Introduction
Breast cancer (BC) was one of the most common types
of cancer in US women in 2017 (1).
Among the different subtypes, triple-negative breast cancer (TNBC)
is defined by the absence of expression of the estrogen receptor
(ER), progesterone receptor and the human epidermal growth factor
receptor-2 or gene amplification (2,3). These
biologic characteristics confer TNBC with greater aggressiveness
and relapse risk along with a worse prognosis compared with other
subtypes of BC (4,5). Limited options for systemic treatment
exist for BC other than chemotherapy (6). BC heterogeneity has limited the
successful development of targeted therapy (7). Currently, there are no approved
targeted therapies for TNBC (8).
The epidermal growth factor receptor (EGFR) is
essential for ductal morphogenesis during the development of normal
mammary glands (9) and its
upregulation in BC has been well documented (10). Previously, researchers report that
the EGFR is commonly upregulated in TNBC compared with other BC
subtypes and is associated with poor prognosis (11–13).
EGFR inhibition is a promising approach for TNBC; however, minimal
benefits have been observed by targeting TNBC in clinical studies
(14,15). The molecular mechanisms for the
insensitivity of EGFR targeted therapy in patients with TNBC remain
unclear (16).
Previously, Wang et al (17) reported a novel ER variant with a
molecular weight of 36 kDa, ER-α36, which is located mainly in the
plasma membrane and cytoplasm. ER-α36 differs from the estrogen
receptor α-66 (ER-α66) as it lacks both transcriptional activation
domains [Activation factor (AF)-1 and AF-2], but has the
DNA-binding domain and partial ligand-binding domains. ER-α36
possesses a unique 27 amino acid domain that replaces the last 138
amino acids encoded by exons 7 and 8 of the ER-α66 gene. ER-α36
lacks intrinsic transcription ability, but mediates non-genomic
estrogen signaling. ER-a36 is generated from a promoter located in
the first intron of the ER-α66 gene, indicating that ER-α36
expression is regulated independently from ER-α66. Τhis is
consistent with the findings that ER-α36 is expressed in cancer
tissue specimens from patients with ER-negative BC and established
ER-negative BC cells that lack ER-α66 expression (18,19). It
has been suggested that ER-α36 may mediate rapid estrogen
signaling, which serves a role in anti-estrogen drug resistance in
ER-positive BC and in chemotherapy resistance in ER-negative BC
(20). ER-α36 mediates rapid
estrogen and antiestrogen signaling and stimulates cell
proliferation through the activation of the mitogen-activated
protein kinase (MAPK/ERK) and the PI3K/AKT signaling pathways
(21).
Icaritin is a prenylflavonoid derivative from the
genus Epimedium that has been used in traditional Chinese
medicine for centuries (22).
Studies have demonstrated that icaritin can be used against
different types of cancer. Icatrin can inhibit the proliferation
and enhance the radio-sensitivity of BC cells (23); induce apoptosis of human endometrial
cancer cells (24); and exhibit
potent proliferation inhibition in chronic myeloid leukemia and
suppress the growth of renal carcinoma cells (25). Recently, Wang et al (26) demonstrated that icaritin can decrease
the expression of the ER-α36 protein in TNBC cells. Thus, it was
speculated that the combined application of icaritin and the EGFR
inhibitor for patients with TNBC may achieve improved results
compared with the individual use of either drug.
In the present study, the function of the ER-α36 in
EGFR targeted therapy-resistant TNBC was investigated. Furthermore,
the efficiency of combination therapy with ER-α36 molecular
inhibitor icaritin and EGFR inhibitor cetuximab for TNBCs was also
evaluated.
Materials and methods
Ethical approval
The study protocol was approved by the Animal Care
and Use Committee of Third Military Medical University (Army
Medical University, Chongqing, China).
Chemicals and antibodies
E2β was purchased from Merck KGaA. The polyclonal
anti-ER-α36 antibody was generated and characterized as described
previously (14). Antibodies against
EGFR (cat. no. 4267), ER-α66 (cat. no. 13258), glyceraldehyde
3-phosphate dehydrogenase (cat. no. 2118), AKT (cat. no. 9272),
GAPDH (cat. no. 2118) and phospho-Akt (Ser473; cat. no. 4060) were
all obtained from Cell Signaling Technology, Inc. Icaritin was
purchased from Shenogen Pharma Group, Ltd., and cetuximab was
obtained from Merck KGaA.
Culture and treatment of cells
MCF-7, MDA-MB-231 and MDA-MB-436 cell lines were
purchased from American Type Culture Collection. The MDA-MB-231
cell line is a well known cell line of highly aggressive, invasive
and poorly differentiated TNBC established in 1978 (27,28). The
MDA-MB-436 cell line is also well known and possesses BRCA1
mutations (29). These cell lines
were chosen as they are well studied, their behavior is highly
predictable. The cells were maintained in DMEM containing 10% fetal
calf serum and 1% penicillin/streptomycin (DMEM and fetal calf
serum were purchased from HyClone; GE Healthcare Life Sciences and
penicillin/streptomycin were purchased from Thermo Fisher
Scientific, Inc.) at 37°C in an incubator containing 5%
CO2. Prior to treatment with E2β and icaritin, cells
were transferred to phenol red-free medium containing 2.5%
charcoal-stripped fetal calf serum (HyClone; GE Healthcare Life
Sciences) and maintained for 24 h.
Establishment of stable cell
lines
MDA-MB-231 and MDA-MB-436 cell lines with the ER-α36
expression knockdown using the short-hairpin (sh) RNA method were
established as described previously (30). The ER-α36 shRNA plasmid, vehicle
plasmid (pRNAT-U6.1/Neo) and anti-ER-α36 antibody were provided by
Dr. Zhao-yi Wang (Department of Medical Microbiology and
Immunology, Creighton University Medical School). Transfection of
the plasmids were performed after cell confluency reached 60%
within 24 h of seeding. Transfection reagent
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for plasmid transfection according to
the manufacturer's instructions. A total of 10 µg
plasmid/1×106 cells was incubated for 12 h at 37°C in a
humidified atmosphere with 5% CO2. At 48 h
post-transfection, the appropriate antibiotic (neomycin;
Sigma-Aldrich; Merck KGaA) was used to screen the transfected cell
lines for 3 weeks, and >20 clones of selected cells were pooled
and termed MDA-MB-231/V and MDA-MB-231/Sh36 or MDA-MB-436/V and
MDA-MB-436/Sh36, respectively. The efficiency of ER-α36 shRNA
plasmid transduction was determined by western blotting using
anti-ER-α36 antibody (1:1,000).
Cell proliferation assay
Cells were seeded in 60-mm petri dishes at a final
concentration of 5×104 cells/dish. After 24 h, the
indicated concentrations of cetuximab (1, 5, 10, 50 and 100 µg/ml),
icaritin (1, 2.5, 5, 7.5 and 10 µM), cetuximab (100 µg/ml) +
icaritin (1, 2.5, 5, 7.5 and 10 µM) or control DMSO were added.
After 7 days of culture, cell numbers were determined using the
Countess II Automated Cell Counter (Thermo Fisher Scientific, Inc).
All cells were maintained at 37°C and 5% CO2 in a
humidified incubator.
Western blotting
Cells were washed twice with cold phosphate-buffered
saline (PBS) and extracted on ice with RIPA buffer (Beyotime
Institute of Biotechnology) containing 1% phenylmethane sulfonyl
fluoride and 1% phosphatase inhibitor cocktail solution (Beyotime
Institute of Biotechnology). Protein concentrations were quantified
using a Bicinchoninic Acid Protein Assay kit (Bio-Rad Laboratories,
Inc.). Cell lysates were boiled for 5 min in sodium dodecyl sulfate
gel-loading buffer and stored at −20°C for western blotting. Cell
lysates containing 50 µg protein were separated using 10% sodium
dodecyl sulfate–polyacrylamide gel electrophoresis and transferred
to polyvinylidene fluoride (PVDF) membranes (EMD Millipore). PVDF
membranes were blocked for 1 h at room temperature with 5% non-fat
milk, and incubated with primary antibody diluted (1:1,000) in 5%
non-fat milk overnight at 4°C. Next, the membranes were incubated
with horseradish peroxidase-conjugated secondary antibody (Cell
Signaling Technology, Inc.; 1:4,000) at room temperature for 1 h
and developed using an ECL Western Blotting Analysis System (GE
Healthcare). GAPDH was used as the control. The density of the
immunoreactive bands was quantified using Image J V1.8 (National
Institutes of Health).
Flow cytometry
Cells (2×105/well) in 6-well plates were
treated with the indicated concentrations of cetuximab, icaritin,
cetuximab + icaritin or control DMSO were added for 24 h, collected
and washed twice in ice-cold PBS. The apoptosis assay was conducted
using an Annexin V-FITC apoptosis detection Kit (Beyotime Institute
of Biotechnology) according to the manufacturer's instructions and
a BD Accuri™ C6 Flow Cytometer (Becton, Dickinson and Company) was
used for fluorescence detection. The results were analyzed using
FlowJo 7.6 software (Becton, Dickinson and Company).
Construction of an orthotopic mouse
model of BC
A suspension of MDA-MB-231 and MDA-MB-436 cells in a
PBS-Matrigel (v/v, 1:1) solution was implanted in the mammary fat
pads of female NOD/SCID mice (n=40; age, 4–6 weeks; and weight:
18–20 g) obtained from the Animal Center of the Third Military
Medical University. Each mouse was inoculated with 1×106
tumor cells. The tumor volume was calculated as length ×
width2/2. After the tumors had reached 6–8 mm in
diameter, mice were grouped randomly (5 per group) and injected
(i.v.) with control (0.9% NaCl), cetuximab (2 mg/kg/week), icaritin
(50 mg/kg/week) or cetuximab + icaritin (equivalent dose/week).
According to the guidelines of IACUC, the mice were euthanized
within 48 h when the diameter of the xenografts reached 1.5 cm.
Statistical analyses
All assays were repeated at least 3 times. Data were
described as the mean ± standard deviation (SD) or standard error
of mean (SEM) as indicated. Two-sided paired Student's t-tests were
used to compare the differences between two groups. One-way ANOVA
was used for the comparison of multiple groups, followed by the
Bonferroni's post-hoc test. Statistical analyses were performed
using SPSS v.19 (IBM Corp). P<0.05 was considered to indicate a
statistically significant difference.
Results
High expression of ER-α36 activates
the PI3K/AKT signaling pathway downstream of the estrogen
receptor
MDA-MB-231 and MDA-MB-436 cell lines expressed high
levels of ER-α36 and EGFR compared with MCF7 of ER-positive cell,
but undetectable levels of the full length ER-α (Fig. 1A). Following treatment with
increasing concentrations of E2β in MDA-MB-231 and MDA-MB-436
cells, western blotting was conducted with a
phosphorylation-specific anti-AKT antibody. Basal AKT
phosphorylation was notably increased in MDA-MB-231 and MDA-MB-436
cells, particularly at E2β 1 µM concentration (Fig. 1B). To determine if ER-α36 mediated
the activation of mitogenic estrogen signaling in these ΤΝΒC cell
lines, a stable knockdown of ER-α36 cells by shRNA in MDA-MB-231
and MDA-MB-436 cells was performed. Western blotting demonstrated
that ER-α36 expression was down-regulated by ~80% in
shRNA-transfected cells compared with control cells (Fig. 1C). E2β treatment failed to induce AKT
phosphorylation in the MDA-MB-231 cell line following ER-α36
knockdown. Similar results were observed in the MDA-MB-436
ER-α36-knockdown cell line (Fig.
1D). These results suggested that estrogen may activate the
downstream PI3K/AKT signaling pathway through ER-α36.
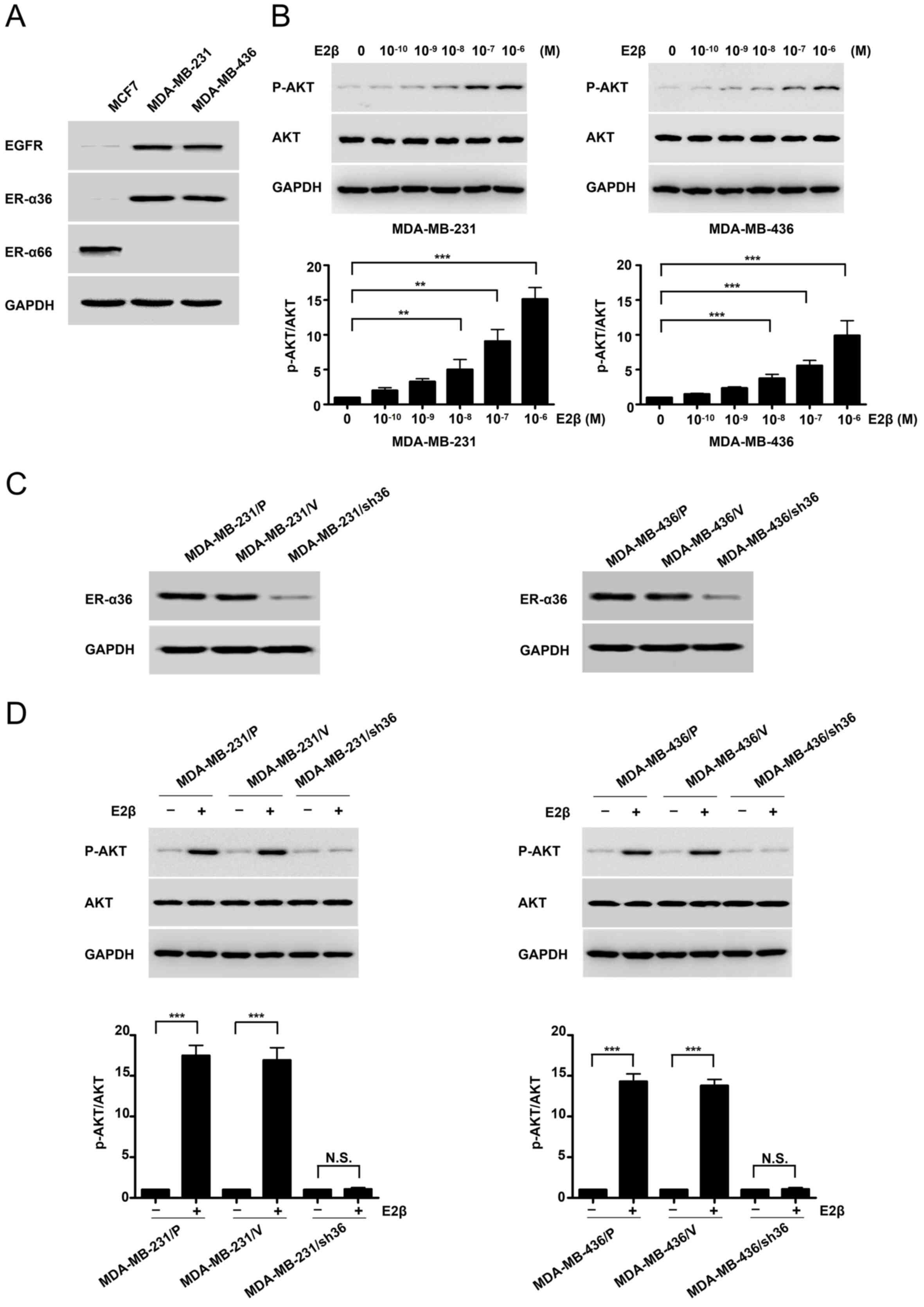 | Figure 1.High expression of ER-α36 mediates
estrogen signaling via the PI3K/AKT signaling pathway in TNBC
cells. (A) Western blots displaying the expression of ER-α66,
ER-α36 and EGFR in an ER-positive breast-cancer cell line (MCF7)
and TNBC cell lines (MDA-MB-231 and MDA-MB-436). (B) MDA-MB-231 and
MDA-MB-436 cells were treated with different concentrations (M) of
estrogen for 30 min, and AKT phosphorylation levels were
investigated by western blotting. (C) Western blots representing
ER-α36 expression in variants of MDA-MB-231 and MDA-MB-436 cells;
parental cells, MDA-MB-231/P and MDA-MB-436/P; control cells
transfected with the empty vector, MDA-MB-231/V and MDA-MB-436/V;
and ER-α36 expression knockdown cells, MDA-MB-231/sh36 and
MDA-MB-436/sh36. (D) Western blots representing the effects of E2β
(1 µM) on the phosphorylation and expression of AKT in variants of
MDA-MB-231 and MDA-MB-436 cells, as well as the fold-change of
p-AKT/AKT. GAPDH was used as a loading control. Data are presented
as the mean ± SEM from three independent experiments. **P<0.01
and ***P<0.001. ER-α36, estrogen receptor α-36; ER-α66, estrogen
receptor α-66; EGFR, epidermal growth factor receptor; TNBC, triple
negative breast cancer; E2β, 17β-estradiol; p, phosphorylated. |
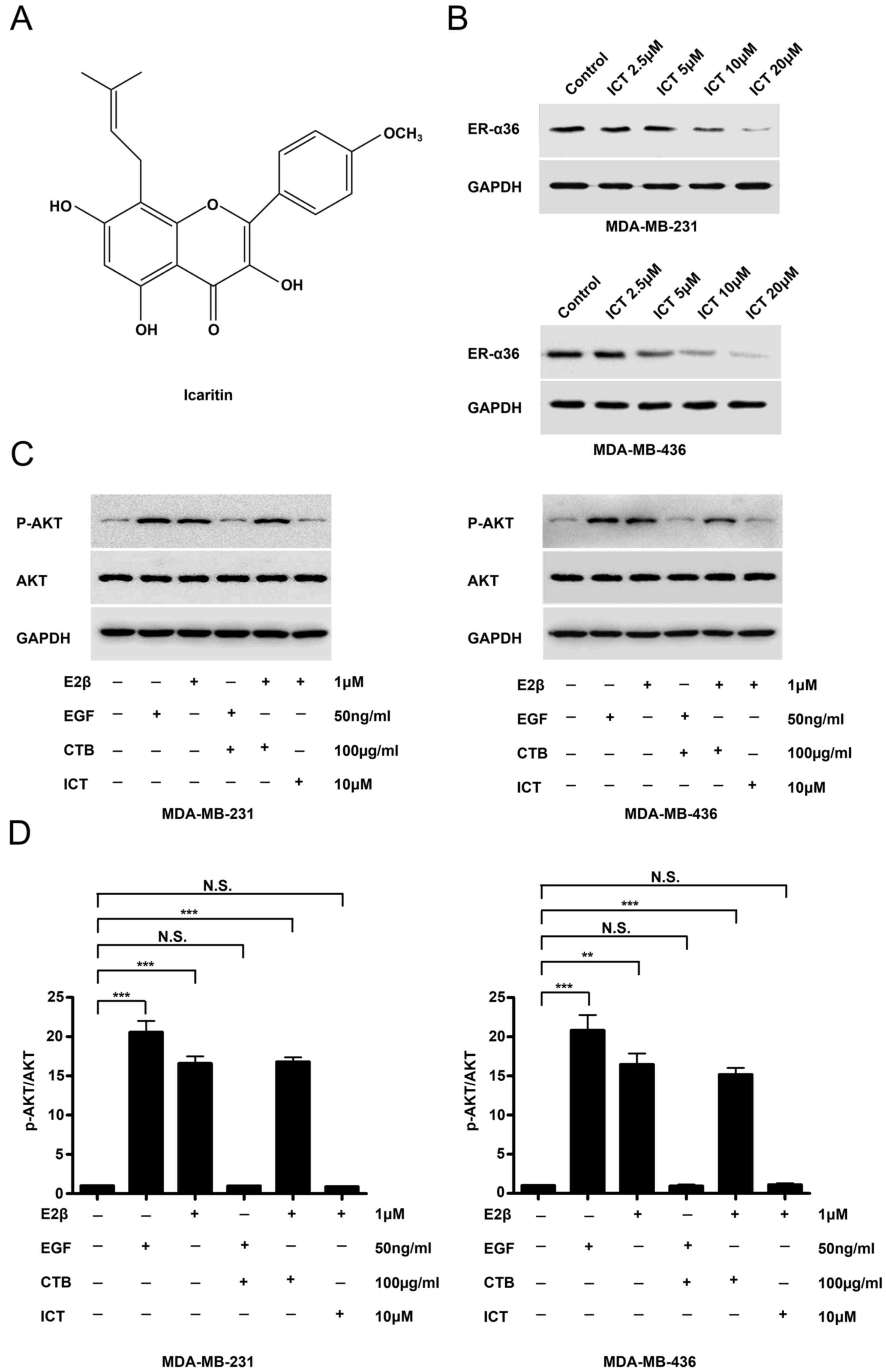
Icaritin downregulates the expression
of ER-α36 and inhibits E2β-stimulated AKT phosphorylation
The structure of icaritin was shown in Fig. 2A. Western blotting revealed that
icaritin treatment potently reduced ER-α36 expression in both TNBC
cell lines (Fig. 2B). The EGFR
inhibitor cetuximab was used to treat MDA-MB-231 and MDA-MB-436
cells for 30 min prior to stimulation with EGF and E2β.
EGF-stimulated AKT phosphorylation was inhibited by the EGFR
inhibitor cetuximab, however, cetuximab failed to influence
E2β-stimulated AKT phosphorylation in the two TNBC cancer cell
lines (Fig. 2C). In icaritin-treated
E2β-stimulated TNBC cells, AKT phosphorylation was inhibited
(Fig. 2C and D). These results
suggested that E-α36-mediated activation of the estrogen signaling
pathway may be associated with EGFR-targeted treatment failure in
TNBC cells.
Cetuximab plus icaritin inhibits TNBC
cell proliferation and induces apoptosis
To determine whether the effects of the combined
application of cetuximab and icaritin on the proliferation of TNBC
cells were stronger compared with cetuximab or icaritin treatment
alone, different concentrations of cetuximab were tested in
MDA-MB-231 cells. The quantity of MDA-MB-231 cells did not decrease
significantly, even in the highest dose group of 100 µg/ml
cetuximab (Fig. 3A). Next,
MDA-MB-231 cell were treated with different concentrations of
icaritin, and the mean percentages of cells were 1 µM, 99.09±4.80%;
2.5 µM, 93.99±6.62%; 5 µM, 83.52±6.77%; 7.5 µM, 66.4%±5.658 and 10
µM, 41.35±7.00% (Fig. 3A). To
identify the combined effects, MDA-MB-231 cells were treated with
cetuximab + icaritin; the mean percentages of cells were as
follows: 100 µg/ml cetuximab + 1 µM icaritin, 94.45±6.58%; ,100
µg/ml cetuximab + 2.5 µM icaritin, 81.84±4.55%; 100 µg/ml cetuximab
+ 5 µM icaritin, 66.01±8.75%; 100 µg/ml cetuximab + 7.5 µM
icaritin, 47.32±5.82%; and 100 µg/ml cetuximab + 10 µM icaritin,
22.99±4.05% (Fig. 3A). The combined
administration of icaritin and cetuximab was more effective in
inhibiting the proliferation of MDA-MB-231 cells at 100 µg/ml
cetuximab + 2.5 µM icaritin compared with icaritin or cetuximab
alone (Fig. 3A). Similarly, the
combination strategy significantly reduced the mean percentages of
cells compared with single drug treatment in MDA-MB-436 cells
(Fig. 3A). These results suggested
that once the EGFR and ER signaling pathways were suppressed
simultaneously, the proliferation of TNBC cells was inhibited more
potently.
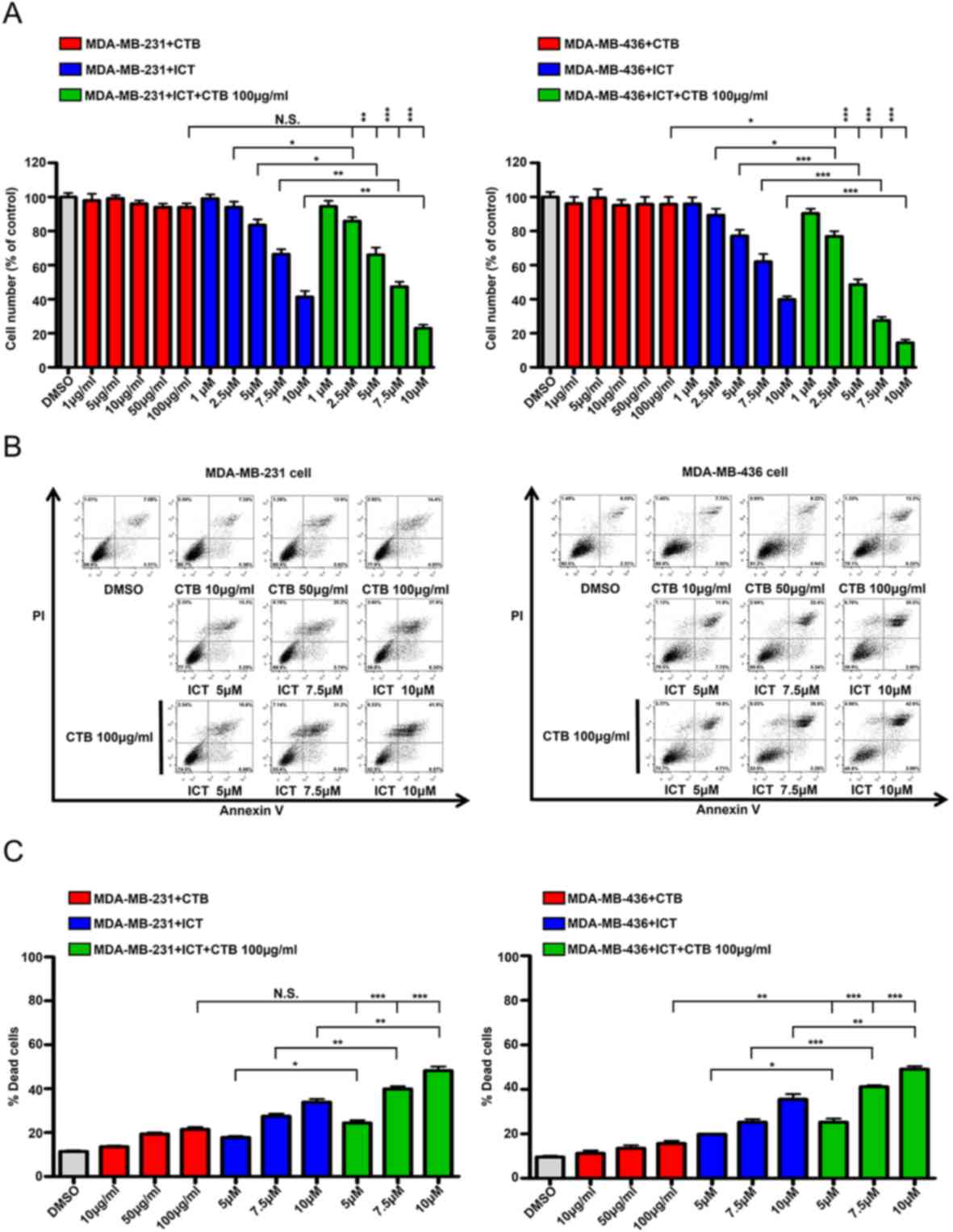 | Figure 3.ICT and CTB co-treatment inhibits the
proliferation and induce the apoptosis of TNBC cells. (A) Cell
numbers (% of control) of MDA-MB-231 and MDA-MB-436 cells compared
between CTB (1, 5, 10, 50 and 100 µg/ml), ICT (1, 2.5, 5, 7.5 and
10 µM) and CTB (100 µg/ml) + ICT (1, 2.5, 5, 7.5 and 10 µM)
treatment for 7 days. (B and C) Apoptosis assay performed using
MDA-MB-231 and MDA-MB-436 cells treated with CTB (10, 50 and 100
µg/ml), ICT (5, 7.5 and 10 µM) and CTB (100 µg/ml) + ICT (5, 7.5
and 10 µM) treatment for 24 h. Data presented as mean ± SD obtained
from three independent experiments. *P<0.05, **P<0.01 and
***P<0.001. CTB, cetuximab; ICT, icaritin; TNBC, triple-negative
breast cancer. |
To ascertain whether this inhibition was caused by
apoptosis, the Annexin V/PI double labeling apoptosis assay was
performed. The percentages of apoptotic cells in the combination
groups were as follows: 100 µg/ml cetuximab + 5 µM icaritin,
24.35±2.14%; 100 µg/ml cetuximab + 7.5 µM icaritin, 39.85±2.26%;
and 100 µg/ml cetuximab + 10 µM icaritin, 48.19±3.34%, which were
higher compared with the cetuximab alone treatment group
(21.47±1.81%) or icaritin gradient treatment group (5 µM icaritin,
17.80±1.15%; 7.5 µM icaritin, 27.45±2.06%; 10 µM icaritin,
33.85±2.53%) (Fig. 3B and C).
Similar results were also obtained using the MDA-MB-436 cell line
(Fig. 3B and C). Together, these
results demonstrated that the combination of cetuximab and icaritin
may more effectively promote the apoptosis of TNBC cells compared
with either drug used alone. (Fig. 3B
and C).
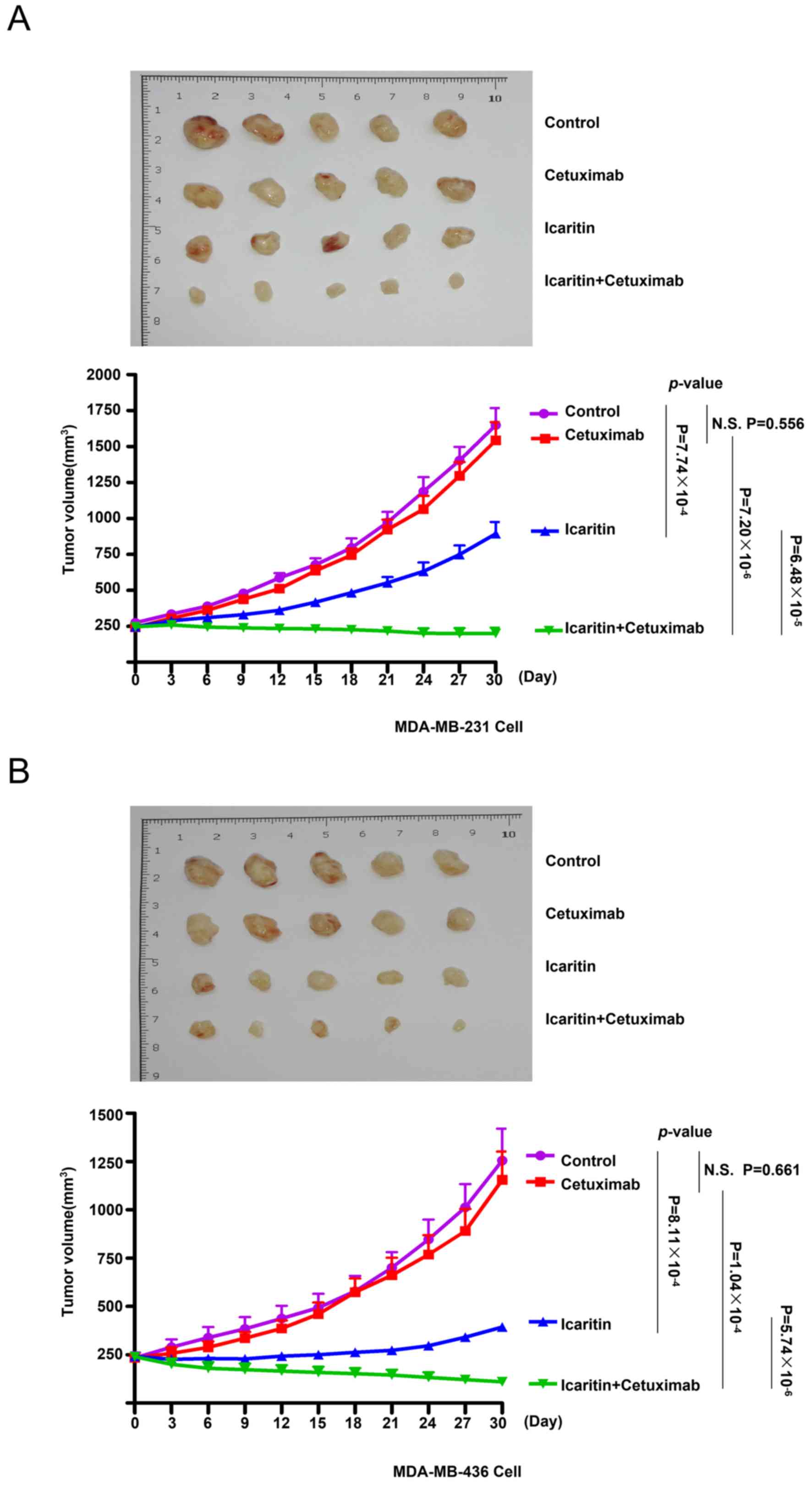
Therapeutic effects of the combined
treatment with cetuximab and icaritin on TNBC cells in vivo
To evaluate the effects of cetuximab monotherapy,
icaritin monotherapy and combined treatment on TNBC cells in
MDA-MB-231 and MDA-MB-436 ×enografts, human BC cell xenografts were
created in immunocompromised NOD/SCID mice using MDA-MB-231 or
MDA-MB-436 cells. The mice were divided into 4 groups: Control,
cetuximab, icaritin and cetuximab + icaritin (Fig. 4A and B). Cetuximab monotherapy was
ineffective in MDA-MB-231 ×enografts [tumor doubling time
(TDT)=12±2 days, N.S.] compared with the control (TDT=12±3 days,
P=0.556) and icaritin monotherapy (TDT=21±3 days, P<0.001;
Fig. 4A) groups. The combined
therapy induced a significant reduction in the tumor growth of
MDA-MB-231 ×enografts compared with cetuximab (P<0.001) or
icaritin (P<0.001) monotherapy. Similar results could be
observed in the xenografts derived from the MDA-MB-436 cell line
(Fig. 4B). These results indicated
that the combination of cetuximab and icaritin exhibited greater
therapeutic effects compared with those elicited by cetuximab
monotherapy or icaritin monotherapy.
Discussion
The EGFR regulates the development of epithelial
tissue and maintains homeostasis. The EGFR is a driver of
tumorigenesis in lung cancer, BC and glioblastoma (31). Inappropriate activation of the EGFR
in cancer results mainly from amplifications and point mutations at
the genomic locus (32).
Experimental and clinical studies (11,33) have
suggested that EGFR expression in TNBC is higher compared with
other subtypes of BC, and that EGFR expression is associated with a
poor prognosis, the 5-year disease free survival (DFS) for
EGFR-positive and EGFR-negative patients were 69.0% and 83.8%,
respectively. DFS was significantly poorer for the EGFR-positive
patients (HR=2.11, P=0.011) (34).
Thus, EGFR inhibition is a promising approach against TNBC.
Although, a variety of EGFR inhibitors have been developed,
clinical studies have reported that the use of cetuximab alone in
the treatment of patients with TNBC did not achieve the expected
results, and most TNBC patients exhibit sustained activation of the
PI3K/AKT signaling pathway downstream of the EGFR, suggesting that
most had alternate mechanisms for pathway activation (35).
Previously, Zhang et al (36) reported that E2β-stimulated
proliferation of ER-negative BC cells is through a novel variant of
the ER, ER-α36. It has also been demonstrated that E2β induced the
physical interaction between ER-α36 and Src, and consequently, the
auto-phosphorylation of Src-Y416 in ER-negative BC cells (37). In the present study, ER-α36 was
upregulated in TNBC cell lines, and it was demonstrated that E2β
serves an important role in activating the downstream PI3K/AKT
signaling pathway by binding to ER-α36. These results are
consistent with those of Tsai et al (38) who reported that E2β could induce
PI3K/AKT phosphorylation in MDA-MB-231 ER-negative cells. Friedl
et al (39) reported that the
malignant growth of MD-MB-231 cells was stimulated by estrogen in
immunodeficient mice. Therefore, the role of estrogen may be beyond
classical activation of ER signaling. These data suggest that
non-genomic and mitogenic estrogen signaling is retained in TNBC
cells (39). Therefore, knowing the
specific signaling pathway is important for therapeutic strategies
targeting ER-α36. Τhe present study demonstrated that icaritin
effectively downregulated the expression of ER-α36, inhibited TNBC
cell proliferation and induced apoptosis. However, the present
study did not demonstrate that the effects of icaritin on the
proliferation and apoptosis of TNBC cells were directly due to
ER-α36 inhibition, future studies are required to ascertain
this.
The present study demonstrated that EGF and estrogen
activated the AKT signaling pathway. In vitro, MDA-MB-231
and MDA-MB-436 cells starved for 24 h in low-concentration serum
and a phenol red-free environment, which has a weak estrogen-like
effect, were used to investigate estrogen activation of the AKT
signaling pathway. Cetuximab alone inhibited the activation of the
AKT signaling pathway induced by EGF. In mice, the presence of
endogenous estrogen activated the AKT signaling pathway, therefore,
cetuximab alone was not sufficient to inhibit the activation of the
AKT signaling pathway and tumor growth. This result was consistent
with the hypothesis of an AKT bypass activation mechanism by the
ER-α36 mediated rapid estrogen signaling pathway in TNBC cells.
In summary, the existence of ER-α36-mediated rapid
estrogen signaling bypass activation AKT signaling pathway was
demonstrated in TNBC cells. This ER-α36 mediated rapid estrogen
signaling pathway is one of the mechanisms for the resistance of
TNBC cells to EGFR-targeted therapy. It was also revealed that the
combination of the ER-α36 molecular inhibitor icaritin and the EGFR
inhibitor cetuximab may more effectively inhibit the proliferation
and promote the apoptosis of TNBC cells compared with either
individual drug. The current data may help to develop novel
therapeutic strategies against TNBC.
Acknowledgements
The authors would like to thank Dr Zhao-yi Wang
(Department of Medical Microbiology and Immunology, Creighton
University Medical School) for donating the plasmid and
antibody.
Funding
This study was supported by grants from the National
Key Research and Development Program of China (grant no.
2016YFA0202104), the National Natural Science Foundation of China
(grant no. 81602730) and the Key Clinical Research Program of
Southwest Hospital (grant nos. SWH2016ZDCX1005, SWH2017ZDCX1003 and
SWH2019TD-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
LY and XZL acquired, analyzed and interpreted data
and drafted the manuscript; XWQ, ZYY and RLC acquired data; SCY, LC
and HJC analyzed and interpreted data, critically revised the
manuscript for intellectual content, obtained funding and
supervised the study; all authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Animal Care
and Use Committee of the Third Military Medical University (Army
Medical University, Chongqing, China).
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lehmann BD, Bauer JA, Chen X, Sanders ME,
Chakravarthy AB, Shyr Y and Pietenpol JA: Identification of human
triple-negative breast cancer subtypes and preclinical models for
selection of targeted therapies. J Clin Invest. 121:2750–2767.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haffty BG, Yang Q, Reiss M, Kearney T,
Higgins SA, Weidhaas J, Harris L, Hait W and Toppmeyer D:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carey LA, Dees EC, Sawyer L, Gatti L,
Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML and Perou
CM: The triple negative paradox: Primary tumor chemosensitivity of
breast cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudis CA and Gianni L: Triple-negative
breast cancer: An unmet medical need. Oncologist. 16 (Suppl
1):S1–S11. 2011. View Article : Google Scholar
|
|
8
|
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z,
Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y and
Chen-Yan Z: Recent treatment progress of triple negative breast
cancer. Prog Biophys Mol Biol. Nov 21–2019.(Epub ahead of print).
PubMed/NCBI
|
|
9
|
Troyer KL and Lee DC: Regulation of mouse
mammary gland development and tumorigenesis by the ERBB signaling
network. J Mammary Gland Biol Neoplasia. 6:7–21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar R and Wang RA: Protein kinases in
mammary gland development and cancer. Microsc Res Tech. 59:49–57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nielsen TO, Hsu FD, Jensen K, Cheang M,
Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler
L, et al: Immunohistochemical and clinical characterization of the
basal-like subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corkery B, Crown J, Clynes M and O'Donovan
N: Epidermal growth factor receptor as a potential therapeutic
target in triple-negative breast cancer. Ann Oncol. 20:862–867.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park HS, Jang MH, Kim EJ, Kim HJ, Lee HJ,
Kim YJ, Kim JH, Kang E, Kim SW, Kim IA and Park SY: High EGFR gene
copy number predicts poor outcome in triple-negative breast cancer.
Mod Pathol. 27:1212–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuda H, Zhang D, Bartholomeusz C,
Doihara H, Hortobagyi GN and Ueno NT: Role of epidermal growth
factor receptor in breast cancer. Breast Cancer Res Treat.
136:331–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gelmon K, Dent R, Mackey JR, Laing K,
McLeod D and Verma S: Targeting triple-negative breast cancer:
Optimising therapeutic outcomes. Ann Oncol. 23:2223–2234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakai K, Hung MC and Yamaguchi H: A
perspective on anti-EGFR therapies targeting triple-negative breast
cancer. Am J Cancer Res. 6:1609–1623. 2016.PubMed/NCBI
|
|
17
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee LM, Cao J, Deng H, Chen P, Gatalica Z
and Wang ZY: ER-alpha36, a novel variant of ER-alpha, is expressed
in ER-positive and -negative human breast carcinomas. Anticancer
Res. 28:479–483. 2008.PubMed/NCBI
|
|
19
|
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J,
Wang T, Fan Z, Fan T, Lin B, et al: Expression of ER-{alpha}36, a
novel variant of estrogen receptor {alpha}, and resistance to
tamoxifen treatment in breast cancer. J Clin Oncol. 27:3423–3429.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang ZY and Yin L: Estrogen receptor
alpha-36 (ER-α36): A new player in human breast cancer. Mol Cell
Endocrinol. 418:193–206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: A variant of estrogen receptor-{alpha},
hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent
membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA.
103:9063–9068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang XJ, Xi YM and Li ZJ: Icaritin: A
novel natural candidate for hematological malignancies therapy.
Biomed Res Int. 2019:48602682019.PubMed/NCBI
|
|
23
|
Hong J, Zhang Z, Lv W, Zhang M, Chen C,
Yang S, Li S, Zhang L, Han D and Zhang W: Icaritin synergistically
enhances the radiosensitivity of 4T1 breast cancer cells. PLoS One.
8:e713472013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Priceman SJ, Xin H, Zhang W, Deng J,
Liu Y, Huang J, Zhu W, Chen M, Hu W, et al: Icaritin inhibits
JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS One.
8:e816572013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu JF, Li ZJ, Zhang GS, Meng K, Kuang WY,
Li J, Zhou XF, Li RJ, Peng HL, Dai CW, et al: Icaritin shows potent
anti-leukemia activity on chronic myeloid leukemia in vitro and in
vivo by regulating MAPK/ERK/JNK and JAK2/STAT3/AKT signalings. PLoS
One. 6:e237202011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang X, Zheng N, Dong J, Liu L and Huang
J: Estrogen receptor-α36 is involved in icaritin induced growth
inhibition of triple-negative breast cancer cells. J Steroid
Biochem Mol Biol. 171:318–327. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brinkley BR, Beall PT, Wible LJ, Mace ML,
Turner DS and Cailleau RM: Variations in cell form and cytoskeleton
in human breast carcinoma cells in vitro. Cancer Res. 40:3118–3129.
1980.PubMed/NCBI
|
|
28
|
Cailleau R, Young R, Olive M and Reeves WJ
Jr: Breast tumor cell lines from pleural effusions. J Natl Cancer
Inst. 53:661–674. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: One tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang L and Wang ZY: Breast cancer cell
growth inhibition by phenethyl isothiocyanate is associated with
down-regulation of oestrogen receptor-alpha36. J Cell Mol Med.
14:1485–1493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Normanno N, De Luca A, Bianco C, Strizzi
L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F and
Salomon DS: Epidermal growth factor receptor (EGFR) signaling in
cancer. Gene. 366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu D, He J, Yuan Z, Wang S, Peng R, Shi
Y, Teng X and Qin T: EGFR expression correlates with decreased
disease-free survival in triple-negative breast cancer: A
retrospective analysis based on a tissue microarray. Med Oncol.
29:401–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carey LA, Rugo HS, Marcom PK, Mayer EL,
Esteva FJ, Ma CX, Liu MC, Storniolo AM, Rimawi MF, Forero-Torres A,
et al: TBCRC 001: Randomized phase II study of cetuximab in
combination with carboplatin in stage IV triple-negative breast
cancer. J Clin Oncol. 30:2615–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang X, Ding L, Kang L and Wang ZY:
Estrogen receptor-alpha 36 mediates mitogenic antiestrogen
signaling in ER-negative breast cancer cells. PLoS One.
7:e301742012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang XT, Ding L, Kang LG and Wang ZY:
Involvement of ER-α36, Src, EGFR and STAT5 in the biphasic estrogen
signaling of ER-negative breast cancer cells. Oncol Rep.
27:2057–2065. 2012.PubMed/NCBI
|
|
38
|
Tsai EM, Wang SC, Lee JN and Hung MC: Akt
activation by estrogen in estrogen receptor-negative breast cancer
cells. Cancer Res. 61:8390–8392. 2001.PubMed/NCBI
|
|
39
|
Friedl A and Jordan VC: Oestradiol
stimulates growth of oestrogen receptor-negative MDA-MB-231 breast
cancer cells in immunodeficient mice by reducing cell loss. Eur J
Cancer. 30A:1559–1564. 1994. View Article : Google Scholar : PubMed/NCBI
|