Introduction
Malignant mesothelioma (MM) is a rare but aggressive
and fatal neoplasm that originates from the thoracic and abdominal
serosal membranes, with malignant peritoneal mesothelioma (MPeM)
representing 7–30% of MM cases (1).
Environmental and occupational exposure to asbestos is associated
with this condition, and the incidence of MPeM in East China is 4.5
cases per million individuals in 2018 (2). Due to a lack of specificity of clinical
symptoms, difficulty in making an early diagnosis and rapid disease
progression with few effective treatments, the median survival
prognosis is ≤12 months from diagnosis (2). Recently, it has been shown that
cytoreductive surgery (CRS), radiotherapy and chemotherapy could
increase the survival of patients with MPeM (3). Therefore, early predictors of prognosis
may help to guide intensive treatment protocols to improve survival
and quality of life for patients with a short life expectancy. Due
to the influence of tumour morphology and radiological markers,
such as TNM stage, it is difficult to fully assess the prognosis of
patients with MPeM.
Ki-67 is a nuclear protein that is detected in the
active phases of the cell cycle (G1, G2, S
and M) but absent in quiescent cells (G0) (4). Therefore, Ki-67 is widely used as a
predictive and prognostic marker in numerous types of cancer, such
as those of the breast (5), lung
(6) and prostate (7). Ki-67 has also been demonstrated to be a
prognostic factor for MM (8).
CD146 is a multifunctional molecule that is involved
in several physiological and pathological processes involving
immunity and angiogenesis, and has also been found to serve a
critical role in cancer progression (9). In a number of tumours, such as melanoma
and gallbladder cancer (10,11), CD146 has been found to promote cancer
progression and migration. In malignant pleural mesothelioma, CD146
has been identified as an indicator of poor prognosis (12). However, only one study was found to
report the effect of CD146 on the prognosis of MPeM (13).
Survivin is encoded by the baculoviral inhibitor of
apoptosis repeat-containing 5 gene and is an important inhibitor of
effector caspases in the apoptosis pathway. It is overexpressed in
a number of tumours, but not in normal differentiated tissues, and
may serve a key role in tumour prognosis (14,15).
Survivin is used to evaluate the prognosis of some tumours
(16,17), such as malignant pleural mesothelioma
(18), but less so in MPeM. The
present study aimed to explore the possible prognostic value of
survivin and CD146 expression for patients with MPeM.
Materials and methods
Patients and tumour tissue
samples
A total of 60 patients diagnosed with MPeM in
Cangzhou Central Hospital (Cangzhou, China) over ~3 years (August
2015-September 2018) were included in the present study. The
inclusion criteria were as follows: i) Newly diagnosed cases that
were not combined with other tumours or other fatal diseases, such
as severe infection, or failure of one or more organs that
seriously affected survival; ii) patients with complete clinical
data; and iii) patients without a history of receiving special
medications, such as aspirin and diclofenac sodium. Patient
demographics, asbestos exposure, treatments and follow-up data were
retrieved from medical records. The histopathological diagnostic
criteria of MPeM were established according to the guidelines for
pathologic diagnosis of malignant mesothelioma (19). Yan et al (20) proposed a novel ‘TNM’ staging system
in 2011, in which MPeM staging was determined according to the
extent of peritoneal disease burden (T), intra-abdominal nodal
metastasis (N), extra-abdominal metastasis (M) and peritoneal
carcinomatosis index (PCI). PCI was defined based on the following
regions: The upper transverse plane, which is the lowest aspect of
the costal margin; the lower transverse plane, which is the
anterior superior iliac spine; and the abdomen, which is divided
into three equal sectors by sagittal planes. The abdomen is divided
into nine abdominopelvic regions (AR-8) by two transverse planes
and two sagittal planes. AR-9 is located in the left upper abdomen,
including the upper jejunum. AR-10 is the lower jejunum located in
the left lower abdomen. AR-11 is the upper ileum located in the
right upper abdomen, and AR-12 is the lower ileum, including the
terminal ileum. For each region, four categories were used to
estimate tumour volume: V0 indicated the absence of cancer at a
particular abdominopelvic or anatomic site; V1 indicated tumour
nodules <0.5 cm in diameter (minimal volume); V2 indicated
tumours 0.5–5 cm in diameter (moderate volume); and V3 indicated
tumours >5 cm in diameter (gross volume). Volume estimates were
determined by the radiologist who performed the CT scan. PCI was
based on lesion size (0–3) and tumour distribution (0–12) to
determine the extent of the disease (0–39). PCI was calculated to
determine the T stage, with scores of 1, 2, 3 and 4 corresponding
to PCI scores of 1–10, 11–20, 21–30 and 31–39, respectively. T1N0M0
was included in stage I disease; T2-3N0M0 represented stage II; and
T4N0M0 and any N/M positive cases were classified as stage III.
Furthermore, 60 peritonitis tissues and 60 normal
peritoneal tissues were selected as control specimen sets. The
Pathology Department of Cangzhou Central Hospital provided tumour,
peritonitis and peritoneal tissue specimens. Tumour and peritonitis
tissue samples were obtained using ultrasound-guided biopsies for
diagnostic purposes before patients received any clinical
treatment. Normal mesothelial cells were taken from the normal
peritoneal tissue of surgical peritoneal specimens.
Immunohistochemical analysis
Tissues were fixed in 4% phosphate-buffered
paraformaldehyde at room temperature for 24 h and embedded in
paraffin. Three consecutive 4 µm-thick tissue sections of each
paraffin block were used for immunohistochemical staining. Before
dewaxing, the paraffin sections were rewarmed (baked in 70°C
incubator for 2 h). Paraffin sections were dewaxed and dehydrated,
using xylene I and xylene II for 5 min, and 100, 85 and 70% ethanol
for 3 min, respectively. In a medical microwave oven, sodium
citrate buffer solution (0.01 mol/l, pH6.0) was heated to 95°C, in
which specimens were incubated for 25 min, and naturally cooled to
room temperature (for ~1 h). Specimens were incubated in 0.03%
H2O2 at 37°C for 15 min to block endogenous
peroxidase activity. Specimens were washed by 0.01 mol/l PBS
(pH=7.4) for 5 min between steps. Specimens were incubated with 50
ul of normal 5–10% goat serum blocking antigen for 15 min at room
temperature. Specimens were incubated overnight at 4°C with primary
antibodies against the following molecules: Survivin (dilution,
1:100; rabbit monoclonal, clone EP119; OriGene Technologies, Inc.;
cat. no. S1130), CD146 (dilution, 1:100; rabbit monoclonal, clone
EP54; OriGene Technologies, Inc.; cat. no. GTX34461) and Ki-67
(dilution, 1:200; rabbit monoclonal, clone EP5; OriGene
Technologies, Inc.; cat. no. GTX16667). After incubation (at room
temperature for 40 min) with peroxidase-conjugated secondary
antibodies (diluted concentration 1:200; Santa Cruz Biotechnology,
Inc.), a Diaminobenzidine Peroxidase Substrate kit (Laboratories,
Inc.) was used to visualise signals (light microscope;
magnification, ×43; Olympus Corporation, put in multiple ×10).
Negative control specimens were processed under the same
conditions, except that blocking liquid (negative control, the
first antibody was replaced by normal serum and stained by
immunohistochemistry S-P method) was used in place of the primary
antibody.
Immunoreactivity evaluation
For evaluation, using the Jiangsu Jieda 801 image
analysis system (Jetta Technology; version no. 801), >200 tumour
cells were scored per field (magnification ×40). Sections of tumour
tissues were scored semi-quantitatively for survivin and CD146 as
follows: 0, <5% immuno-positive cells; 1+, 5–25% immuno-positive
cells; 2+, 26–50% immuno-positive cells; 3+, >50%
immuno-positive cells. Score 0 was defined as negative, and scores
1+, 2+ and 3+ were defined as positive, which corresponds to grade
4 expression. The Ki-67 labelling index (Ki-67LI) was determined by
the number of positive cells per 500 tumour cells: Lower Ki-67LI,
≤15%; and higher Ki-67LI, >15%, which corresponds to grade 2
expression.
Statistical analysis
Correlations between parameters were tested by
calculating the Spearman's rank correlation coefficient (rate).
Kaplan-Meier analysis was used to calculate the overall cumulative
probability of survival, and the log-rank test was used to assess
differences in survival. Overall survival (OS) was measured from
the date of initial diagnosis to the date of last follow-up
examination or mortality (median). Univariate analysis was
performed to assess the association between prognostic factors and
survival (rate). Prognostic factors that were identified as
significant in the univariate analysis were included in the
multivariate analysis using the Cox proportional hazards model
(rate). The nomogram was formulated using the ‘rms’ version 5.1–4
package in R version 3.5.2 software (R Foundation for Statistical
Computing; www.r-project.org) as a tool to
predict the prognosis of MPeM and forest maps to show the hazard
ratios (HRs) of independent prognostic factors. P<0.05 was
considered to indicate a statistically significant difference;
however, P<0.10 was considered statistically significant in
univariate and multivariate analyses. The performance of the
nomogram was estimated using a calibration curve. The predictive
accuracy of the model was estimated using the concordance index
(C-index). Statistical analyses were performed using SPSS v22.0
(IBM Corp.) and R version 3.5.2 software. Packages, including
‘survival’, ‘nomogramEx’, ‘rms’ and ‘survminer’ were used. The
version number of survival, nomogramEx, rms and survminer
respectively is 2.44-1, 2.0, 5.1–3 and 0.4.3.
Results
Patients
In total, 60 patients were evaluated in the present
study, comprising 22 men and 38 women (1:1.73). The median age at
diagnosis was 62 years (range, 42–84). Asbestos exposure was
documented for 86.7% of patients. Epithelioid and non-epithelioid
tumours were found in 30 cases each (50%). The mean PCI was 27.5
(range, 3–39). According to the novel ‘TNM’ staging system, five
patients (8.3%) had stage I, 47 patients (78.3%) had stage II and
eight patients (13.3%) had stage III MPeM. A total of 38 patients
received tumour-directed treatment with systemic or local abdominal
chemotherapy, whereas the remaining patients received best
supportive care (BSC), mainly due to comorbidities, advanced
disease stage or poor performance status. The median OS was 9.25
months (range, 1–48 months). Five patients were still alive at the
time of the final analysis. Clinical information is detailed in
Table I.
 | Table I.Demographic patient characteristics
(n=60). |
Table I.
Demographic patient characteristics
(n=60).
| Factors | Value or no. of
patients |
|---|
| Age, years |
|
|
Median | 62 |
|
Range | 42-84 |
| Sex, n |
|
|
Male | 22 |
|
Female | 38 |
| Asbestos exposure,
n |
|
| + | 52 |
| − | 8 |
| Histological type,
n |
|
|
Epithelioid | 30 |
|
Non-epithelioid | 30 |
| PCI, n |
|
|
≤30 | 30 |
|
>30 | 30 |
| TNM stage, n |
|
| Stage
I | 5 |
| Stage
II | 47 |
| Stage
III | 8 |
| Treatment |
|
|
BSC | 22 |
|
Chemotherapy | 38 |
| Ki67 |
|
|
≤0.15 | 30 |
|
>0.15 | 30 |
| Survivin, n |
|
|
<5% | 26 |
|
5-25% | 21 |
|
26-50% | 8 |
|
>50% | 5 |
| CD146, n |
|
|
<5% | 29 |
|
5-25% | 18 |
|
26-50% | 10 |
|
>50% | 3 |
Associations between survivin, CD146
and Ki-67 expression and clinicopathological parameters
Survivin and CD146 expression levels are detailed in
Table I. Staining of CD146 was
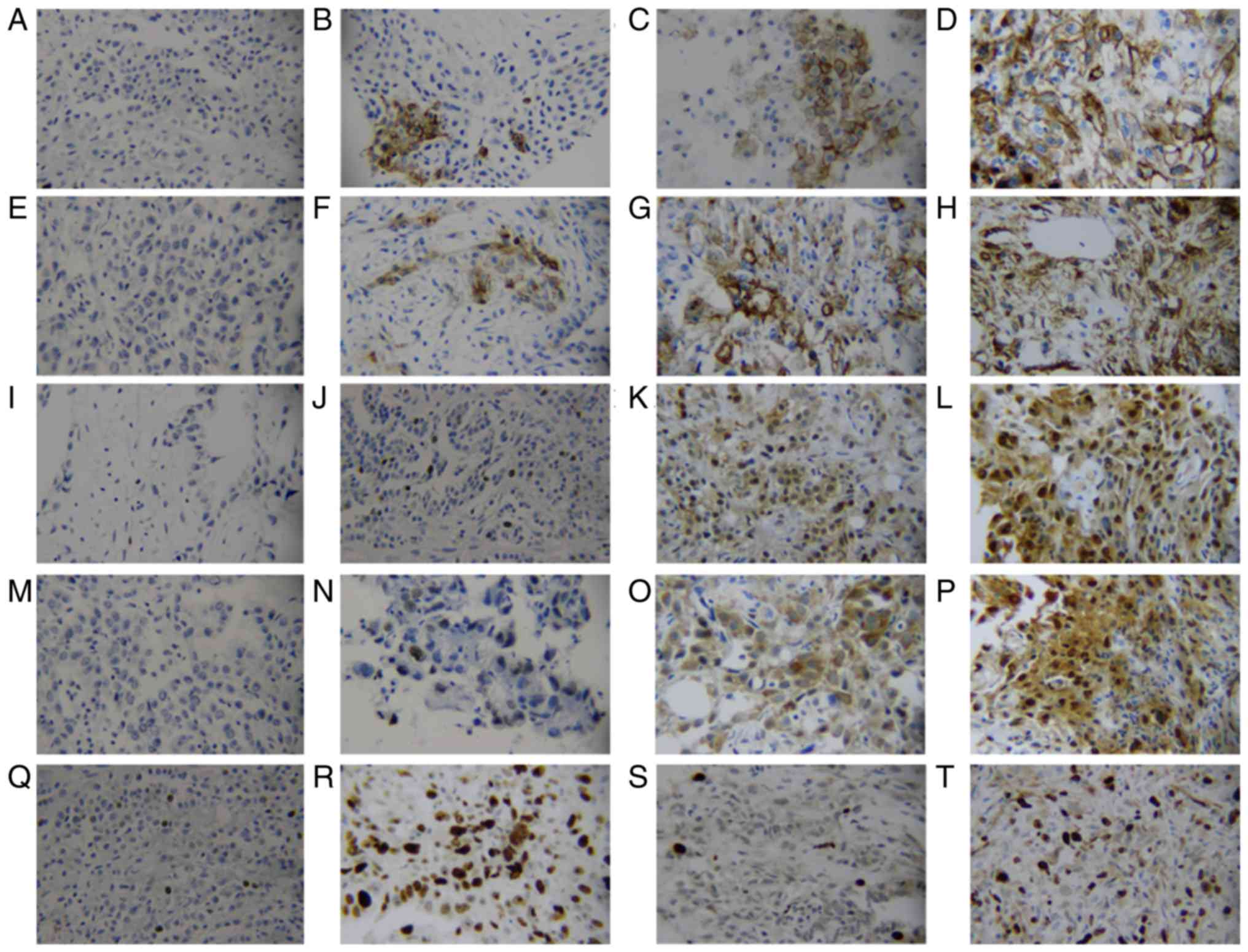
observed in the cytoplasm and cell membrane (Fig. 1A-H), and staining of survivin was
observed in the nucleus and cytoplasm (Fig. 1I-P), while Ki-67 staining was only
nuclear (Fig. 1Q-T). Carcinomas
expressed survivin (≥5%) in 34 (56.67%) of 60 specimens, and CD146
(≥5%) was detected in 31 (51.67%) of 60 specimens. Spearman's rho
analysis revealed that survivin and CD146 expression were both
correlated with Ki-67LI (r=0.425, P=0.001; r=0.362, P=0.004,
respectively; Table II). Survivin,
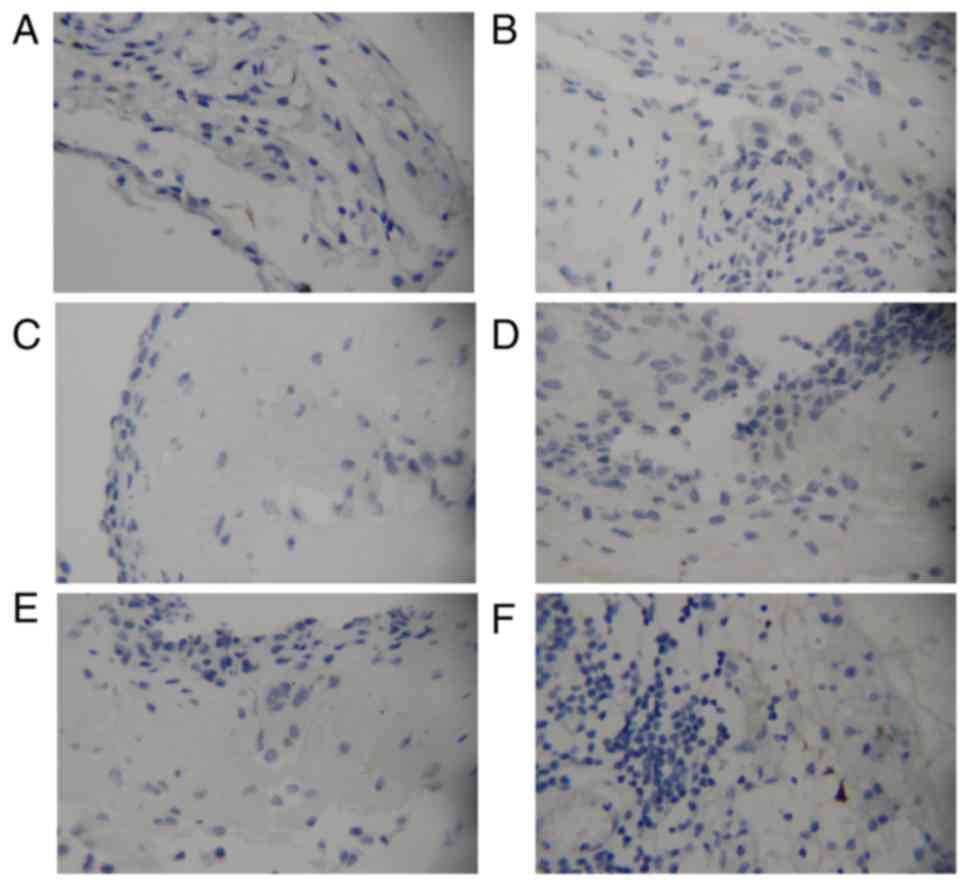
CD146 and Ki-67 expression in normal mesothelium (Fig. 2A, C and E) and specimens from
patients suffering from peritonitis (Fig. 2B, D and F) were negative. Mesothelial
cells and lymphocytes, identified in peritonitis tissue specimens,
exhibited no staining for the aforementioned proteins.
 | Table II.Association and differences of
survivin and CD146 expression with clinicopathologic parameters and
Ki-67LI in patients with malignant peritoneal mesothelioma. |
Table II.
Association and differences of
survivin and CD146 expression with clinicopathologic parameters and
Ki-67LI in patients with malignant peritoneal mesothelioma.
|
|
| Survivin | CD146 |
|---|
|
|
|
|
|
|---|
|
|
| Reactive grade,
n |
| Reactive grade,
n |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | No. | 0 | 1+ | 2+ | 3+ | P-value | 0 | 1+ | 2+ | 3+ | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
|
|
|
|
|
|
≤62 | 31 | 14 | 10 | 3 | 4 | 1.000 | 14 | 11 | 5 | 1 | 0.881 |
|
>62 | 29 | 12 | 11 | 5 | 1 |
| 15 | 7 | 5 | 2 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
|
|
Male | 22 | 11 | 4 | 5 | 2 | 0.400 | 10 | 7 | 4 | 1 | 0.774 |
|
Female | 38 | 15 | 17 | 3 | 3 |
| 19 | 11 | 6 | 2 |
|
|
Histologicaltype |
|
|
|
|
|
|
|
|
|
|
|
|
Epithelioid | 30 | 11 | 12 | 5 | 2 | 0.424 | 12 | 11 | 6 | 1 | 0.322 |
|
Non-epithelioid | 30 | 15 | 9 | 3 | 3 |
| 17 | 7 | 4 | 2 |
|
| Ki67 |
|
|
|
|
|
|
|
|
|
|
|
|
≤0.15 | 30 | 19 | 8 | 2 | 1 | 0.001 | 20 | 6 | 4 | 0 | 0.004 |
|
>0.15 | 30 | 7 | 13 | 6 | 4 |
| 9 | 12 | 6 | 3 |
|
Survival analysis
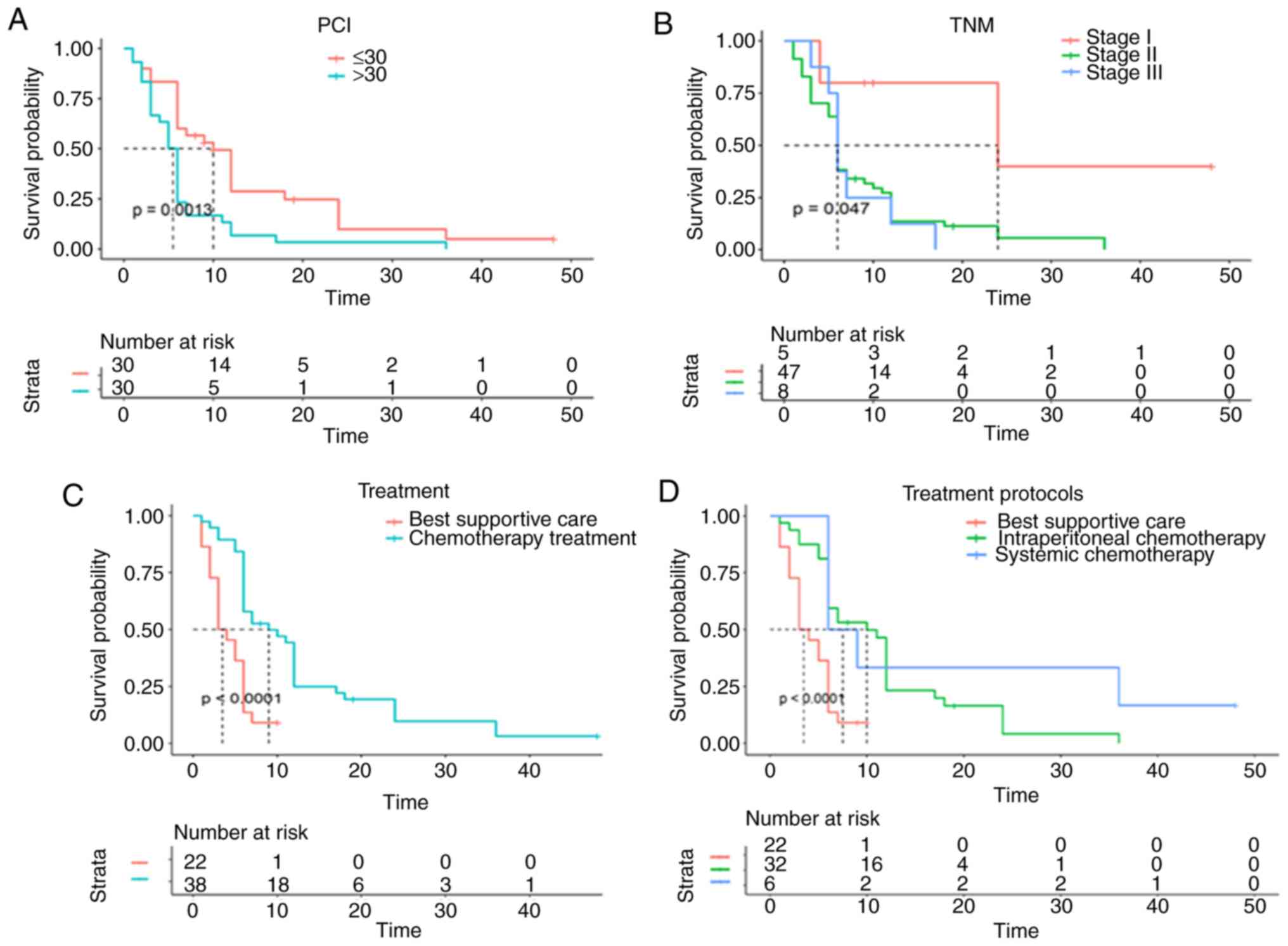
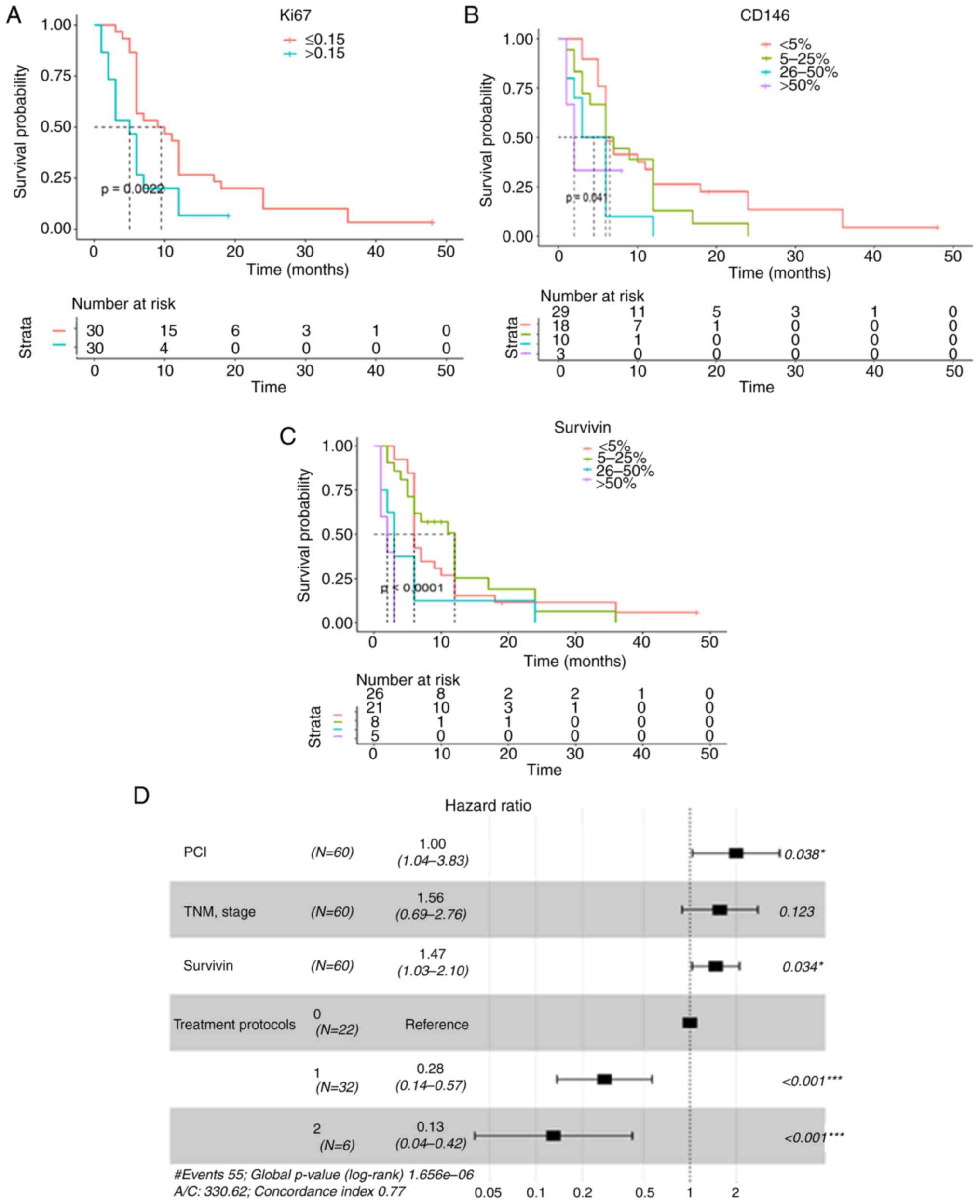
Kaplan-Meier analysis and univariate Cox regression
analysis demonstrated that a lower PCI, stage I, chemotherapy
treatment (Fig. 3A-D; Table III) and a lower Ki-67LI had
significantly positive effects on OS in patients with MPeM
(Fig. 4A; Table III). In addition, a lower level of
survivin expression was significantly associated with improved OS
in grade 4 patients (P<0.000; Fig.
4C; Table III), while high
CD146 expression was associated with poor MPeM prognosis (P=0.041;
Fig. 4B). All factors were included
in the multivariate Cox analysis, in which a lower PCI (HR=1.99;
95% CI, 1.04–3.83; P=0.038), lower survivin expression (HR=1.47;
95% CI, 1.03–2.10; P=0.034), and treatment protocols, including
intraperitoneal chemotherapy (HR=0.28; 95% CI, 0.14–0.57;
P<0.001) and systemic chemotherapy (HR=0.13; 95% CI, 0.04–0.42;
P<0.001) retained independent prognostic significance, with a
positive effect on OS (Table III).
TNM stage (P=0.123) was also included in the forest maps (with
P=0.05 as the cut-off point), and the results are shown in Fig. 4D. High PCI and expression of survivin
indicated poor prognosis in patients with MPeM. Intraperitoneal and
systemic chemotherapy had a statistically positive effect on
overall survival. Treatment protocol was a disordered
multivariable, and treatment protocols = 0 was the reference.
 | Table III.Univariate and multivariate analyses
of factors affecting OS in patients with malignant peritoneal
mesothelioma. |
Table III.
Univariate and multivariate analyses
of factors affecting OS in patients with malignant peritoneal
mesothelioma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex (female vs.
male) | 0.66 | (0.37–1.17) | 0.110 |
|
|
|
| Age (≤62 vs.
>62) | 1.00 | (0.59–1.70) | 0.990 |
|
|
|
| Asbestos exposure
(yes vs. no) | 0.96 | (0.43–2.14) | 0.900 |
|
|
|
| Histological
type | 1.21 | (0.70–2.09) | 0.450 |
|
|
|
| (Epithelioid vs.
Non-epithelioid) |
|
|
|
|
|
|
| PCI (≤30 vs.
>30) | 2.18 | (1.26–3.76) | 0.001 | 1.99 | (1.04–3.83) | 0.044 |
| TNM Stage (I vs. II
vs. III) | 1.61 | (1.05–2.73) | 0.047 | 1.56 | (0.89–2.76) | 0.123 |
| Treatment (BSC vs.
chemotherapy treatment) | 0.30 | (0.16–0.57) | <0.000 |
|
|
|
| Ki-67 (≤15% vs.
>15%) | 2.19 | (1.24–3.89) | 0.007 |
|
|
|
| Survivin (negative
vs. positive) | 1.65 | (1.17–2.32) | <0.000 | 1.47 | (1.03–2.10) | 0.034 |
| CD146 (negative vs.
positive) | 1.48 | (1.08–2.03) | 0.041 |
|
|
|
| Treatment
protocol |
|
| <0.000 |
|
| <0.001 |
| Best
supportive care | 1.00 |
|
| 1.00 |
|
|
|
Intraperitoneal
chemotherapy | 0.32 | (0.17–0.62) |
| 0.28 | (0.14–0.57) | <0.001 |
|
Systemic chemotherapy | 0.20 | (0.07–0.59) |
| 0.13 | (0.04–0.42) | <0.001 |
Construction and validation of the
nomogram
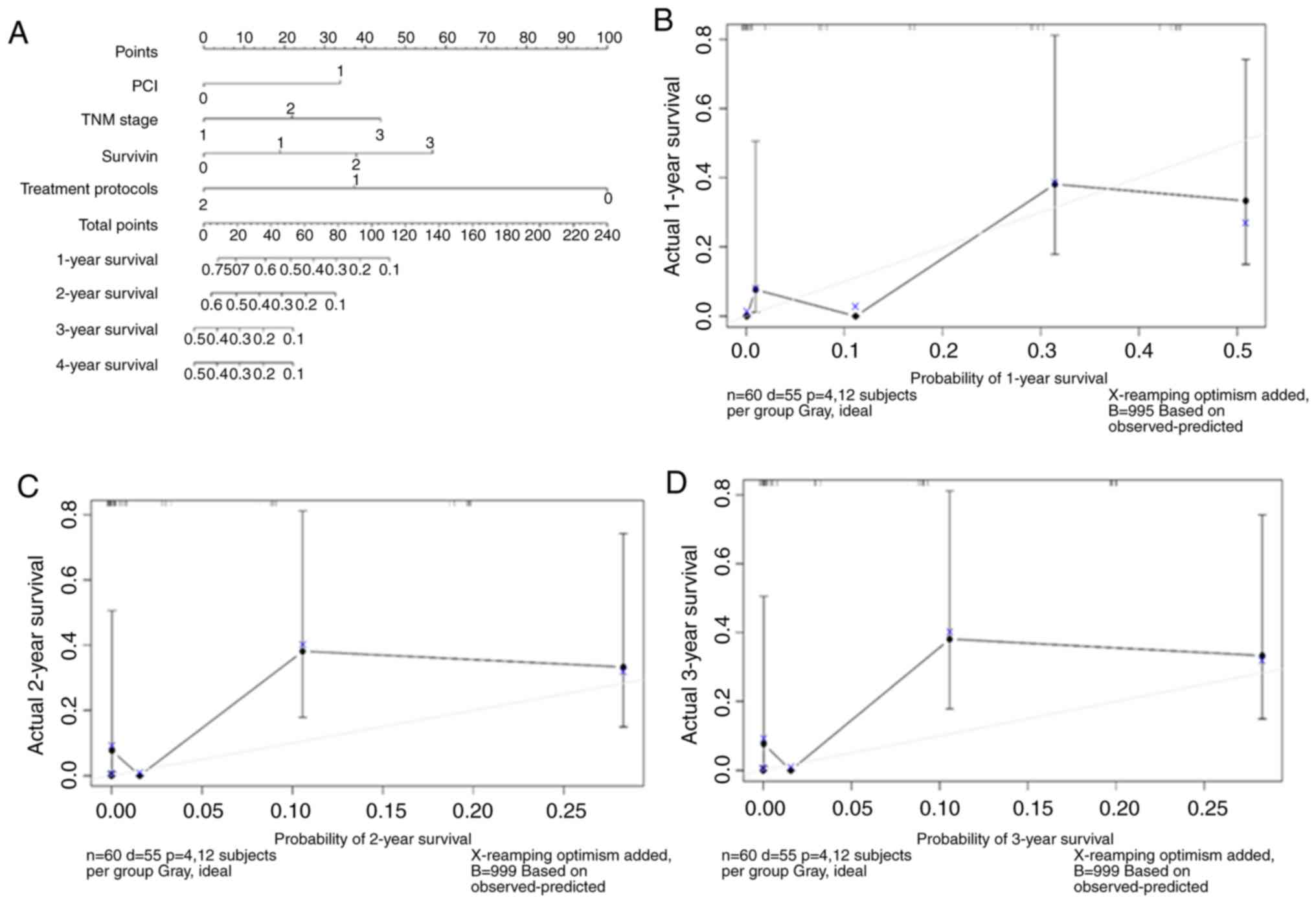
The graphical calculator or nomogram uses line
scores to assist the clinician in quickly estimating individualised
patient-specific OS (Fig. 5A). PCI,
TNM stage, survivin and treatment protocols were incorporated into
the calculator. PCI was divided into two categories: ≤30 and
>30; TNM stage was divided into three categories: Stage I, stage
II and stage III; survivin was divided into four categories:
<5%, 5–25%, 26–50% and >50%; treatment protocols were divided
into three categories: BSC, intraperitoneal chemotherapy and
systemic chemotherapy. The model determined the estimated values of
1-, 2- and 3-year OS by simply adding up the corresponding scores
of the four factors to calculate the total score. In addition, the
performance of the nomogram was graphically evaluated using a
calibration curve (Fig. 5B-D). The
C-index was 0.77, and the predicted line overlapped well with the
reference line, demonstrating good performance of the nomogram.
Discussion
The worldwide incidence of MPeM continues to rise,
partly because of its association with asbestos exposure. Although
MPeM is traditionally considered resistant to antitumour therapy,
some patients exhibit a good response to CRS with hyper-thermic
intraperitoneal chemotherapy (HIPEC) or multidisciplinary therapy
(3). Therefore, it is important to
identify prognostic factors that can predict who will benefit from
these treatments. Some of the predictive factors for OS in patients
with MPeM include age, sex, histologic type and grade, lymphatic
metastasis, and imaging staging (2,15,21).
Clinical imaging examinations mainly include CT and MRI, which do
not convey pathological information and cannot fully assess the
prognosis of MPeM. Individual studies have also identified blood
neutrophil-to-lymphocyte ratio (22)
and glucose transporter 1 expression (23) as predictors of survival.
Numerous studies (24–26) have
suggested that age is a prognostic factor for MPeM, and older age
suggests poor prognosis. In general, the prognosis of patients over
65 is worse than that of patients under 65 years of age (27). Research has revealed that sex and
clinical stage are independent prognostic factors for OS in
malignant pleural mesothelioma (28). MPeM can be divided into epithelioid,
sarcomatoid and biphasic types; among the aforementioned, the
epithelioid subtype is associated with an improved outcome.
Prognosis of the non-epithelioid subtypes (including sarcomatoid
and biphasic) is extremely poor (24,26). The
results of the present study demonstrated that age, sex and
histopathological typing were not associated with MPeM prognosis
using a univariate analysis. In future studies, the sample size
could be increased and further research should be conducted to
confirm the association between age, histological type and
prognosis. Among the patients in the present study, 63.3% of the
patients were female, the asbestos exposure rate was 86.7% in all
patients included. In the present study, the high asbestos exposure
rate and the frequent incidence of disease in women could be
attributed to the practice of hand-spinning asbestos yarn in the
1970s, when the workers were mainly teenage girls.
Yan et al (20) created a clinicopathologic staging
system that emphasizes the prognostic importance of tumour volume
and distribution within the peritoneal cavity, lymph node
involvement and extra-abdominal metastases. A high PCI has been
shown to be a poor prognostic factor in MPeM in a study (20). In the present study, Kaplan-Meier and
univariate Cox regression analyses showed that both a lower PCI and
stage I tumours had significantly positive effects on OS, while
multivariate Cox regression analysis did not confirm the
associations between stage I and OS. All patients in the present
study received internal conservative treatment, and the PCI and TNM
grades were based on CT imaging, which may be different from
previous surgical grades. In future studies, the sample size and
treatment methods should be improved to verify the aforementioned
results.
Research has suggested that CRS combined with HIPEC
should be considered as standard treatment for patients diagnosed
with MPeM (29). For numerous
patients with MPeM who are unable to tolerate or unwilling to
undergo surgery, clinicians usually provide supportive treatment or
chemotherapy. Currently, the first-line clinical systemic therapy
is pemetrexed combined with cisplatin or carboplatin (30). In the present study, tumour-directed
treatment, especially systemic chemotherapy with pemetrexed alone
or in combination with cisplatin and intraperitoneal chemotherapy
with cisplatin, had a significantly positive effect on OS in
MPeM.
Ki-67 is an indicator of tumour replication. High
expression of Ki-67 indicates active tumour growth. Numerous
studies (27,31) have indicated that high levels of
Ki-67 result in a poor prognosis. Patients with MPeM with high
Ki-67 expression and a high PCI have an average survival time of 10
months (20). A multicentre study
reported that the Ki-67 index is an independent prognostic factor
for epithelioid rather than non-epithelioid malignant pleural
mesothelioma (8). Pillai et
al (32) examined the expression
of Ki-67 in 42 MPeM tumours and concluded that high Ki-67
expression is associated with poor survival. In the present study,
Ki-67 expression was tumour-specific and was negative in normal
mesothelium and peritonitis specimens. Univariate analysis showed
that lower Ki-67 expression suggested an improved prognosis in
patients with MPeM, while multivariate analysis did not confirm
this association.
CD146 is a cell adhesion molecule that participates
in several physiological and pathological processes, such as signal
transduction, cell migration, angiogenesis and immune responses. It
has become an increasingly important molecule, especially as a
novel biomarker for angiogenesis and cancer. Zeng et al
(33) found that CD146 expression is
significantly associated with late stage tumours and poor prognosis
in breast cancer. In addition, CD146 is significantly associated
with advanced tumour stage in malignant melanoma (34) and mesothelioma (35). The results of the present study
revealed that CD146 expression was correlated with Ki-67 expression
and that there was no CD146 expression in mesothelial cells and
peritonitis tissues. Moreover, univariate analysis suggested that
lower CD146 expression was associated with improved prognosis in
patients with MPeM. These results suggested that CD146 was related
to the prognosis of MPeM, but this correlation was not confirmed
using the multivariate analysis.
A number of studies have shown that the expression
of nuclear survivin is related to cell proliferation, advanced
disease and poor clinical outcome (18,27).
Overexpression of survivin suggests poor prognosis in numerous
types of cancer, such as gallbladder cancer and pancreatic ductal
adenocarcinoma (36,37). In addition, elevated concentrations
of survivin in pleural fluid are associated with shorter survival
in patients with malignant pleural effusion (16). Meerang et al (38) reported that high survivin labelling
index is an indicator of poor prognosis in patients with malignant
pleural mesothelioma. However, other studies identified no
association between survivin and disease outcome (39,40).
At present, there have only been a few reports on
the association between survivin and MPeM prognosis (41,42). The
present study revealed that survivin was expressed in 34/60 (56.7%)
mesothelioma specimens in a tumour-specific manner. Spearman's rho
analysis revealed a significant correlation between survivin
expression and Ki-67LI (r=0.425; P=0.001), confirming that survivin
expression was associated with cell proliferation, which was
consistent with the results of Bitanihirwe et al (43). Furthermore, the univariate and
multivariate analyses showed that a lower level of survivin
expression was significantly associated with improved OS
(P<0.0001; P=0.034). The aforementioned results suggest that
survivin is important in predicting the prognosis of MPeM. Other
studies have shown that patients with MM with an improved response
to tumour-directed treatment have higher survivin expression in
their tumours (20,44). This indicator may offer theoretical
guidance for providing improved clinical treatment for different
patients.
The present study constructed a clinical assessment
tool (nomogram) with a high accuracy (C-index, 0.77) for predicting
survival in patients with MPeM. This tool provided a simple
graphical display of predicted survival based on clinical
parameters that affect MPeM OS: PCI, TNM stage, survivin and
treatment protocols. Estimation of survival may individualise
patient treatment and follow-up, including influencing the extent
of surgical and systemic therapy, and the frequency of diagnostic
imaging. This model should be validated with additional data and in
a prospective manner, and future refinement will likely improve its
predictive accuracy.
In conclusion, PCI, tumour-directed treatment
(including intraperitoneal and systemic chemotherapy), and survivin
levels appear to serve vital roles in influencing survival time.
These factors were incorporated into a nomogram to predict outcomes
in MPeM, providing a useful tool for clinicians to personalise
treatment in this poor prognosis population.
Acknowledgements
The authors would like to thank Professor Mark
Abramovitz for editing the English language of a draft of this
manuscript.
Funding
The present study was funded by Cang Zhou Finance
Bureau (grant no. 1213018ZD).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZha and GZhe conceived and designed the study. DLY
and YFL analysed and interpreted the data. GZha wrote, edited and
reviewed the manuscript. All authors gave final approval for
publication. GZha takes full responsibility for the work as a
whole, including the study design, access to data and the decision
to submit and publish the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of CangZhou Central Hospital (approval no. 2012-012-01)
and was carried out according to the Declaration of Helsinki. All
participants signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Zandwijk N, Clarke C, Henderson D,
Musk AW, Fong K, Nowak A, Loneragan R, McCaughan B, Boyer M, Feigen
M, et al: Guidelines for the diagnosis and treatment of malignant
pleural mesothelioma. J Thorac Dis. 5:E254–E307. 2013.PubMed/NCBI
|
|
2
|
Hui S, Guo-Qi Z, Xiao-Zhong G, Chun-Rong
L, Yu-Fei L and Dong-Liang-Y: IMP3 as a prognostic biomarker in
patients with malignant peritoneal mesothelioma. Hum Pathol.
81:138–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexander HR, Bartlett DL, Pingpank JF,
Libutti SK, Royal R, Hughes MS, Holtzman M, Hanna N, Turner K,
Beresneva T and Zhu Y: Treatment factors associated with long-term
survival after cytoreductive surgery and regional chemotherapy for
patients with malignant peritoneal mesothelioma. Surgery.
153:779–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerdes J, Li L, Schlueter C, Duchrow M,
Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E and Flad HD:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
5
|
Cuzick J, Dowsett M, Pineda S, Wale C,
Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, et al:
Prognostic value of a combined estrogen receptor, progesterone
receptor, Ki-67, and human epidermal growth factor receptor 2
immunohistochemical score and comparison with the genomic health
recurrence score in early breast cancer. J Clin Oncol.
29:4273–4278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Ramnath N, Moysich KB, Asch HL,
Swede H, Alrawi SJ, Huberman J, Geradts J, Brooks JS and Tan D:
Prognostic significance of MCM2, Ki-67 and gelsolin in non-small
cell lung cancer. BMC Cancer. 6:2032006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zellweger T, Günther S, Zlobec I, Savic S,
Sauter G, Moch H, Mattarelli G, Eichenberger T, Curschellas E,
Rüfenacht H, et al: Tumour growth fraction measured by
immunohistochemical staining of Ki67 is an independent prognostic
factor in preoperative prostate biopsies with small-volume or
low-grade prostate cancer. Int J Cancer. 124:2116–2123. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghanim B, Klikovits T, Hoda MA, Lang G,
Szirtes I, Setinek U, Rozsas A, Renyi-Vamos F, Laszlo V, Grusch M,
et al: Ki67 index is an independent prognostic factor in
epithelioid but not in non-epithelioid malignant pleural
mesothelioma: A multicenter study. Br J Cancer. 112:783–792. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang G, Zhang L, Zhu Q, Bai D, Zhang C
and Wang X: CD146 promotes metastasis and predicts poor prognosis
of hepatocellularcarcinoma. J Exp Clin Cancer Res. 35:382016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lei X, Guan CW, Song Y and Wang H: The
multifaceted role of CD146/MCAM in the promotion of melanoma
progression. Cancer Cell Int. 15:32015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Yang ZL, Liu JQ, Jiang S and Miao
XY: Identification of CD146 expression, angiogenesis, and
lymphangiogenesis as progression, metastasis and poor-prognosis
related markers for gallbladder adenocarcinoma. Tumour Biol.
33:173–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato A, Torii I, Okamura Y, Yamamoto T,
Nishigami T, Kataoka TR, Song M, Hasegawa S, Nakano T, Kamei T and
Tsujimura T: Immunocytochemistry of CD146 is useful to discriminate
between malignant pleural mesothelioma and reactive mesothelium.
Mod Pathol. 23:1458–1466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okazaki Y, Nagai H, Chew SH, Li J,
Funahashi S, Tsujimura T and Toyokuni S: CD146 and insulin-like
growth factor 2 mRNA-binding protein 3 predict prognosis of
asbestos-induced rat mesothelioma. Cancer Sci. 104:989–995. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goričar K, Kovač V, Franko A, Dodič-Fikfak
M and Dolžan V: Serum surviving levels and outcome of chemotherapy
in patients with maligna-nt mesothelioma. Dis Markers.
2015:3167392015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 15:5000–5005.
2008. View Article : Google Scholar
|
|
16
|
Arellano-Orden E, Romero-Romero B,
Sánchez-López V, Martín-Juan J, Rodríguez-Panadero F and
Otero-Candelera R: Survivin is a negative prognostic factor in
malignant pleural effusion. Eur J Clin Invest. 48:102018.
View Article : Google Scholar
|
|
17
|
Contis J, Lykoudis PM, Goula K, Karandrea
D and Kondi-Pafiti A: Survivinn expression as an independent
predictor of overall survival in pancreatic adenocarcinoma. J
Cancer Res Ther. 14 (Suppl):S719–S723. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gordon GJ, Mani M, Mukhopadhyay L, Dong L,
Edenfield HR, Glickman JN, Yeap BY, Sugarbaker DJ and Bueno R:
Expression patterns of inhibitor of apoptosis proteins in malignant
pleural mesothelioma. J Pathol. 211:447–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Husain AN, Colby T, Ordonez N, Krausz T,
Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac
LR, et al: Guidelines for pathologic diagnosis of malignant
mesothelioma: 2012 update of the consensus statement from the
international mesothelioma interest group. Arch Pathol Lab Med.
137:647–667. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan TD, Deraco M, Elias D, Glehen O,
Levine EA, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH, et
al: A novel tumor-node-metastasis (TNM) staging system of diffuse
malignant peritoneal mesothelioma using outcome analysis of a
multi-institutional database. Cancer. 117:1855–1863. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vigneswaran WT, Kircheva DY,
Ananthanarayanan V, Watson S, Arif Q, Celauro AD, Kindler HL and
Husain AN: Amount of epithelioid differentiation is a predictor of
survival in malignant pleural mesothelioma. Ann Thorac Surg.
103:962–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin W, Zheng G, Yang K, Song H and Liang
Y: Analysis of prognostic factors of patients with malignant
peritoneal mesothelioma. World J Surg Oncol. 16:442018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hommell-Fontaine J, Isaac S, Passot G,
Decullier E, Traverse-Glehen A, Cotte E, You B, Mohamed F, Gilly
FN, Glehen O and Berger F: Malignant peritoneal mesothelioma
treated by cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy: Is GLUT1 expression a major prognostic factor? A
preliminary study. Ann Surg Oncol. 20:3892–3898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verma V, Sleightholm RL, Rusthoven CG,
Koshy M, Sher DJ, Grover S and Simone CB II: Malignant peritoneal
mesothelioma: National practice patterns, outcomes, and predictors
of survival. Ann Surg Oncol. 25:2018–2026. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shavelle R, Vavra-Musser K, Lee J and
Brooks J: Life expectancy in pleural and peritoneal mesothelioma.
Lung Cancer Int. 2017:27825902017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leinwand JC, Taub RN, Chabot JA and Kluger
MD: Two-Stage cytoreductive surgery and intraperitoneal
chemotherapy for diffuse malignant peritoneal mesothelioma:
Predictors of overall survival in an intention-to-treat series. Ann
Surg Oncol Dec. 12:102019.
|
|
27
|
Magge D, Zenati MS, Austin F, Mavanur A,
Sathaiah M, Ramalingam L, Jones H, Zureikat AH, Holtzman M, Ahrendt
S, et al: Malignant peritoneal mesothelioma: Prognostic factors and
oncologic outcome analysis. Ann Surg Oncol. 21:1159–1165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takamori S, Toyokawa G, Shimokawa M,
Kinoshita F, Kozuma Y, Matsubara T, Haratake N, Akamine T, Hirai F,
Seto T, et al: The c-reactive protein/albumin ratio is a novel
significant prognostic factor in patients with malignant pleural
mesothelioma: A retrospective multi-institutional study. Ann Surg
Oncol. 25:1555–1563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Helm JH, Miura JT, Glenn JA, Marcus RK,
Larrieux G, Jayakrishnan TT, Donahue AE, Gamblin TC, Turaga KK and
Johnston FM: Cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy for malignant peritoneal mesothelioma: A systematic
review and meta-analysis. Ann Surg Oncol. 22:1686–1693. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levý M, Boublíková L, Büchler T and Šimša
J: Treatment of malignant peritoneal mesothelioma. Klin Onkol.
32:333–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feldman AL, Libutti SK, Pingpank JF,
Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ,
Kleiner DE and Alexander HR: Analysis of factors associated with
outcome in patients with malignant peritoneal mesothelioma
undergoing surgical debulking and intraperitoneal chemotherapy. J
Clin Oncol. 21:4560–4567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pillai K, Pourgholami MH, Chua TC and
Morris DL: Ki67-BCL2 index in prognosis of malignant peritoneal
mesothelioma. Am J Cancer Res. 3:411–423. 2013.PubMed/NCBI
|
|
33
|
Zeng Q, Li W, Lu D, Wu Z, Duan H, Luo Y,
Feng J, Yang D, Fu L and Yan X: CD146, an epithelial-mesenchymal
transition inducer, is associated with triple-negative breast
cancer. Proc Natl Acad Sci USA. 109:1127–1132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Q, Zhang B, Zhao X, Zhang Y, Liu Y and
Yan X: Blockade of adhesion molecule CD146 causes pregnancy failure
in mice. J Cell Physiol. 215:621–626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bidlingmaier S, He J, Wang Y, An F, Feng
J, Barbone D, Gao D, Franc B, Broaddus VC and Liu B: Identification
of MCAM/CD146 as the target antigen of a human monoclonal antibody
that recognizes both epithelioid and sarcomatoid types of
mesothelioma. Cancer Res. 69:1570–1577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nigam J, Chandra A, Kazmi HR, Singh A,
Gupta V, Parmar D and Srivastava MK: Expression of serum survivin
protein in diagnosis and prognosis of gallbladder cancer: A
comparative study. Med Oncol. 31:1672014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren YQ, Zhang HY, Su T, Wang XH and Zhang
L: Clinical significance of serum survivin in patients with
pancreatic ductal adenocarcinoma. Eur Rev Med Pharmacol Sci.
18:3063–3068. 2014.PubMed/NCBI
|
|
38
|
Meerang M, Bérard K, Friess M, Bitanihirwe
BK, Soltermann A, Vrugt B, Felley-Bosco E, Bueno R, Richards WG,
Seifert B, et al: Low merlin expression and high survivin labeling
index are indicators for poor prognosis in patients with malignant
pleural mesothelioma. Mol Oncol. 10:1255–1265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hmeljak J, Erčulj N, Dolžan V, Kern I and
Cör A: BIRC5 promoter SNPs do not affect nuclear survivin
expression and survival of malignant pleural mesothelioma patients.
J Cancer Res Clin Oncol. 137:1641–1651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kleinberg L, Lie AK, Flørenes VA, Nesland
JM and Davidson B: Expression of inhibitor-of-apoptosis protein
family members in malignant mesothelioma. Hum Pathol. 38:986–994.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De Cesare M, Cominetti D, Doldi V,
Lopergolo A, Deraco M, Gandellini P, Friedlander S, Landesma Y,
Kauffman MG, Shacham S, et al: Anti-Tumor activity of selective
inhibitors of XPO1/CRM1-mediated nuclear export in diffuse
malignant peritoneal mesothelioma: The role of survivin.
Oncotarget. 6:13119–13132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zaffaroni N, Costa A, Pennati M, De Marco
C, Affini E, Madeo M, Erdas R, Cabras A, Kusamura S and Baratti D:
Survivin is highly expressed and promotes cell survival in
malignant peritoneal mesothelioma. Cell Oncol. 29:453–466.
2007.PubMed/NCBI
|
|
43
|
Bitanihirwe BK, Meerang M, Friess M,
Soltermann A, Frischknecht L, Thies S, Felley-Bosco E, Tsao MS,
Allo G, de Perrot M, et al: PI3K/mTOR signaling in mesothelioma
patients treated with induction chemotherapy followed by
extrapleural pneumonectomy. J Thorac Oncol. 9:239–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hmeljak J, Erčulj N, Dolžan V, Pižem J,
Kern I, Kovač V, Cemažar M and Cör A: Is survivin expression
prognostic or predictive in malignant pleural mesothelioma.
Virchows Arch. 462:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|