Introduction
Colorectal cancer (CRC) is responsible for >1.8
million new colorectal cancer cases and 881,000 cases of
CRC-associated mortality worldwide each year (according of GLOBOCAN
2018). It was also the third most commonly diagnosed cancer in both
men and women in the United States, in 2017 (1,2).
Evidence has suggested that different biological pathways are
involved in CRC development, particularly the type that originated
from the precursor lesions termed ‘polyps’ (3). Currently, histology is used to classify
these lesions as adenomatous or serrated polyps (4). The adenoma subdivisions include
villous, tubulovillous or tubuluar, and serrated polyps, which are
then subdivided into hyperplastic polyps, sessile serrated polyps
or traditional serrated adenomas (4).
Early detection of CRC results in improved
prognostic outcomes for patients, and late diagnosis leads to
challenges in treatment (5,6). Currently, researchers are striving to
identify highly specific novel biological markers for the
non-invasive and easy diagnosis of CRC (7–9).
Previous studies have indicated that long non-coding RNAs (lncRNAs)
are involved in different biological processes of cells,
particularly in gene expression via the regulation of transcription
and post-transcriptional processing as well as chromatin
modification. They can also potentially contribute to tumor
progression and metastasis in different types of tissue (10–12). p53
mutations occur in 40–50% of sporadic cases of CRC, resulting in
direct deactivation of the affected gene (13). Emerging evidence has suggested that
lncRNAs may regulate the p53 gene or p53 targets (14). Metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1) is a highly conserved lncRNA
and is overexpressed in various types of human cancer (15). It has been revealed that p53 is a
downstream mediator of MALAT1 activity, and the decrease in MALAT1
levels leads to the activation of p53 (16). In addition, the 1-kb sequence
upstream of regulator of reprogramming (ROR) is a p53-binding site
that induces ROR expression. ROR expression suppresses p53 to
maintain cellular homeostasis (17).
Cyclin-dependent kinase inhibitor 1 (CDKN1A), also known as p21,
mediates p53-dependent growth arrest (18). LincRNA-p21 is induced by p53 and is
expressed ~15 kb upstream of the CDKN1A (p21) on chromosome 17
(15).
Although there are preliminary data that support the
involvement of the aforementioned lncRNAs on p53 regulation, their
usage as predictive biomarkers in early detection and management of
CRC is not currently well known in screening programs (14,15).
Although detection of p53 at the protein level using
immunohistochemistry could demonstrate this association, it could
not determine the mediation of lncRNAs in this pathway.
Colon tissue homeostasis occurs in connection with
the function of LincRNAs and other cellular regulatory systems.
Understanding the associations between the p53 regulatory system
and colon tissue-specific lincRNAs could aid in identifying the
primary lesion and CRC tumor etiology. An increased knowledge of
the aberrant expression levels of these lncRNAs and the p53 gene in
the colon neoplasm could provide evidence for the potential
biomarkers targeting future novel therapeutic approaches. The
present study hypothesized that CRC-associated lincRNA-p21,
lincRNA-RoR and MALAT1 may serve as prognostic and therapeutic
targets in patients with CRC.
Materials and methods
Patients
A total of 125 volunteers with suspected colon
polyps and CRC were subjected to colonoscopy. Non-Iranian patients
and those who were negative for colon polyps and CRC were excluded
(53 volunteers). The final number of patients included in the
present study was 72. Finally, this cross-sectional study was
performed on a total of 72 patients (46 polyps and 26 tumor
tissues), who were referred to the Research Institute for
Gastroenterology and Liver Diseases, Shahid Beheshti University of
Medical Sciences (Tehran, Iran) between August, 2014 and 2016,
enrolled in the present study. The mean age ± standard deviation of
the patients with polyps and tumors was 49.71±17.54 and 59.84±18.87
years, respectively.
The patients were of Iranian descent and provided
written informed consent for the present study prior to the
sampling procedure. Biopsies were performed during the colonoscopy
and diagnoses were confirmed following evaluation by pathologists.
Detailed clinicopathological parameters provided the following data
on each polyp lesion and invasive colon cancer subject in patients
with polyps [including age, body mass index (BMI), sex, smoking
status, ethnicity, education, constipation, diarrhea, anemia,
weight loss, bleeding from the anus, family history, polyp type,
polyp location, number of polyps and polyp size] and patients with
tumors (including age, sex, ethnicity, family history, tumor site,
grade, tumor stage, tumor size, radiotherapy and adjuvant therapy)
from patient records. The tumor stage was determined using
Tumor-Node-Metastasis (TNM) classification of the Union for
International Cancer Control (UICC) (19). The protocol for the present study
conformed to the ethical guidelines of The Declaration of Helsinki
(1975) as reflected by the approval from the Ethics Committee of
the Gastroenterology and Liver Diseases Research Center, Shahid
Beheshti University of Medical Sciences (Date of approval: May 15,
2014, under the ethical code IR.SBMU.RIGLD.1393.Code 765). The
non-tumorous tissue samples were provided with a 10 cm margin from
the tumor tissue. The tissue samples were frozen in liquid nitrogen
immediately following surgical removal and stored at −80°C.
Reverse transcription-quantitative
(RT-q) PCR and PCR analysis
RNA was extracted from 72 tissue samples (46 polyps
and 26 tumor tissues) and paired adjacent normal tissues from the
patients included in the present study using an RNeasy Mini kit
(50) (Qiagen GmbH), according to the manufacturer's protocol. RNA
integrity, quantity and quality were determined using 1% standard
agarose gel electrophoresis and a NanoDrop™ 2000 system (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). RNA was stored at
−70°C prior to use. cDNA was synthesized using 1 μg samples of RNA,
and the RNAs Revert Aid RT Reverse Transcription kit (Thermo Fisher
Scientific, Inc.), following the adjustment of the concentrations,
according to the manufacturer's protocol. qPCR was performed using
a PCR cycler (Rotor-Gene Q MDx; Qiagen GmbH). cDNA fragments were
used as templates to amplify the lincRNA-p21, lincRNA-RoR, MALAT1
and p53 genes using SYBR® Premix Ex Taq™ (Takara Bio,
Inc.), according to the manufacturer's protocol. The experimental
protocol was performed as follows: i) Thermocycling conditions
consisted of an initial activation step for 30 sec at 95°C, 40
cycles at 95°C for 5 sec and 60°C for 35 sec; and ii) melting curve
analysis. A duplicate, no template control, consisting of water,
was included in every run for each primer pair to test for DNA
contamination in buffers as well as solutions and to assess the
primer-dimers.
To select the best normalizing gene, the previous
study by Kheirelseid et al (20) was reviewed and the variation of Cq in
several randomly selected samples using primers targeting B2M,
GAPDH and β-actin was also compared. The results of these
comparisons confirmed the use of B2M gene as a normalizer
endogenous gene.
The primer sequences for lincRNA-p21, lincRNA-ROR,
MALAT1, p53 and B2M, were as follows: lincRNA-p21: Forward:
5’-GGGTGGCTCACTCTTCTGGC-3’, and reverse:
5’-TGGCCTTGCCCGGGCTTGTC-3’; lincRNA-ROR: Forward,
5’-CCAGGACAATGAAACCAC-3’, and reverse: 5’-AGGAGC CCAAAGTAACAG-3’;
MALAT1: Forward, 5’-GGTAACGATGGTGTCGAGGTC-3’, and reverse:
5’-CCAGCATTACAGTTCTTGAACATG-3’; p53: Forward:
5’-TCTAGAGCCACCGTCCAGG-3’, and reverse: 5’-ACGCTAGGATCTGACTGCG-3’;
and B2M: Forward: 5’-TGCTGTCTCCATGTTTGATGTATCT-3’ and reverse:
5’-TCTCTGCTCCCCACCTCTAAGT-3’. The 2-ΔΔCq method was used
to determine the expression fold changes (tumor vs. normal)
(21). In addition, patients were
divided into low-expression (≤median) and high-expression
(>median) groups. The median cut-off value of 1.5 fold was
used.
Statistical analysis
Data was plotted and statistical analysis was
performed using GraphPad Prism (v.5.04; GraphPad Software, Inc.),
and the significance was determined using paired t-test and one-way
analysis of variance test, with a Tukey's multiple comparison
post-hoc test in which P<0.05 was considered to indicate a
statistically significant difference. Differences in
clinicopathological outcome between groups were assessed using the
SPSS software (v.19.0; IBM Corp.). The association between p53 and
lncRNA expression was assessed via linear regression. The receiver
operating characteristics (ROC) curve was constructed to describe
diagnostic specificity and sensitivity, and was performed using the
GenEx program (v.6.1; MultiD Analsyses AB).
Results
General statistical information
CRC was characterized in 36.1% of the patients
(26/72), and polyps were detected in 63.9% (46/72). In those
patients with polyps in the evaluations (46 in total), the mean age
of the patients was 49.71±17.54 years with a mean BMI of
25.74±3.42, 24 patients (52.2%) were male, 9/46 (25.7%) were
smokers, 3/46 (8.6%) consumed alcohol and 9/46 (25.7%) had a
positive family history for gastrointestinal polyps. The most
common type of polyp was adenomatous polyp in 23 (50.0%) patients,
followed by hyperplastic and inflammatory polyps in 13 (28.3%) and
10 (21.7%) patients, respectively. The most common site of the
polyps was the recto-sigmoid in 17 (37.0%) patients; followed by
the transverse and descending colon, each in 9 (19.6%) patients;
ascending colon in 6 (13.0%) and cecum in 3 (6.5%) patients. In the
majority of cases [31 (67.4%) patients] the number of polyps was
<5 and their size was 5–10 mm. The most common symptom in these
patients was constipation (34.8%) followed by bleeding from the
anus (32.6%), anemia (21.7%), diarrhea (19.6%) and weight loss
(17.4%).
In those patients with colon cancer (26 cases in
total), the mean age was 59.84±18.87 years, 18 patients (69.2%)
were female, 5 (19.2)% were smokers and 5 (19.2%) had family
history of colon cancer. In 16 patients (61.5%), the site of the
cancer was the colon, whereas in 10 others (38.5%), it was the
rectum. In 12, 10 and 4 patients, the tumor grade was determined as
II, I and III, respectively. The tumor stage was III, I and II in
12, 10 and 4 cases, respectively. In 18 (69.2%) of the cases, the
size of the tumor was ≥5 cm and 5 (19.2%) and 6 (23.1%) patients
underwent radiotherapy and adjuvant therapy, respectively. The
cancer patients' data were limited than those of the polyp
patients, including BMI, consumed alcohol, constipation, bleeding
from the anus, anemia, diarrhea and weight loss.
Expression of lncRNAs in the samples
of patients
In order to investigate the expression of MALAT1,
lincRNA-p21, lincRNA-ROR and p53 genes in the colon cancer tissues,
a qPCR analysis was performed on 66 neoplastic and adjacent
non-neoplastic tissues (46 with polyps and 20 with tumor tissue).
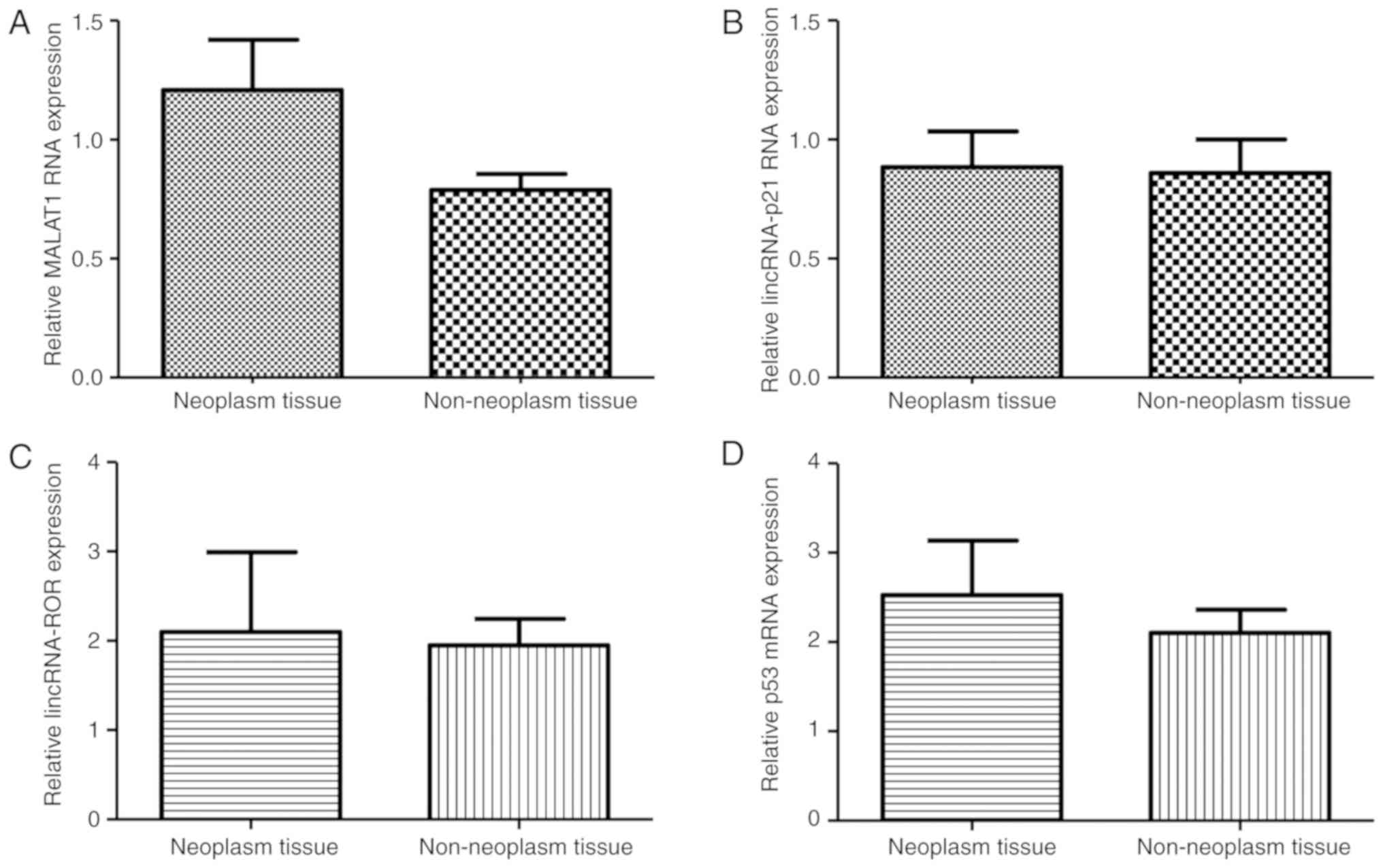
The results indicated that there were no significant differences in
the expression of MALAT-1, lincRNA-p21, lincRNA-ROR and p53 between
the aforementioned tissues (Fig.
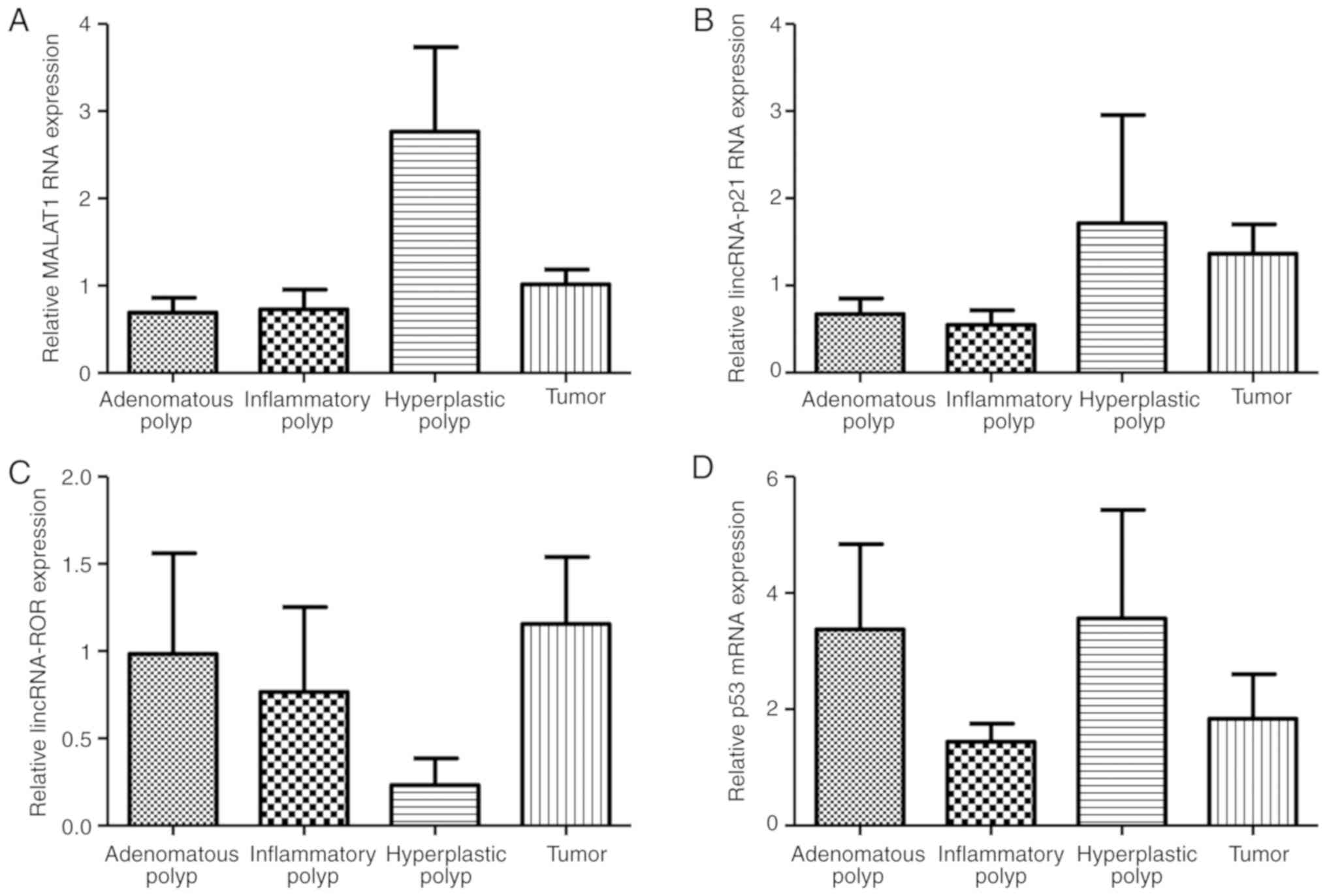
1A-D). The extent of the gene expression of the lncRNAs was
compared between the polyp (adenomatous, inflammatory and
hyperplastic types) and colon cancer tissues. The level of
expression of the MALAT1 gene revealed a statistically significant
difference between the different polyp types and the tumor tissue
(P=0.0028; Fig. 2A). However, there
was no statistically significant difference identified in the
expression of lincRNA-p21, lincRNA-RoR and p53 mRNA between the
polyp and colon cancer tissues (P>0.05; Fig. 2B-D). Patients were divided into two
groups, consisting of colon cancer tissue and colon polyps, and the
association between the MALAT1, lincRNA-ROR and lincRNA-P21 genes
was evaluated (Table I).
 | Table I.Evaluation between MALAT1,
lincRNA-ROR and lincRNA-P21 expression in tumor tissue and polyp
tissue. |
Table I.
Evaluation between MALAT1,
lincRNA-ROR and lincRNA-P21 expression in tumor tissue and polyp
tissue.
|
| MALAT1 | LincRNA-ROR | LincRNA-p21 |
|---|
|
|
|
|
|
|---|
| Variables | Low, n (%) | High, n (%) | Low, n (%) | High, n(%) | Low, n (%) | High, n (%) |
|---|
| Polyp tissue | 34 (73.9) | 12 (26.1) | 37 (80.4) | 9 (19.6) | 32 (69.6) | 14 (30.4) |
| Tumor tissue | 15 (57.7) | 11 (42.3) | 18 (69.2) | 8 (30.8) | 13 (50.0) | 13 (50.0) |
| P-value |
| 0.156 |
| 0.282 |
| 0.1 |
Associations between the expression of
lncRNAs and clinical characteristics
In order to further evaluate the role of MALAT1,
lincRNA-p21, lincRNA-RoR and p53 in colon cancer and polyps, the
associations between the transcript levels of the genes and several
clinicopathological features were also investigated (Tables II and III).
 | Table II.Association between
clinicopathological characteristics and MALAT1, lincRNA-ROR and
lincRNA-P21 expression in the 46 patients with colon polyps. |
Table II.
Association between
clinicopathological characteristics and MALAT1, lincRNA-ROR and
lincRNA-P21 expression in the 46 patients with colon polyps.
|
| MALAT1 | LncRNA-ROR | LncRNA-p21 | p53 |
|---|
|
|
|
|
|
|
|---|
| Variables | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Age,
yearsa |
|
| 0.939 |
|
| 0.112 |
|
| 0.191 |
|
| 0.957 |
|
<50 | 12 (41.4) | 4 (40.0) |
| 15 (46.9) | 1 (14.3) |
| 14 (46.7) | 2 (22.2) |
| 5 (41.7) | 11 (40.7) |
|
|
≥50 | 17 (58.6) | 6 (60.0) |
| 17 (53.1) | 6 (85.7) |
| 16(53.3) | 7 (77.8) |
| 7 (58.3) | 16 (59.3) |
|
| BMIa |
|
| 0.608 |
|
| 0.313 |
|
| 0.489 |
|
| 0.656 |
|
Underweight, ≤18.5 | 1 (5.0) | 1 (16.7) |
| 1 (4.3) | 1 (33.3) |
| 1 (4.8) | 1 (20.0) |
| 1 (16.7) | 1 (5.0) |
|
| Normal
weight, 18.6–24.9 | 6 (30.0) | 1 (16.7) |
| 6 (26.1) | 1 (33.3) |
| 5 (23.8) | 2 (40.0) |
| 2 (33.3) | 5 (25.0) |
|
|
Overweight, 25–29.9 | 11 (55.0) | 4 (66.7) |
| 14 (60.9) | 1 (33.3) |
| 13 (61.9) | 2 (40.0) |
| 3 (50.0) | 1 (60.0) |
|
| Obese,
≥30 | 2 (10.0) | − |
| 2 (8.7) | − |
| 2 (9.5) | − |
| − | 2 (10.0) |
|
| Sex |
|
| 0.619 |
|
| 0.207 |
|
| 0.403 |
|
| 0.403 |
|
Male | 17 (50.0) | 7 (58.3) |
| 21 (56.8) | 3 (33.3) |
| 18 (56.3) | 6 (42.9) |
| 6 (42.9) | 18 (56.3) |
|
|
Female | 17 (50.0) | 5 (41.7) |
| 16 (43.2) | 6 (66.7) |
| 14 (43.8) | 8 (57.1) |
| 8 (57.1) | 14 (43.8) |
|
|
Smokinga |
|
| 0.330 |
|
| 0.639 |
|
| 0.847 |
|
| 0.781 |
|
Yes | 8 (29.6) | 1 (12.5) |
| 7 (24.1) | 2 (33.3) |
| 7 (25.0) | 2 (28.6) |
| 2 (22.2) | 7 (26.9) |
|
| No | 19 (70.4) | 7 (87.5) |
| 22 (75.9) | 4 (66.7) |
| 21 (75.0) | 5 (71.4) |
| 7 (77.8) | 19 (73.1) |
|
|
Ethnicitya |
|
| 0.228 |
|
| 0.654 |
|
| 0.644 |
|
| 0.626 |
|
Turkish | 2 (7.4) | 1 (12.5) |
| 3 (10.3) | − |
| 2 (7.1) | 1 (14.3) |
| − | 3 (11.5) |
|
|
Persian | 22 (81.5) | 5 (62.5) |
| 22 (75.9) | 5
(83.3) |
| 21 (75.0) | 6 (85.7) |
| 7 (77.8) | 20 (76.9) |
|
|
Lurs | 1 (3.7) | 2 (25.0) |
| 2 (6.9) | 1 (16.7) |
| 3 (10.7) | − |
| 1 (11.1) | 2 (7.7) |
|
|
Kurd | 2 (7.4) | − |
| 2 (6.9) | − |
| 2 (7.1) | − |
| 1 (11.1) | 1 (3.8) |
|
|
Educationa |
|
| 0.865 |
|
| 0.817 |
|
| 0.865 |
|
| 0.492 |
|
Academic | 13 (46.4) | 3 (42.9) |
| 13 (44.8) | 3 (50.0) |
| 13 (46.4) | 3 (42.9) |
| 5 (55.6) | 11 (42.3) |
|
|
Non-academic | 15 (53.6) | 4 (57.1) |
| 16 (55.2) | 3 (50.0) |
| 15 (53.6) | 4 (57.1) |
| 4 (44.4) | 15 (57.7) |
|
| Constipation |
|
| 0.902 |
|
| 0.497 |
|
| 0.447 |
|
| 0.152 |
|
Yes | 12 (35.3) | 4 (33.3) |
| 12 (32.4) | 4 (44.4) |
| 10 (31.3) | 6 (42.9) |
| 7 (50.0) | 9 (28.1) |
|
| No | 22 (64.7) | 8 (66.7) |
| 25 (67.6) | 5 (55.6) |
| 22 (68.8) | 8 (57.1) |
| 7 (50.0) | 23 (71.9) |
|
| Diarrhea |
|
| 0.768 |
|
| 0.099 |
|
| 0.833 |
|
| 0.308 |
|
Yes | 7 (20.6) | 2 (16.7) |
| 9 (24.3) | − |
| 6 (18.8) | 3 (21.4) |
| 4 (28.6) | 5 (15.6) |
|
| No | 27 (79.4) | 10 (83.3) |
| 28 (75.7) | 9 (100.0) |
| 26 (81.3) | 11 (78.6) |
| 10 (71.4) | 27 (84.4) |
|
| Anemia |
|
| 0.190 |
|
| 0.389 |
|
| 0.418 |
|
| 0.129 |
|
Yes | 9 (26.5) | 1 (8.3) |
| 9 (24.3) | 1 (11.1) |
| 8 (25.0) | 2 (14.3) |
| 5 (35.7) | 5 (15.6) |
|
| No | 25 (73.5) | 11 (91.7) |
| 28 (75.7) | 8 (88.9) |
| 24 (75.0) | 12 (85.7) |
| 9 (64.3) | 27 (84.4) |
|
| Weight loss |
|
| 0.419 |
|
| 0.159 |
|
| 0.713 |
|
| 0.633 |
|
Yes | 5 (14.7) | 3 (25.0) |
| 5 (13.5) | 3 (33.3) |
| 6 (18.8) | 2 (14.3) |
| 3 (21.4) | 5 (15.6) |
|
| No | 29 (85.3) | 9 (75.0) |
| 32 (86.5) | 6 (66.7) |
| 26 (81.3) | 12 (85.7) |
| 11 (78.6) | 27 (84.4) |
|
| Bleeding from the
anus |
|
| 0.171 |
|
| 0.959 |
|
| 0.080 |
|
| 0.699 |
|
Yes | 13 (38.2) | 2 (16.7) |
| 12 (32.4) | 3 (33.3) |
| 13 (40.6) | 2 (14.3) |
| 4 (28.6) | 11 (34.4) |
|
| No | 21 (61.8) | 10 (83.3) |
| 25 (67.6) | 6 (66.7) |
| 19 (59.4) | 12 (85.7) |
| 10 (71.4) | 21 (65.6) |
|
| Family history of
colon polypsa |
|
| 0.958 |
|
| 0.639 |
|
| 0.246 |
|
| 0.136 |
|
Yes | 7 (25.9) | 2 (25.0) |
| 7 (24.1) | 2 (33.3) |
| 6 (21.4) | 3 (42.9) |
| 4 (44.4) | 5 (19.2) |
|
| No | 20 (74.1) | 6 (75.0) |
| 22 (75.9) | 4 (66.7) |
| 22 (78.6) | 4 (57.1) |
| 5 (55.6) | 21 (80.8) |
|
| Consumption of
vegetables |
|
| 0.126 |
|
| 0.976 |
|
| 0.509 |
|
| 0.509 |
|
Low | 12 (46.2) | 1 (14.3) |
| 11 (39.3) | 2 (40.0) |
| 11 (42.3) | 2 (28.6) |
| 2 (28.6) | 11 (42.3) |
|
|
High | 14 (53.8) | 6 (85.7) |
| 17 (60.7) | 3 (60.0) |
| 15 (57.7) | 5 (71.4) |
| 5 (71.4) | 15 (57.7) |
|
| Consumption of red
meata |
|
| 0.943 |
|
| 0.743 |
|
| 0.943 |
|
| 0.943 |
|
Low | 4 (15.4) | 1 (14.3) |
| 4 (14.3) | 1 (20.0) |
| 4 (15.4) | 1 (14.3) |
| 1 (14.3) | 4 (15.4) |
|
|
High | 22 (84.6) | 6 (85.7) |
| 24 (85.7) | 4 (80.0) |
| 22 (84.6) | 6 (85.7) |
| 6 (85.7) | 22 (84.6) |
|
| Consumption of
sodaa |
|
| 0.641 |
|
| 0.516 |
|
| 0.641 |
|
| 0.390 |
|
Low | 13 (50.0) | 4 (57.1) |
| 15 (53.6) | 2 (40.0) |
| 13 (50.0) | 4 (57.1) |
| 2 (28.6) | 15 (57.7) |
|
|
Moderate | 3 (11.5) | − |
| 3 (10.7) | − |
| 3 (11.5) | − |
| 1 (14.3) | 2 (7.7) |
|
|
High | 10 (38.5) | 3 (42.9) |
| 10 (35.7) | 3 (60.0) |
| 10 (38.5) | 3 (42.9) |
| 4 (57.1) | 9 (34.6) |
|
| Consumption of fast
fooda |
|
| 0.385 |
|
| 0.605 |
|
| 0.346 |
|
| 0.922 |
|
Low | 18 (69.2) | 3 (42.9) |
| 17 (60.7) | 4 (80.0) |
| 15 (57.7) | 6 (85.7) |
| 4 (57.1) | 17 (65.4) |
|
|
Moderate | 3 (11.5) | 1 (14.3) |
| 4 (14.3) | − |
| 4 (15.4) | − |
| 1 (14.3) | 3 (11.5) |
|
|
High | 5 (19.2) | 3 (42.9) |
| 7 (25.0) | 1 (20.0) |
| 7 (26.9) | 1 (14.3) |
| 2 (28.6) | 6 (23.1) |
|
|
Exercisea |
|
| 0.279 |
|
| 0.976 |
|
| 0.833 |
|
| 0.126 |
|
Yes | 17 (65.4) | 3 (42.9) |
| 17 (60.7) | 3 (60.0) |
| 16 (61.5) | 4 (57.1) |
| 6 (85.7) | 14 (53.8) |
|
| No | 9 (34.6) | 4 (57.1) |
| 11 (39.3) | 2 (40.0) |
| 10 (38.5) | 3 (42.9) |
| 1 (14.3) | 12 (46.2) |
|
| Type of polyp |
|
| 0.024 |
|
| 0.409 |
|
| 0.434 |
|
| 0.727 |
|
Adenomatous | 19 (82.6) | 4 (17.4) |
| 17 (73.9) | 6 (26.1) |
| 14 (60.9) | 9 (39.1) |
| 6 (26.1) | 17 (73.9) |
|
|
Hyperplastic | 6 (46.2) | 7 (53.8) |
| 12 (92.3) | 1 (7.7) |
| 10 (76.9) | 3 (23.1) |
| 4 (30.8) | 9 (69.2) |
|
|
Inflammatory | 9 (90.0) | 1 (10.0) |
| 8 (80.0) | 2 (20.0) |
| 8 (80.0) | 2 (20.0) |
| 4 (40.0) | 6 (60.0) |
|
| Location of
polypa |
|
| 0.542 |
|
| 0.170 |
|
| 0.098 |
|
| 0.439 |
|
Cecum | 2 (6.1) | 1 (9.1) |
| 2 (5.7) | 1 (11.1) |
| 2 (6.5) | 1 (7.7) |
| 2 (14.3) | 1 (3.3) |
|
|
Ascending colon | 5 (15.2) | 1 (9.1) |
| 3 (8.6) | 3 (33.3) |
| 2 (6.5) | 4 (30.8) |
| 1 (7.1) | 5 (16.7) |
|
|
Transverse colon | 8 (24.2) | 1 (9.1) |
| 9 (25.7) | − |
| 9 (29.0) | − |
| 2 (14.3) | 7 (23.3) |
|
|
Descending colon | 5 (15.2) | 4 (36.4) |
| 8 (22.9) | 1 (11.1) |
| 6 (19.4) | 3 (23.1) |
| 2 (14.3) | 7 (23.3) |
|
|
Recto-sigmoid | 13 (39.4) | 4 (36.4) |
| 13 (37.1) | 4 (44.4) |
| 12 (38.7) | 5 (38.5) |
| 7 (50.0) | 10 (33.3) |
|
| Number of
polyps |
|
| 0.022 |
|
| 0.999 |
|
| 0.613 |
|
| 0.199 |
| ≤5 | 26 (76.5) | 5 (41.7) |
| 25 (67.6) | 6 (66.7) |
| 21 (65.6) | 10 (71.4) |
| 12 (85.7) | 19 (59.4) |
|
|
5–10 | 4 (11.8) | 6 (50.0) |
| 8 (21.6) | 2 (22.2) |
| 7 (21.9) | 3 (21.4) |
| 1 (7.1) | 9 (28.1) |
|
|
≥10 | 4 (11.8) | 1 (8.3) |
| 4 (10.8) | 1 (11.1) |
| 4 (12.5) | 1 (7.1) |
| 1 (7.1) | 4 (12.5) |
|
| Size of
polypa |
|
| 0.563 |
|
| 0.225 |
|
| 0.537 |
|
| 0.836 |
| ≤5
mm | 5 (20.0) | 3 (37.5) |
| 7 (25.9) | 1 (16.7) |
| 6 (22.2) | 2 (33.3) |
| 2 (22.2) | 6 (25.0) |
|
| 5–10
mm | 10 (40.0) | 3 (37.5) |
| 12 (44.4) | 1 (16.7) |
| 10 (37.0) | 3 (50.0) |
| 3 (33.3%) | 10 (41.7%) |
|
| ≥10
mm | 10 (40.0) | 2 (25.0) |
| 8 (29.6) | 4 (66.7) |
| 11 (40.7) | 1 (16.7) |
| 4 (44.4) | 8 (33.3) |
|
 | Table III.Association between
clinicopathological characteristics and MALAT1, lincRNA-ROR and
lincRNA-P21 expression in 26 patients with colon cancer tissue. |
Table III.
Association between
clinicopathological characteristics and MALAT1, lincRNA-ROR and
lincRNA-P21 expression in 26 patients with colon cancer tissue.
|
| MALAT1 | LncRNA-ROR | LncRNA-p21 | p53 |
|---|
|
|
|
|
|
|
|---|
| Variables | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Age, years |
|
| 0.664 |
|
| 0.877 |
|
| 0.352 |
|
| 0.940 |
|
<50 | 3 (20.0) | 3 (27.3) |
| 4 (22.2) | 2 (25.0) |
| 2 (15.4) | 4 (30.8) |
| 2 (22.2) | 4 (23.5) |
|
|
≥50 | 12 (80.0) | 8 (72.7) |
| 14 (77.8) | 6 (75.0) |
| 11 (84.6) | 9 (69.2) |
| 7 (77.8) | 13 (76.5) |
|
| Sex |
|
| 0.597 |
|
| 0.620 |
|
| 1 |
|
| 0.492 |
|
Male | 4 (26.7) | 4 (36.4) |
| 5 (27.8) | 3 (37.5) |
| 4 (30.8) | 4 (30.8) |
| 2 (22.2) | 6 (35.3) |
|
|
Female | 11 (73.3) | 7 (63.6) |
| 13 (72.2) | 5 (62.5) |
| 9 (69.2) | 9 (69.2) |
| 7 (77.8) | 11 (64.7) |
|
|
Ethnicitya |
|
| 0.592 |
|
| 0.086 |
|
| 0.462 |
|
| 0.293 |
|
Turkish | 3 (27.3) | 3 (37.5) |
| 2 (16.7) | 4 (57.1) |
| 2 (25.0) | 4 (36.4) |
| 2 (28.6) | 4 (33.3) |
|
|
Persian | 6 (54.5) | 3 (37.5) |
| 8 (66.7) | 1 (14.3) |
| 4 (50.0) | 5 (45.5) |
| 5 (71.4) | 4 (33.3) |
|
|
Lurs | 2 (18.2) | 1 (12.5) |
| 1 (8.3) | 2 (28.6) |
| 1 (12.5) | 2 (18.2) |
| − | 3 (25.0) |
|
|
Kurd | − | 1 (12.5) |
| 1 (8.3) | − |
| 1 (12.5) | − |
| − | 1 (8.3) |
|
| Family history of
colon cancer |
|
| 0.261 |
|
| 0.619 |
|
| 0.135 |
|
| 0.018 |
|
Yes | 4 (26.7) | 1 (9.1) |
| 3 (16.7) | 2 (25.0) |
| 1 (7.7) | 4 (30.8) |
| 4 (44.4) | 1 (5.9) |
|
| No | 11 (73.3) | 10 (90.9) |
| 15 (83.3) | 6 (75.0) |
| 12 (92.3) | 9 (69.2) |
| 5 (55.6) | 16 (94.1) |
|
| Tumor site |
|
| 0.851 |
|
| 0.420 |
|
| 1 |
|
| 0.648 |
|
Colon | 9 (60.0) | 7 (63.6) |
| 12 (66.7) | 4 (50.0) |
| 8 (61.5) | 8 (61.5) |
| 5 (55.6) | 11 (64.7) |
|
|
Rectum | 6 (40.0) | 4 (36.4) |
| 6 (33.3) | 4 (50.0) |
| 5 (38.5) | 5 (38.5) |
| 4 (44.4) | 6 (35.3) |
|
| Grade |
|
| 0.121 |
|
| 0.528 |
|
| 0.255 |
|
| 0.771 |
| I | 6 (40.0) | 4 (36.4) |
| 8 (44.4) | 2 (25.0) |
| 6 (46.2) | 4 (30.8) |
| 3 (33.3) | 7 (41.2) |
|
| II | 5 (33.3) | 7 (63.6) |
| 7 (38.9) | 5 (62.5) |
| 4 (30.8) | 8 (61.5) |
| 5 (55.6) | 7 (41.2) |
|
|
III | 4 (26.7) | − |
| 3 (16.7) | 1 (12.5) |
| 3 (23.1) | 1 (7.7) |
| 1 (11.1) | 3 (17.6) |
|
| Stage |
|
| 0.338 |
|
| 0.057 |
|
| 0.231 |
|
| 0.771 |
| I | 6 (40.0) | 4 (36.4) |
| 9 (50.0) | 1 (12.5) |
| 7 (53.8) | 3 (23.1) |
| 3 (33.3) | 7 (41.2) |
|
| II | 1 (6.7) | 3 (27.3) |
| 1 (5.6) | 3 (37.5) |
| 2 (15.4) | 2 (15.4) |
| 1 (11.1) | 3 (17.6) |
|
|
III | 8 (53.3) | 4 (36.4) |
| 8 (44.4) | 4 (50.0) |
| 4 (30.8) | 8 (61.5) |
| 5 (55.6) | 7 (41.2) |
|
| Size of tumor |
|
| 0.165 |
|
| 0.671 |
|
| 0.395 |
|
| 0.492 |
| <5
cm | 3 (20.0) | 5 (45.5) |
| 6 (33.3) | 2 (25.0) |
| 5 (38.5) | 3 (23.1) |
| 2 (22.2) | 6 (35.3) |
|
| ≥5
cm | 12 (80.0) | 6 (54.5) |
| 12 (66.7) | 6 (75.0) |
| 8 (61.5) | 10 (76.9) |
| 7 (77.8) | 11 (64.7) |
|
| Radiotherapy |
|
| 0.058 |
|
| 0.619 |
|
| 0.619 |
|
| 0.778 |
|
Yes | 1 (6.7) | 4 (36.4) |
| 3 (16.7) | 2 (25.0) |
| 2 (15.4) | 3 (23.1) |
| 2 (22.2) | 3 (17.6) |
|
| No | 14 (93.3) | 7 (63.6) |
| 15 (83.3) | 6 (75.0) |
| 11 (84.6) | 10 (76.9) |
| 7 (77.8) | 14 (82.4) |
|
| Adjuvant
therapya |
|
| 0.407 |
|
| 0.015 |
|
| 0.038 |
|
| 0.760 |
|
Yes | 4 (36.4) | 2 (20) |
| 2 (13.3) | 4 (66.7) |
| 1 (9.1) | 5 (50.0) |
| 2 (33.3) | 4 (26.7) |
|
| No | 7 (63.6) | 8 (80.0) |
| 13 (86.7) | 2 (33.3) |
| 10 (90.9) | 5 (50.0) |
| 4 (66.7) | 11 (73.3) |
|
The MALAT-1 relative expression groups demonstrated
a statistically significant difference between the polyp type and
polyp number (Table II). The
statistical analyses between these two groups revealed a
significant association between the p53 transcript levels and
family history (P=0.018). LincRNA-ROR and lincRNA-p21 expression
was significantly associated with adjuvant therapy (P=0.015 and
0.038, respectively; Table III).
No significant associations were identified between the transcript
level groups and other clinicopathological variables (Tables II and III).
Relative expression of lncRNAs and p53
in individual samples
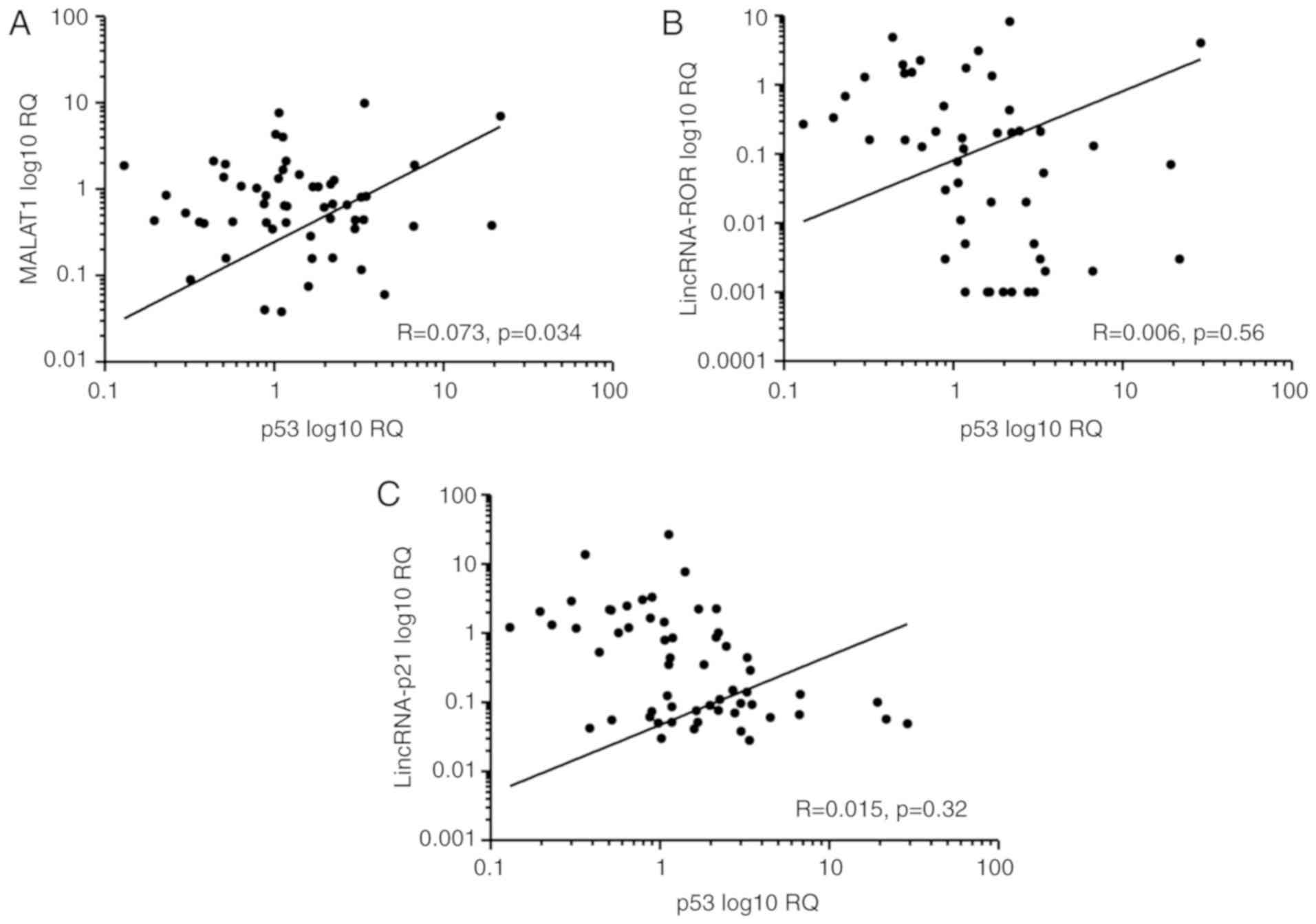
In order to determine whether any association was
present between the expression of the LncRNAs and the p53 gene, the
relative expression of these genes was compared in each set of the
samples. A significant association was observed between the levels
of MALAT1 and p53 in neoplastic tissues (R=0.073; P=0.034; Fig. 3A), but there was no significant
association between the levels of lincRNA-ROR and p53 (R=0.006;
P=0.56; Fig. 3B), or lincRNA-p21 and
p53 (R=0.015; P=0.32; Fig. 3C).
Evaluation of MALAT-1, lincRNA-p21,
lincRNA-ROR and p53 in neoplastic tissue as predictive
CRC-associated biomarkers
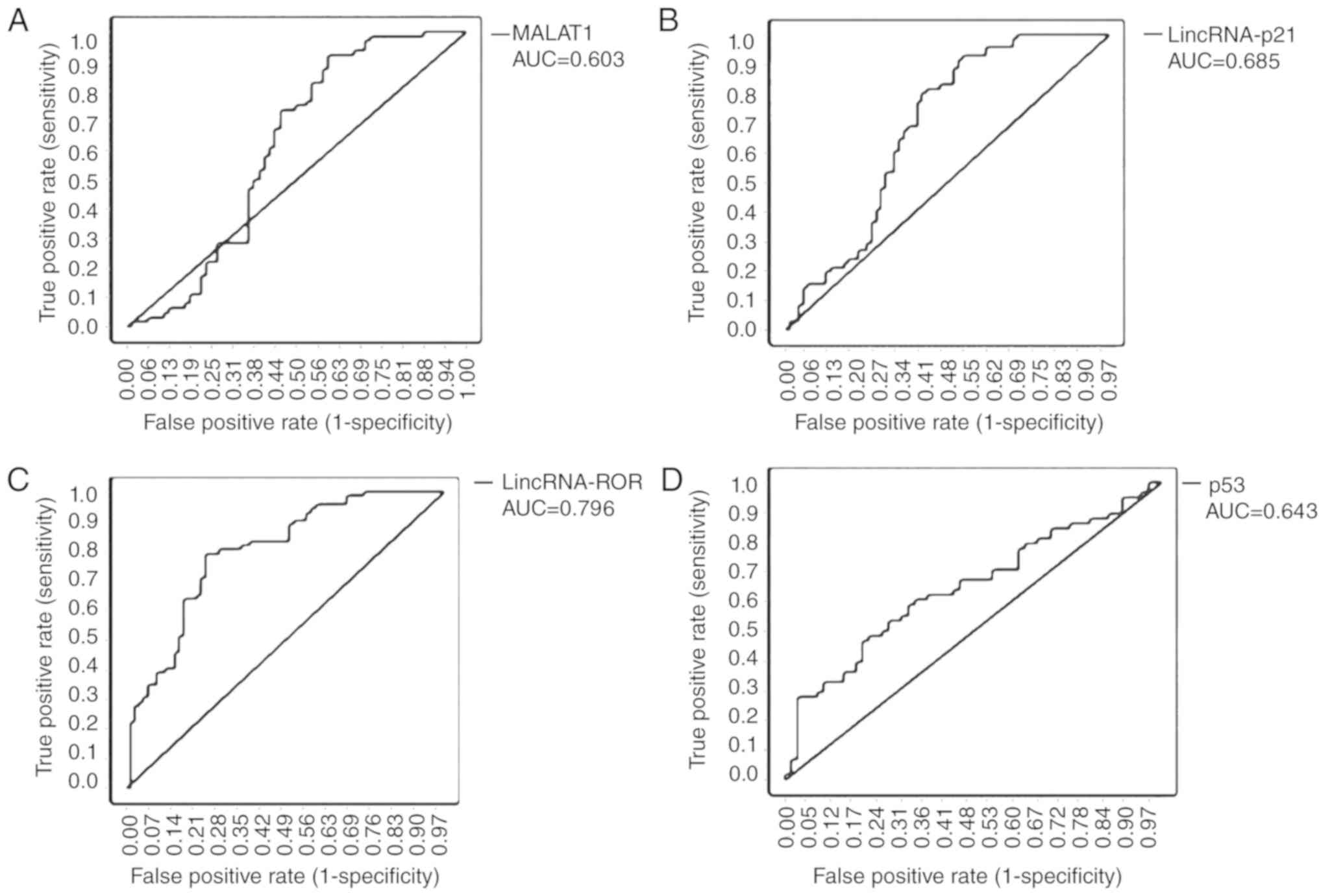
To investigate the characteristics of MALAT-1,
lincRNA-p21, lincRNA-ROR and p53 as potential biomarkers for CRC,
the ROC curves and the area under the ROC curves (AUC) were
generated and calculated for 66 samples from patients with CRC and
healthy adjacent tissues. The ROC curves indicated a strong
separation between the patients with CRC and the healthy adjacent
tissue group, with an AUC of 0.603 [95% confidence interval (CI),
0.501–0.706; P<0.05] for MALAT1, 0.685 (95% CI, 0.595–0.775;
P<0.001) for lincRNA-p21, 0.796 (95% CI, 0.723–0.868;
P<0.001) for lincRNA-ROR and 0.643 (95% CI, 0.542–0.744;
P<0.05) for p53 (Fig. 4A-D;
Table IV).
 | Table IV.Diagnosis value between neoplastic
tissue and adjacent tissue. |
Table IV.
Diagnosis value between neoplastic
tissue and adjacent tissue.
| Variables | MALAT1 | LincRNA-ROR | LincRNA-p21 | p53 |
|---|
| Cut-off | −1.4142 | −2.9084 | −1.3672 | −0.2797 |
| Specificity, % | 75 | 81.9 | 77.5 | 67.2 |
| Sensitivity, % | 50 | 63.9 | 59.2 | 53.4 |
| AUC | 0.603 | 0.796 | 0.685 | 0.643 |
| 95% CI | 0.501±0.706 | 0.723±0.867 | 0.595±0.775 | 0.542±0.744 |
Discussion
Colonoscopy is the gold standard method for the
diagnosis of colon cancer, but the technique is invasive and
expensive (22). Early detection of
colorectal neoplasms is important since it decreases mortality and
increases the survival rate in patients with CRC (23). Identifying a biomarker to detect
cancer in the early stages is, therefore, a major objective in this
field. Although 70% of the human genome is transcribed into RNA,
only a limited amount of RNAs encode proteins (24). The majority of lncRNAs have conserved
sequences that lead to the concept that suggests lncRNA networks
may possess a significant function in biological processes
(25). For example, lncRNA
regulation is involved in CRC progression, proliferation,
apoptosis, differentiation, invasion and metastasis (26). The lncRNA function and expression
patterns have been reported to be different in different cell types
(27). Therefore, it is important to
investigate lncRNA in the colon polyp and tumor to identify the
potential biomarkers that can detect the disease in the early
stages. More importantly, the molecular function and the biology of
p53-regulated lncRNAs should be determined in the colorectal
neoplastic tissue. In the present study, the expression of MALAT1,
lincRNA-p21 and lincRNA-ROR was determined from the network of
p53-regulated LncRNAs, as well as the clinicopathological features
of colon polyps and tumor tissues. The results indicated that
lincRNA-ROR expression did not reveal any association with
radiotherapy sensitivity, but that a significant association did
exist between the higher expression of lincRNA-ROR in patients
receiving adjuvant therapy. According to a previous study by Yang
et al (28) the knockdown of
lincRNA-ROR improved sensitivity to radiotherapy in patients with
CRC by preventing cell viability and promoting apoptosis. Further
studies should be performed on the potential post-transcriptional
regulatory mechanisms, with treatments other than radiotherapy.
Indeed, previous studies have indicated that lincRNA-ROR did not
regulate p53 protein levels in unstressed cells (from intracellular
or extracellular stresses, e.g., DNA damage) and increased the
level of lincRNA-ROR-suppressed p53; on the other hand, p53
activation can influence lincRNA-ROR expression by an
autoregulatory negative feedback loop, which maintains cellular
homeostasis (14,15). According to the results of the
present study, there was no significant association between the
expression of p53 and lincRNA-ROR in the colorectal neoplasm
tissue; therefore, the present study hypothesized that this
inconsistency could be due to the pathological diversity of the
tissues of the patients. Gene expression analysis revealed no
significant alteration of MALAT1 in the colon neoplastic tissue in
comparison with the adjacent normal tissue. However, the present
results demonstrate that the MALAT1 level was higher in patients
with hyperplastic polyps when compared with those with other types
of polyps and tumors. In addition, a association between MALAT1
expression and number of polyps was observed in the present study.
MALAT1 is a functional lncRNA that was first reported in the
invasive non-small cell carcinoma and overexpressed in a number of
other types of cancer tissue, indicating that MALAT1 was associated
with hyperproliferation, invasion and metastasis (15,29–31). To
the best of our knowledge, however, the present study is the first
to investigate the association between the MALAT1 RNA level and the
different types of colon polyp and clinicopathological
features.
CRC cases frequently progress from non-cancerous
growths, called polyps, to malignant adenocarcinomas and distant
metastases; therefore, CRC is known as a ‘silent’ disease, whose
early diagnosis may aid the initiation of early treatment and
increase survival rates (32). The
results of the present study provide further evidence of the
importance of MALAT1 as an early CRC detector and as a prognostic
marker. In neoplastic tissues, the analysis revealed that there was
an association between the expression of MALAT1 and the p53 gene.
Several studies support the potential role of MALAT1 in regulating
cell proliferation, invasion and tumor formation in different types
of tumor (33,34). According to a previous study by
Tripathi et al (16), the
destruction of MALAT1 led to the activation of p53 and its targets.
Nevertheless, the study of Tripathi et al (16) indicated that when MALAT1 was depleted
in the HeLa, U2OS and WI-38-VA13 tumorigenic cell lines, possessing
poor functional of p53, tumor suppressor p16 and retinoblastoma
protein, the cells did not undergo G1 or G1/S arrest, and exhibited
normal S-phase progression. Their data indicate cell cycle defects
and the downregulation of the E2F target genes on MALAT1 depletion
occurs only in specific cell lines (e.g., human dermal fibroblasts)
(16). With regard to the function
of MALAT1 in specific cells, the present study hypothesized that an
interaction between MALAT1 and p53 exists in colorectal
neoplasms.
Previous studies have assessed the expression of
lincRNA-p21 with various types of cancer (18,35,36).
However, few studies report the influence of lincRNa-p21 on CRC. In
addition, further studies revealed that lincRNA-p21 was associated
with malignancy progression, and contributed to the treatment and
prognosis of the tumor (18,37,38). The
results of the present study indicated that the relative expression
of lincRNA-p21 was significantly associated with adjuvant therapy
in the CRC tissue. A recent independent study by Wang et al
(39), revealed that the increase in
lincRNA-p21 inhibited the stability and/or translation of
β-catenin. The inhibition of β-catenin leads to the suppression of
the Wnt signaling pathway, which promotes cell apoptosis and
increases the radiosensitivity for CRC (39). Another study by Zhao et al
(40) indicated that lincRNA-p21
levels were significantly increased following surgical treatment in
comparison with the time prior to surgery. Despite these results,
further studies with a higher sample size are required to
investigate the mechanism of this association between treatment
models and the aberrant expression of lincRNA-P21.
In conclusion, the results of the present study
suggest an interaction between lincRNA-ROR, MALAT1, lncRNA-p21 and
certain clinicopathological features, which appear to serve an
important role in tumorigenesis, development and influencing the
response of cancer cells in treatments.
The results of the present study identified the
association between the aberrant expression of lincRNA-p21 and
lincRNA-ROR and adjuvant therapy in the CRC tissue. In addition, a
association was observed between the MALAT1 level and the type of
colon polyp, as well as the number of polyps in the patients. The
results of the present study provide further evidence towards the
importance of MALAT1 as both an early CRC detector and as a
prognostic marker. Low sample size in this cross-sectional study
affected the association, and thus, further studies on the same
groups of patients are required in order to prove increased lncRNA
levels in association with the increased risk of CRC
development.
Acknowledgements
Not applicable.
Funding
The present study was supported by Research
Institute for Gastroenterology and Liver Diseases, Shahid Beheshti
University of Medical Sciences (grant no, RIGLD765).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HAA, SI and VC conceived and designed the study. MA,
VC and RM performed the experiments. VC, RM, SI, HAA, and MA
contributed to the interpretation of the data. VC, and HAA wrote
the original draft of the manuscript. SI, MA and HAA revised the
manuscript. HAA acquired funding All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of the Gastroenterology and Liver Diseases Research
Center, Shahid Beheshti University of Medical Science, Tehran,
Iran. The protocol conforms with the ethical guidelines of The
Declaration of Helsinki (1975).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tannapfel A, Neid M, Aust D and Baretton
G: The origins of colorectal carcinoma: Specific nomenclature for
different pathways and precursor lesions. Dtsch Arztebl Int.
107:760–766. 2010.PubMed/NCBI
|
|
4
|
Jass JR: Gastrointestinal polyposes:
Clinical, pathological and molecular features. Gastroenterol Clin
North Am. 36:927–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Printz C: Colorectal cancer incidence
increasing in young adults. Cancer. 121:1912–1913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krishnamurthy A, Kankesan J, Wei X, Nanji
S, Biagi JJ and Booth CM: Chemotherapy delivery for resected
colorectal cancer liver metastases: Management and outcomes in
routine clinical practice. Eur J Surg Oncol. 43:364–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yates LR and Campbell PJ: Evolution of the
cancer genome. Nat Rev Genet. 13:795–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meyerson M, Gabriel S and Getz G: Advances
in understanding cancer genomes through second-generation
sequencing. Nat Rev Genet. 11:685–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaleshi V, Haghighi MM, Savabkar S, Zali
N, Vahedi M, Khanyaghma M, Javadi GR, Asadzade H and Zali MR:
Correlation between the EGF gene intronic polymorphism, rs2298979,
and colorectal cancer. Oncol Lett. 6:1079–1083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu X, Sood AK, Dang CV and Zhang L: The
role of long noncoding RNAs in cancer: The dark matter matters.
Curr Opin Genet Dev. 48:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafiee A, Riazi-Rad F, Havaskary M and
Nuri F: Long noncoding RNAs: Regulation, function and cancer.
Biotechnol Genet Eng Rev. 34:153–180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Egranov SD, Yang L and Lin C:
Molecular mechanisms of long noncoding RNAs-mediated cancer
metastasis. Genes Chromosomes Cancer. 58:200–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li XL, Zhou J, Chen ZR and Chng WJ: P53
mutations in colorectal cancer - molecular pathogenesis and
pharmacological reactivation. World J Gastroenterol. 21:84–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang A, Xu M and Mo Y-Y: Role of the
lncRNA-p53 regulatory network in cancer. J Mol Cell Biol.
6:181–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaudhary R and Lal A: Long noncoding RNAs
in the p53 network. Wiley Interdiscip Rev RNA. 8:e14102017.
View Article : Google Scholar
|
|
16
|
Tripathi V, Shen Z, Chakraborty A, Giri S,
Freier SM, Wu X, Zhang Y, Gorospe M, Prasanth SG, Lal A and
Prasanth KV: Long noncoding RNA MALAT1 controls cell cycle
progression by regulating the expression of oncogenic transcription
factor B-MYB. PLoS Genet. 9:e10033682013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang A, Zhou N, Huang J, Liu Q, Fukuda K,
Ma D, Lu Z, Bai C, Watabe K and Mo YY: The human long non-coding
RNA-RoR is a p53 repressor in response to DNA damage. Cell Res.
23:340–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Liang H, Yang H, Zhou K, Xu L, Liu
J, Lai B, Song L, Luo H, Peng J, et al: LincRNa-p21: Function and
mechanism in cancer. Med Oncol. 34:982017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
New Jersey, NY: 2011
|
|
20
|
Kheirelseid EA, Chang KH, Newell J, Kerin
MJ and Miller N: Identification of endogenous control genes for
normalisation of real-time quantitative PCR data in colorectal
cancer. BMC Mol Biol. 11:122010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2− ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vatandoost N, Ghanbari J, Mojaver M, Avan
A, Ghayour-Mobarhan M, Nedaeinia R and Salehi R: Early detection of
colorectal cancer: From conventional methods to novel biomarkers. J
Cancer Res Clin Oncol. 142:341–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie X, Tang B, Xiao Y-F, Xie R, Li BS,
Dong H, Zhou JY and Yang SM: Long non-coding RNAs in colorectal
cancer. Oncotarget. 7:5226–5239. 2016.PubMed/NCBI
|
|
25
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo J, Qu J, Wu D-K, Lu Z-L, Sun Y-S and
Qu Q: Long non-coding RNAs: A rising biotarget in colorectal
cancer. Oncotarget. 8:22187–22202. 2017.PubMed/NCBI
|
|
27
|
Yamada A, Yu P, Lin W, Okugawa Y, Boland
CR and Goel A: A RNA-Sequencing approach for the identification of
novel long non-coding RNA biomarkers in colorectal cancer. Sci Rep.
8:5752018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang P, Yang Y, An W, Xu J, Zhang G, Jie J
and Zhang Q: The long noncoding RNA-ROR promotes the resistance of
radiotherapy for human colorectal cancer cells by targeting the
p53/miR-145 pathway. J Gastroenterol Hepatol. 32:837–845. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji P, Diederichs S, Wang W, Böing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin β4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun D, Li X, He Y, Li W, Wang Y, Wang H,
Jiang S and Xin Y: YAP1 enhances cell proliferation, migration, and
invasion of gastric cancer in vitro and in vivo. Oncotarget.
7:81062–81076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Konishi H, Ichikawa D, Yamamoto Y, Arita
T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, et
al: Plasma level of metastasis-associated lung adenocarcinoma
transcript 1 is associated with liver damage and predicts
development of hepatocellular carcinoma. Cancer Sci. 107:149–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gellad ZF and Provenzale D: Colorectal
cancer: National and international perspective on the burden of
disease and public health impact. Gastroenterology. 138:2177–2190.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Lu W, Xu J, Shi Y, Zhang H and Xia
D: Prognostic value of long non-coding RNA MALAT1 in cancer
patients. Tumour Biol. 37:897–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong Y, Liang G, Yuan B, Yang C, Gao R and
Zhou X: MALAT1 promotes the proliferation and metastasis of
osteosarcoma cells by activating the PI3K/Akt pathway. Tumour Biol.
36:1477–1486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Wei G, Xia H, Yu H, Tang Q and Bi
F: Down regulation of lincRNA-p21 contributes to gastric cancer
development through Hippo-independent activation of YAP.
Oncotarget. 8:63813–63824. 2017.PubMed/NCBI
|
|
36
|
Wang X, Xu Y, Wang X, Jiang C, Han S, Dong
K, Shen M and Xu D: LincRNA-p21 suppresses development of human
prostate cancer through inhibition of PKM 2. Cell Prolif.
50:e123952017. View Article : Google Scholar
|
|
37
|
Tang SS, Zheng BY and Xiong XD:
LincRNA-p21: Implications in human diseases. Int J Mol Sci.
16:18732–18740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Paepe B, Lefever S and Mestdagh P: How
long noncoding RNAs enforce their will on mitochondrial activity:
Regulation of mitochondrial respiration, reactive oxygen species
production, apoptosis, and metabolic reprogramming in cancer. Curr
Genet. 64:163–172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X,
Ma Z, Li X and Zhang Y: LincRNA-p21 enhances the sensitivity of
radiotherapy for human colorectal cancer by targeting the
Wnt/β-catenin signaling pathway. Oncol Rep. 31:1839–1845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao W, Song M, Zhang J, Kuerban M and
Wang H: Combined identification of long non-coding RNA CCAT1 and
HOTAIR in serum as an effective screening for colorectal carcinoma.
Int J Clin Exp Pathol. 8:14131–14140. 2015.PubMed/NCBI
|