB signaling
pathways by miR-146. In 2011, Garcia et al (41) reported that miR-146a could promote
the proliferation and invasion of triple-negative breast cancer
cells by downregulating BRCA1. In 2014, Forloni et al
(42) reported that miR-146a could
promote the development and progression of melanoma by activating
the Notch signaling pathway. In 2013, Chen et al (37) indicated that upregulation of miR-146a
significantly decreased the expression of EGFR in NSCLC cell lines.
In 2014, Cornett and Lutz (43) found that miR-146a could target
cyclooxygenase 2 in NSCLC. In 2016, Li et al (14) showed that miR-146a could inhibit the
proliferation and cell cycle progression of NSCLC cells by
targeting cyclin D1 and cyclin D2. In this study, the downstream
target genes of miR-146a were predicted using the bioinformatics
tools Target Scan, Pictar, and miRDB, which indicated that miR-146a
may regulate IRAK1 and TRAF6 by binding to the 3′-UTR of IRAK1 and
TRAF6. In 2013, Hung et al (34) reported that miR-146a enhanced the
carcinogenicity of oral cancer by targeting IRAK1 and TRAF6
simultaneously. However, the role of IRAK1 and TRAF6 as miR-146a
target genes in NSCLC, to the best of our knowledge, has not been
reported.
In this study, the target genes were verified by the
dual-luciferase reporter assay system, and the results showed the
activity ratios of firefly luciferase to Renilla luciferase
in A549 cells transfected with miR-146a-mimics + IRAK1-WT and
miR-146a-mimics + TRAF6-WT to be significantly lower than those
transfected with miR146a-nc+IRAK1-WT and miR146a-nc + TRAF6-WT. In
the control groups, however, the activity ratios in A549 cells
transfected with miR-146a-nc + IRAK1-MUT and miR146a-mimics +
IRAK1-WT, and miR-146a-mimics + TRAF6-MUT and miR146a-nc +
TRAF6-MUT, showed no significant difference. The above results
confirmed that miR-146a could specifically bind to the 3′-UTR of
IRAK1 and TRAF6, therefore, blocking the
transcription of the luciferase gene and decreasing the ratio of
luciferase activity. However, when the 3′-UTR of IRAK1 and
TRAF6 were mutated, the binding sites of miR-146a were
destroyed, and the transcription of the luciferase gene was not
affected, so the ratio of luciferase activity did not differ
significantly. This indicated that IRAK1 and TRAF6
are the target genes directly regulated by miR-146a.
In this study, the effects of miR-146a on mRNA and
protein levels were also verified by RT-qPCR and western blot
analysis, respectively. The results indicated that, in the mimics
group, the relative expressions of IRAK1 and TRAF6 mRNA and the
relative gray values of proteins were lower and weaker than those
in the nc and mock groups (P<0.05), which further confirmed that
miR-146a can simultaneously target IRAK1 and TRAF6 activities and
therefore act as a tumor suppressor. Additionally, in 2006, Taganov
et al (44) reported that
miR-146a could bind to IRAK1 and TRAF6, which encode the adaptor
molecules between Toll-like receptors (TLRs) and NF-κB, inhibiting
the expression of IRAK1 and TRAF6, therefore reducing the
downstream NF-κB activity after TLR activation. Labbaye and
Testa (45) confirmed the
aforementioned results in 2012. In 2015, Park et al
(46) and Yousefzadeh et al
(47) also reported that miR-146a
inhibits the activation of the NF-κB pathway by inhibiting the
expression of IRAK1 and TRAF6, reducing the production of
inflammatory cells and preventing the immune system from
overreacting. Therefore, in lung cancer miR-146a may increase
activation of the NF-κB signaling pathway by targeting IRAK1 and
TRAF6. Further investigation on the specific mechanisms involved is
required.
In conclusion, the expression of miR-146a in human
lung adenocarcinoma cells decreased significantly, so it may play a
role as a tumor suppressor gene. The upregulation of miR-146a
significantly inhibited the proliferation, growth and metastasis of
human lung adenocarcinoma cell line A549 and promoted apoptosis.
miR-146a likely exerts its biological functions by targeting the
activity of IRAK1 and TRAF6 genes, therefore
affecting the downstream signaling pathways. However, whether
miR-146a also regulates other target genes to inhibit the activity
of lung cancer cells requires further study. Further exploration
and understanding of the mechanism by which miR-146a acts in lung
adenocarcinoma may provide new effective targets for clinical
molecular therapy for lung cancer.
Acknowledgements
Not applicable.
Funding
This work is partially supported by the Medical
Elite Cultivation Program of Fujian (grant no., 2013-ZQN-JC-14),
The Fourth Round of Fujian Provincial Joint Tackling Project
between Health and Education-Joint Research Fund of National Health
and Family Planning Commission of China (grant no., WKJ2016-2-30),
the Fujian Medical Innovation Project (grant no., 2014-CX-14) and
the Science Technology Innovation Joint Project Foundation of
Fujian Province (grant no. 2016Y9028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and TL conceived the study, and analyzed and
interpreted the data. FY performed the experiments, analyzed data
and wrote the manuscript. SZ and WX performed the experiments and
analyzed data. SY designed experiments, analyzed the data, polished
the manuscript and guided the reply to the comments. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Voltaggio L, Cimino-Mathews A, Bishop JA,
Argani P, Cuda JD, Epstein JI, Hruban RH, Netto GJ, Stoler MH,
Taube JM, et al: Current concepts in the diagnosis and pathobiology
of intraepithelial neoplasia: A review by organ system. CA Cancer J
Clin. 66:408–436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berghmans T, Pasleau F, Paesmans M,
Bonduelle Y, Cadranel J, Cs Toth I, Garcia C, Giner V, Holbrechts
S, Lafitte JJ, et al: Surrogate markers predicting overall survival
for lung cancer: ELCWP recommendations. Eur Respir J. 39:9–28.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feber A, Xi L, Luketich JD, Pennathur A,
Landreneau RJ, Wu M, Swanson SJ, Godfrey TE and Litle VR: MicroRNA
expression profiles of esophageal cancer. J Thorac Cardiovasc Surg.
135:255–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cufer T and Knez L: Update on systemic
therapy of advanced non-small-cell lung cancer. Expert Rev
Anticancer Ther. 14:1189–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Domingues D, Turner A, Silva MD, Marques
DS, Mellidez JC, Wannesson L, Mountzios G and de Mello RA:
Immunotherapy and lung cancer: Current developments and novel
targeted therapies. Immunotherapy. 6:1221–1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casaluce F, Sgambato A, Sacco PC,
Palazzolo G, Maione P, Rossi A, Ciardiello F and Gridelli C:
Emerging drugs targeting PD-1 and PD-L1: Reality or hope? Expert
Opin Emerg Drugs. 19:557–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CM, Chu WY, Wong DL, Tsang HF, Tsui NB,
Chan CM, Xue VW, Siu PM, Yung BY, Chan LW and Wong SC: Current and
future molecular diagnostics in non-small-cell lung cancer. Expert
Rev Mol Diagn. 15:1061–1074. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slezak-Prochazka I, Durmus S, Kroesen BJ
and van den Berg A: MicroRNAs, macrocontrol: Regulation of miRNA
processing. RNA. 16:1087–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eder M and Scherr M: MicroRNA and lung
cancer. N Engl J Med. 352:2446–2448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vinci S, Gelmini S, Pratesi N, Conti S,
Malentacchi F, Simi L, Pazzagli M and Orlando C: Genetic variants
in miR-146a, miR-149, miR-196a2, miR-499 and their influence on
relative expression in lung cancers. Clin Chem Lab Med.
49:2073–2080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li YL, Wang J, Zhang CY, Shen YQ, Wang HM,
Ding L, Gu YC, Lou JT, Zhao XT, Ma ZL and Jin YX: miR-146a-5p
inhibits cell proliferation and cell cycle progression in NSCLC
cell lines by targeting CCND1 and CCND2. Oncotarget. 7:59287–59298.
2016.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W and Kong L, Ni Q, Lu Y, Ding W,
Liu G, Pu L, Tang W and Kong L: miR-146a ameliorates liver
ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS
One. 9:e1015302014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santra M, Zhang ZG, Yang J, Santra S,
Chopp M and Morris DC: Thymosin β4 up-regulation of microRNA-146a
promotes oligodendrocyte differentiation and suppression of the
Toll-like proinflammatory pathway. J Biol Chem. 289:19508–19518.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Selvamani SP, Mishra R and Singh SK:
Chikungunya virus exploits miR-146a to regulate NF-κB pathway in
human synovial fibroblasts. PLoS One. 9:e1036242014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zochbauer-Muller S, Gazdar AF and Minna
JD: Molecular pathogenesis of lung cancer. Annu Rev Physiol.
64:681–708. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Makeyev EV and Maniatis T: Multilevel
regulation of gene expression by microRNAs. Science. 319:1789–1790.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Van Den Berg A, Mols J and Han J:
RISC-target interaction: Cleavage and translational suppression.
Biochim Biophys Acta. 1779:668–677. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dillhoff M, Wojcik SE and Bloomston M:
MicroRNAs in solid tumors. J Surg Res. 154:349–354. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vychytilova-Faltejskova P and Slaby O:
Circulating blood-borne microRNAs as biomarkers in solid tumors.
Exp Suppl. 106:75–122. 2015.PubMed/NCBI
|
|
24
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martinez P, Martinez-Marti A, Navarro A,
Cedres S and Felip E: Molecular targeted therapy for early-stage
non-small-cell lung cancer: Will it increase the cure rate? Lung
cancer. 84:97–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zavala V, Perez-Moreno E, Tapia T, Camus M
and Carvallo P: miR-146a and miR-638 in BRCA1-deficient triple
negative breast cancer tumors, as potential biomarkers for improved
overall survival. Cancer Biomark. 16:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomokuni A, Eguchi H, Tomimaru Y, Wada H,
Kawamoto K, Kobayashi S, Marubashi S, Tanemura M, Nagano H, Mori M
and Doki Y: miR-146a suppresses the sensitivity to interferon-alpha
in hepatocellular carcinoma cells. Biochem Biophys Res Commun.
414:675–680. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kao YY, Tu HF, Kao SY, Chang KW and Lin
SC: The increase of oncogenic miRNA expression in tongue
carcinogenesis of a mouse model. Oral Oncol. 51:1103–1112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu R, Yi B, Wei S, Yang WH, Hart KM,
Chauhan P, Zhang W, Mao X, Liu X, Liu CG and Wang L:
FOXP3-miR-146-NF-κB axis and therapy for precancerous lesions in
prostate. Cancer Res. 75:1714–1724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiang W, Wu X, Huang C, Wang M, Zhao X,
Luo G, Li Y, Jiang G, Xiao X and Zeng F: PTTG1 regulated by
miR-146a-3p promotes bladder cancer migration, invasion, metastasis
and growth. Oncotarget. 8:664–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma W, Zhao X, Liang L, Wang G, Li Y, Miao
X and Zhao Y: miR-146a and miR-146b promote proliferation,
migration and invasion of follicular thyroid carcinoma via
inhibition of ST8SIA4. Oncotarget. 8:28028–28041. 2017.PubMed/NCBI
|
|
33
|
Zhang X, Li D, Li M, Ye M, Ding L, Cai H,
Fu D and Lv Z: MicroRNA-146a targets PRKCE to modulate papillary
thyroid tumor development. Int J Cancer. 134:257–267. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hung PS, Liu CJ, Chou CS, Kao SY, Yang CC,
Chang KW, Chiu TH and Lin SC: miR-146a enhances the oncogenicity of
oral carcinoma by concomitant targeting of the IRAK1, TRAF6 and
NUMB genes. PLoS One. 8:e799262013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saba R, Sorensen DL and Booth SA:
MicroRNA-146a: A dominant, negative regulator of the innate immune
response. Front Immunol. 5:5782014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang RJ, Zheng YH, Wang P and Zhang JZ:
Serum miR-125a-5p, miR-145 and miR-146a as diagnostic biomarkers in
non-small cell lung cancer. Int J Clin Exp Pathol. 8:765–771.
2015.PubMed/NCBI
|
|
37
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Greve J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang WM and Liu JC: Effect and molecular
mechanism of mir-146a on proliferation of lung cancer cells by
targeting and regulating MIF gene. Asian Pac J Trop Med. 9:806–811.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thomson DW, Bracken CP and Goodall GJ:
Experimental strategies for microRNA target identification. Nucleic
Acids Res. 39:6845–6853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Vandenboom TG II, Wang Z, Kong D,
Ali S, Philip PA and Sarkar FH: miR-146a suppresses invasion of
pancreatic cancer cells. Cancer Res. 70:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Garcia AI, Buisson M, Bertrand P, Rimokh
R, Rouleau E, Lopez BS, Lidereau R, Mikaelian I and Mazoyer S:
Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in
triple negative sporadic breast cancers. EMBO Mol Med. 3:279–290.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Forloni M, Dogra SK, Dong Y, Conte D Jr,
Ou J, Zhu LJ, Deng A, Mahalingam M, Green MR and Wajapeyee N:
miR-146a promotes the initiation and progression of melanoma by
activating Notch signaling. Elife. 3:e014602014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cornett AL and Lutz CS: Regulation of
COX-2 expression by miR-146a in lung cancer cells. RNA.
20:1419–1430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Labbaye C and Testa U: The emerging role
of MIR-146A in the control of hematopoiesis, immune function and
cancer. J Hematol Oncol. 5:132012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yousefzadeh N, Alipour MR and Soufi FG:
Deregulation of NF-κB-miR-146a negative feedback loop may be
involved in the pathogenesis of diabetic neuropathy. J Physiol
Biochem. 71:51–58. 2015. View Article : Google Scholar : PubMed/NCBI
|