Introduction
Cervical cancer (CC) is a prevelant female
malignancy with a high mortality. In 2012, there were 527,600 newly
onset cases and 265,700 death cases globally (1). With advance in the screening
technologies and therapeutic strategies, the incidence and
mortality of CC are on the decline (2). Nevertheless, the overall survival of CC
patients is poor (3). Effective
hallmarks that predict the occurrence and progression of CC are
lacking. Currently, squamous cell carcinoma antigen (SCC-Ag) is
commonly applied for diagnosing CC (4). However, the sensitivity and specificity
of SCC-Ag are relatively low, which markedly restricts its clinical
application. It is necessary and urgent to uncover efficient
diagnostic markers for CC.
Long non-coding RNAs (lncRNAs) are widely expressed
in mammals and regulate gene expression. They are able to mediate
various cellular behavior (5,6). lncRNAs
influence the occurrence and progression of tumors as oncogenes or
tumor-suppressor genes (7). It is
reported that lncRNAs form different types of complexes alongside
many factors, thus directly regulating downstream genes and
affecting malignant phenotypes of tumor cells (8). Several abnormally expressed lncRNAs
have been identified in CC. These lncRNAs may be utilized as
diagnostic and prognostic hallmarks (9). In this study, we explored the
biological function of lncRNA PICART1 in the malignant progression
of CC and the potential mechanism.
Patients and methods
Sample collection and ethical
statements
Sixty paired CC tissues and paracancerous tissues
were surgically harvested from CC patients admitted to Weifang
People's Hospital (Weifang, China) from March 2016 to October 2018.
Tumor node metastasis (TNM) staging and tumor size of enrolled CC
patients were recorded. None of these patients were preoperatively
treated. This study was approved by the Medical Ethics Committee of
Weifang People's Hospital and informed consent was received from
each subject.
RNA extraction and qRT-PCR
Cells and tissues were lysed using TRIzol method
(Invitrogen; Thermo Fisher Scientific, Inc.) for reverse
transcription of RNAs using the PrimeScript RT reagent Kit
(Takara). RNA was quantified using spectrometer and subjected to
qRT-PCR following SYBR Premix Ex Taq™ (Takara). Relative level of
target gene was determined by the 2−∆∆ct method.
Cell culture and transfection
CC cell lines (HeLa, C4-1, SiHa, Caski) and normal
cervical endothelial cell line (Etc1/E6E7) were obtained from
American Type Culture Collection (ATCC) (Manassas). Cell culture
was conducted using Roswell Park Memorial Institute 1640
(RPMI-1640) medium (HyClone; GE Healthcare) with 10% fetal bovine
serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) in an incubator
with 5% CO2 at 37°C. For cell transfection, 1.5 ml of
serum-free medium and 0.5 ml of Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) containing transfection vectors
were applied in each well of a 6-well plate. Fresh medium was
replaced 4–6 h later. Transfected cells for 24–48 h were collected
for determination.
Cell Counting Kit-8 (CCK-8) assay
Cell density was adjusted to 2×103
cells/100 µl and cells were inoculated in a 96-well plate. At the
appointed times, cells per well were incubated with 10 µl of CCK-8
solution (CCK-8, Dojindo Molecular Technologies, Inc.). Absorbance
(450 nm) was recorded by a microplate reader (Bio-Rad Laboratories,
Inc.).
Transwell assay
Suspension (1.0×105 cells/ml) was
prepared and subjected to serum starvation for 12 h. Suspension
(200 µl/well) was applied in the upper side of Transwell chamber
(EMD Millipore) pre-coated with Matrigel. In the lower side, 700 µl
of medium containing 10% FBS was applied. After 48 h of incubation,
cells invaded to the lower side were subjected to fixation in
methanol for 15 min, crystal violet staining for 20 min and cell
counting using a microscope. Invasive cells were counted in 5
randomly selected fields per sample. Migration assay was performed
in the same way except for Matrigel pre-coating.
Determination of subcellular
distribution
Cytoplasmic and nuclear RNAs were extracted using
the PARIS kit (Invitrogen; Thermo Fisher Scientific, Inc.) and
subjected to qRT-PCR. U6 was the internal reference of nucleus and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was that of
cytoplasm.
RNA immunoprecipitation (RIP)
Cells were treated according to the procedures of
Millipore Magna RIP™ RNA-Binding Protein Immunoprecipitation kit
(EMD Millipore). Cell lysate was incubated with antibodies or
anti-IgG antibody at 4°C for 6 h. A protein-RNA complex was
captured and digested with 0.5 mg/ml proteinase K containing 0.1%
sodium dodecyl sulphate (SDS) to extract RNA. The magnetic beads
were repeatedly washed with RIP washing buffer to remove
non-specific adsorption as much as possible. Finally, the extracted
RNA was subjected to mRNA level determination using qRT-PCR.
Chromatin immunoprecipitation
(ChIP)
Cells were subjected to cross-link with 1%
formaldehyde at room temperature for 10 min into small fractions
with 200–300 bp. Subsequently, cells were lysed and sonicated for
30 min. Finally, the sonicated lysate was immuno-precipitated with
anti-TCF21, anti-ARID1A or anti-IgG. Purified immunoprecipitated
chromatins were subjected to qRT-PCR.
Western blotting
Cells were lysed using radioimmunoprecipitation
assay (RIPA) and extracted protein was quantified by bicinchoninic
acid (BCA) method (Beyotime Institute of Biotechnology).
Electrophoresis was conducted for transferring proteins on a
polyvinylidene fluoride (PVDF) membranes (EMD Millipore). After 2-h
blockage of non-specific sites in 5% skim milk, membranes were
reacted with primary and secondary antibodies. Band exposure was
achieved by electrochemiluminescence (ECL) and analyzed by Image
Software (National Institutes of Health).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
22.0 statistical software (IBM Corp.) was used for data analysis.
Data were expressed as mean ± standard deviation (mean ± SD).
Intergroup data were compared using the t-test. Kaplan-Meier curves
were employed for survival analysis. Pearson's correlation analysis
was conducted for evaluating the relationship between two genes.
P<0.05 was considered to indicate a statistically significant
difference.
Results
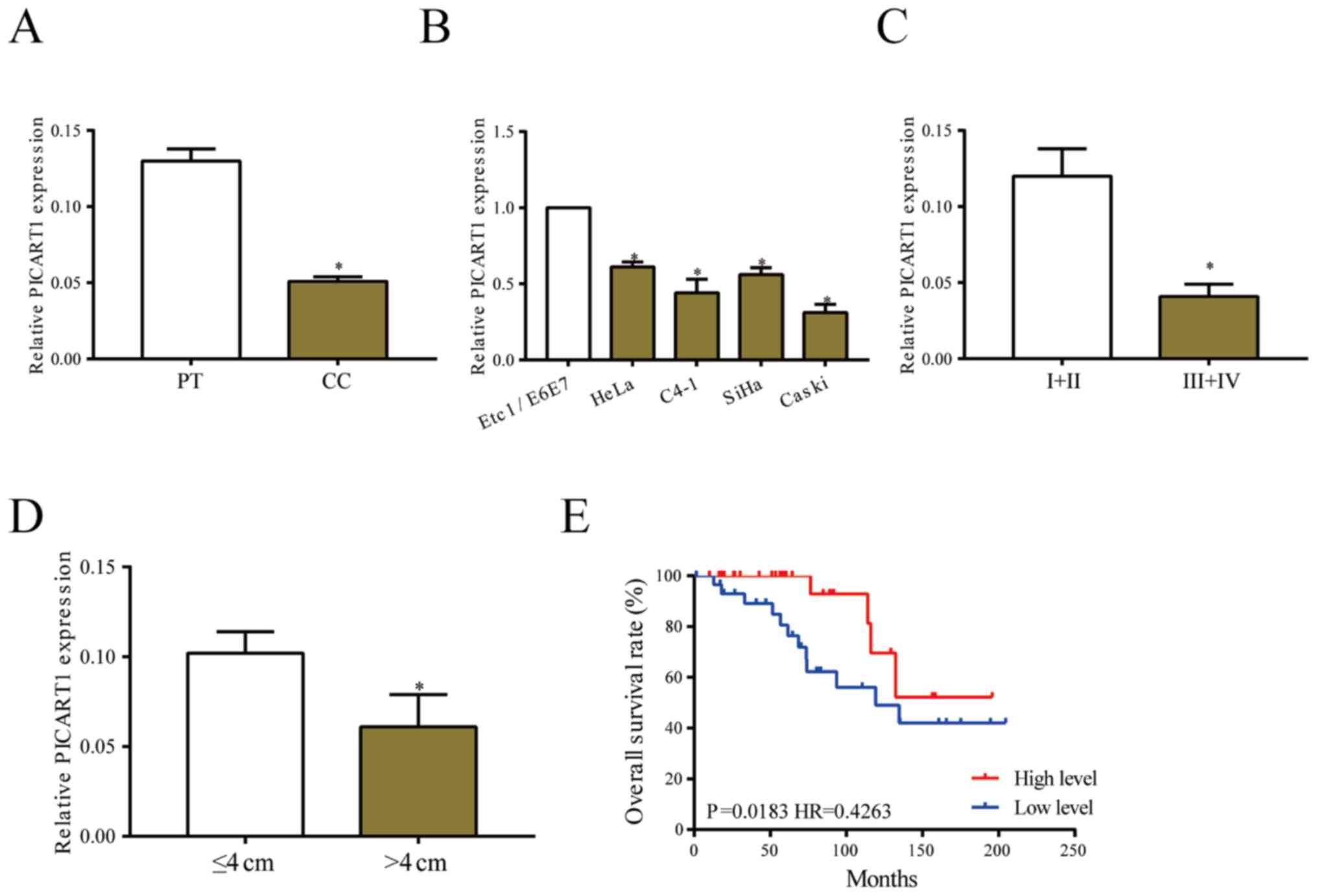
PICART1 is downregulated in CC
Expression level of PICART1 was determined in 60
paired CC tissues. PICART1 was downregulated in CC tissues relative
to paracancerous ones (Fig. 1A).
Identically, its level remained lower in CC cell lines than that of
normal cervical endothelial cell line (Fig. 1B). PICART1 was found to be closely
related to clinical characteristics of CC patients. Relative to CC
patients in stage I+II, those in stage III+IV presented lower level
of PICART1 (Fig. 1C). Lower
abundance of PICART1 was observed in CC tissues larger than 4 cm in
tumor size (Fig. 1D). Survival
analysis showed worse prognosis in CC patients with low level of
PICART1 (Fig. 1E).
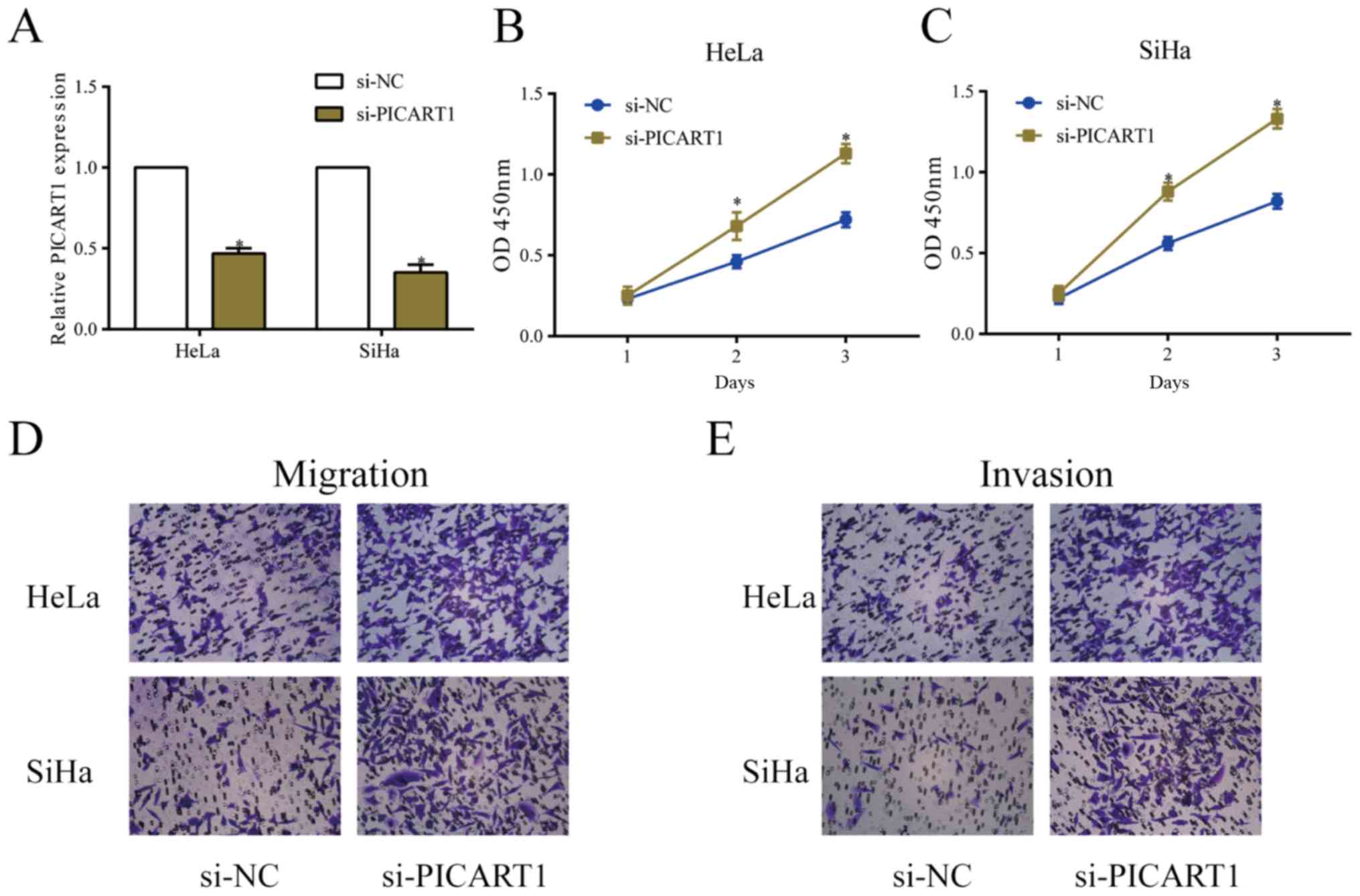
Silence of PICART1 accelerates
proliferative, migratory and invasive abilities of CC
We constructed si-PICART1 and tested its
transfection efficacy. Transfection of si-PICART1 greatly
downregulated PICART1 level in HeLa and SiHa cells (Fig. 2A). The viability in CC cells was
markedly elevated after silence of PICART1 (Fig. 2B and C). Similarly, the migratory and
invasive abilities of CC cells were accelerated after transfection
of si-PICART1 (Fig. 2D and E).
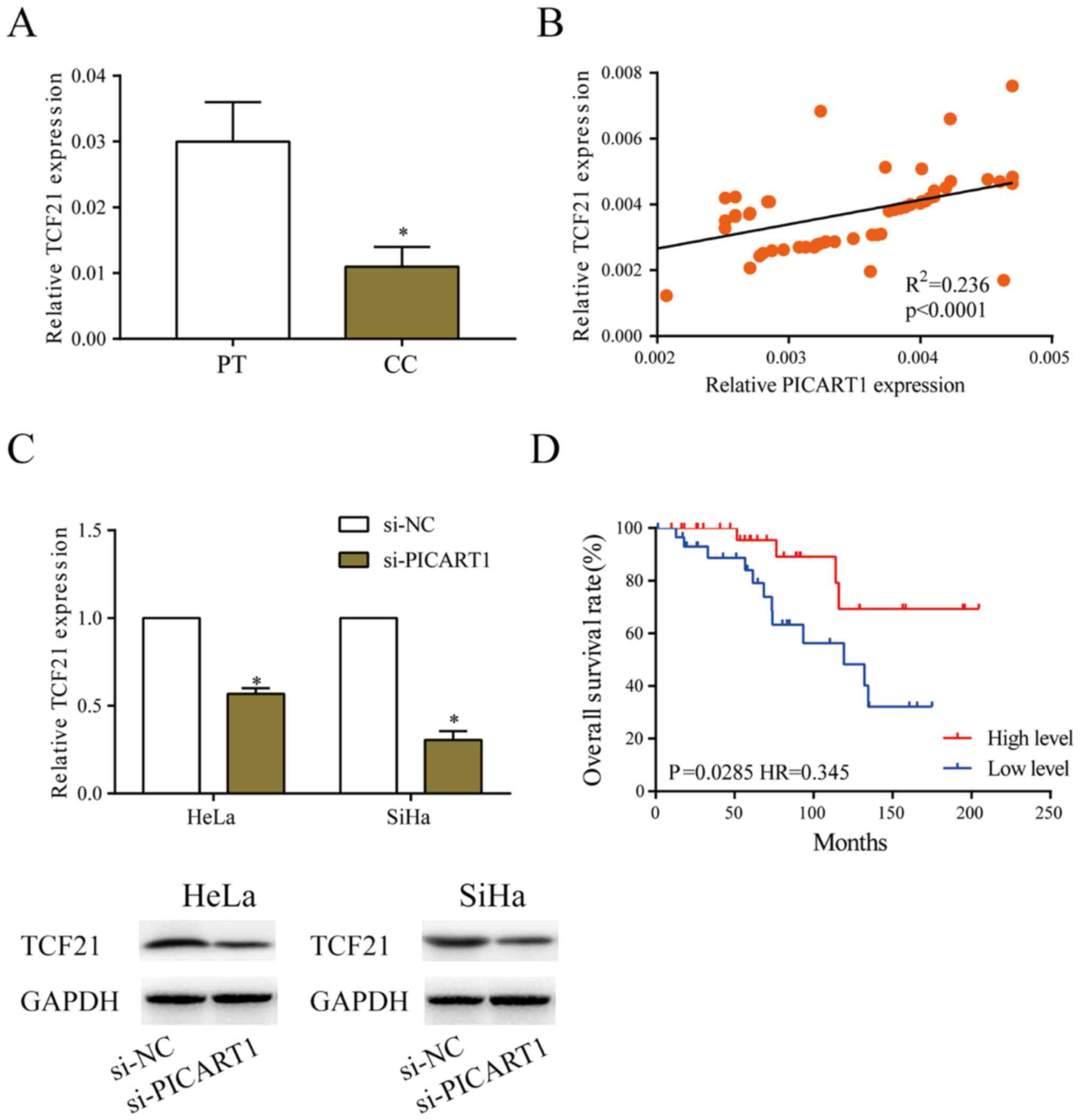
PICART1 positively regulates TCF21
level
Compared with paracancerous tissues, TCF21
expression was low in CC tissues (Fig.
3A). A positive correlation between expression levels of TCF21
and PICART1 was observed in CC tissues (R2=0.236,
P<0.001, Fig. 3B). Transfection
of si-PICART1 downregulated both mRNA and protein level of TCF21 in
HeLa and SiHa cells (Fig. 3C).
Kaplan-Meier curves revealed worse prognosis in CC patients
expressing low level of TCF21 (Fig.
3D). It is believed that TCF21 is closely related to the
malignant progression of CC.
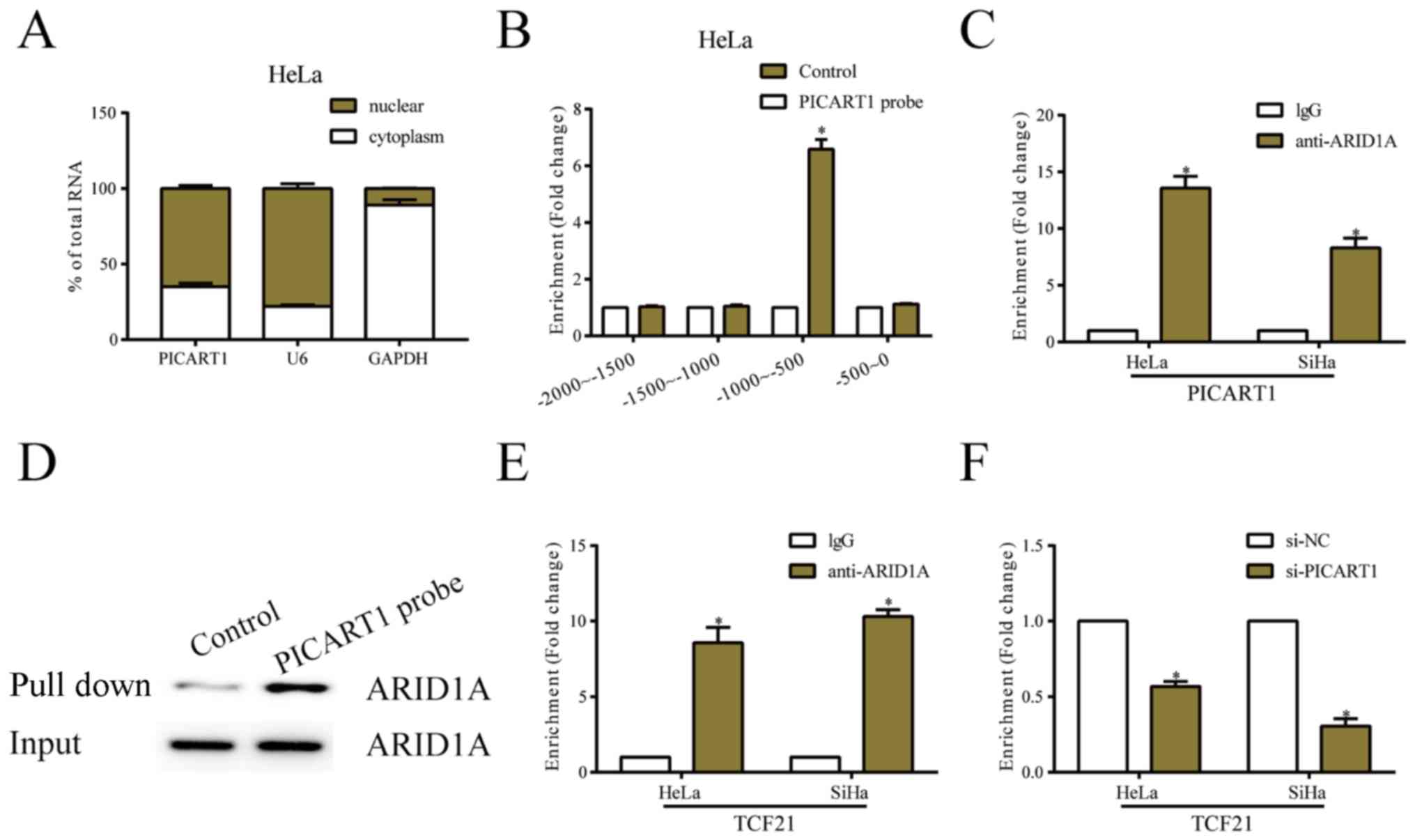
PICART1 recruites ARID1A to activate
TCF21 expression
Subcellar distribution analysis showed that PICART1
was mainly expressed in nuclear fraction of HeLa cells (Fig. 4A). We next explored the possible
functions of PICART1 and TCF21 in the progression of CC. Analysis
of the DNA sequences in TCF21 promoter region showed a remarkable
enrichment of PICART1 from −1000 to −500 bp (Fig. 4B). Moreover, the interaction between
PICART1 and ARID1A was also identified (Fig. 4C and D). Potential binding sites were
verified among PICART, ARID1A and TCF21. ChIP assay confirmed the
decreased enrichment of ARID1A in TCF21 promoter region after
PICART1 knockdown (Fig. 4E and
F).
Discussion
CC ranks fourth in the morbidity and mortality of
malignant tumors throughout the world. The prognosis of CC is
unsatisfactory. Many genes, factors and pathways are involved in
tumorigenesis of CC. It is of great significance to seek effective
diagnostic and prognostic markers for CC.
A large number of observational studies have been
conducted to identify the potential role of lncRNAs related to CC.
For example, serum level of lncRNA PVT1 is upregulated in CC
patients, which exerts diagnostic potential with 71.6 sensitivity
and 98.8% specificity (10). lncRNA
XLOC_010588 is downregulated in CC patients and predicts poor
prognosis (11). In this study,
lncRNA PICART1 was downregulated in CC tissues and cell lines.
Silence of PICART1 accelerated the proliferative, migratory and
invasive abilities of HeLa and SiHa cells.
TCF21 was found to be the target gene of PICART1.
TCF21, also known as Pod-1, capsuling and epicardin, mediates cell
differentiation and apoptosis by binding to DNAs in the
developmental process (12). TCF21
is mainly expressed in the embryonic mesenchymal cells around the
kidney, heart, lung and gastrointestinal epithelial developmental
areas (13,14). Its level rapidly decreases after
birth except for that in kidney, heart, lung and spleen stromal
cells (15). Antisense inhibition of
TCF21 has been reported to disrupt epithelial differentiation and
branching morphogenesis in epithelial cells in mouse embryonic
kidneys, suggesting that TCF21 could influence the process of EMT
(16). TCF21 deficiency in the
kidney results in decreased glomerulogenesis and tubular formation
(17). Notably, transfection of
TCF21 siRNA in mesenchymal progenitor cells from mouse kidney
stimulates proliferative and migratory abilities, as well as
downregulates expression of smooth muscle and myofibroblast
secreted proteins (15). In clinical
trials, ARID1A mutation is believed to be closely related to poor
prognosis of ovarian cancer patients, indicating its
tumor-suppressor role (18,19). ARID1A protects genomic stability and
antagonizes the carcinogenic role of EZH2 in clear cell carcinoma
of ovary (20,21). In addition, ARID1A as a priority
guides SWI/SNF complexes H3K27ac- and H3K4me1-labeled enhancers to
interact with multiple transcriptional factors (22). Owing to the dislocation of SWI/SNF
complexes to enhancers, ARID1A deficiency astimulates the
progression of adenocarcinoma of colon in mice. Herein, our study
found that PICART1 recruited ARID1A to activate TCF21 expression,
thus alleviating the progression of CC. It is noteworthy that
PICART1 shows ability to mediate a series of downstream genes. RNA
sequencing of downstream targets after silencing of PICART1 is
necessary.
In conclusion, lncRNA PICART1 is downregulated in CC
tissues, and closely related to disease progression. PICART1
recruits ARID1A to activate TCF21 expression, thus alleviating the
malignant progression of cervical cancer. It is believed that
PICART1 may be utilized as a new drug target for CC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and RH designed the study and performed the
experiments, YZ and XD collected the data, RH and XD analyzed the
data, YZ and RH prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Signed informed
consents were obtained from the patients and/or the guardians.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian JDC and Liang L: Involvement of
circular RNA SMARCA5/microRNA-620 axis in the regulation of
cervical cancer cell proliferation, invasion and migration. Eur Rev
Med Pharmacol Sci. 22:8589–8598. 2018.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salvatici M, Achilarre MT, Sandri MT,
Boveri S, Vanna Z and Landoni F: Squamous cell carcinoma antigen
(SCC-Ag) during follow-up of cervical cancer patients: Role in the
early diagnosis of recurrence. Gynecol Oncol. 142:115–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Solans L, Gonzalo-Asensio J, Sala C,
Benjak A, Uplekar S, Rougemont J, Guilhot C, Malaga W, Martín C and
Cole ST: The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion
system in Mycobacterium tuberculosis. PLoS Pathog. 10:e10041832014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwartz S, Bernstein DA, Mumbach MR,
Jovanovic M, Herbst RH, León-Ricardo BX, Engreitz JM, Guttman M,
Satija R, Lander ES, et al: Transcriptome-wide mapping reveals
widespread dynamic-regulated pseudouridylation of ncRNA and mRNA.
Cell. 159:148–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lei B, Xu SP, Liang XS, Li YW, Zhang JF,
Zhang GQ and Pang D: Long non-coding RNA MVIH is associated with
poor prognosis and malignant biological behavior in breast cancer.
Tumour Biol. 37:5257–5264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jalali S, Bhartiya D, Lalwani MK,
Sivasubbu S and Scaria V: Systematic transcriptome wide analysis of
lncRNA-miRNA interactions. PLoS One. 8:e538232013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chi S, Shen L, Hua T, Liu S, Zhuang G,
Wang X, Zhou X, Wang G and Wang H: Prognostic and diagnostic
significance of lncRNAs expression in cervical cancer: A systematic
review and meta-analysis. Oncotarget. 8:79061–79072. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JP, Yang XJ, Xiao L and Wang Y: Long
noncoding RNA PVT1 as a novel serum biomarker for detection of
cervical cancer. Eur Rev Med Pharmacol Sci. 20:3980–3986.
2016.PubMed/NCBI
|
|
11
|
Liao LM, Sun XY, Liu AW, Wu JB, Cheng XL,
Lin JX, Zheng M and Huang L: Low expression of long noncoding
XLOC_010588 indicates a poor prognosis and promotes proliferation
through upregulation of c-Myc in cervical cancer. Gynecol Oncol.
133:616–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hidai H, Bardales R, Goodwin R,
Quertermous T and Quertermous EE: Cloning of capsulin, a basic
helix-loop-helix factor expressed in progenitor cells of the
pericardium and the coronary arteries. Mech Dev. 73:33–43. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Richardson JA and Olson EN:
Capsulin: A novel bHLH transcription factor expressed in epicardial
progenitors and mesenchyme of visceral organs. Mech Dev. 73:23–32.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quaggin SE, Vanden Heuvel GB and Igarashi
P: Pod-1, a mesoderm-specific basic-helix-loop-helix protein
expressed in mesenchymal and glomerular epithelial cells in the
developing kidney. Mech Dev. 71:37–48. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plotkin M and Mudunuri V: Pod1 induces
myofibroblast differentiation in mesenchymal progenitor cells from
mouse kidney. J Cell Biochem. 103:675–690. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quaggin SE, Schwartz L, Cui S, Igarashi P,
Deimling J, Post M and Rossant J: The basic-helix-loop-helix
protein pod1 is critically important for kidney and lung
organogenesis. Development. 126:5771–5783. 1999.PubMed/NCBI
|
|
17
|
Cui S, Schwartz L and Quaggin SE: Pod1 is
required in stromal cells for glomerulogenesis. Dev Dyn.
226:512–522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katagiri A, Nakayama K, Rahman MT, Rahman
M, Katagiri H, Nakayama N, Ishikawa M, Ishibashi T, Iida K,
Kobayashi H, et al: Loss of ARID1A expression is related to shorter
progression-free survival and chemoresistance in ovarian clear cell
carcinoma. Mod Pathol. 25:282–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bitler BG, Aird KM, Garipov A, Li H,
Amatangelo M, Kossenkov AV, Schultz DC, Liu Q, Shih IeM,
Conejo-Garcia JR, et al: Synthetic lethality by targeting EZH2
methyltransferase activity in ARID1A-mutated cancers. Nat Med.
21:231–238. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dykhuizen EC, Hargreaves DC, Miller EL,
Cui K, Korshunov A, Kool M, Pfister S, Cho YJ, Zhao K and Crabtree
GR: BAF complexes facilitate decatenation of DNA by topoisomerase
IIα. Nature. 497:624–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathur R, Alver BH, San Roman AK, Wilson
BG, Wang X, Agoston AT, Park PJ, Shivdasani RA and Roberts CW:
ARID1A loss impairs enhancer-mediated gene regulation and drives
colon cancer in mice. Nat Genet. 49:296–302. 2017. View Article : Google Scholar : PubMed/NCBI
|