Introduction
Pancreatic carcinoma is a highly malignant
gastrointestinal cancer with a poor prognosis and a low 5-year
survival rate (1). Recently, the
incidence and mortality rates of pancreatic cancer have steadily
increased in the United States, within Europe and in China
(1–4). According to the American Cancer
Society, there were ~55,440 novel pancreatic cancer diagnoses and
~44,330 pancreatic cancer-associated mortalities in 2018 in the USA
(4,5). By 2030, pancreatic cancer is expected
to become the second leading cause of cancer-associated mortality
in the USA (1). Meanwhile, in 2015,
the China Cancer Center demonstrated that pancreatic cancer ranks
8th in terms of incidence among male patients with a malignancy
(2). The mortality rate of all types
of cancer in Beijing and Shanghai ranks fifth (2,3).
Pancreatic cancer often presents with lesions that invade adjacent
blood vessels, such as mesenteric and splenic vessels, with the
patient losing the opportunity of surgical resection (6). Therefore, patients with pancreatic
cancer have limited treatment options and a poor quality of life.
To improve the quality of life and prolong survival in such
patients, local ablative procedures are used to treat pancreatic
carcinoma (7). These treatments
include irreversible electroporation (IRE) (8), radiofrequency ablation (RFA) (9) and high-intensity focused ultrasound
(HIFU) (10), which have been widely
used in the past few years, achieving good therapeutic effects in
pancreatic carcinoma.
HIFU is a non-invasive procedure for the ablative
treatment of localized tumors. The basic principle is that
ultrasound is focused at the focal region and produces biological
effects, including thermal, cavitation and mechanical effects, to
achieve thermal ablation of the target tissue, with pathological
changes, including coagulative necrosis (11). HIFU has been widely used in the
treatment of uterine fibroids and hepatocellular carcinoma, with
good therapeutic results (12).
Previous studies have confirmed that HIFU therapy effectively
alleviates cancer-associated abdominal pain, reduces tumor volume
and may confer an additional survival benefit (11,13–23).
However, vascular complications caused by this treatment have been
reported, including secondary occlusion of the superior mesenteric
artery (24) and portal vein
thrombosis (25). The present study
aimed to evaluate the safety of HIFU therapy by assessing blood
vessel events in patients with pancreatic cancer.
Materials and methods
Patients and lesions
The present observational single-center study was
approved by The Ethics Committee of the Second Affiliated Hospital
of Chongqing Medical University (Chongqing, China; approval no.
12/2018). Every patient provided written informed consent before
treatment initiation. Between April 2018 and April 2019, 15
patients with pancreatic carcinoma (Union for International Cancer
Control (UICC) stage II–IV) (6) were
enrolled, including 6 males and 9 females (mean age, 39–81 years;
median age, 65±11 years). According to the TNM staging system
(6), a total of two patients were
diagnosed as stage II, six patients as stage III and seven patients
were diagnosed as stage IV. Of the 15 patients, 7 had tumors of the
pancreatic head and 8 had tumors in the body and/or tail of the
pancreas. The inclusion criteria were: i) Confirmed diagnosis of
pancreatic carcinoma (6); ii) tumor
invading a blood vessel; and iii) distance between the pancreatic
tumor and peripancreatic blood vessel <1 cm. The exclusion
criteria were: i) Non-eligibility for general anesthesia; ii)
calcification of blood vessels invaded by tumor lesions; iii)
Child-Pugh class C (26); iv)
hemorrhage and other severe diseases, such as severe heart failure,
cerebrovascular disease and renal insufficiency and v) poorly
managed diabetes.
All patients underwent CT or MRI and were assessed
using color Doppler flow imaging (CDFI) to evaluate the vascular
hemodynamic changes of peripancreatic arterial and venous vessels
pre-treatment and at 1 week and 1-month post-HIFU treatment. The
primary blood vessels around the pancreas included the splenic
artery, splenic vein, superior mesenteric artery, superior
mesenteric vein (SMV), hepatic artery, celiac artery and portal
vein (PV). The hemodynamic parameters of each blood vessel were
measured using CDFI, including mean blood flow velocity, peak
systolic blood flow velocity (PSV), vascular resistance index (RI),
vascular pulsatility index (PI), vascular diameter and vascular
blood flow. Combined with pre- and post-HIFU treatment imaging
modalities, such as MRI and CT, changes in blood vessel shape and
inner diameter were assessed and vessel occlusion, thrombosis and
hemorrhage were recorded.
Color Doppler ultrasound
examination
An APLI0 500TUS-A500 color Doppler ultrasound system
(TOSHIBA) was used with a wide-band convex array probe with a
center frequency of 3.5 MHz and a mechanical index of
0.06–0.15.
Ultrasound examination was performed by an
experienced sonographer. All patients with pancreatic cancer
underwent ultrasound examination in the fasting state, using a
3.0–5.0 MH variable convex transducer. The operating steps were
similar to the routine abdominal scan method (27), followed by abdominal parenchymal
organs, the biliary system and retroperitoneal large blood vessels
and their primary branches. There was final focus on the location,
size, echo, boundary and blood flow of the lesion. Image quality
was improved by excluded artifact interference, adopting an
appropriate graded compression scan and focusing on observation and
recording the relationship between pancreatic tumor lesions and
blood vessels around the pancreas. The clearest slice was selected
and the probe was fixed. The mean blood flow rate, systolic blood
flow velocity peak, vascular resistance index, vascular pulsation
index and vessel diameter at the closest point from the deep
surface of the tumor lesion were measured.
HIFU therapy
HIFU was performed using a Model-JC Focused
Ultrasound Tumor Therapeutic system (Chongqing Haifu Medical
Technology Co., Ltd.) equipped with a diagnostic ultrasound probe
(3.50–5.00 MHz) for real-time guidance and a therapeutic transducer
(focal length, 10–25; diameter, 10–30 cm) operating at 0.5–2 MHz.
The focal region was an ellipsoid with short and long axes of 3 and
8 mm, respectively.
Prior to HIFU treatment, all patients underwent
colonic lavage with liquid food, laxatives and cleansing enema to
protect the gastrointestinal tract in front of the target area. The
gastric tube was then placed and gastric juice changes were
observed with a vacuum suction device to prevent gastrointestinal
damage and reduce the occurrence of postoperative pancreatitis,
until 1–2 days after surgery. Skin preparation in the treated area
was performed by degreasing with 75% ethanol and degassing with a
vacuum aspirator to avoid skin burns. During HIFU treatment, the
patient was placed in the prone position after general anesthesia.
According to the proposed HIFU treatment plan, real-time ultrasound
monitoring was used to determine tumor location and size, carefully
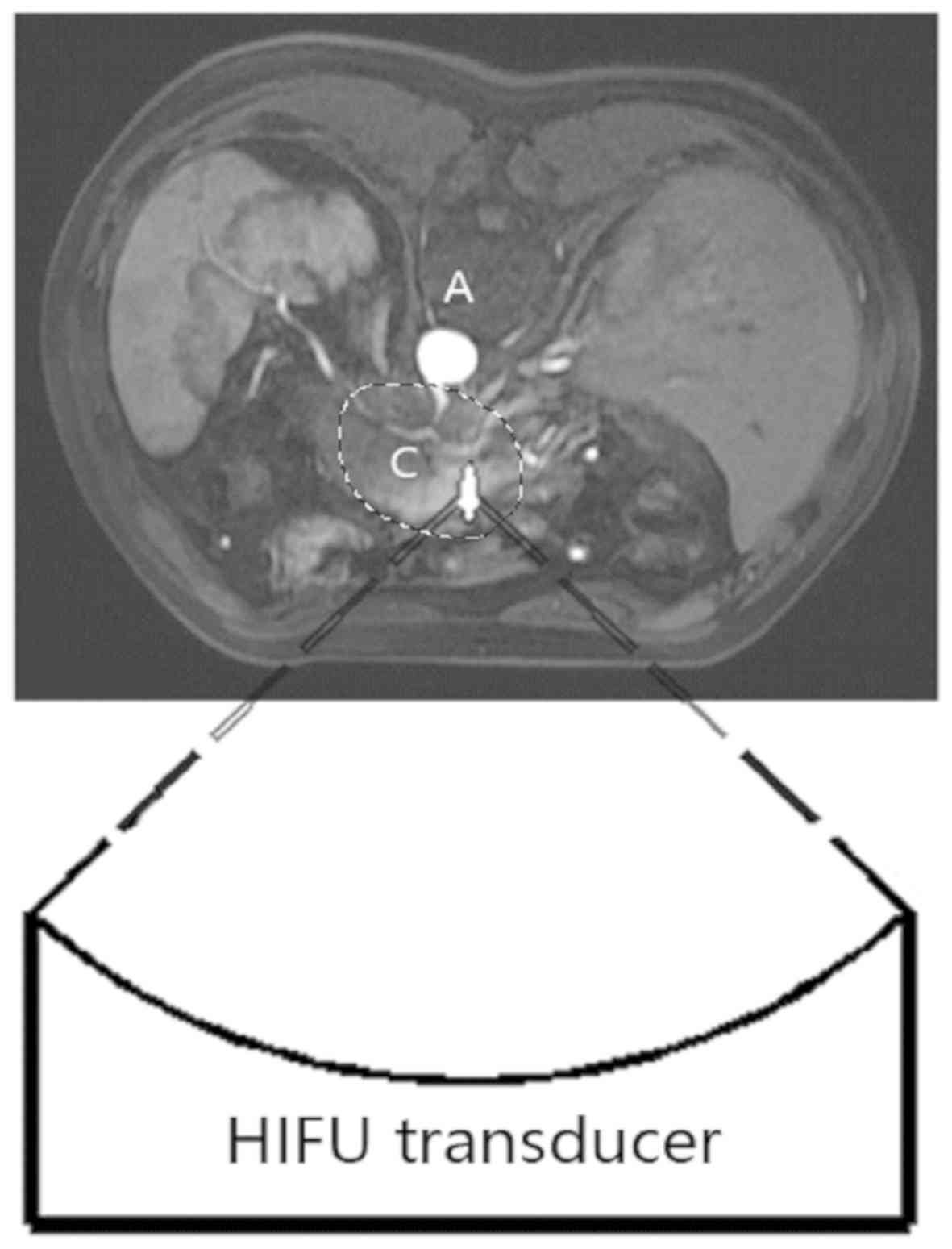
identifying lesions invading blood vessels, as shown in Fig. 1.
The lesions were divided into slices 5-mm-thick and
focus was placed on the deepest layer of a slice containing the
maximum tumor area. A safety gap of ~15 mm was maintained in case
the target area was close to important organs, including large
blood vessels or the gastrointestinal tract and near the tumor
edge. The target area of the tumor lesion was locally ablated from
the deepest region to the surface and the treatment was repeated
for each slice until the tumor was completely ablated. In the
course of treatment, point scan was the main method at a power of
100–400 W. Notable signs of effective HIFU sonication included the
presence of massive gray-scale changes (MGSCs) or an increase in
gray-scale throughout the target area. Treatment was complete when
the tumor volume was fully covered by MGSCs. The treatment
parameters and patient characteristics are shown in Table I.
 | Table I.Baseline characteristics of patients
with pancreatic cancer and who underwent high-intensity focused
ultrasound treatment. |
Table I.
Baseline characteristics of patients
with pancreatic cancer and who underwent high-intensity focused
ultrasound treatment.
| Characteristic | n |
|---|
| Total number of
patients | 15 |
| Sex |
|
|
Male | 6 |
|
Female | 9 |
| Age, years (median
± SD) | 65±11 |
| Site of pancreatic
disease |
|
|
Head | 7 |
| Body or
tail | 8 |
| CA19-9 |
|
| + | 15 |
| − | 0 |
| TNM stage |
|
| II | 2 |
|
III | 6 |
| IV | 7 |
| Intervention
duration, min (mean ± SD) | 81±37 |
| Therapeutic
sonication duration, | 725±370 |
| sec (mean ± SD)
duration (seconds) |
|
| Total energy, J
(mean ± SD) | 185,075±95,176 |
| Average power, W
(mean ± SD) | 295±54 |
| Lesion ablation
rate, % | 68.4% |
Data collection
A total of 15 patients with pancreatic cancer were
treated with HIFU. According to the imaging manifestations of
pancreatic cancer invading peripancreatic blood vessels, the
relationship between blood vessels and the tumor were divided into
two groups: i) Normal pancreatic tissue between the tumor and blood
vessels, with the tumor <1 cm from major blood vessels; and ii)
tumor adjacent to or surrounding the blood vessel. The association
between tumor and blood vessel was recorded. All patients underwent
CT or MRI and underwent abdominal blood vessel CDFI to analyze
hemodynamic parameters. The follow-up period of the present study
was between June 2018 and September 2019. Follow-up examinations
included abdominal blood vessel CDFI, and CT or MRI every 1 or 2
months.
Evaluation of therapeutic efficacy and
pain relief
The treatment efficacy was evaluated using contrast
enhanced CT or MRI 1 week post-HIFU treatment and pain relief
pre-treatment and at 1 week after treatment was assessed using the
NRS pain score table (numerical rating scale of 0–10, with 0
indicating ‘no pain’ and 10 reflecting ‘maximum imaginable pain’)
(7). Based on images obtained using
contrast enhanced CT or MRI, no enhancement area was observed in
the tumor lesion, which was considered to be completely ablated and
necrotic. Blood vessel adverse events were defined as non-perfusion
or partial perfusion. The rate of lesion ablation was calculated
based on preoperative and postoperative contrast enhanced CT or
MRI. 3D Image Processing software (version 1.0; Chongqing Haifu
Medical Technology Co., Ltd.) was used to delineate the tumor and
determine the tumor volume, non-perfused volume (NPV) and lesion
ablation rate (%) as NPV/tumor volume ×100.
Statistical analysis
Data were analyzed using SPSS version 22.0 software
(IBM Corp.). Data are presented as the mean ± standard deviation.
Preoperative and postoperative samples were compared using a paired
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical characteristics
All patients successfully completed the HIFU
treatment procedure for pancreatic cancer and no clinical
complications, such as persistent severe abdominal pain,
gastrointestinal bleeding, obstructive jaundice and peritoneal
irritation, were observed. The specific invaded and adjacent blood
vessels are presented in Table II.
A total of 33 blood vessels were invaded by tumor lesion and 22
were within 1 cm of the lesion, which were well identified using
imaging. Compared with preoperative NRS pain score (4.0±2.0),
postoperative NRS pain score (1.6±1.3) was significantly reduced
(P<0.01), indicating pain relief after the operation. The lesion
ablation rate was 68.4%, which was calculated using 3D image
processing software, and the tumor marker CA19-9 was positive in
all patients (Table I), which is
useful for the diagnosis and treatment of the disease (6).
 | Table II.Information on the association
between tumor lesions and blood vessels in patients with pancreatic
cancer, treated with high-intensity focused ultrasound (n=15). |
Table II.
Information on the association
between tumor lesions and blood vessels in patients with pancreatic
cancer, treated with high-intensity focused ultrasound (n=15).
| Blood vessel | Number of vessels
invaded | Number of vessels
within 1 cm |
|---|
| Splenic vein | 9 | 1 |
| Splenic artery | 7 | 3 |
| Superior mesenteric
vein | 6 | 4 |
| Superior mesenteric
artery | 3 | 6 |
| Portal vein | 4 | 4 |
| Celiac artery | 2 | 2 |
| Hepatic artery | 2 | 2 |
| Total | 33 | 22 |
Hemodynamic data analysis
Specific arterial and venous hemodynamic parameters
(PSV, MV, VF, PI, RI and VD) are presented in Tables III and IV. As presented in Table III, comparing the PI and RI of
arterial vessels before and after HIFU treatment, there were no
significant differences (P>0.05). Furthermore, there was no
significant change in the PSV, MV, VF and VD of arterial blood
vessels after HIFU compared with pre-treatment values (P>0.05).
There was no significant change in the PSV, MV, VF and VD of venous
blood vessels after HIFU compared with pre-treatment values
(Table IV; P>0.05). These data
indicated that the functions of these vessels had no obvious
changes after HIFU treatment.
 | Table III.Hemodynamics of tumor invasion or
adjacent main arterial blood before and after HIFU treatment. |
Table III.
Hemodynamics of tumor invasion or
adjacent main arterial blood before and after HIFU treatment.
|
|
| PSV, cm/s | MV, m/s | VF, l/min | PI | RI | VD, cm |
|---|
|
|
|
|
|
|
|
|
|
|---|
| BV | n | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value |
|---|
| SPA | 10 | 108±50 | 89±50 | 0.24 | 32±16 | 27±16 | 0.27 | 0.7±0.2 | 0.5±0.2 | 0.91 | 0.3±0.2 | 0.3±0.2 | 0.16 | 1.5±0.7 | 1.1±0.5 | 0.12 | 0.3±0.2 | 0.3±0.2 | 0.52 |
| SMA | 9 | 101±60 | 124±32 | 0.19 | 21±15 | 26±7 | 0.21 | 0.6±0.4 | 0.8±0.1 | 0.06 | 0.3±0.2 | 0.3±0.2 | 0.50 | 2.2±1.4 | 2.6±0.8 | 0.11 | 0.4±0.2 | 0.4±0.2 | 0.66 |
| CA | 4 | 128±27 | 118±38 | 0.24 | 32±5 | 34±11 | 0.56 | 0.9±0.3 | 0.7±0.5 | 0.80 | 0.5±0.2 | 0.5±0.2 | 0.88 | 1.4±0.6 | 1.4±0.6 | 0.29 | 0.5±0.1 | 0.5±0.1 | 0.85 |
| HA | 4 | 78±72 | 76±77 | 0.83 | 44±46 | 37±36 | 0.35 | 0.4±0.3 | 0.4±0.3 | 0.53 | 0.2±0.1 | 0.1±0.1 | 0.30 | 0.6±0.5 | 1.3±1.2 | 0.20 | 0.2±0.2 | 0.3±0.2 | 0.53 |
 | Table IV.Hemodynamics of tumor invasion or
adjacent main venous blood before and after HIFU treatment. |
Table IV.
Hemodynamics of tumor invasion or
adjacent main venous blood before and after HIFU treatment.
|
|
| PSV, cm/s | MV, cm/s | VF, l/min | VD, cm |
|---|
|
|
|
|
|
|
|
|---|
| BV | n | Before | After | P-value | Before | After | P-value | Before | After | P-value | Before | After | P-value |
|---|
| SPV | 10 | 33±39 | 19±17 | 0.12 | 15±18 | 7±6 | 0.12 | 0.1±0.1 | 0.1±0.1 | 0.05a | 0.3±0.3 | 0.3±0.2 | 0.20 |
| SMV | 10 | 30±66 | 20±35 | 0.36 | 13±29 | 9±4 | 0.40 | 0.1±0.1 | 0.1±0.1 | 0.42 | 0.3±0.4 | 0.3±0.4 | 0.86 |
| PV | 10 | 17±10 | 19±15 | 0.63 | 6±4 | 6±5 | 0.80 | 0.3±0.2 | 0.3±0.3 | 0.60 | 0.9±0.4 | 0.7±0.4 | 0.12 |
Adverse effects of HIFU treatment
The postoperative complications in patients included
fever (n=1) and skin numbness in the treatment area (n=1). Both
patients improved following symptomatic treatment. No imaging
changes of adjacent vessels, such as stenosis and occlusion, were
observed in imaging data. No blood vessel adverse events were
observed within 1 week after HIFU treatment and during follow-up.
The results demonstrated that splenic vessels were the most
frequently invaded blood vessels in pancreatic tumors (Table II). Following comparison of data
from all patients, a 63-year-old female patient with pancreatic
cancer, whose MRI showed that the tumor invaded the splenic vein
and was adjacent to the splenic artery in the tail of the pancreas
(Fig. 2Aa), which was similar to
most pancreatic cancer patients in our study and had more
representative data and had certain research value. Finally, the
incidences of avascular adverse events and associated complications
in pancreatic cancer treated with HIFU were lower compared with
other local ablation methods, such as RFA and IRE (Table V).
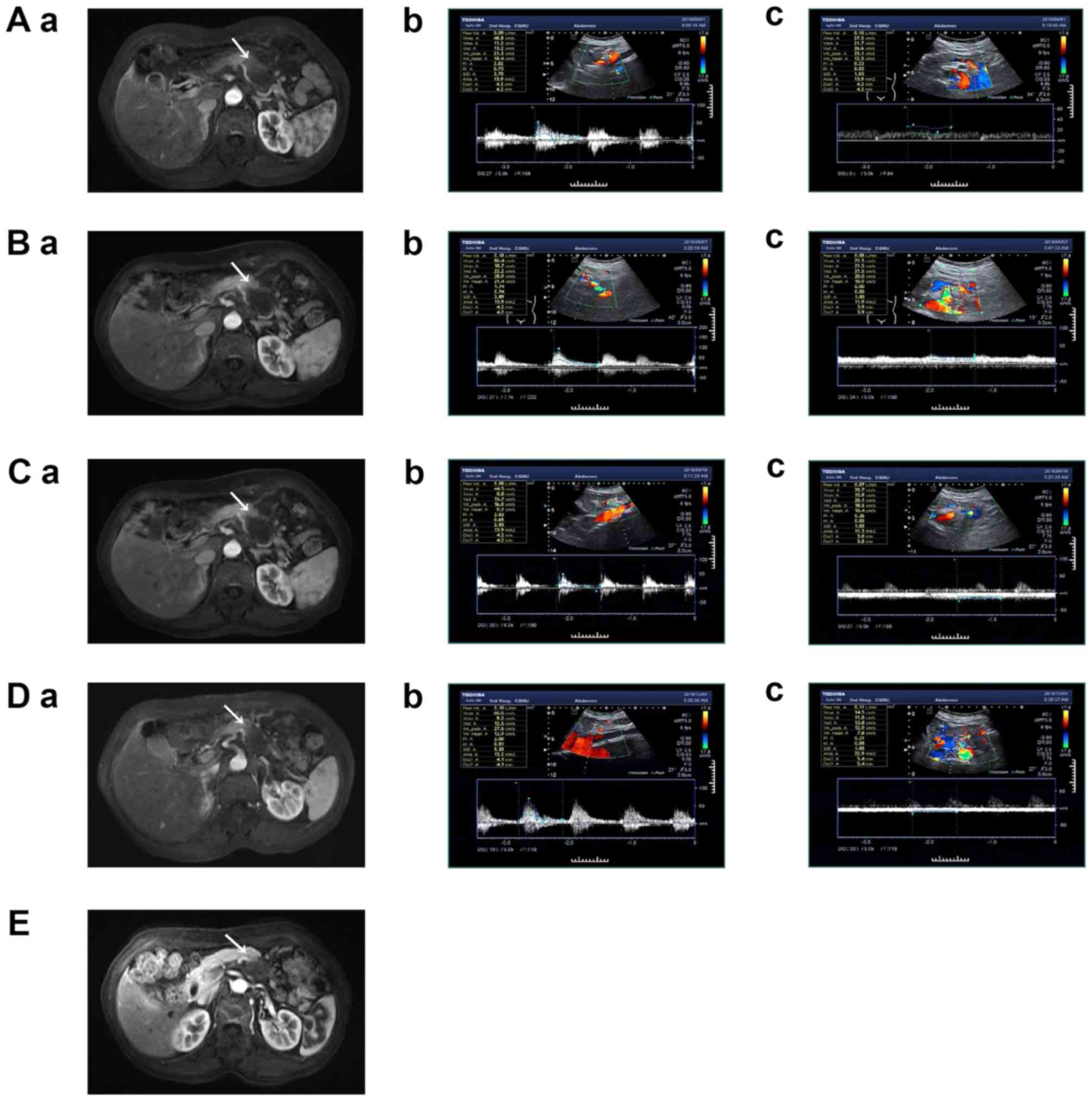 | Figure 2.A 63-year-old female patient with
pancreatic cancer in the tail of pancreas was evaluated
radiologically (A) before HIFU, (B) at 1 week, (C) at 1 month, (D)
at 4 months and (E) 10 months after HIFU, respectively. Arrows
indicate a tumor lesion in the tail of the pancreas. Before HIFU,
(Aa) enhanced MRI showed that the tumor invaded the splenic vein
and was adjacent to the splenic artery. CDFI images showed normal
blood flow in (Ab) splenic artery and (Ac) splenic vein. At 1week
after HIFU, (Ba) enhanced MRI showed no significant change in the
morphology of splenic blood vessels, and no overt abnormal changes
of hemodynamics of the (Bb) splenic artery and (Bc) vein in CDFI
images. Follow-up 1 month after HIFU, (Ca) enhanced MRI showed that
splenic vessel filling was normal in the enhancement phase, with no
vascular stenosis or occlusion. There was no significant change in
hemodynamics of the (Cb) splenic artery and (Cc) vein. At 4 months
after HIFU, (Da) the vascular morphology of the splenic vein and
the splenic artery were normal, and the hemodynamics of the (Db)
splenic artery and (Dc) vein vessels were similar to those of the
previous follow-up. At 10 months after HIFU, (E) enhanced MRI
showed smaller lesion volumes compared with preoperative values,
with no vascular stenosis or occlusion. There were no CDFI images
due to insufficient intestinal preparation and intestinal gas
interference. MRI, magnetic resonance imaging; HIFU, high-intensity
focused ultrasound; CDFI, color doppler flow imaging. |
 | Table V.Selected studies assessing
high-intensity focused ultrasound, radiofrequency ablation and
irreversible electroporation in pancreatic cancer. |
Table V.
Selected studies assessing
high-intensity focused ultrasound, radiofrequency ablation and
irreversible electroporation in pancreatic cancer.
| A, HIFU |
|---|
|
|---|
| Author, year | Patients, n | Access | Median
survival | Pain reduction,
% | Complication
rate | Vascular
complications | Other
complications | (Refs.) |
|---|
| Zhou et al,
2014 | 3,022 (996 stage
III/962 stage IV) | MR/US guidance | 10.0 months | 71.3 | 9.7 | Portal vein
Thrombosis (n=1); GI bleeding (n=1); | Skin burn (n=62)
Fever (n=27) Acute pancreatitis (n=15) Pancreatic pseudocyst (n=1)
Acute amylase (n=48) Jaundice (n=6) GI dysfunction (n=36)
Other | (10) |
| Marinova et al,
2016 | 20 | US guidance | No record | 75 | No record | No record | Transient
subcutaneous edema (n=1), minor skin burns (n=1) | (13) |
| Marinova et al,
2018 | 50 (19 stage
III) | US guidance | 8.3 months after
HIFU | 80 | 26 | No record | Pancreatic lipase
increase (n=4), Induration of subcutaneous fat tissue (≤2 cm)
(n=9) | (14) |
| Wang et al,
2013 | 224 (86 stage
III) | US guidance | No record | No record | 5.4 | No record | Superficial
second-degree skin burns (n=2), deep second to third degree skin
burns (n=1), minor skin burns (n=9) | (15) |
| Strunk et al,
2018 | 50 | US guidance | 16.2 months | No record | 6 | Tumor-associated
vascular (superior mesenteric vein, portal vein, or splenic vein)
occlusion (n=3) | No record | (16) |
| Xiong et al,
2009 | 89 (39 stage
III) | US guidance | 11.2 months | 78.6 | 11.2 | No record | Superficial second
degree skin burns (n=3); Subcutaneous sclerosis (n=6); Pancreatic
pseudocyst (n=1) | (17) |
| Sofuni et al,
2014 | 30 (16 stage
III) | US guidance | No record | 66.7 | 10 | No record | Pancreatic
pseudocyst (n=2); Mild pancreatitis (n=1) | (18) |
| Anzidei et al,
2014 | 6 | MR guidance | No record | 100 | No record | No record | No record | (19) |
| Strunk et al,
2016 | 15 (6 stage III/9
stage IV) | US guidance | 6 months after
HIFU | 40 | No record | No record | Skin burns (n=1)
Subcutaneous sclerosis | (21) |
| Shi et al,
2017 | 71 (stage III) | US guidance | No record | 92.9 | No record | No record | Skin burning pain;
Rib pain; Visceral pain | (22) |
| Dababou et al,
2017 | 729 | MR/US guidance | No record | 81 | No record | Portal vein
thrombosis | Skin burn; mild
pancreatitis; pancreaticoduodenal fistula. | (23) |
| Vidaljove et al,
2015 | 43 | US guidance | 12.5 months | No record | 11.3 | No record | Minor skin burns
(n=12); grade III skin burns (n=2) pancreatitis with
gastro-intestinal bleeding (n=1) | (32) |
| Zhao et al,
2017 | 38 (stage III) | US guidance | 10.3 months | No record | 25 | No record | Abdominal pain
(n=12); Fever (n=9); Elevated CRP (n=8); Leucopenia (n=4); Elevated
amylase (n=1); Other (n=4); | (33) |
| Ji et al, 2018 | 87 | US guidance | No record | No record | 28 | No record | Fatigue (n=14);
Abdominal pain (n=7); Fever (n=7); Nausea (n=5); Rash (n=4) | (34) |
|
| B, RFA |
|
| Author,
year | Patients,
n | Access | Median
survival | Pain reduction,
% | Complication
rate | Vascular
complications | Other
complications | (Refs.) |
|
| Cantore et al,
2012 | 107 | Surgical (via
laparotomy) | 25.6 months | No record | 17.8 | Portal vein
thrombosis (n=5) | Pancreatic fistulas
(n=6), acute pancreatitis (n=3), duodenal injury (n=3), other | (9) |
| Girelli et al,
2010 | 50 | Surgical (via
laparotomy) with US guidance | No record | 69 | 18 | Portal vein
thrombosis (n=4) | Pancreatic fistulas
(n=2), duodenal bleeding (n=2), severe pancreatitis (n=1) | (42) |
| Girelli et al,
2013 | 100 | Surgical (via
laparotomy) with US guidance | 20 months | 100 | 15 | Portal vein
thrombosis. (n=4) | Acute pancreatitis
(n=3), pancreatic fistulas (n=3), duodenal ulcer (n=2), lymphatic
fistula (n=1), n abdominal fluid (n=2) | (43) |
| Frigerio et al,
2013 | 57 | US guidance | 19 months | No record | 14 | No record | Pancreatic
fistulas, duodenal injury, gastric ulcer, jaundice, other | (49) |
| D'Onofrio et al,
2017 | 18 | Percutaneous with
US guidance | No record | No record | No record | No record | No record | (50) |
|
| C, IRE |
|
| Author,
year | Patients,
n | Access | Median
survival | Pain reduction,
% | Complication
rate | Vascular
complications | Other
complications | (Refs.) |
|
| Martin et al,
2015 | 200 | Urgical With
intraoperative US Guidance | 24.9 months | No record | 37 | Hepatic arterial
thrombosis and nonocclusive superior mesenteric vein/portal vein
thrombosis | Infection,
pancreatitis, pancreatic fistulas, other | (8) |
| Mansson et al,
2016 | 24 | Percutaneous With
US guidance | 7 months after
IRE | No record | 64 | Portal vein
thrombosis (n=2); Superior mesenteric vein thrombosis (n=1). | Infection (n=5),
pancreatitis (n=2), duodenum ulcer bleeding (n=1), gastric
retention (n=1); small bleeding (n=1); | (44) |
| Yan et al,
2016 | 25 | Surgical With
intraoperative US Guidance | No record | No record | 36 | Portal vein
thrombosis (n=1); | Upper
gastrointestinal hemorrhage (n=1), pancreatic fistula (n=3), acute
pancreatitis (n=1), delayed gastric emptying (n=1),
gastrointestinal obstructions (n=2) | (45) |
| Scheffer et al,
2017 | 25 | Percutaneous With
US guidance | 11 months after
IRE | No increase | 40 | High-grade superior
mesenteric artery stenosis (n=1); | Pancreatitis (n=1),
bleeding from duodenal ulcer (n=1), abscess (n=1), biliary
obstruction and fistula (n=2), nausea, vomiting, diarrhea, delayed
gastric emptying, abdominal pain, (n=6), pneumonia (n=1) | (46) |
| Kluger et al,
2016 | 50 | Surgical | 12.3 months | No record | 30 | Portal vein
thrombosis (n=2) | Upper
gastrointestinal bleeding (n=3), visceral ulcerations/perforations
(n=1), intraperitoneal hemorrhage (n=3), other | (47) |
| Narayanan et al,
2017 | 50 | CT Guidance | 14.2 months after
IRE | No record | 20 | No record | Abdominal pain
(n=7), pancreatitis (n=1), sepsis (n=1), gastric leak (n=1) | (48) |
| Zhang et al,
2017 | 21 | US/CT Guidance | No record | No record | No record | No record | Hypoglycemia (n=1),
hypokalemia (n=1), chest tightness and high blood pressure (n=1),
premature ventricular contractions (n=1) | (51) |
Discussion
Pancreatic cancer is highly malignant and lacks a
typical set of early stage symptoms. Most patients are diagnosed
with advanced disease and are usually not eligible for surgical
treatment due to tumor invasion of mesenteric roots and arterial
vessels, or because of liver and peritoneal metastasis. As a
result, the 5-year survival rate is very low and decreases year by
year (6). As a non-invasive
treatment modality, HIFU has achieved good therapeutic results in
the treatment of various benign and malignant tumors. Asian and
European patients benefit from survival and pancreatic cancer
related pain relief after HIFU treatment (28). It was first reported in 2000 that
HIFU successfully ablates and treats pancreatic cancer (29). Several studies have confirmed the
safety and efficacy of HIFU in the treatment of pancreatic cancer
(10,14–16,19–25,29–34). The
most common complications observed following HIFU treatment of
pancreatic cancer include skin burns (10,15,17,21–23,30–32),
pancreatitis (10,18,23,31,32),
duodenal fistula (23,31) and obstructive jaundice (19,34).
Vascular adverse events occur infrequently, including vascular
complications of secondary occlusion of superior mesenteric artery
(24) and PV thromboses (25). To the best of our knowledge, no cases
of vessel rupture and bleeding have been described. Previous
studies have shown that adjacent blood vessels are safe from HIFU
ablation of the tumors near large hepatic and PVs in the liver
(35,36). Zhang et al (35) reported that HIFU could safely and
effectively ablate lesions close to large blood vessels without
damage to such vessels, with no blood vessel adverse events
observed. In addition, HIFU ablation of pancreatic cancer is safe
for peripancreatic blood vessels. Strunk et al (16) reported that 94% of patients showed no
patency change in associated vessels after HIFU treatment of
locally pancreatic cancer with tumor invasion and encasement of
blood vessels. Meanwhile, no vascular adverse events were
recorded.
The aforementioned studies focused on the imaging
changes of blood vessels assessed using CT/MRI and did not assess
vascular function using hemodynamics analysis using CDFI. The
purpose of the present study was to evaluate the effect of HIFU
treatment on vascular function by measuring preoperative and
postoperative hemodynamic parameters of peripheral blood vessels in
pancreatic cancer cases using CDFI, observing the shape changes of
blood vessels using imaging, to determine potential adverse events
of adjacent blood vessels after HIFU treatment of pancreatic
cancer.
In the present study, vascular shape assessment in
all patients revealed that splenic vessels, superior mesenteric
vessels and PVs were mainly involved. Based on images assessing
potential filling defects in the analyzed blood vessels, occlusion
or thrombosis was ruled out. The blood vessels associated with the
tumors were observed and their inner diameters were measured.
Finally, the imaging data obtained before and after HIFU treatment
were compared. There were no shape changes of blood vessels, as
well as no vascular adverse events, such as vascular occlusion,
thrombosis and rupture of blood vessels. These results corroborated
previous studies (16,35).
Based on the data obtained in the present study,
hemodynamic parameters reflected the functions of peripancreatic
blood vessels. Among hemodynamic indexes, PI and RI reflect the
resistance of arterial vessels. Specifically, PI denotes blood
vessel wall elasticity, while RI directly reflects resistance to
blood flow (37). The elasticity of
the associated vessels did not change significantly following HIFU
treatment. PSV reflects the degree of vascular filling, indicating
whether there is a change in blood supply to distal tissues and
organs (38). In the present study,
all patients received preoperative and postoperative CDFI
examinations and the hemodynamic parameters of pancreatic tumor
lesions and adjacent blood vessels were analyzed. In addition, a
comparative analysis of venous and arterial blood vessels was
performed. These data also indicated that HIFU treatment had no
significant influence on the function of peripancreatic blood
vessels and did not affect peripancreatic tissues or organs.
There were fewer complications and side effects
after tumor lesion ablation in the present study compared with
previous reports (14,28,33,34,39,40).
Meanwhile, no notable clinical symptoms and manifestations of
mesenteric vascular occlusion, such as persistent abdominal pain
and peritoneal irritation, were observed. Pain was significantly
reduced in most patients after HIFU treatment, but acute pain
occurred immediately post-HIFU in one patient. It was observed that
the transient stimulation reaction of HIFU ablation to the pancreas
improves after symptomatic analgesia and significantly reduced pain
on postoperative day 2. The NRS pain score was significantly
reduced compared with the preoperative results. The present study
hypothesized that HIFU ablation of pancreatic tumor lesions could
control tumor growth and reduce tumor compression to relieve pain,
whereas HIFU thermal ablation has been reported to cause damage to
peripheral nerves of the pancreas, thereby blocking pain nerve
impulses (22,25,28).
In previous studies, it was observed that HIFU
ablation of malignant tumors has some effects on adjacent tissues.
In a study of preoperative HIFU ablation for borderline resectable
pancreatic cancer, the patient underwent surgical resection 1 week
after HIFU treatment (41). Wang
et al (41) observed faintly
yellow burn marks on the vessel wall adjacent to the tumor lesion
(the anterior-lateral part of the junction of the portal vein and
the SMV) and normal vasoactive activity. These results are similar
to those previous experiments reporting that HIFU effectively
causes coagulative necrosis of the tissue near large blood vessels
and that ablation at 0–5 mm close to the blood vessel may cause
damage to the vessel wall, but such damage is reversible and could
self-resolve within about 1 week (36). The present study separately evaluated
the hemodynamic parameters of adjacent arterial and venous blood
vessels, as well as vascular function. It was hypothesized that
weak venous vessel wall and tumor compression or invasion of the
vein slows the blood flow rate, increasing the likelihood of
thrombosis (25). However, the blood
flow rate of arterial vessels is high, combined with good vascular
elasticity. Compared with venous vessels, arterial vessels have
lower probability of thrombosis and partial narrowing of blood
vessels is more likely to occur. This is consistent with findings
from Strunk et al (16). In
addition, physicians should be aware of the tumor squeezing blood
vessels or tumor invasion of blood vessels during the HIFU therapy.
It is understood that tumor compression or invasion of blood
vessels reduces blood flow rate and favors coagulation (25). Meanwhile, the compression effect of
contraction on blood vessels after tumor ablation, as well as the
cavitation effect caused by ultrasound radiation, could damage the
intima of blood vessels, inducing vasospasm and thrombosis and
eventually causing partial occlusion of blood vessels (18).
Compared with other local ablation methods (RFA and
IRE), HIFU has lower incidence rates of vascular adverse events and
associated complications in pancreatic cancer. Portal vein
thrombosis is the most common vascular-associated complication of
pancreatic cancer following RFA and IRE treatment (8,9,42–51). In
related studies, patients with pancreatic cancer received RFA and
IRE and the primary complications included acute pancreatitis
(8,9,42–46,48),
abdominal pain (46,48), pancreatic fistula (8,9,42,43,45,49),
duodenal ulcer or perforation (9,42–44,49),
gastrointestinal bleeding (42,44–47) and
biliary fistula (46). In HIFU, the
main complications included acute pancreatitis, skin burn,
abdominal pain (22,33,34) and
elevated amylase expression levels (14,21,33,34),
whereas vascular adverse events are rarely recorded and patients
can recover and be discharged within a short time (24,25). In
addition, HIFU treatment utilizes ultrasound without involving the
use of needles, electrodes, probes or similar items, therefore HIFU
is safer and less invasive compared with other local ablation
methods and can be performed in patients with tumors near vessels,
the intestine or the bile duct stent (7). In addition, HIFU treatment could avoid
potential complications caused by puncture, especially bleeding and
metastasis in the puncture channel (16). Therefore, HIFU ablation of pancreatic
cancer treatment is beneficial.
The present study was limited by the small number of
patients. The morbidity of pancreatic cancer is relatively lower
compared with other gastrointestinal malignancies, therefore there
were not large numbers of patients with pancreatic cancer suitable
to be involved in the present single-center study. Future studies
should include a larger number of selected patients. For example,
tumor size and location were not considered as exclusion criteria
and vascular hemodynamic data immediately after surgery were not
available due to general anesthesia. Due to the short follow-up
time of the present study, the long-term survival rate of patients
was not assessed.
In conclusion, the present single-center study
assessed the shape and hemodynamics of related vessels before and
after HIFU treatment and no significant changes were found, with
blood vessels maintaining their normal function. No adverse
vascular events were associated with HIFU therapy in pancreatic
cancer. Therefore, it was concluded that HIFU therapy for
pancreatic cancer has no deleterious effects on peripancreatic
arterial and venous blood vessels.
Acknowledgments
Not applicable.
Funding
This study was financially supported by the Yuzhong
District Research Program of Basic Research and Frontier Technology
of Chongqing (grant no. 20170412), the Chongqing Research Program
of Special Program Social Insurance and People's Livelihood (grant
no. cstc2017shmsA130027) and the Chongqing Research Program of
Clinical Evaluation and Application Demonstration of Innovative
Medical Devices (grant no. cstc2015jcsf10002-14).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, HZ, KZ and DDD designed the present study. XG,
YY, JZ, WY and LR performed the literature review. XG and CJ
analyzed the data. XG and KZ drafted the initial manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Second Affiliated Hospital of Chongqing Medical
University (Chongqing, China) and performed in accordance with The
Declaration of Helsinki. Written informed consent was provided by
all patients prior to the study start (approval no. 12/2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ashktorab H, Kupfer SS, Brim H and
Carethers JM: Racial disparity in gastrointestinal cancer risk.
Gastroenterology. 153:910–923. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun YS, Pawlik TM and Vauthey JN: 8th
Edition of the AJCC cancer staging manual: Pancreas and
hepatobiliary cancers. Ann Surg Oncol. 25:845–847. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rombouts SJ, Vogel JA, van Santvoort HC,
van Lienden KP, van Hillegersberg R, Busch OR, Besselink MG and
Molenaar IQ: Systematic review of innovative ablative therapies for
the treatment of locally advanced pancreatic cancer. Br J Surg.
102:182–193. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin RC II, Kwon D, Chalikonda S,
Sellers M, Kotz E, Scoggins C, McMasters KM and Watkins K:
Treatment of 200 locally advanced (stage III) pancreatic
adenocarcinoma patients with irreversible electroporation: Safety
and efficacy. Ann Surg. 262:486–494; discussion 492–494. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantore M, Girelli R, Mambrini A, Frigerio
I, Boz G, Salvia R, Giardino A, Orlandi M, Auriemma A and Bassi C:
Combined modality treatment for patients with locally advanced
pancreatic adenocarcinoma. Br J Surg. 99:1083–1088. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y: High-intensity focused ultrasound
treatment for advanced pancreatic cancer. Gastroenterol Res Pract.
2014:1–11. 2014. View Article : Google Scholar
|
|
11
|
Wu F: High intensity focused ultrasound: A
noninvasive therapy for locally advanced pancreatic cancer. World J
Gastroenterol. 20:16480–16488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu F, Wang ZB, Chen WZ, Wang W, Gui Y,
Zhang M, Zheng G, Zhou Y, Xu G, Li M, et al: Extracorporeal high
intensity focused ultrasound ablation in the treatment of 1,038
patients with solid carcinomas in China: An overview. Ultrason
Sonochem. 11:149–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marinova M, Strunk HM, Rauch M, Henseler
J, Clarens T, Brüx L, Dolscheid-Pommerich R, Conrad R, Cuhls H,
Radbruch L, et al: High-intensity focused ultrasound (HIFU) for
tumor pain relief in inoperable pancreatic cancer: Evaluation with
the pain sensation scale (SES). Schmerz. 31:31–39. 2016.(In
German). View Article : Google Scholar
|
|
14
|
Marinova M, Huxold HC, Henseler J, Mücke
M, Conrad R, Rolke R, Ahmadzadehfar H, Rauch M, Fimmers R,
Luechters G, et al: Clinical effectiveness and potential survival
benefit of US-guided high-intensity focused ultrasound therapy in
patients with advanced-stage pancreatic cancer. Ultraschall Med.
40:625–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Zhu H, Meng Z, Chen Z, Lin J, Shen
Y and Gao H: Safety evaluation of high-intensity focused ultrasound
in patients with pancreatic cancer. Onkologie. 36:88–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strunk H, Lützow, Henseler J, Mücke M,
Rauch M, Marx C, Schild HH and Marinova M: Mesenteric vessel
patency following HIFU therapy in patients with locally invasive
pancreatic cancer. Ultraschall Med. 39:650–658. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiong LL, Hwang JH, Huang XB, Yao SS, He
CJ, Ge XH, Ge HY and Wang XF: Early clinical experience using high
intensity focused ultrasound for palliation of inoperable
pancreatic cancer. JOP. 10:123–129. 2009.PubMed/NCBI
|
|
18
|
Sofuni A, Moriyasu F, Sano T, Itokawa F,
Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Ikeuchi N, Tanaka R, et
al: Safety trial of high-intensity focused ultrasound therapy for
pancreatic cancer. World J Gastroenterol. 20:9570–9577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anzidei M, Marincola BC, Bezzi M,
Brachetti G, Nudo F, Cortesi E, Berloco P, Catalano C and Napoli A:
Magnetic resonance-guided high-intensity focused ultrasound
treatment of locally advanced pancreatic adenocarcinoma:
Preliminary experience for pain palliation and local tumor control.
Invest Radiol. 49:759–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Y, Ying X, Hu X, Zhao J, Fang X, Wu M,
Chen TZ and Shen H: Influence of high intensity focused ultrasound
(HIFU) treatment to the pancreatic function in pancreatic cancer
patients. Pak J Pharm Sci. 28 (Suppl 3):S1097–S1100. 2015.
|
|
21
|
Strunk HM, Henseler J, Rauch M, Mücke M,
Kukuk G, Cuhls H, Radbruch L, Zhang L, Schild HH and Marinova M:
Clinical use of high-intensity focused ultrasound (HIFU) for tumor
and pain reduction in advanced pancreatic cancer. Rofo.
188:662–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Y, Ying X, Hu X and Shen H: Pain
management of pancreatic cancer patients with high-intensity
focused ultrasound therapy. Pak J Pharm Sci. 30 (Suppl
1):S303–S307. 2017.
|
|
23
|
Dababou S, Marrocchio C, Rosenberg J,
Bitton R, Pauly KB, Napoli A, Hwang JH and Ghanouni P: A
meta-analysis of palliative treatment of pancreatic cancer with
high intensity focused ultrasound. J Ther Ultrasound. 5:92017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei W, Jie T and Huiyi Y: Ablation effects
of high-intensity focused ultrasound therapy on pancreatic cancer.
Chin J Ultrasound Med. 76–79. 2007.
|
|
25
|
Orsi F, Zhang L, Arnone P, Orgera G,
Bonomo G, Vigna PD, Monfardini L, Zhou K, Chen W, Wang Z and
Veronesi U: High-intensity focused ultrasound ablation: Effective
and safe therapy for solid tumors in difficult locations. AJR Am J
Roentgenol. 195:W245–W252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christensen E, Schlichting P, Fauerholdt
L, Gluud C, Andersen PK, Juhl E, Poulsen H and Tygstrup N:
Prognostic value of Child-Turcotte criteria in medically treated
cirrhosis. Hepatology. 4:430–435. 2010. View Article : Google Scholar
|
|
27
|
Jia L, Binsheng F, Rongqin Z, et al: Value
of ultrasonography in preoperative evaluation of vascular invasion
and resectability of pancreatic cancer. Chin J Hepatic Surg.
410–413. 2018.
|
|
28
|
Kun Z, Dimitrov DD, Andreev TV, Liang W,
Feradova H, Gorchev AG, Hui Z and Zhibiao W: One-year survival of
inoperable pancreatic cancer treated by high intensity focused
ultrasound: A retrospective multiple-center clinical trial in China
and Bulgaria. J Chongqing Med Univ. 40:378–382. 2015.
|
|
29
|
Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai
J, Li KQ, Jin CB, Xie FL and Su HB: Feasibility of US-guided
high-intensity focused ultrasound treatment in patients with
advanced pancreatic cancer: Initial experience. Radiology.
236:1034–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bin HU, Wei LV and Dan W: Effect of HIFU
on relieving the pain of advanced pancreatic cancer. J
Hepatopancreatobiliary Surg. 105–108. 2014.
|
|
31
|
Sung HY, Jung SE, Cho SH, Zhou K, Han JY,
Han ST, Kim JI, Kim JK, Choi JY, Yoon SK, et al: Long-term outcome
of high-intensity focused ultrasound in advanced pancreatic cancer.
Pancreas. 40:1080–1086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vidal-Jove J, Perich E, Jaen A and
Castillo Manuel AD: Ultrasound guided high intensity focused
ultrasound (USgHIFU) for malignant tumors: Survival advantage in
stage III and IV pancreatic cancer. J Ther Ultrasound. 3 (Suppl
1):O792015. View Article : Google Scholar
|
|
33
|
Zhao J, Zhao F, Shi Y, Deng Y, Hu X and
Shen H: The efficacy of a new high intensity focused ultrasound
therapy for locally advanced pancreatic cancer. J Cancer Res Clin
Oncol. 143:2105–2111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ji Y, Zhang Y, Zhu J, Zhu L, Zhu Y, Hu K
and Zhao H: Response of patients with locally advanced pancreatic
adenocarcinoma to high-intensity focused ultrasound treatment: A
single-center, prospective, case series in China. Cancer Manag Res.
10:4439–4446. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H,
Chen W, Bai J and Wang Z: High-intensity focused ultrasound (HIFU):
Effective and safe therapy for hepatocellular carcinoma adjacent to
major hepatic veins. Eur Radiol. 19:437–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang F, He M, Liu Y, Huang X, Zhang L,
Bai J and Wang Z: Pathological observation after MRI guided high
intensity focused ultrasound therapy for ablating the liver tissues
adjacent to goat portal vein. Sheng Wu Yi Xue Gong Cheng Xue Za
Zhi. 28:666–669. 2011.(In Chinese). PubMed/NCBI
|
|
37
|
Mingdong J, Jin B and Zhibiao W:
Hemodynamic changes of the atheromatous abdominal aorta of rabbits
treated with high intensity focused ultrasound. J Clin Ultrasound
Med. 196–198. 2004.
|
|
38
|
Marik PE, Monnet X and Teboul JL:
Hemodynamic parameters to guide fluid therapy. Ann Intensive Care.
1:12011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jung SE, Cho SH, Jang JH and Han JY:
High-intensity focused ultrasound ablation in hepatic and
pancreatic cancer: Complications. Abdom Imaging. 36:185–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao HF, Wang K, Meng ZQ, Chen Z, Lin JH,
Zhou ZH, Wang P, Shi WD and Sheng YH: High intensity focused
ultrasound treatment for patients with local advanced pancreatic
cancer. Hepatogastroenterology. 60:1906–1910. 2013.PubMed/NCBI
|
|
41
|
Wang G and Zhou D: Preoperative ultrasound
ablation for borderline resectable pancreatic cancer: A report of
30 cases. Ultrason Sonochem. 27:694–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Girelli R, Frigerio I, Salvia R, Barbi E,
Tinazzi Martini P and Bassi C: Feasibility and safety of
radiofrequency ablation for locally advanced pancreatic cancer. Br
J Surg. 97:220–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Girelli R, Frigerio I, Giardino A, Regi P,
Gobbo S, Malleo G, Salvia R and Bassi C: Results of 100 pancreatic
radiofrequency ablations in the context of a multimodal strategy
for stage III ductal adenocarcinoma. Langenbecks Arch Surg.
398:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mansson C, Brahmstaedt R, Nilsson A,
Nygren P and Karlson BM: Percutaneous irreversible electroporation
for treatment of locally advanced pancreatic cancer following
chemotherapy or radiochemotherapy. Eur J Surg Oncol. 42:1401–1406.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yan L, Chen YL, Su M, Liu T, Xu K, Liang
F, Gu WQ and Lu SC: A single-institution experience with open
irreversible electroporation for locally advanced pancreatic
carcinoma. Chin Med J (Engl). 129:2920–2925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Scheffer HJ, Vroomen LG, de Jong MC,
Melenhorst MC, Zonderhuis BM, Daams F, Vogel JA, Besselink MG, van
Kuijk C, Witvliet J, et al: Ablation of locally advanced pancreatic
cancer with percutaneous irreversible electroporation: Results of
the phase I/II PANFIRE study. Radiology. 282:585–597. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kluger MD, Epelboym I, Schrope BA,
Mahendraraj K, Hecht EM, Susman J, Weintraub JL and Chabot JA:
Single-institution experience with irreversible electroporation for
T4 pancreatic cancer: First 50 patients. Ann Surg Oncol.
23:1736–1743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Narayanan G, Hosein PJ, Beulaygue IC,
Froud T, Scheffer HJ, Venkat SR, Echenique AM, Hevert EC,
Livingstone AS, Rocha-Lima CM, et al: Percutaneous image-guided
irreversible electroporation for the treatment of unresectable,
locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol.
28:342–348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Frigerio I, Girelli R, Giardino A, Regi P,
Salvia R and Bassi C: Short term chemotherapy followed by
radiofrequency ablation in stage III pancreatic cancer: Results
from a single center. J Hepatobiliary Pancreat Sci. 20:574–577.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
D'Onofrio M, Crosara S, De Robertis R,
Butturini G, Salvia R, Paiella S, Bassi C and Mucelli RP:
Percutaneous radiofrequency ablation of unresectable locally
advanced pancreatic cancer: Preliminary results. Technol Cancer Res
Treat. 16:285–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Y, Shi J, Zeng J, Alnagger M, Zhou
L, Fang G, Long X, Pan Z, Li Y, Chen J, et al: Percutaneous
irreversible electroporation for ablation of locally advanced
pancreatic cancer: Experience From a Chinese institution. Pancreas.
46:e12–e14. 2017. View Article : Google Scholar : PubMed/NCBI
|