Introduction
Esophageal neuroendocrine neoplasms (ENENs) are
exceedingly uncommon but increasingly prevalent malignancies with
high heterogeneity. In 1997, only three patients (0.05%) were
documented in the esophagus among 6,122 cases of gastrointestinal
carcinoid tumors (1); In Korea,
ENENs only accounted for 1.3–1.4% of the gastrointestinal NENs
reported from 2012 to 2014 (2,3). They
are characterized by distinct histological features and tumor
behavior compared with esophageal adenocarcinoma (EAC) and
esophageal squamous cell carcinoma (ESCC) (4,5). Owing
to its rarity, only a few cases concerning the ENENs have been
reported in the literature in the previous two decades, with most
focusing on the clinicopathological characteristics (3,5–7) and treatment (8,9). As a
result, a staging system that can accurately provide prognostic
information and stratify patients by risk are far from being well
established up to date. The Tumor-Node-Metastasis (TNM)
classification is the most frequently used indicator of outcomes
for malignancies (10,11). Several studies have indicated that
the 7th American Joint Committee on Cancer (AJCC) TNM staging
system for EACC may also be applied to describe ENENs (5,6).
However, this classification for ENENs never achieved widespread
acceptance, due to the relatively small population size. More
recently, the 8th AJCC staging system on esophageal cancer, which
was introduced in 2016, has been recommended to replace the old
version. The current (8th) TNM classification presents three
classifications separately for both EAC and ESCC: The classic
pathological stage groups (pTNM) expanded from the seventh edition
foundation, the newly introduced postneoadjuvant pathologic stage
groups (ypTNM), and clinical stage groups (cTNM) (Tables SI and SII) (12–14). The
definition of T was optimized in the 8th classification, and the
descriptors of N and M remained unchanged. The current N
classification has already incorporated the number of lymph nodes
with metastasis into the staging protocols. The N classification
for solid cancer of various origins, including
gastroenteropancreatic neuroendocrine neoplasms, only distinguishes
between node-negative and node-positive disease, and the lymph
nodes status was demonstrated to exhibit good discrimination of
prognosis (15–17). However, to the best of our knowledge,
the prognostic value of both the 8th AJCC staging system and lymph
nodes status has not been validated in ENENs, particularly within
large population-based samples.
In the present study, population-based data was used
to validate the current TNM staging systems, and a new modified
staging classification which adopted the revised N definition was
tested that would address the weaknesses associated with the
staging system and predict the prognosis of patients with ENENs
more accurately and quickly.
Patients and methods
Patients and data collection
The data used in the present study was retrieved
between 1973 and 2015 (months unavailable) from the Surveillance,
Epidemiology, and End Results registry (SEER) database (seer.cancer.gov) of the US National Cancer Institute.
Eligible patients were those who were diagnosed with pathologically
confirmed ENENs (Appendix S1). The
demographics and tumor variables (race, sex, age, location, grade,
survival months and SEER cause-specific death classification) were
retrieved from SEER. A tumor location (upper, middle and lower) was
assigned for each patient on the basis of retrospective review of
SEER data. The data on primary tumor size, depth of invasion, local
extension, lymph node involvement and presence/absence of distant
metastases were used to determine the TNM categories (Appendix S1). Individuals with unknown
follow-up information, unknown cancer stage at diagnosis and who
had presented with other primary tumors were excluded. The patients
with unknown extent of tumor or lymph node status were included in
the study if they had distant metastases. A total of 300 eligible
patients with pathologically confirmed ENENs were identified from
the SEER database. A total of 63 patients who had other primary
cancer types, 45 patients with unknown cancer stage at diagnosis
and one patient with unknown follow-up information, were excluded;
leaving a final cohort of 191 patients in the study (Table I).
 | Table I.Baseline clinicopathological
characteristics. |
Table I.
Baseline clinicopathological
characteristics.
| Characteristic | SEER, n=191 | Multicentric
database, n=51 |
|---|
| Median age, years
(IQR) | 65.0 (57.5–72.0) | 60.0 (53.5–67.5) |
| Sex, n (%) |
|
|
| Male | 159 (83.2) | 34 (66.7) |
|
Female | 32 (16.8) | 17 (33.3) |
| Race, n (%) |
|
|
|
White | 165 (86.4) | 0 (0.0) |
|
Black | 13 (6.8) | 0 (0.0) |
|
Other | 13 (6.8) | 51 (100.0) |
| Location, n (%) |
|
|
|
Upper | 11 (5.8) | 9 (17.6) |
|
Middle | 30 (15.7) | 21 (41.2) |
|
Lower | 108 (56.5) | 16 (31.4) |
|
Unknown | 42 (22.0) | 5 (9.8) |
| Grade, n (%) |
|
|
|
G1-G2 | 5 (2.6) | 0 (0.0) |
|
G3-G4 | 151 (79.1) | 36 (70.6) |
|
Unknown | 35 (18.3) | 15 (29.4) |
| Median tumor size, cm
(IQR) | 6.2 (4.0–8.0) | 3.9 (3.0–5.0) |
| Median Ki67,
(IQR) | NR | 0.80 (0.70–0.90) |
| Cell morphology, n
(%) | NR |
|
| Small
cell |
| 17 (33.3) |
| Non-small
cell |
| 34 (66.7) |
| T
statusa, n (%) |
|
|
| T1 | 31/66 (47.0) | 10/33 (30.3) |
| T2 | 7/66 (10.6) | 14/33 (42.4) |
| T3 | 19/66 (28.8) | 9/33 (27.3) |
|
T4a | 2/66 (3.0) | 0/33 (0.0) |
|
T4b | 7/66 (10.6) | 0/33 (0.0) |
| N
statusa, n (%) |
|
|
| N0 | 38/66 (57.6) | 13/33 (39.4) |
| N1 | 21/66 (31.8) | 11/33 (33.30) |
| N2 | 6/66 (9.1) | 8/33 (24.2) |
| N3 | 1/66 (1.5) | 1/33 (3.0) |
| Regional lymph
nodes involvement statusa, n (%) |
|
|
|
Negative | 38/66 (57.6) | 13/33 (39.4) |
|
Positive | 28/66 (42.4) | 20/33 (60.6) |
| M status, n
(%) |
|
|
| M0 | 66 (34.6) | 33 (64.7) |
| M1 | 125 (65.4) | 18 (35.5) |
| pTNM for ESCC, n
(%) |
|
|
| I | 23
(12.0) | 5 (9.8) |
|
IA | 8
(4.2) | 0 (0.0) |
|
IB | 15 (7.8) | 5 (9.8) |
| II | 19 (9.9) | 10 (19.6) |
|
IIA | 7
(3.7) | 6 (11.8) |
|
IIB | 12 (6.2) | 4 (7.8) |
|
III | 16 (8.4) | 17 (33.3) |
|
IIIA | 4
(2.1) | 8 (15.7) |
|
IIIB | 12 (6.3) | 9 (17.6) |
| IV | 133 (69.6) | 19 (37.3) |
|
IVA | 8
(4.2) | 1 (2.0) |
|
IVB | 125 (65.4) | 18 (35.3) |
| pTNM for EAC, n
(%) |
|
|
| I | 23 (12.0) | 5 (9.8) |
|
IA | 8 (4.2) | 0 (0.0) |
|
IB | 1 (0.5) | 1 (2.0) |
|
IC | 14 (7.3) | 4 (7.8) |
| II | 19 (9.9) | 10 (19.6) |
|
IIA | 2 (1.0) | 6 (11.8) |
|
IIB | 17 (8.9) | 4 (7.8) |
|
III | 16 (8.4) | 17 (33.3) |
|
IIIA | 4 (2.1) | 8 (15.7) |
|
IIIB | 12 (6.3) | 9 (17.6) |
| IV | 133 (69.6) | 19 (37.3) |
|
IVA | 8 (4.2) | 1 (2.0) |
|
IVB | 125 (65.4) | 18 (35.3) |
| cTNM for ESCC, n
(%) |
|
|
| I | 31 (16.2) | 7 (13.7) |
| II | 15 (7.9) | 13 (25.5) |
|
III | 10 (5.2) | 12 (23.5) |
| IV | 135 (70.7) | 19 (37.3) |
|
IVA | 10 (5.2) | 1 (2.0) |
|
IVB | 125 (65.4) | 18 (35.3) |
| cTNM for EAC, n
(%) |
|
|
| I | 23 (12.0) | 5 (9.8) |
| II | 10 (5.2) | 8 (15.7) |
|
IIA | 8 (4.2) | 2 (3.9) |
|
IIB | 2 (1.0) | 6 (11.8) |
|
III | 19 (9.9) | 11 (21.6) |
| IV | 139 (72.8) | 27 (52.9) |
|
IVA | 14 (7.3) | 9 (17.6) |
|
IVB | 125 (65.4) | 18 (35.3) |
| Modified staging
system, n (%) |
|
|
| I | 23 (12.0) | 5 (9.8) |
| II | 11 (5.8) | 8 (15.7) |
|
III | 25 (13.1) | 20 (39.2) |
| IV | 132 (69.1) | 18 (35.3) |
|
IVA | 7 (3.7) | 0 |
|
IVB | 125 (65.4) | 18 (35.3) |
| Median follow-up
time, months (IQR) | 6.0 (2.0–16.0) | 7.0 (4.0–16.0) |
| Median DSS time,
months (95% CI) | 9.0 (6.6–11.4) | 16.0
(11.5–20.5) |
| Median DSS time for
limited disease, months (95% CI) | 22.0
(15.2–28.8) | 19.0
(13.9–24.1) |
| Median DSS time for
extensive disease, months (95% CI) | 6.0 (4.3–7.7) | 6.0 (4.4–7.6) |
Three Chinese centers provided data on the ENENs for
the validation analysis: Wuhan Union Hospital (Wuhan; between
January 2011 and June 2018; n=16), Hubei Cancer Hospital
(Wuhan; from January 2009 to December 2016; n=6), and Wuhan
Tongji Hospital (Wuhan; from Jan 1, 2012, to Jun 1, 2017,
N=29). The inclusion and exclusion criteria were identical
to those used in the development cohort. A total of 51 patients
from the multicentric database were included in the validation
analysis. In addition to the demographics and tumor variables
aforementioned, the Ki67 and cell morphology were also investigated
Reports with no mention of small-cell morphology present or
large-cell morphology were grouped into the non-small-cell
classification. The classification of cancer location was based on
the distance from the epicenter of the tumor to the incisors as
follows: Upper (>15 and ≤24 cm); middle (>24 and ≤33 cm); and
lower (>33 and ≤42 cm). Approval for the study protocol was
obtained by the Institutional Ethics Committee of the Tongji
Medical College, Huazhong University of Science and Technology.
Written informed consent was provided all the patients for their
data to be used for research.
Statistical analysis
Descriptive statistics were used to report the
demographic characteristics of enrolled patients and stage
distribution. The limited disease was defined as a neoplasm without
distant metastases, and the extensive disease was defined as a
neoplasm that transfer to parts of the body remote from the primary
tumor. Since patients with distant metastasis were categorized in
the advanced stage, only the T and N statuses of patients with
limited disease were reported. Patients who succumbed to causes
other than cancer were censored at their date of death.
Disease-specific survival (DSS) was estimated via the Kaplan-Meier
method and the log-rank test was used to compare the survival
curves between groups using SPSS software (version 22.0; IBM
Corp.). The T, N and M categories were combined according to the
current stage groups for EAC and ESCC. The potential overlaps in
outcomes among groups were further investigated by modification of
the current staging classifications. Multivariate analysis was
performed to determine which N classifications were able to
independently predict outcomes using the Cox proportional hazards
regression. On the basis of the current findings, a Nr
classification (Nr0, negative lymph nodes; Nr1, positive lymph
nodes) was proposed and the survival for all possible T and Nr
combinations (such as T1Nr0M0, T1Nr1M0 and T2Nr0M0), was estimated;
thus, a modified staging system for ENENs was devised. The Cox
proportional hazards model was used to evaluate the association
between each staging classification and DSS after adjusting for
race, sex, age, tumor grade and tumor location. Only race, sex and
age were adjusted in the Cox regression model for pTNM, given that
the grade and tumor location had been assigned to the 8th AJCC
pathological staging classifications. Hazard ratios (HRs) and 95%
confidence intervals (CIs) were calculated. Concordance indices
were calculated to evaluate the discriminatory powers of the
staging systems. A concordance index (C-index) of 1 represents
perfect discrimination, and a C-index of 0.5 denoted agreement by
chance alone. Analyses were performed using SPSS (version 22.0; IBM
Corp.), RStudio [version 1.1.456; (18)] and R [version 3.5.1; (19)] software. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient and tumor characteristics
A final cohort of 191 patients from the SEER
database were included in the study (Table I). The median age at diagnosis was
65.0 years (range, 57.5–72.0 years) with a male to female ratio of
5.0. The majority of tumors were located in the middle (15.7%) or
lower (56.5%) esophagus, and 79.1% of the tumors were poorly
differentiated. A total of 125 (65.4%) patients had metastatic
disease. Of the entire study population, the median DSS was 9.0
months. The median DSS for limited disease and extensive disease
were 22.0 and 6.0 months, respectively.
The median age for the multicentric database was
60.0 years (range, 53.5–67.5 years) with a male to female ratio of
2.0. Notably, ~73% of patients had a tumor located in the middle
(41.2%) or lower (31.4%) of esophagus and 70.6% of the tumors were
poorly differentiated. Small cell carcinoma accounted for 33.3% of
all types of ENENs in the present study. A total of 18 (35.3%)
patients had metastatic disease. The median DSS times for the whole
cohort, limited disease and extensive disease were 16.0, 19.0 and
6.0 months, respectively (Table
I).
Pathologic stage groups and
survival
Survival curves according to current pTNM were
analyzed. As presented in Table I,
the proportion of stage groups without further subgroup
classifications for EAC and ESCC was the same. The distribution was
as follows in the SEER database; Stage I (12.0%); stage II (9.9%);
stage III (8.4%) and stage IV (69.6%). Multicenter series was as
follows; Stage I (9.8%); stage II (19.6%); stage III (33.3%) and
stage IV (37.3%). The Kaplan-Meier analysis revealed progressively
poorer DSS with more advanced stages in both EAC and ESCC [median
DSS: Stage I (80.1 months); stage II (25.6 months); stage III (16.3
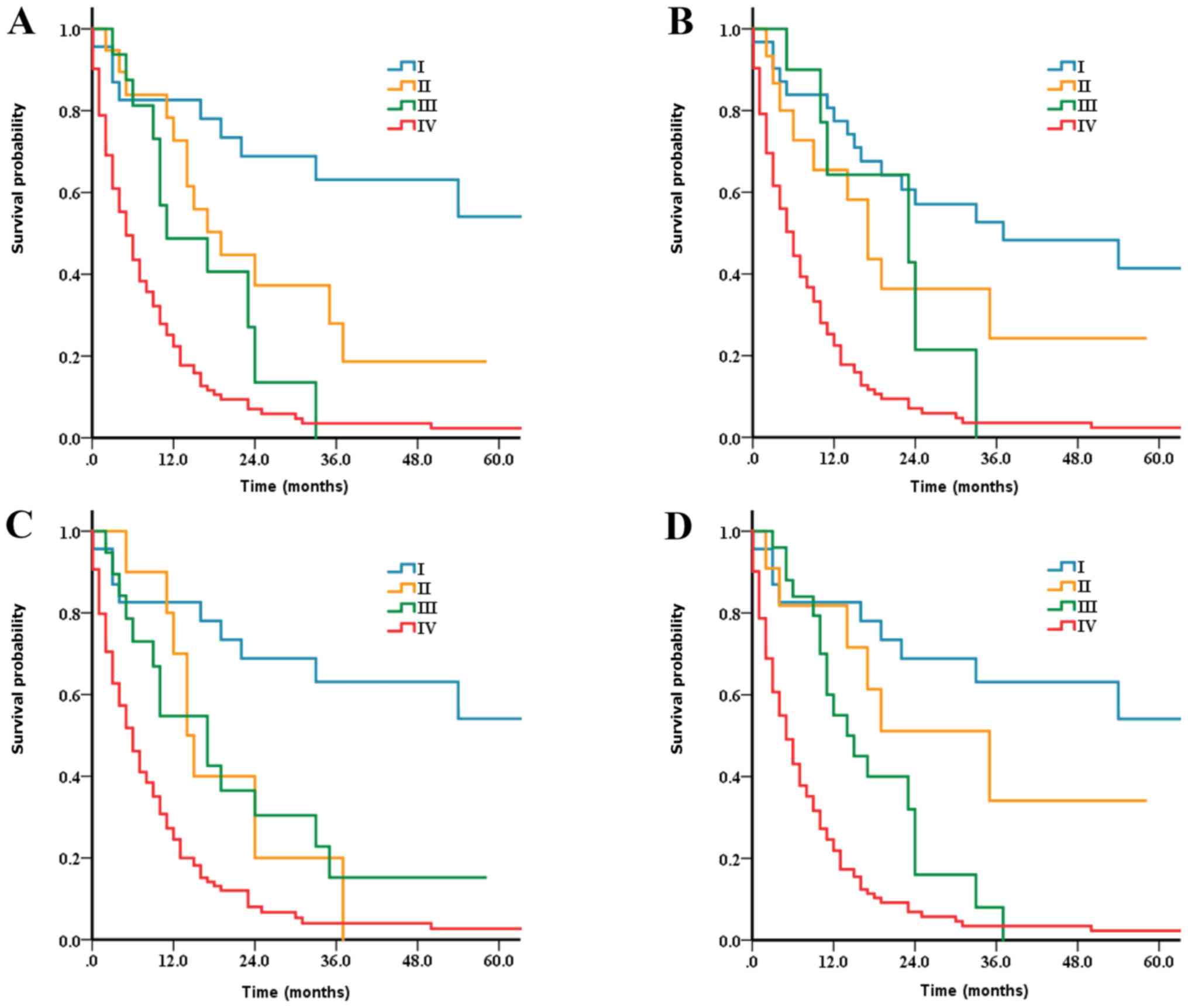
months) and stage IV (9.6 months); Fig.
1A], with overlaps between stage II and stage III disease for
the SEER series (P=0.177 for stage II vs. stage III). For the
multicentric series (Fig. 2A), there
were also overlaps in the survival of patients with stages II and
III disease (P=0.402). A significant increase in HRs was observed
in stages I–III in the SEER series compared with stage IV, via
multivariate analysis (Table II).
Furthermore, multivariate analysis in the multicentric series also
demonstrated increased risk of mortality of the patients in stages
I–IV (Table III).
 | Table II.Multivariate analysis of prognostic
factors from SEER database. |
Table II.
Multivariate analysis of prognostic
factors from SEER database.
|
| pTNM for ESCC and
EAC | cTNM for ESCC | cTNM for EAC | Modified staging
system |
|---|
|
|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 1.01
(0.997–1.03) | 0.110 | 1.01
(0.995–1.03) |
0.176 | 1.01
(0.993–1.03) | 0.243 | 1.01
(0.99–1.027) | 0.219 |
| Sex |
|
|
|
|
|
|
|
|
|
Male | 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Female | 0.75
(0.45–1.24) | 0.485 | 0.75
(0.46–1.24) |
0.267 | 0.78
(0.47–1.30) | 0.337 | 0.76
(0.46–1.26) | 0.283 |
| Race |
|
|
|
|
|
|
|
|
|
Other | 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Black | 1.17
(0.46–2.97) | 0.748 | 1.11
(0.43–2.85) |
0.824 | 1.09
(0.43–2.80) | 0.851 | 1.24
(0.48–3.20) | 0.658 |
|
White | 1.64
(0.85–3.18) | 0.139 | 1.86
(0.94–3.71) |
0.077 | 1.68
(0.84–3.36) | 0.140 | 1.82
(0.91–3.63) | 0.088 |
| Location | Not included |
|
|
|
|
|
|
|
|
Upper |
|
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Middle |
|
| 0.77
(0.35–1.71) |
0.525 | 0.71
(0.32–1.56) | 0.393 | 0.69
(0.31–1.52) | 0.354 |
|
Lower |
|
| 0.49
(0.25–0.96) |
0.038 | 0.43
(0.22–0.84) | 0.013 | 0.46
(0.23–0.90) | 0.023 |
|
Unknown |
|
| 0.71
(0.35–1.44) |
0.340 | 0.64
(0.31–1.31) | 0.224 | 0.66
(0.32–1.35) | 0.255 |
| Grade | Not included |
|
|
|
|
|
|
|
|
G1-G2 |
|
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
G3-G4 |
|
| 2.50
(0.59–10.59) |
0.212 | 1.99
(0.47–8.45) | 0.352 | 2.13
(0.50–9.04) | 0.305 |
| GX |
|
| 2.06
(0.47–9.02) |
0.336 | 1.86
(0.42- 8.15) | 0.412 | 1.82
(0.41–7.98) | 0.428 |
| Tumor size
(cm) | 0.995
(0.93–1.06) | 0.890 | 0.996
(0.93–1.06) |
0.911 | 0.992
(0.93–1.06) | 0.811 | 0.99
(0.93–1.06) | 0.782 |
| Stage |
|
|
|
|
|
|
|
|
| I | 0.12
(0.06–0.25) | <0.001 | 0.18
(0.10–0.32) | <0.001 | 0.13
(0.06–0.27) | <0.001 | 0.12
(0.06–0.25) | <0.001 |
| II | 0.28
(0.15–0.50) | <0.001 | 0.31
(0.16–0.60) | <0.001 | 0.41
(0.19–0.86) | 0.019 | 0.22
(0.09–0.50) | <0.001 |
|
III | 0.41
(0.22–0.77) | 0.006 | 0.31
(0.13–0.71) |
0.006 | 0.40
(0.22–0.71) | 0.002 | 0.39
(0.23–0.66) | <0.001 |
| IV | 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
 | Table III.Multivariate analysis of prognostic
factors from multicentric series. |
Table III.
Multivariate analysis of prognostic
factors from multicentric series.
|
| pTNM for ESCC and
EAC | cTNM for ESCC | cTNM for EAC | Modified staging
system |
|---|
|
|
|
|
|
|
|---|
| Characteristic | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | 1.02
(0.97–1.07) | 0.394 | 1.03
(0.99–1.09) | 0.162 | 1.06
(1.00–1.11) | 0.034 | 1.05
(0.99–1.11) | 0.079 |
| Sex |
|
|
|
|
|
|
|
|
|
Male | 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Female | 1.30
(0.54–3.14) | 0.564 | 1.51
(0.59–3.88) | 0.388 | 1.71
(0.66–4.43) | 0.266 | 1.72
(0.66–4.51) | 0.271 |
| Location | Not included |
|
|
|
|
|
|
|
|
Upper |
|
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Middle |
|
| 0.32
(0.08–1.27) | 0.105 | 0.20
(0.05–0.82) | 0.026 | 0.28
(0.07–1.07) | 0.063 |
|
Lower |
|
| 0.16
(0.03–0.99) | 0.048 | 0.10
(0.02–0.48) | 0.004 | 0.17
(0.03–0.88) | 0.035 |
|
Unknown |
|
| 0.20
(0.03–1.25) | 0.085 | 0.16
(0.03–1.02) | 0.052 | 0.20
(0.03–1.22) | 0.082 |
| Grade | Not included |
|
|
|
|
|
|
|
|
G3-G4 |
|
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
|
Unknown |
|
| 1.43
(0.56–3.69) | 0.457 | 1.28
(0.52–3.18) | 0.594 | 1.02
(0.39–2.64) | 0.968 |
| Tumor size, cm | 1.02
(0.69–1.51) | 0.919 | 0.95
(0.63–1.44) | 0.805 | 0.98
(0.66–1.46) | 0.929 | 0.996
(0.65–1.52) | 0.986 |
| Stage |
|
|
|
|
|
|
|
|
| I | <0.001
(0.000-inf) | 0.997 | 0.04
(0.004–0.44) | 0.009 | <0.001
(0.000-inf) | 0.998 | <0.001
(0.000-inf) | 0.998 |
| II | 0.24
(0.07–0.84) | 0.026 | 0.38
(0.12–1.22) | 0.104 | 0.32
(0.09–1.15) | 0.081 | 0.28
(0.06–1.37) | 0.116 |
|
III | 0.46
(0.17–1.24) | 0.125 | 0.49
(0.11–2.15) | 0.343 | 0.47
(0.15–1.49) | 0.199 | 0.51
(0.16–1.64) | 0.259 |
| IV | 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
| 1 (Ref) |
|
Clinical stage groups and
survival
According to the current cTNM for ESCC, it is
notable that overlaps in DSS were observed between stage I and II
diseases, and between stage II and III diseases for both the SEER
series (Fig. 1B) and the
multicentric database (Fig. 2B).
Patients with stage II disease and stage III disease exhibited
similar survival (median DSS time from SEER series: 17 vs. 23
months, P=0.992; median DSS from multicentric series: 19 vs. 19
months, P=0.697). Compared with stage IV disease, the HR of stage
II was comparable to that of stage III in both SEER series (Stages
II and III HRs, 0.31 and 0.31, respectively; Table II) and multicentric series (stages
II and III HRs, 0.38 and 0.49, respectively; Table III) by multivariate analysis.
According to the current cTNM for EAC, overlaps were
also revealed between the stage II and III diseases for both the
SEER series (Fig. 1C) and the
multicentric database (Fig. 2C).
Compared with stage IV disease, the HRs for stages III, II and I
diseases were 0.40 (95% CI, 0.22–0.71; P=0.002), 0.41 (95% CI,
0.19–0.86; P=0.019) and 0.13 (95% CI, 0.06–0.27; P<0.001),
respectively (Table II). Thus, the
risk of death increased in stage II, decreased from stage IV to III
and decreased further in stage I for the patients in the SEER
series. There was no significant difference in HR between stages II
and III for the multicentric series; compared with stage II: Stage
III (HR, 1.46; 95% CI, 0.36–5.87; P=0.598) (Table III).
Grouping of ENENs by lymph node
status
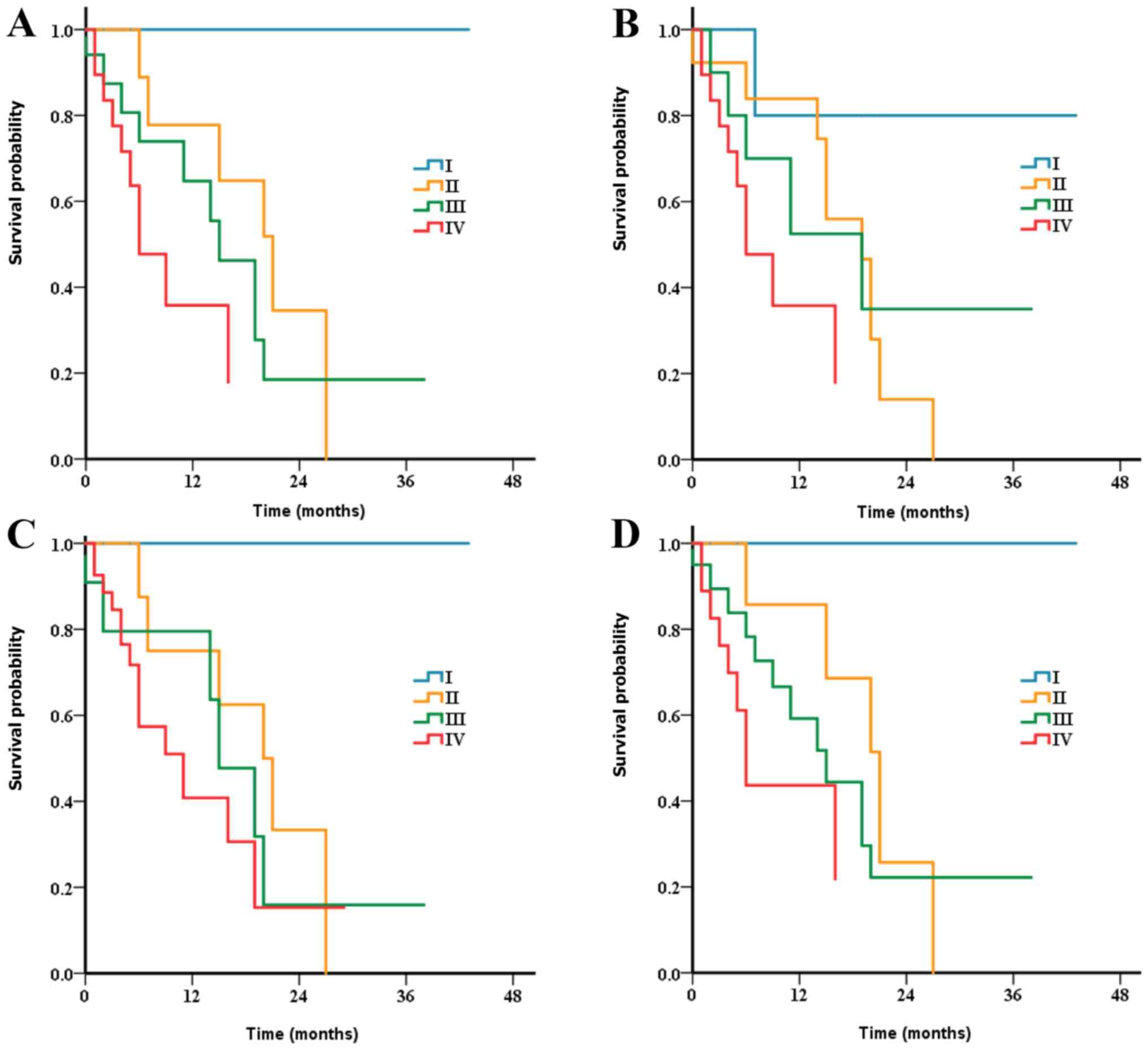
The Kaplan-Meier analysis exhibited no worsening DSS
as the number of positive lymph nodes increased for both SEER
series and multicentric series (Fig.
S1). Further multivariate analysis revealed no increased HRs of
death from stage N0 to stage N3 for the patients without metastases
from the SEER series; compared with stage N0 disease: N1 (HR, 2.70;
95% CI, 1.33–5.49; P=0.006); N2 (HR, 0.83; 95% CI, 0.18–3.92;
P=0.817); and N3 (HR <0.001, P=0.997) (Table SIII). However, patients with Nr0
exhibited a significantly improved DSS time compared with patients
with Nr1 disease according to the univariate and multivariate
analysis for both two cohorts (Table
SIV and SV).
Proposal and validation of new TNrM
stage groups
The Kaplan-Meier survival curves, including all T,
Nr and M combinations, indicated multiple overlaps across the
groups for patients with ENENs from the SEER series (Fig. S2). These curves demonstrated that
the best outcome was attained by patients with T1Nr0 disease
(1-year survival, 82.6%; 2-year survival, 68.8%), which was defined
as stage I. The next best similar survival between patients with
T2Nr0 and T3Nr0 disease (1-year survival, 100.0 and 77.8%,
respectively; 2-year survival, 50.0 and 51.9%, respectively;
P=0.946) indicates that the two stages should be defined as stage
II disease. There was an overlap of survival in patients with T1Nr1
(1-year survival, 62.5%; 2-year survival, 18.8%) and T2Nr1 (1-year
survival, 40.0%; 2-year survival, 20.0%) disease (P=0.417), as well
as in patients with T2Nr1 and T3Nr1 disease (1-year survival,
61.7%; 2-year survival, 20.6%) (P=0.108). In addition, patients
with T4aNr1 disease had similar survival compared with stage T3Nr1
disease (P=0.205). The results suggest that the new staging system
should be modified to adopt the definition of stage III disease
(T1-3Nr1M0 and T4aNr0-1M0). Patients with T4b disease (1-year
survival, 0%; 2-year survival, 0%) or M1 disease (1-year survival,
23.3%; 2-year survival, 7.3%) exhibited the poorest survival, which
should therefore be defined as stage IV. Thus, new modified staging
definitions were adopted (Table
SVI), which were mostly similar to the staging classifications
for the gastrointestinal neuroendocrine neoplasms. Overall, DSS was
significantly different across stages for both SEER series
(P<0.001) and multicentric database (P=0.034) (Figs. 1D and 2D) with persistent overlaps in combined
stage. There was an expected decrease in survival times as tumor
stage increased with HRs of 0.12 (95% CI, 0.06–0.25), 0.22 (95% CI,
0.09–0.50), and 0.39 (95% CI, 0.23–0.66) for stages I, II, and III,
respectively, compared with stage IV in the patients from SEER
database (Table II). Decreased DSS
time was also observed for stages I–III compared with stage IV, for
the cohort from multicentric database, with HRs of <0.001 (95%
CI, 0.000-inf), 0.28 (95% CI, 0.06–1.37), and 0.51 (95% CI,
0.16–1.64), respectively, (Table
III).
The C-indices of different staging systems are
presented in Table SVII. The
respective C- indices using the 8th pTNM and the modified staging
systems for patients with ENENs were as follows: SEER series; 0.656
(95% CI, 0.607–0.705) and 0.659 (95% CI, 0.610–0.708),
respectively; multicentric database, 0.69 (95% CI, 0.561–0.819) and
0.688 (95% CI, 0.561–0.815), respectively.
Discussion
Currently, there is no specific staging system for
the ENENs, and the present study was the first consolidation of a
data-based process for the revision of the 8th AJCC staging
classification both for EAC and ESCC to assess DSS in this
exceedingly uncommon and increasingly prevalent cancer type
(1–3). The proposed system may help to better
discriminate prognosis of patients with ENENs and guide the
evaluation of current and novel treatments for this disease.
Due to the unavailability of the information on
postneoadjuvant therapy from the SEER database, the 8th AJCC ypTNM
were not used to assess the prognostic value. The analysis revealed
that the 8th AJCC pTNM system has an improved potential for the
discrimination of prognosis compared with the current AJCC cTNM
systems. It is unlikely that stages II and III represent distinct
prognostic categories in view of the fact that survival outcomes
are not significant for both groups according to the current cTNM
systems. However, further subgroup analysis suggested the
consistency of the outcomes among the substages became unclear
based on the pTNM systems (Figs. S3
and S4). Consequently, the present
study revealed that the existing TNM staging classifications may
not optimally distinguish survival outcomes among patients with
ENENs. Moreover, the clinical stage grouping prior to treatment
decision has received increasing attention since the wide
application of modern imaging and endoscopic technologies, thus
warranting further modifications and validation of an easier and
more accurate cTNM staging groups for ENENs. In addition,
multivariate analysis indicated that the lymph node status
(excluding the number of lymph nodes with metastasis) was a
significant prognostic factor, supporting the adoption of the Nr
definition in the modified staging system. Therefore, a modified
cTNM to the 8th AJCC staging classifications was proposed, which
maintains the T and M definitions but adopts a new Nr definition,
similar to the gastrointestinal staging classifications (stage I,
T1N0M0; stage II, T2-T3N0M0; stage III, T1-T3N1MO and T4NanyM0; and
stage IV, TanyNanyM1). The modified staging system was confirmed
using two relatively large ENEN series, and the risk of mortality
uniformly progressed from class I to IV for both cohorts. Moreover,
the discrimination ability for tumor-associated mortality (measured
by the C-index) was slightly better or comparable for the modified
staging system, relative to the pTNM system. Due to the increased
application of computed tomographic scans and endoscopic
technologies, it was relatively easy to assess the regional lymph
node involvement status. However, given that some patients did not
have lymph node dissection or presented with insufficient lymph
node dissection, the number of positive lymph nodes was difficult
to assess. Consequently, the current findings suggest that the
modified staging classification may be more suitable and easier for
ENENs and can be adopted in clinical practice in the future.
Currently, there is no consensus on the guidelines
for the management of ENENs due to their rarity and heterogeneity.
The establishment of an accurate and easy staging is the first step
in optimizing treatment and outcomes for patients with ENENs.
Accumulating evidence has supported the application of surgical
resection as an effective treatment option for patients with ENEN
with limited disease. Notably, cT4b tumors with invasion of the
heart, great vessels trachea, or adjacent organs including liver,
pancreas, lung and spleen are considered to be unresectable
(20). Therefore, the modified
staging groups may help to distinguish the patients with
unresectable disease from the patients without distant metastasis.
Moreover, the present population-based study enabled us to detect
differences in outcomes, particularly among patients with limited
disease without lymph nodes metastasis, who have a relatively good
prognosis and may not experience rapid disease progression, which
provides a theoretical basis for future stratified treatment.
Moreover, the median DSS time for limited disease in the present
study ranged from 19–22 months, and for extensive disease, the
median DSS was ~6 months, which supports a study on small cell
carcinoma of esophagus reported by Luo et al (15).
The present study has several limitations. The
rarity of ENETs makes it difficult to accumulate a large cohort for
evaluation, which may explain the existence of overlaps among
stages using the modified staging system results in a decrease of
statistical power to detect significant differences among
substages. The present study was also limited by its retrospective
nature. Therefore, additional prospective studies with a larger
sample size will be required to validate the modified staging
system.
In conclusion, although the 8th AJCC pTNM
classification allows for the discrimination of clinical outcomes
in patients with ENENs, the present findings suggested that this
system should be further refined. A modified clinical staging
system was proposed by maintaining the T and M definitions of the
current AJCC staging system and adopting the Nr classification,
which demonstrated a significant association with DSS. Although the
modified staging system may be more accurate and easier in
predicting the prognosis of ENENs, the current findings still need
to be replicated in other cohorts before adoption in clinical
practice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant no. 81874061), Chinese Society
of Clinical Oncology-Shiyao Cancer Research Fund (grant no.
Y-sy2019-009), Hubei Provincial Natural Science Foundation Guiding
Project (grant no. 2018CFC846) and The 7th Wuhan Young and
Middle-aged Backbone Talent of Medical Training Project 2019 (grant
nos. 2019 and 87).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
All authors conceived and designed the present study
and drafted the initial manuscript. YC, GP, SY, ZL and TZ provided
the study material and patient information. HW, YC, GP, SY and YZ
acquired the data. Statistical analysis was performed by HW, YC,
GP, HM, ZL and TZ. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Approval for the study protocol was obtained by the
Institutional Ethics Committee of the Tongji Medical College,
Huazhong University of Science and Technology (approval no. S1172).
Written informed consent was obtained from all the patients for
their data to be used for research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ENENs
|
esophageal neuroendocrine
neoplasms
|
|
SEER
|
Surveillance, Epidemiology, and End
Results registry
|
|
AJCC
|
American Joint Committee on Cancer
|
|
DSS
|
disease-specific survival
|
|
TNM
|
Tumor-Node-Metastasis
|
|
pTNM
|
pathological Tumor-Node-Metastasis
stage groups
|
|
Nr
|
revised N
|
|
EAC
|
esophageal adenocarcinoma
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
ypTNM
|
postneoadjuvant pathologic stage
groups
|
|
cTNM
|
clinical stage groups
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
C-index
|
concordance index
|
References
|
1
|
Modlin IM and Sandor A: An analysis of
8305 cases of carcinoid tumors. Cancer. 79:813–829. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gastrointestinal Pathology Study Group of
Korean Society of Pathologists, ; Cho MY, Kim JM, Sohn JH, Kim MJ,
Kim KM, Kim WH, Kim H, Kook MC, Park DY, et al: Current trends of
the incidence and pathological diagnosis of gastroenteropancreatic
neuroendocrine tumors (GEP-NETs) in Korea 2000–2009: Multicenter
study. Cancer Res Treat. 44:157–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CG, Lim YJ, Park SJ, Jang BI, Choi SR,
Kim JK, Kim YT, Cho JY, Yang CH, Chun HJ, et al: The clinical
features and treatment modality of esophageal neuroendocrine
tumors: A multicenter study in Korea. BMC Cancer. 14:5692014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang KL, Yang Q, Cleary KR, Swisher SG,
Correa AM, Komaki R, Ajani JA, Rashid A, Hamilton SR and Wu TT: The
significance of neuroendocrine differentiation in adenocarcinoma of
the esophagus and esophagogastric junction after preoperative
chemoradiation. Cancer. 107:1467–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang Q, Wu H, Nie L, Shi J, Lebenthal A,
Chen J, Sun Q, Yang J, Huang L and Ye Q: Primary high-grade
neuroendocrine carcinoma of the esophagus: A clinicopathologic and
immunohistochemical study of 42 resection cases. Am J Surg Pathol.
37:467–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng HY, Ni PZ, Wang YC, Wang WP and Chen
LQ: Neuroendocrine carcinoma of the esophagus: Clinical
characteristics and prognostic evaluation of 49 cases with surgical
resection. J Thorac Dis. 8:1250–1256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maru DM, Khurana H, Rashid A, Correa AM,
Anandasabapathy S, Krishnan S, Komaki R, Ajani JA, Swisher SG and
Hofstetter WL: Retrospective study of clinicopathologic features
and prognosis of high-grade neuroendocrine carcinoma of the
esophagus. Am J Surg Pathol. 32:1404–1411. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Deng HY, Li G, Luo J, Li XR, Alai G and
Lin YD: The role of surgery in treating resectable limited disease
of esophageal neuroendocrine carcinoma. World J Surg. 42:2428–2436.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okuma HS, Iwasa S, Shoji H, Takashima A,
Okita N, Honma Y, Kato K, Hamaguchi T, Yamada Y and Shimada Y:
Irinotecan plus cisplatin in patients with extensive-disease poorly
differentiated neuroendocrine carcinoma of the esophagus.
Anticancer Res. 34:5037–5041. 2014.PubMed/NCBI
|
|
10
|
Rice TW, Ishwaran H, Ferguson MK,
Blackstone EH and Goldstraw P: Cancer of the esophagus and
esophagogastric junction: An eighth edition staging primer. J
Thorac Oncol. 12:36–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rindi G, Falconi M, Klersy C, Albarello L,
Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A,
Doglioni C, et al: TNM staging of neoplasms of the endocrine
pancreas: Results from a large international cohort study. J Natl
Cancer Inst. 104:764–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rice TW, Ishwaran H, Hofstetter WL, Kelsen
DP, Apperson-Hansen C and Blackstone EH; Worldwide Esophageal
Cancer Collaboration Investigators, : Recommendations for
pathologic staging (pTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:897–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rice TW, Ishwaran H, Kelsen DP, Hofstetter
WL, Apperson-Hansen C and Blackstone EH; Worldwide Esophageal
Cancer Collaboration Investigators, : Recommendations for
neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus
and esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:906–912. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rice TW, Ishwaran H, Blackstone EH,
Hofstetter WL, Kelsen DP and Apperson-Hansen C; Worldwide
Esophageal Cancer Collaboration Investigators, : Recommendations
for clinical staging (cTNM) of cancer of the esophagus and
esophagogastric junction for the 8th edition AJCC/UICC staging
manuals. Dis Esophagus. 29:913–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo G, Javed A, Strosberg JR, Jin K, Zhang
Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, et al: Modified
staging classification for pancreatic neuroendocrine tumors on the
basis of the American joint committee on cancer and European
neuroendocrine tumor society systems. J Clin Oncol. 35:274–280.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim MK, Warner RR, Roayaie S, Harpaz N,
Ward SC, Itzkowitz S and Wisnivesky JP: Revised staging
classification improves outcome prediction for small intestinal
neuroendocrine tumors. J Clin Oncol. 31:3776–3781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin JA, Warner RR, Wisnivesky JP and
Kim MK: Improving survival prognostication of
gastroenteropancreatic neuroendocrine neoplasms: Revised staging
criteria. Eur J Cancer. 76:197–204. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
RStudio Team, . RStudio: Integrated
Development for R. RStudio, Inc., Boston, MA, 2015. http://www.rstudio.com/
|
|
19
|
R Core Team, . R: a language and
environment for statistical computing. R Foundation for Statistical
Computing, Vienna, 2012. http://www.R-project.org/
|
|
20
|
Cuccurullo V and Mansi L: AJCC cancer
staging handbook: From the AJCC cancer staging manual (7th
edition). Eur J Nuclear Med Mol Imaging. 38:408. 2011. View Article : Google Scholar
|