Introduction
Malignant pleural mesothelioma, an aggressive tumor,
is commonly caused by exposure to asbestos with a median latency of
22.8 years (1). The incidence of
malignant mesothelioma is predicted to reach a peak around 2020 in
Europe (2) and around 2030 in Japan
(3), and has not yet declined this
century in the United States (4).
The incidence of malignant mesothelioma in developing countries is
predicted to increase due to their heavy use of asbestos without
restriction (5).
Because many cases of malignant pleural mesothelioma
are detected at an advanced stage and are thus unresectable by
surgery, systemic anticancer chemotherapy is the first choice of
treatment. The present standard regimen of chemotherapy for
advanced malignant pleural mesothelioma is a combination of
pemetrexed and cisplatin; however, the median survival of patients
treated with this regimen scarcely exceeds 12 months (6).
MicroRNAs (miRNAs) are short non-coding,
single-strand RNAs that suppress gene expression by binding to the
3′-untranslated region of their target mRNAs (7). Recently, several studies reported
different miRNAs that play important roles in the pathogenesis of
various human cancers as onco-miRNAs or tumor suppressor miRNAs
(8–10).
In a previous study, we investigated the expression
profiles of miRNAs in mesothelioma cell lines and found some to be
significantly down- or upregulated (11). In that study, microRNA-18a (miR-18a)
was one of the upregulated miRNAs. Further, miR-18a has been
identified as an onco-miRNA associated with many human malignancies
including glioblastoma (12),
esophageal cancer (13), and
non-small cell lung cancer (14).
The current study was performed to elucidate the biological
function of miR-18a in mesothelioma.
Materials and methods
Mesothelioma cell lines
Four human mesothelioma cell lines were used in this
study. Two cell lines (ACC-MESO1 and ACC-MESO4) were purchased from
the RIKEN BioResearch Center (Tsukuba, Japan) (15) and the other two (CRL-5915 and
CRL-5946) were purchased from the American Type Culture Collection.
Cells were cultured with Roswell Park Memorial Institute 1640
medium with GlutaMax added, containing 10% fetal bovine serum,
sodium pyruvate, kanamycin, and fungizone (all purchased from
Thermo Fisher Scientific). Cells were maintained in a 5%
CO2 incubator at 37°C.
Transient transfection of mesothelioma
cells
The miR-18a inhibitor (mirVana; has-miR-18a-3p
MH12264) and a negative control miRNA inhibitor (mirVana; negative
control #1) were purchased from Thermo Fisher Scientific.
Mesothelioma cells at 60–80% confluence were transfected with 50 nM
of the miR-18a or negative control inhibitor using Lipofectamine
RNAiMAX (Thermo Fisher Scientific) in Opti-Mem Reduced Serum Medium
(Thermo Fisher Scientific) according to the manufacturer's
recommended protocols.
Cell proliferation assay
After transfection, mesothelioma cell lines were
incubated in growth medium in a 96-well plate. The proliferation
rate was determined at 24, 48, and 72 h with the Cell Titer Glo 2.0
reagent (Promega KK), which measures the number of viable cells
relative to their ATP level, using a GloMax Explore microplate
reader (Promega) according to the manufacturer's recommended
protocols.
Colony formation assay
Mesothelioma cell lines transfected with the miRNA
inhibitor or control were seeded into collagen-coated 6-well plates
at a density of 500 cells/well and incubated in growth medium for
three weeks. Cellmatrix Type I-A (Nitta Gelatin) was used for
collagen coating. Colonies were stained by Cell Stain (EMD
Millipore) and counted.
Wound scratch assay
The migration ability of mesothelioma cells was
analyzed by a wound scratch assay. miRNA inhibitor- and
control-transfected cells were incubated in collagen-coated 24-well
plates. After making wounds with 1 ml micropipette tips, floating
cells were removed by washing twice with fresh growth medium.
Microscopic images were obtained at 0, 12, and 24 h (ACC-MESO1
cells), or at 0, 24, and 48 h (ACC-MESO4, CRL-5915, and CRL-5946
cells). The wound area was measured using TScratch software
(16).
Cell invasion assay
Mesothelioma cell lines were incubated with the
miRNA or control inhibitor in FluoroBlok culture inserts with 8-µm
pores (BD Biosciences) and coated with Geltrex Matrigel (Thermo
Fisher Scientific) according to the manufacturers' protocols.
Invaded cells were measured at 24 or 48 h after incubation with the
miRNA inhibitor.
Apoptosis and necrosis assays
Mesothelioma cells were incubated with the miRNA or
control inhibitor in 96-well plates for 24 h, and the RealTime Glo
Annexin V Apoptosis Assay reagent (Promega) was added to the cells
after transfection. Relative levels of apoptosis and necrosis were
measured by analyzing luminescence and fluorescence with a GloMax
microplate reader according to the manufacturer's recommended
protocol.
Chemosensitivity to pemetrexed and
cisplatin
Mesothelioma cells transfected with the miRNA or
control inhibitor were seeded into 96-well plates containing 0 to
50 µM cisplatin or 0–100 µM pemetrexed (both purchased from
Fujifilm Wako Pure Chemical Corporation). Viable cells were
measured with the Cell Titer Glo 2.0 reagent (Promega) 72 h after
the addition of chemical agents.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from cells transfected with the
miRNA inhibitor or control using a Maxwell RSC simplyRNA Cells kit
and analyzed on a Maxwell RSC Instrument (Promega) according to the
manufacturer's instructions. The extracted RNA was
reverse-transcribed with SuperScript IV VILO Master Mix (Thermo
Fisher Scientific) and amplified using PowerUp SYBR Green Master
Mix (Thermo Fisher Scientific) on an AriaMax Real-Time PCR System
(Agilent Technologies). Relative expression levels were calculated
according to the comparative CT (ΔΔCT) method (17). Expression levels were normalized
against the expression level of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). The following primers were used: CDKN2D
forward, 5′-TCACACTGCTGTGGTCAGCTTT-3′, reverse,
5′-AGGATGTCCACGAGGTCCTGA-3′, GAPDH forward,
5′-ACAACTTTTGGTATCATGGAAGG-3′, and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Statistical analysis
All experiments were conducted at least three times.
Experimental data are presented as means ± standard deviation.
Statistical significance of differences between two groups was
analyzed with an unpaired Student's t-test. Statistical
significance was set at P<0.05.
Results
Inhibition of miR-18a reduces
mesothelioma cell proliferation
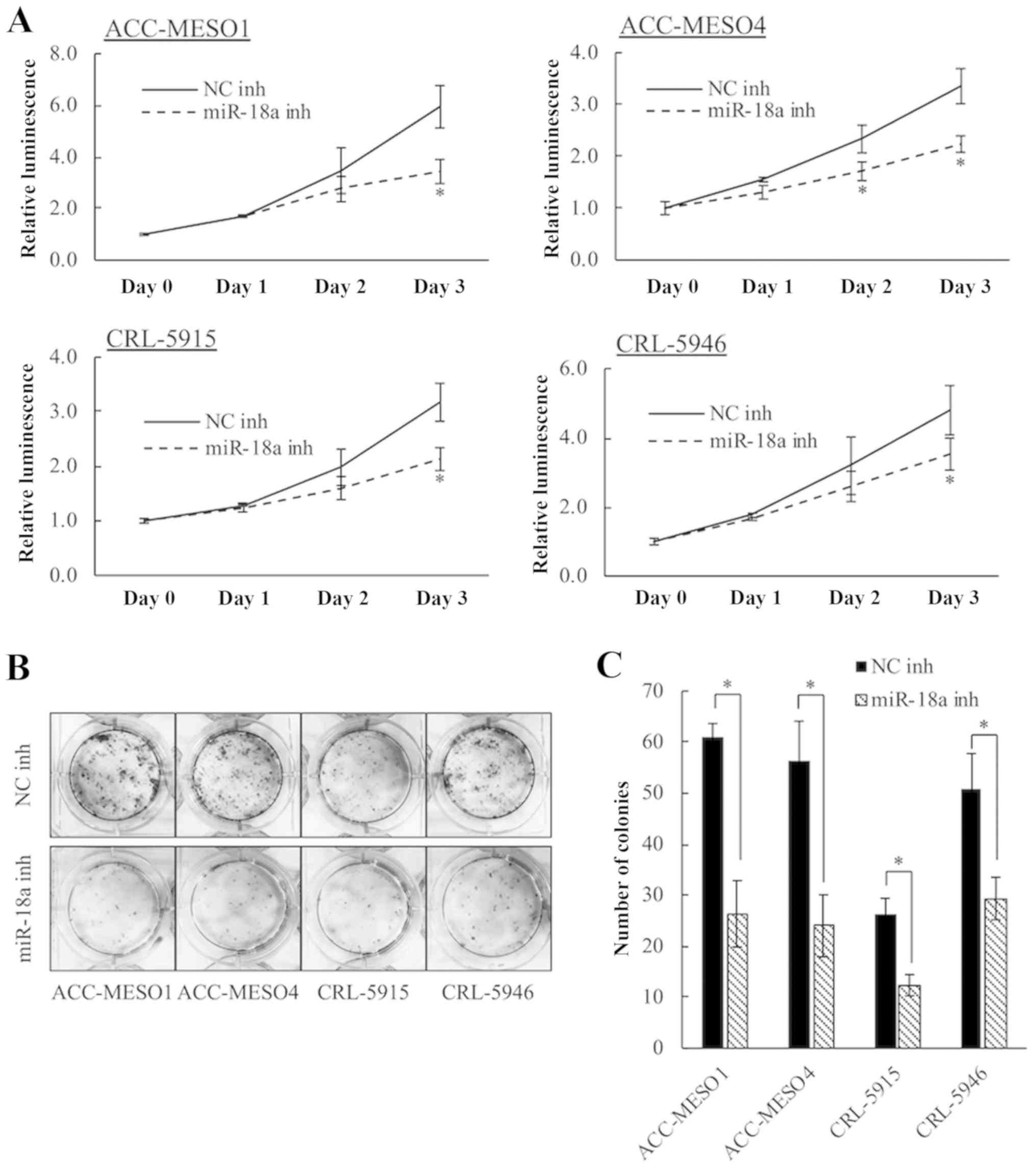
Inhibition of miR-18a significantly decreased
proliferation of mesothelioma cells compared to that by the
negative control inhibitor in all four cell lines (Fig. 1A). After 3 days, inhibition of
miR-18a significantly reduced viability by 42.3% in ACC-MESO1,
33.5% in ACC-MESO4, 32.9% in CRL-5915, and 27.5% in CRL-5946 cells.
The role of miR-18a in mesothelioma cell proliferation was also
evaluated by the colony formation assay (Fig. 1B and C). Inhibition of miR-18a
significantly reduced the colony forming ability of all four cell
lines.
miR-18a inhibition upregulates CDKN2D
expression in mesothelioma cell lines
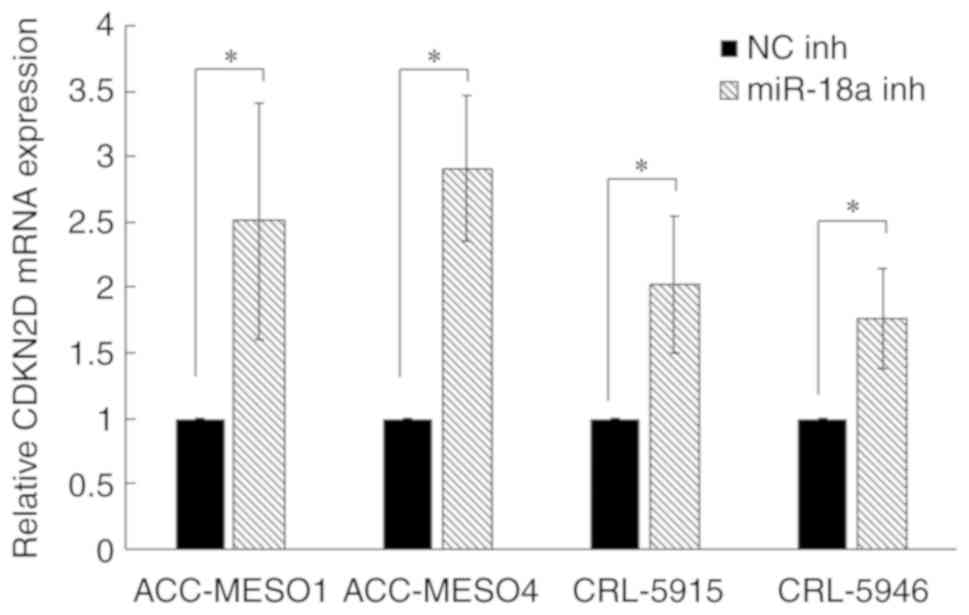
To understand the mechanism of miR-18a in inhibiting
mesothelioma cell growth, we searched for its target gene using the
online miRNA target database, Target Scan Human 7.2 (www.targetscan.org). CDKN2D was identified as a
target gene of miR-18a. Furthermore, RT-qPCR analysis showed that
inhibition of miR-18a significantly upregulated the expression of
CDKN2D (Fig. 2).
Inhibition of miR-18a reduces
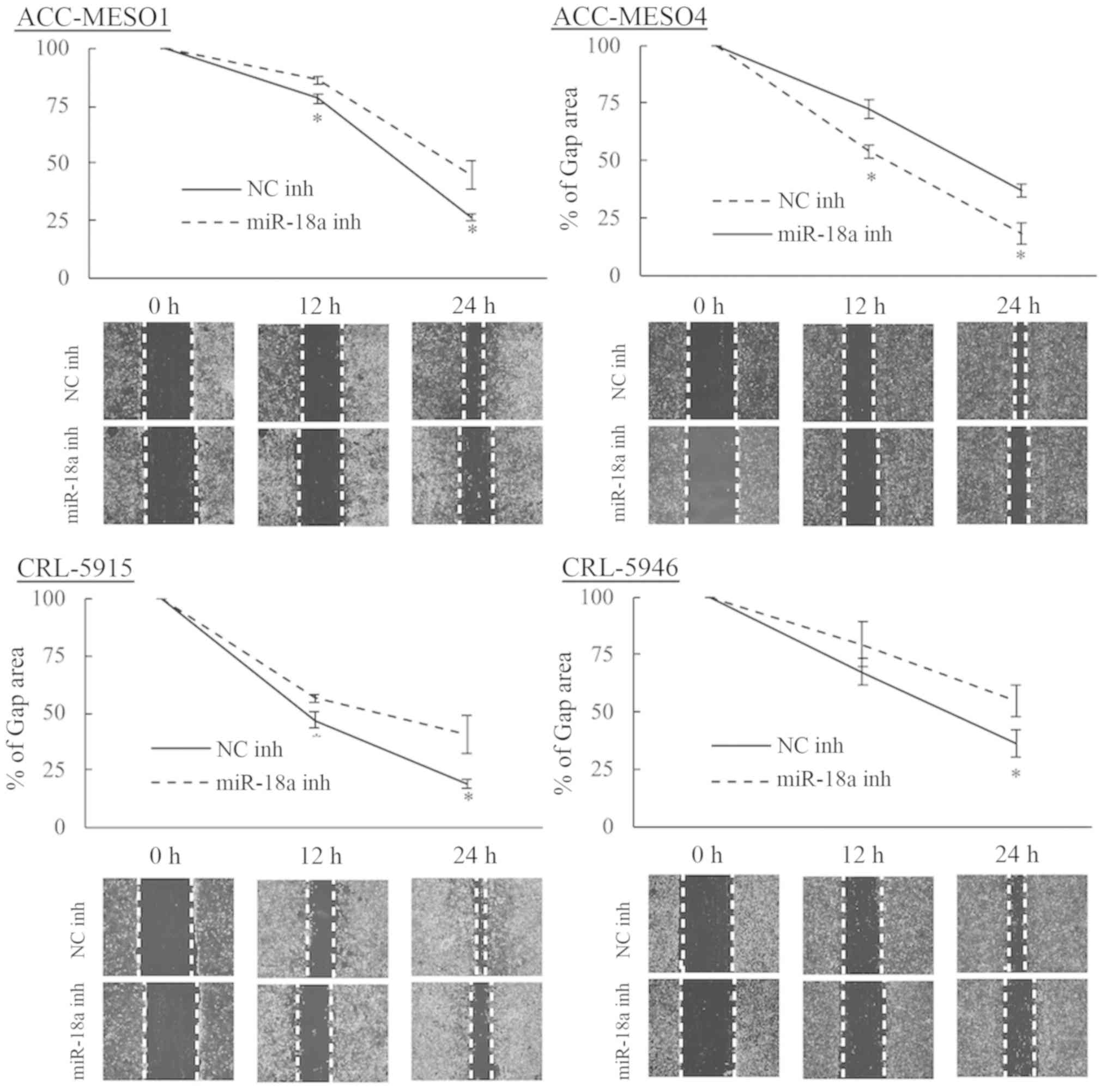
mesothelioma cell migration
Mesothelioma cells transfected with the miR-18a
inhibitor exhibited lower migration rates compared to those
transfected with the negative control inhibitor in all four cell
lines (Fig. 3). At 24 h, inhibition
of miR-18a reduced the migration of ACC-MESO1 cells by 41.0%, and
at 48 h inhibition of miR-18a reduced the migration of ACC-MESO4,
CRL-5915, and CRL-5946 cells by 50.5, 53.0, and 33.7%,
respectively. Mesothelioma cell invasion was not significantly
changed by inhibiting miR-18a (data not shown).
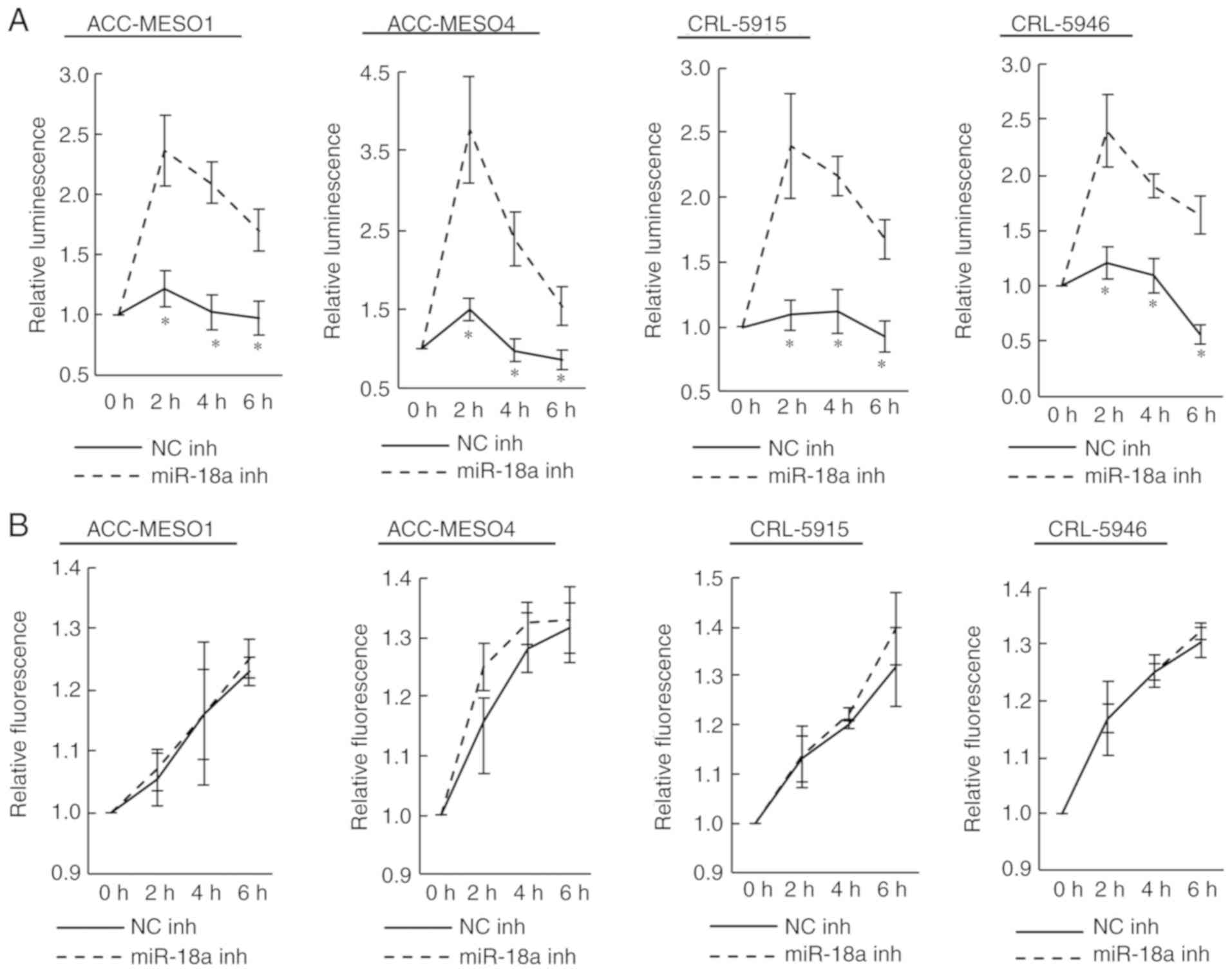
Inhibition of miR-18a increases
apoptosis, but not necrosis, in mesothelioma cell lines
Transfection of the miR-18a inhibitor significantly
increased the extent of apoptosis compared to that caused by the
negative control inhibitor (Fig.
4A). Notably, ACC-MESO4 cells transfected with the miR-18a
inhibitor exhibited over a three times increase in apoptosis
compared to cells transfected with the negative control. However,
no obvious change was observed in the extent of necrosis (Fig. 4B).
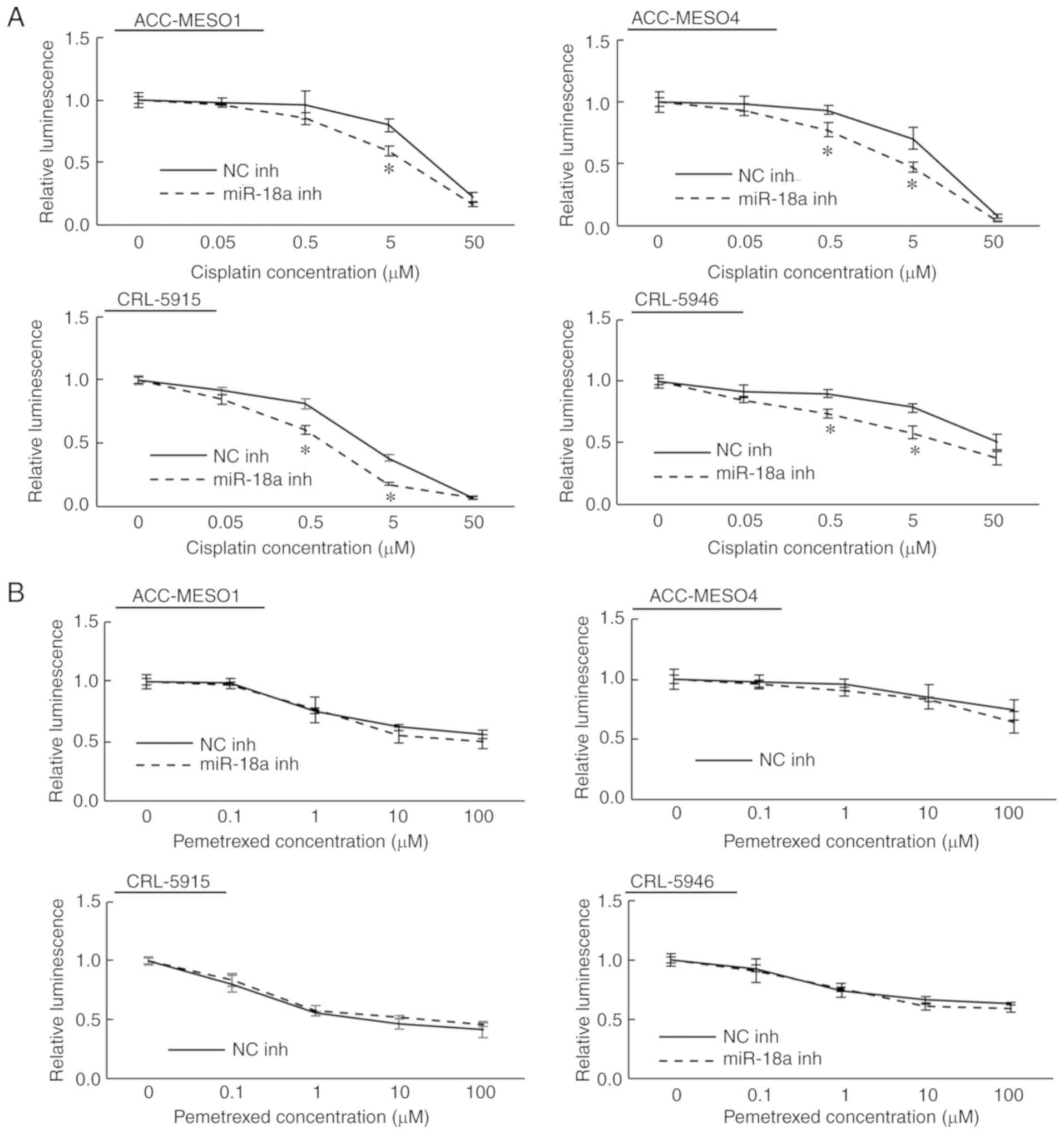
Inhibition of miR-18a increases the
sensitivity of mesothelioma cells to cisplatin, but not
pemetrexed
In the chemosensitivity assay, CRL-5915 cells were
more sensitive to both cisplatin and pemetrexed than the other
three cell lines (ACC-MESO1, ACC-MESO4, and CRL-5946). We also
found that transfection with the miR-18a inhibitor significantly
enhanced sensitivity to cisplatin independent of the original
sensitivity (Fig. 5A). At 0.5 µM
cisplatin, transfection of the miR-18a inhibitor reduced viability
by 10.9, 16.0, 20.6, and 16.3% in ACC-MESO1, ACC-MESO4, CRL-5915,
and CRL-5946 cells, respectively (statistically significant in
ACC-MESO4, CRL-5915, and CRL-5946 cells). At 5 µM cisplatin,
transfection of the miR-18a inhibitor reduced viability by 20.5,
23.3, 20.5, and 19.7% in ACC-MESO1, ACC-MESO4, CRL-5915, and
CRL-5946 cells, respectively (statistically significant in all four
cell lines). In this experiment, no obvious change in pemetrexed
sensitivity was observed by transfection of the miR-18a inhibitor
in cell lines relatively sensitive or resistant to this drug
(Fig. 5B).
Discussion
miRNAs are short, non-coding RNAs that perform a
variety of functions through incomplete binding to the
3′-untranslated region of a target gene (18). Because many miRNAs regulate important
cell functions, such as proliferation and invasion, some have been
researched as therapeutic agents for various human malignancies
(19).
Several studies have focused on the role of miRNAs
in the progression of mesothelioma cells (20,21).
Johnson et al (22) found
that a miR-137 mimic inhibited the proliferation, invasion, and
migration of mesothelioma cells, and Williams et al
(23) found that miR-13 reduced
proliferation, and increased apoptosis and necrosis of mesothelioma
cells by downregulating MCL1. Further, we demonstrated previously
that miR-1 and miR-214 inhibited mesothelioma cell progression by
suppressing PIM1 (11,24), and miR-182 and miR-183 promoted
mesothelioma cell progression by suppressing FOXO1 (25).
In our previous comprehensive analysis of miRNA
expression by RT-qPCR using TaqMan Low Density Array Human miRNA
Cards, both miR-18a-3p and miR-18a-5p were upregulated in malignant
mesothelioma cell lines (ACC-MESO1, ACC-MESO4, CRL-2081, CRL-5820,
CRL-5826, CRL-5915, and CRL-5946) compared to non-neoplastic
mesothelial tissues (11). He et
al (26) found that miR-18a-5p
promoted mesothelioma cell proliferation by downregulating PIAS3,
but little is known about the function of miR-18a-3p in
mesothelioma cells. In the present study, we showed that inhibition
of miR-18a-3p reduced proliferation and migration, but increased
apoptosis of mesothelioma cells. miR-16 is the most extensively
investigated miRNA in malignant mesothelioma and inhibits
mesothelioma cell growth and enhances sensitivity to epidermal
growth factor receptor inhibition (27). A clinical phase 1 trial using
TargomiR, a miR-16-based miRNA mimic that targets the epidermal
growth factor receptor, has been conducted. The objective partial
response rate observed was 5% and the stable disease rate 67%
(28). Thus, miRNA-based therapeutic
targeting of malignant mesothelioma is promising.
In this study, we found that CDKN2D, a target
gene of miR-18a-3p in malignant mesothelioma, was upregulated. The
CDKN2D gene (p19INK4d) is located on chromosome
19p13, and its gene product negatively regulates the cell cycle by
preventing the activation of CDK4 and CDK6 (29,30).
Thus, CDK4 represses the proliferation of non-small cell lung
cancer (31). However, further
research on the target genes is needed to understand the mechanism
of miR-18a-3p in terms of migration, apoptosis, and
chemosensitivity to cisplatin in malignant mesothelioma.
Recent studies have demonstrated that some miRNAs
play important roles, not only in cellular proliferation and
invasion, but also in chemosensitivity. For example, miR-362-5p and
miR-613 suppress chemosensitivity to cisplatin in gastric cancer
(32,33). Additionally, a correlation between
the expression levels of miR-25, miR-145, and miR-210, and the
effectiveness of pemetrexed maintenance treatment in lung
adenocarcinoma, has been observed (34). Current standard chemotherapy for
advanced stage malignant mesothelioma includes a combination of
pemetrexed and cisplatin, but median survival remains only about 12
months, even though there is an approximately 40% response rate
(6). This suggests that mesothelioma
cells have chemoresistance to these anticancer agents. Moody et
al (35) demonstrated that the
loss of miR-31 enhanced sensitivity of malignant mesothelioma cells
to cisplatin. Moreover, transfection of miR-145 and miR-379/411
induced chemosensitivity to pemetrexed in mesothelioma (36,37). In
the current study, we found that inhibition of miR-18a enhanced
chemosensitivity to cisplatin in all four cell lines tested,
including ACC-MESO1, ACC-MESO4, and CRL-5946 cells that showed only
a slight decrease in viability at 5 µM. These results
indicate that miR-18a targeted therapy may have benefits for
patients with cisplatin-resistant mesothelioma. However, to clarify
the detailed mechanism of miR-18a-3p in mesothelioma cell
progression and chemosensitivity, further research is needed.
In conclusion, this study demonstrated that
inhibition of miR-18a-3p significantly reduced mesothelioma
progression and promoted chemosensitivity to cisplatin. Therefore,
miR-18a is a potential therapeutic target for malignant
mesothelioma.
Acknowledgements
The authors would like to thank Ms. Yukari Go and
Mr. Tatsuya Nakagawa (Department of Pathology, Hiroshima
University, Hiroshima, Japan) for their excellent technical
assistance, and Ms Naomi Fukuhara (Department of Pathology,
Hiroshima University, Hiroshima, Japan) for administrative
support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, VJA and YT designed the study. VJA and YT
supervised and facilitated the study. RS, KK, YK, TK and YF
performed the experiments. RS analyzed the data. RS and VJA
interpreted the results and wrote the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frost G: The latency period of
mesothelioma among a cohort of British asbestos workers
(1978–2005). Br J Cancer. 109:1965–1973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peto J, Decarli A, La Vecchia C, Levi F
and Negri E: The European mesothelioma epidemic. Br J Cancer.
79:666–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Myojin T, Azuma K, Okumura J and Uchiyama
I: Future trends of mesothelioma mortality in Japan based on a risk
function. Ind Health. 50:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keshava HB, Tang A, Siddiqui HU, Raja S,
Raymond DP, Bribriesco A, Stevenson J, Murthy SC and Ahmad U:
Largely Unchanged annual incidence and overall survival of pleural
mesothelioma in the USA. World J Surg. 43:3239–3247. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joshi TK and Gupta RK: Asbestos in
developing countries: Magnitude of risk and its practical
implications. Int J Occup Med Environ Health. 17:179–185.
2004.PubMed/NCBI
|
|
6
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu KL, Tsai YM, Lien CT, Kuo PL and Hung
AJ: The roles of MicroRNA in lung cancer. Int J Mol Sci.
20:E16112019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SN, Chang R, Lin LT, Chern CU, Tsai
HW, Wen ZH, Li YH, Li CJ and Tsui KH: MicroRNA in ovarian cancer:
Biology, pathogenesis, and therapeutic opportunities. Int J Environ
Res Public Health. 16:E15102019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Banelli B, Forlani A, Allemanni G,
Morabito A, Pistillo MP and Romani M: MicroRNA in glioblastoma: An
overview. Int J Genomics. 2017:76390842017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amatya VJ, Mawas AS, Kushitani K, Mohi
El-Din MM and Takeshima Y: Differential microRNA expression
profiling of mesothelioma and expression analysis of miR-1 and
miR-214 in mesothelioma. Int J Oncol. 48:1599–1607. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song Y, Wang P, Zhao W, Yao Y, Liu X, Ma
J, Xue Y and Liu Y: miR-18a regulates the proliferation, migration
and invasion of human glioblastoma cell by targeting neogenin. Exp
Cell Res. 324:54–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Lei C, Fan J and Wang J: miR-18a
promotes cell proliferation of esophageal squamous cell carcinoma
cells by increasing cylin D1 via regulating PTEN-PI3K-AKT-mTOR
signaling axis. Biochem Biophys Res Commun. 477:144–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao H, Liu Y, Liang P, Wang B, Tan H,
Zhang Y, Gao X and Gao J: TP53TG1 enhances cisplatin sensitivity of
non-small cell lung cancer cells through regulating miR-18a/PTEN
axis. Cell Biosci. 8:232018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Usami N, Fukui T, Kondo M, Taniguchi T,
Yokoyama T, Mori S, Yokoi K, Horio Y, Shimokata K, Sekido Y and
Hida T: Establishment and characterization of four malignant
pleural mesothelioma cell lines from Japanese patients. Cancer Sci.
97:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hanna J, Hossain GS and Kocerha J: The
potential for microRNA therapeutics and clinical research. Front
Genet. 10:4782019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Truini A, Coco S, Genova C, Mora M, Dal
Bello MG, Vanni I, Alama A, Rijavec E, Barletta G, Biello F, et al:
Prognostic and therapeutic implications of MicroRNA in malignant
pleural mesothelioma. Microrna. 5:12–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo Russo G, Tessari A, Capece M, Galli G,
de Braud F, Garassino MC and Palmieri D: MicroRNAs for the
diagnosis and management of malignant pleural mesothelioma: A
literature review. Front Oncol. 8:6502018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnson TG, Schelch K, Cheng YY, Williams
M, Sarun KH, Kirschner MB, Kao S, Linton A, Klebe S, McCaughan BC,
et al: Dysregulated expression of the microRNA miR-137 and its
target YBX1 contribute to the invasive characteristics of malignant
pleural mesothelioma. J Thorac Oncol. 13:258–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams M, Kirschner MB, Cheng YY, Hanh
J, Weiss J, Mugridge N, Wright CM, Linton A, Kao SC, Edelman JJ, et
al: miR-193a-3p is a potential tumor suppressor in malignant
pleural mesothelioma. Oncotarget. 6:23480–23495. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mawas AS, Amatya VJ, Suzuki R, Kushitani
K, Mohi El-Din MM and Takeshima Y: PIM1 knockdown inhibits cell
proliferation and invasion of mesothelioma cells. Int J Oncol.
50:1029–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki R, Amatya VJ, Kushitani K, Kai Y,
Kambara T and Takeshima Y: miR-182 and miR-183 promote cell
proliferation and invasion by targeting FOXO1 in mesothelioma.
Front Oncol. 8:4462018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He T, McColl K, Sakre N, Chen Y, Wildey G
and Dowlati A: Post-transcriptional regulation of PIAS3 expression
by miR-18a in malignant mesothelioma. Mol Oncol. 12:2124–2135.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schelch K, Kirschner MB, Williams M, Cheng
YY, van Zandwijk N, Grusch M and Reid G: A link between the
fibroblast growth factor axis and the miR-16 family reveals
potential new treatment combinations in mesothelioma. Mol Oncol.
12:58–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Zandwijk N, Pavlakis N, Kao SC, Linton
A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey
DL, et al: Safety and activity of microRNA-loaded minicells in
patients with recurrent malignant pleural mesothelioma: A
first-in-man, phase 1, open-label, dose-escalation study. Lancet
Oncol. 18:1386–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roussel MF: The INK4 family of cell cycle
inhibitors in cancer. Oncogene. 18:5311–5317. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bartkova J, Rajpert-De Meyts E, Skakkebaek
NE, Lukas J and Bartek J: Deregulation of the G1/S-phase control in
human testicular germ cell tumours. APMIS. 111:252–265; discussion
265-256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin S, Wang MJ and Tseng KY:
Polypyrimidine tract-binding protein induces p19(Ink4d) expression
and inhibits the proliferation of H1299 cells. PLoS One.
8:e582272013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei X, Gao M, Ahmed Y, Gao M, Liu W, Zhang
Y, Xie X, Zhao Q, Wang H and Gu K: MicroRNA-362-5p enhances the
cisplatin sensitivity of gastric cancer cells by targeting
suppressor of zeste 12 protein. Oncol Lett. 18:1607–1616.
2019.PubMed/NCBI
|
|
33
|
Xue M, Li G, Sun P, Zhang D, Fang X and Li
W: MicroRNA-613 induces the sensitivity of gastric cancer cells to
cisplatin through targeting SOX9 expression. Am J Transl Res.
11:885–894. 2019.PubMed/NCBI
|
|
34
|
Shi SB, Wang M, Tian J, Li R, Chang CX and
Qi JL: MicroRNA 25, microRNA 145, and microRNA 210 as biomarkers
for predicting the efficacy of maintenance treatment with
pemetrexed in lung adenocarcinoma patients who are negative for
epidermal growth factor receptor mutations or anaplastic lymphoma
kinase translocations. Transl Res. 170:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moody HL, Lind MJ and Maher SG:
MicroRNA-31 regulates chemosensitivity in malignant pleural
mesothelioma. Mol Ther Nucleic Acids. 8:317–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cioce M, Ganci F, Canu V, Sacconi A, Mori
F, Canino C, Korita E, Casini B, Alessandrini G, Cambria A, et al:
Protumorigenic effects of mir-145 loss in malignant pleural
mesothelioma. Oncogene. 33:5319–5331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamamoto K, Seike M, Takeuchi S, Soeno C,
Miyanaga A, Noro R, Minegishi Y, Kubota K and Gemma A: miR-379/411
cluster regulates IL-18 and contributes to drug resistance in
malignant pleural mesothelioma. Oncol Rep. 32:2365–2372. 2014.
View Article : Google Scholar : PubMed/NCBI
|