Introduction
Colorectal cancer is the third most commonly
diagnosed cancer and fourth leading cause of cancer-related deaths
(1). Colorectal cancer is associated
with a high risk of metastasis and recurrence despite an increased
availability of diagnostic and therapeutic strategies. To treat
patients with colorectal cancer, an approach that selectively
targets cancer cells without damaging normal cells and which
minimizes the risk of perforating the intestinal barrier is needed
(2).
Photodynamic therapy (PDT) can be used to treat
colorectal cancer. PDT is considered as a complementary therapy
aimed at preventing tumor recurrence after surgical resection of
colorectal cancer (3), making it a
suitable approach for continuous removal of small fractions of
tumors (4). PDT has been reported to
be effective for treating aggressive colon cancer showing a high
level of the vascular endothelial growth factor, which promotes
tumor growth through angiogenesis (5). It has also been reported that PDT is an
effective alternative treatment for drug-resistant colorectal
cancer (6). PDT uses a
modality-based photosensitizer, which selectively affects cancer
cells, using excitation and light-absorption in the presence of
oxygen to produce a high concentration of reactive oxygen species
(ROS), such as singlet oxygen and other free radicals (7–9). The
resulting damage cannot be overcome by the antioxidant system to
protect the cell from oxidative damage, leading to necrosis,
apoptosis, or autophagy of the target cell and tissue (10,11).
Chlorin e6 (Ce6) is a second-generation photosensitizer with strong
absorption in the red spectrum and can be easily synthesized from
chlorophyll. Irradiated Ce6 produced singlet oxygen which rapidly
induces tumor cell death. Significant clinical benefits have been
obtained with Ce6-mediated PDT (Ce6-PDT) in the treatment of
various cancers including melanoma, bladder, and colorectal cancer
(12).
Autophagy is a cellular pathway that removes damaged
organelles and aggregates of lysosomal degradation to maintain the
stability of the intracellular environment (13,14).
Autophagy contributes to the prolonged survival of tumor cells,
whereas defects in autophagy play a critical role in tumorigenesis
(15,16). However, the exact function of
autophagy in PDT for colorectal cancer treatment is unclear. A
recent study showed that the hypoxic environment produced by PDT
can induce autophagy in tumor cells (17). This suggests that autophagy is a form
of adaptation of the nutritional environment of rapidly growing
tumor cells in response to hypoxia. Oxidative stress has been shown
to correlate with increasing protein toxicity, linking loss of
protein, accumulation of ROS, and protein aggregate formation.
Concurrently, p62 gene expression is increased by oxidative stress
through activation of the transcription factor NF-E2-related factor
2 (18).
Furthermore, p62 is a multifunctional adapter
protein involved in selective autophagy, cellular signaling
pathways, and tumorigenesis (19,20).
Overexpression of p62 is likely related to tumorigenesis and has
been observed in many types of tumors (13,21–23),
such as chemotherapy-resistant epithelial cell carcinoma (24). Analysis of autophagy-deficient mice
showed that autophagy acts as a tumor suppressor by removing p62
(25). Moreover, abnormal expression
of p62 was found in different types of cancer, suggesting a
functional relationship between p62 accumulation and cancer
progression (26). However, the role
of p62 in the antitumor effects of PDT remains unclear.
This study was conducted to investigate the role of
p62 in the antitumor effect of PDT for colorectal cancer. To
understand whether p62 is directly related to PDT efficacy, we
established a colorectal cancer cell line stably overexpressing p62
and tested PDT efficacy using an in vitro system. In
vivo studies of p62-overexpressing cells were conducted to
confirm the antitumor effects in xenograft mouse models. We found
that PDT showed better effects in p62 overexpressing cells. Our
findings suggest that p62 improves the antitumor efficacy of
PDT.
Materials and methods
Materials
Chlorin e6 (Ce6) was obtained from Frontier
Scientific, Inc.. Thiazolyl blue tetrazolium bromide (MTT) was from
Sigma-Aldrich. Mouse monoclonal antibodies to p62 and β-actin were
from Santa Cruz Biotechnology. 5-Fluorouracil (5-FU) and oxalitin
were obtained from Sigma-Aldrich. Anti-p62 was obtained from
Novusbio. Anti-HA was obtained from Santa Cruz Biotechnology.
Anti-caspase 3 was obtained from Cell Signaling Technology.
p62 mRNA levels in colorectal cancer
cell lines
After downloading RNA-sequencing data for 58 human
colon colorectal cancer cell lines from the cancer cell line
Encyclopedia (https://portals.broadinstitute.org/ccle), the relative
fold-changes in p62 mRNA levels were categorized according to the
classification of cells and graphically plotted.
Cell culture and in vitro photodynamic
treatment
SW480, HCT116, LoVo, and DLD1 cells were maintained
in atmosphere of 5% CO2 in RPMI medium (Genedepot)
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin solution. The cells were incubated with or
without Ce6 (0–400 nM) for 6 h at 37°C. The cells were then
photoirradiated using a diode laser-emitting red light at a
wavelength of 670 nm (equipment from L2K Co., Ltd.). The power
density at the illumination area was 800 mW/cm2 and the
total light dose was 4 J/cm2. The cells were harvested
at 4, 8, and 24 h.
Cell viability assay
Cells were cultured overnight on 96-well culture
plates (1×104 cells/well). Cells undergoing PDT were
incubated for 4 h with 0.5 mg/ml thiazolyl blue tetrazolium
bromide. Converted MTT formazan crystals were treated with dimethyl
sulfoxide (Sigma). Absorbance at 540 nm was measured with a
microplate reader.
Immunoblotting
Cells were lysed in buffer containing 20 mM HEPES
(pH 7.0), 1% Triton X-100, 150 mM NaCl, 10% glycerol, 2 mM EGTA, 1
mM EDTA, 1 mM glycerol 2-phosphate, 1 µg/ml leupeptin, 1 µg/µl
aprotinin, 1 mM AEBSF, 50 mM NaF, and 1 mM
Na3VO4. The proteins, separated by SDS-PAGE,
were transferred to a nitrocellulose membrane using an
electrophoresis tank. After the membrane was incubated with
specific antibodies, the signal was enhanced with chemiluminescence
reagents (Genedepot) and then measured with a LAS-3000
(Fujifilm).
Stable cell establishment in
colorectal cancer cells using retrovirus
To prepare SW480/p62, DLD1/p62, LoVo/p62, and
HCT116/p62 cells, a retrovirus encoding HA-p62 genes was produced
using the pMSCV-GFP vector. HEK293T cells were transfected with the
pMSCV-GFP-HA-p62, pgag-pol, and pVSV-G, using Lipofectamine 2000
(Life Technologies). After 48 h, the media containing retroviruses
were collected and filtered to remove cell debris. Cells were
infected and inoculated with the p62 retrovirus and then cells
expressing p62 were selected with puromycin (Thermo Fisher
Scientific).
Knockdown of p62 expression with shRNA
and siRNA
To knock down p62 expression in colorectal cancer
cell lines, the cells were transfected with negative control short
interfering RNA (siRNA) or p62 siRNAs (Santa Cruz Biotechnology)
using Lipofectamine 2000. Cells were also transfected with negative
control short hairpin RNA (shRNA) or p62 shRNAs (Santa Cruz
Biotechnology) using Lipofectamine 2000.
Immunocytochemical assay
Cells were cultured on a glass coverslip. Glass
coverslips of confluent cells were washed twice with
phosphate-buffered saline (PBS). Cells were fixed for 20 min at
room temperature in 4% paraformaldehyde in PBS. After rinsing with
PBS, cells were incubated with 2% FBS/PBS containing 0.05% Triton
X-100 for 30 min to block nonspecific staining. After washing with
PBS, the cells were incubated overnight with an anti-HA antibody in
2% FBS/PBS, containing 0.05% Triton X-100 (1:200). After washing
with PBS, the cells were incubated with secondary Alexa-647
antibody in 2% FBS/PBS containing 0.05% Triton X-100 for 1 h. The
cells were monitored by fluorescence microscopy (Axiovert 200 MAT,
Zeiss).
Xenograft model and PDT
Four-week-old male BALB/c nude mice were used for
the in vivo study. All facilities were approved by the Association
for Assessment and Accreditation of Laboratory Animal Care. The
present animal care and use protocol was reviewed and approved by
the Institutional Animal Care and Use Committee (IACUC) in College
of Medicine, Hanyang University of Korea (approval no. 2019-0043A).
SW480/CTRL and LoVo/p62 cells (0.5×106) were inoculated
subcutaneously in 100 µl of PBS. The mice were then divided into
treatment and control groups. The treatment group consisted of two
subgroups: the control group and PDT group (670 nm).
Fluorometer analysis of intracellular
photosensitizer levels
A total of 1×105 cells per well (2-ml
cell suspension of SW480, HCT116, LoVo, and DLD1) were seeded in
6-well plates. The medium was then replaced with 2 ml of fresh
medium, containing 5 µM Ce6, and incubated for 1–24 h. The
solutions were removed, and the cells were rinsed with PBS (1 ml).
Afterward, the cells were harvested and centrifuged at 1,500 rpm
for 5 min. Pellets were subsequently washed with PBS (1 ml) and
centrifuged again. The fluorescence of the supernatants was
measured with a Synergy MX fluorometer (BioTek) with excitation and
emission wavelengths of 500 and 670 nm, respectively. A calibration
curve was then used to calculate the concentrations of Ce6 per
10,000 cells.
Statistical analysis
Data are presented as the mean ± SEM. ANOVA and
Turkey's post hoc test was used to compare the means between three
or more groups. Student's t-test was performed to compare the means
between two groups. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using GraphPad Prism 8 (GraphPad Software).
Results
Relationship between p62 and PDT
treatment effects
We examined whether p62 protein enhances PDT
efficacy in colorectal cancer. First, we checked the level of p62
expression in colorectal cancer cells using Cancer Cell Line
Encyclopedia RNA sequencing data (27). The expression level of p62 was high
in most colorectal cancer cells regardless of the distribution of
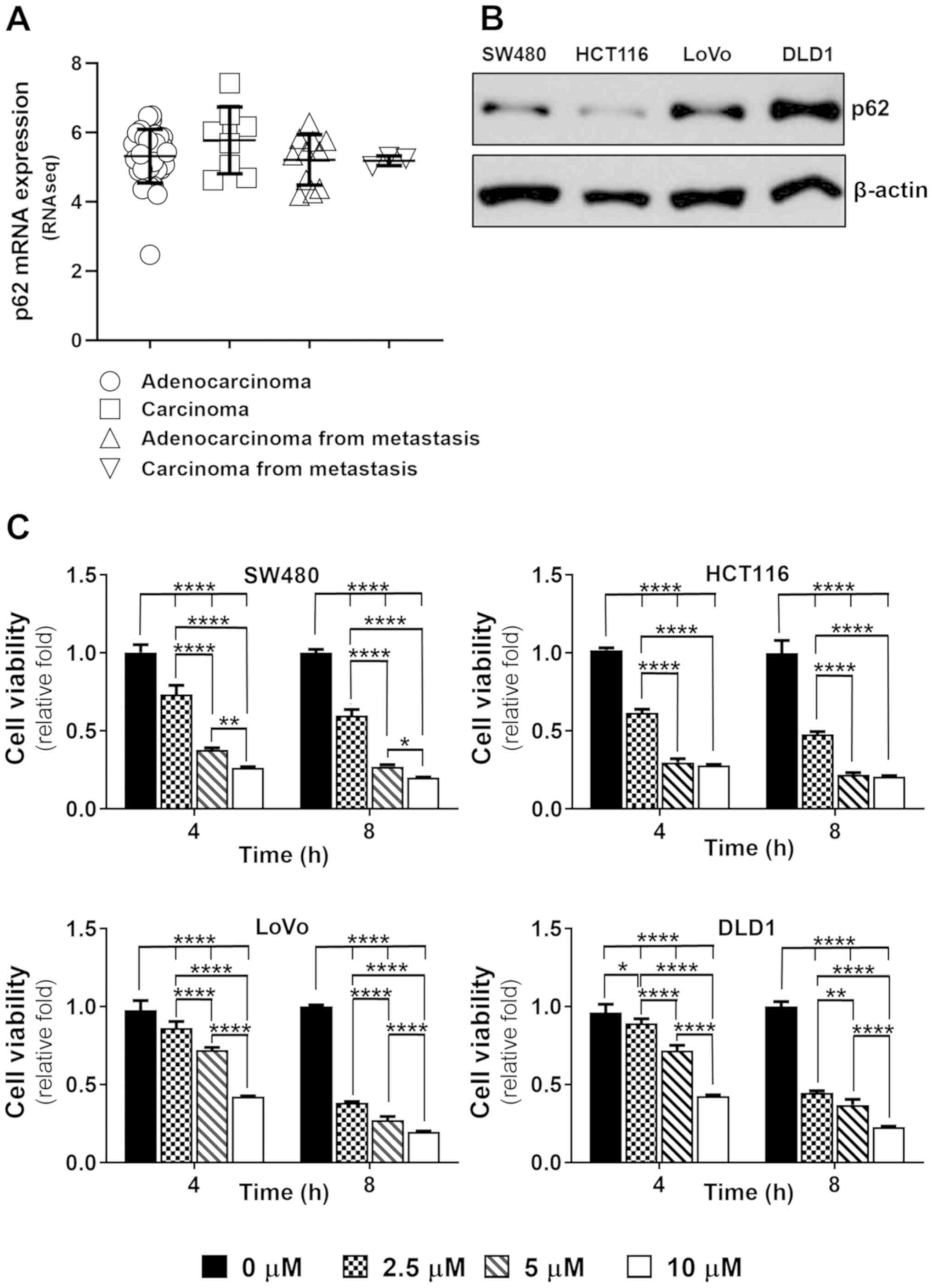
adenocarcinoma, carcinoma, and metastatic cells (Fig. 1A). To determine whether there were
differences in the expression of p62 according to the
classification of colorectal cancer cells, we examined p62 protein
expression in adenocarcinoma, SW480 and DLD1, carcinoma, HCT116,
adenocarcinoma from metastasis, and LoVo cells. We checked the
levels of p62 protein expression for each type of colorectal
cancer. LoVo and DLD1 cells showed higher p62 levels compared to
SW480 and HCT116 cells; there were no differences in the expression
of p62 according to cell type (Fig.
1B). To measure the cellular uptake of photosensitizer, we
indirectly examined the amount of Ce6 remaining in the cell by
fluorometry analysis over time (Fig.
S1). The amount of Ce6 remaining in the cell varied between the
colorectal cancer cell types. SW480 cells showed a high residual
amount of Ce6 until after 9 h. HCT116 cells exhibited the highest
levels of Ce6 at 6 h, and not much remained after that. LoVo cells
showed high levels of residual Ce6 at all time points. Overall, the
residual amount of Ce6 in DLD1 cells was similar to that in other
cells, however, the amount remaining after 6 h was low. Therefore,
PDT after 6 h incubation showed high intracellular uptake of Ce6 in
most cells. To understand whether there were differences in PDT
efficacy depending on p62 expression levels, we tested the effect
of PDT on colorectal cancer cell lines. At 4 h after irradiation,
PDT treatment effects differed depending on the p62 expression
level. Cell lines with low p62 expression, SW480 and HCT116, showed
decreased cell viability in time- and Ce6 concentration-dependent
manners (Fig. 1C). Cells with high
p62 expression, LoVo and DLD1, showed low therapeutic effects at 4
h after PDT application. There was no difference in PDT efficacy
depending on the p62 expression level at 8 h after PDT in most cell
lines.
Inhibition of p62 expression is not
related to the PDT effect
The effects of PDT on p62 in colorectal cancer cells
were variable. To understand the role of p62 in the effectiveness
of PDT, the p62 gene was knocked down in the colorectal cancer
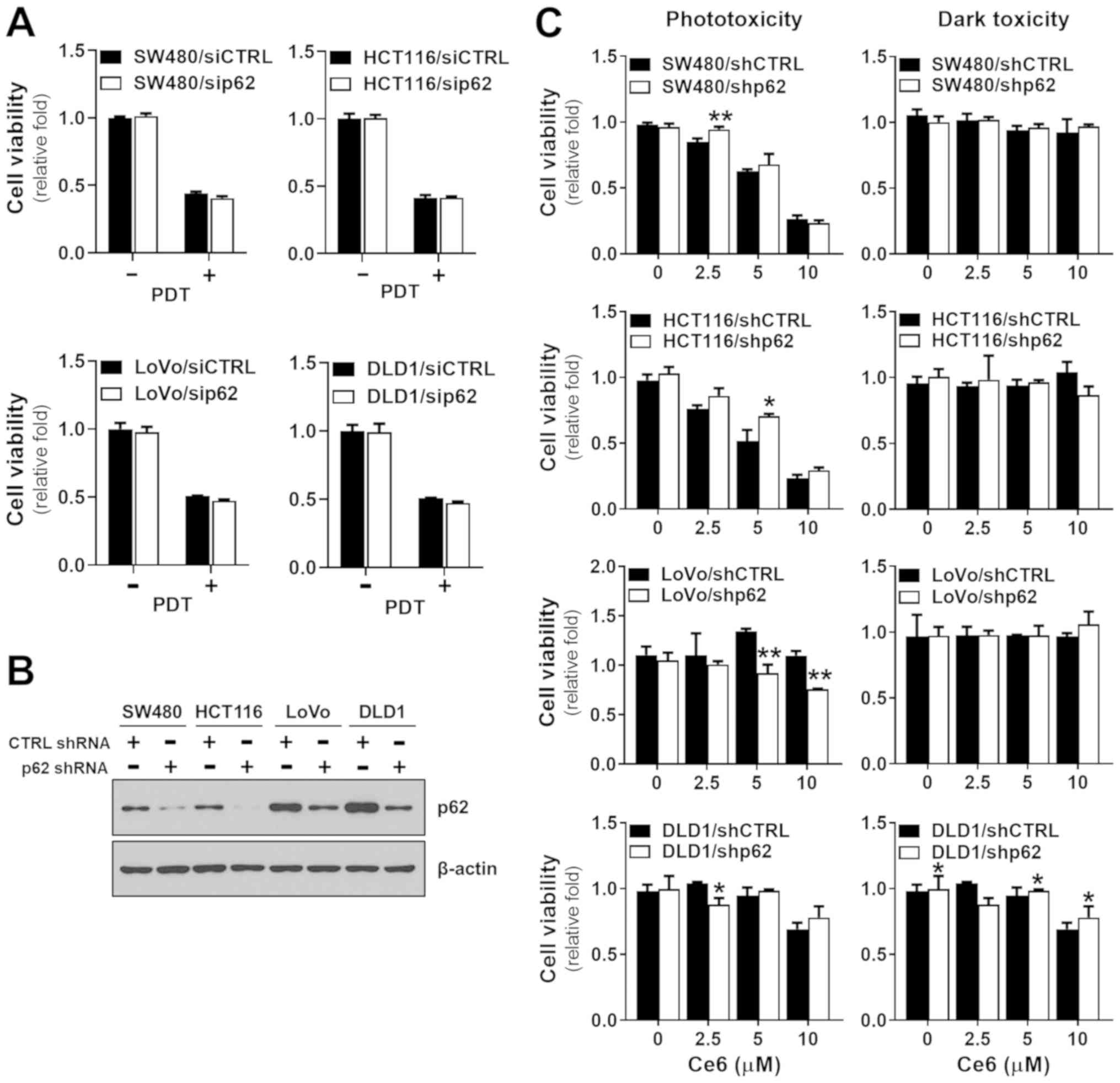
cells. We inhibited p62 gene expression using p62 siRNA before
subjecting the cells to PDT. The effect of p62 silencing in SW480,
HCT116, LoVo, and DLD1 cells was assayed by immunoblotting with
anti-p62 antibody (Fig. S2). There
were no differences in the effects of PDT between control siRNA
cells and p62 siRNA-treated colorectal cancer cells (Fig. 2A). To confirm this result, we further
examined p62 knockdown cell lines using shRNA to knock down p62,
which was verified by western blotting (Fig. 2B) before shp62 cells were subjected
to PDT. The viability of SW480 and HCT116 cells was slightly
decreased following p62 protein expression by shp62 (Fig. 2C). The effect of PDT on HCT116 cells
was significantly reduced at 5 µM Ce6. However, LoVo and DLD1
cells, which showed relatively high levels of p62 at baseline and
which remained high after inhibition, showed higher susceptibility
to PDT. Overall, when p62 was low at baseline and p62 expression
was strongly inhibited, the effect of PDT was decreased, whereas
when p62 was high at baseline and p62 expression was only slightly
inhibited, the effect of PDT was increased, suggesting that the p62
expression level is an important factor determining PDT efficacy in
colorectal cancer cells.
p62 overexpression elevates PDT
efficacy in colorectal cancer cells
We tested whether the p62 expression level affects
PDT, as the data presented above suggested that inhibition of p62
expression had no effects on PDT of colorectal cancer cells. To
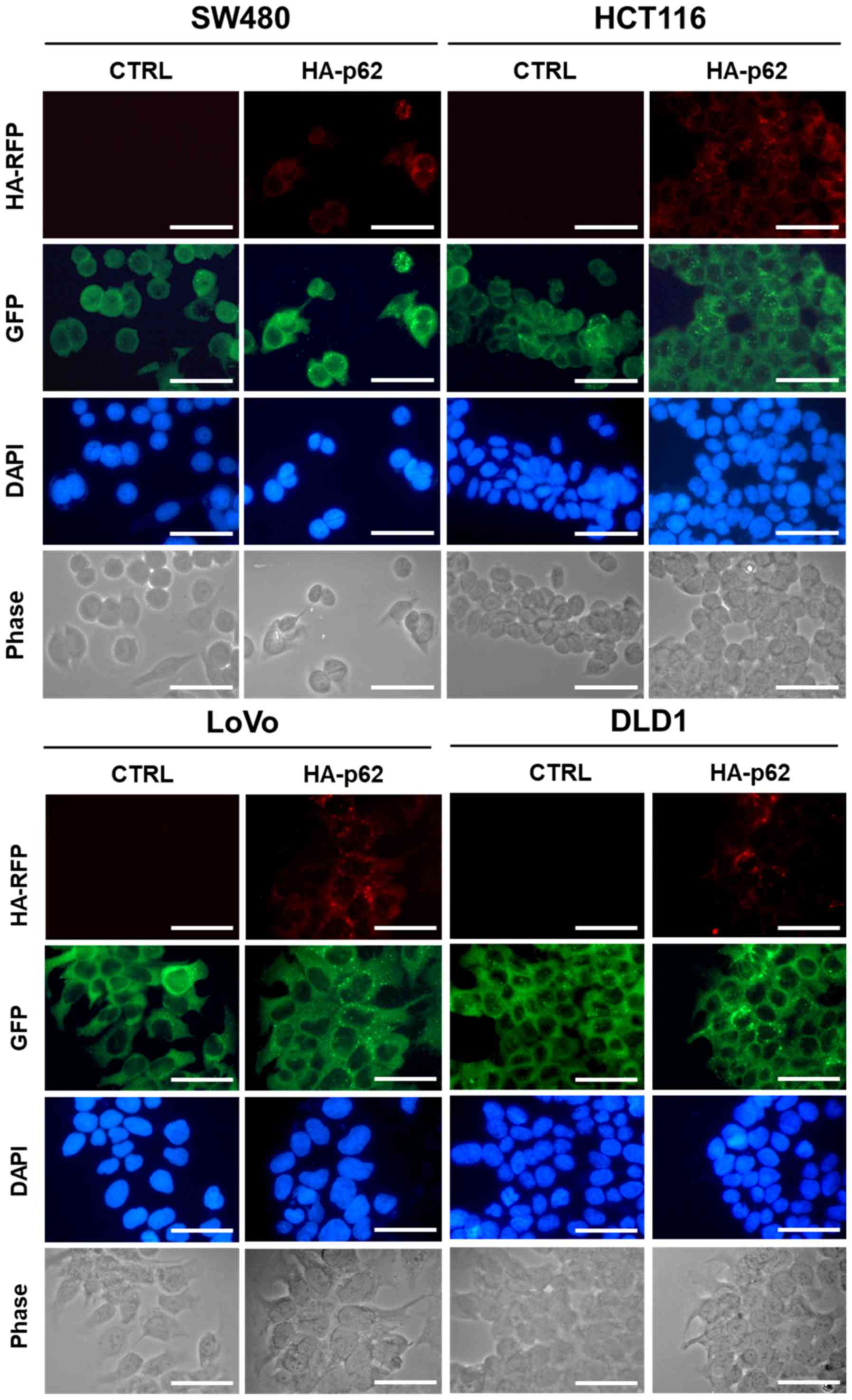
confirm the intracellular expression level of p62, we also
conducted immunocytochemistry analysis using anti-p62 antibody and
anti-HA antibody to crosscheck the HA-tagged p62 expression level.
Immunocytochemistry analysis showed that p62 was expressed mainly
in the cytosol (Fig. 3). To confirm
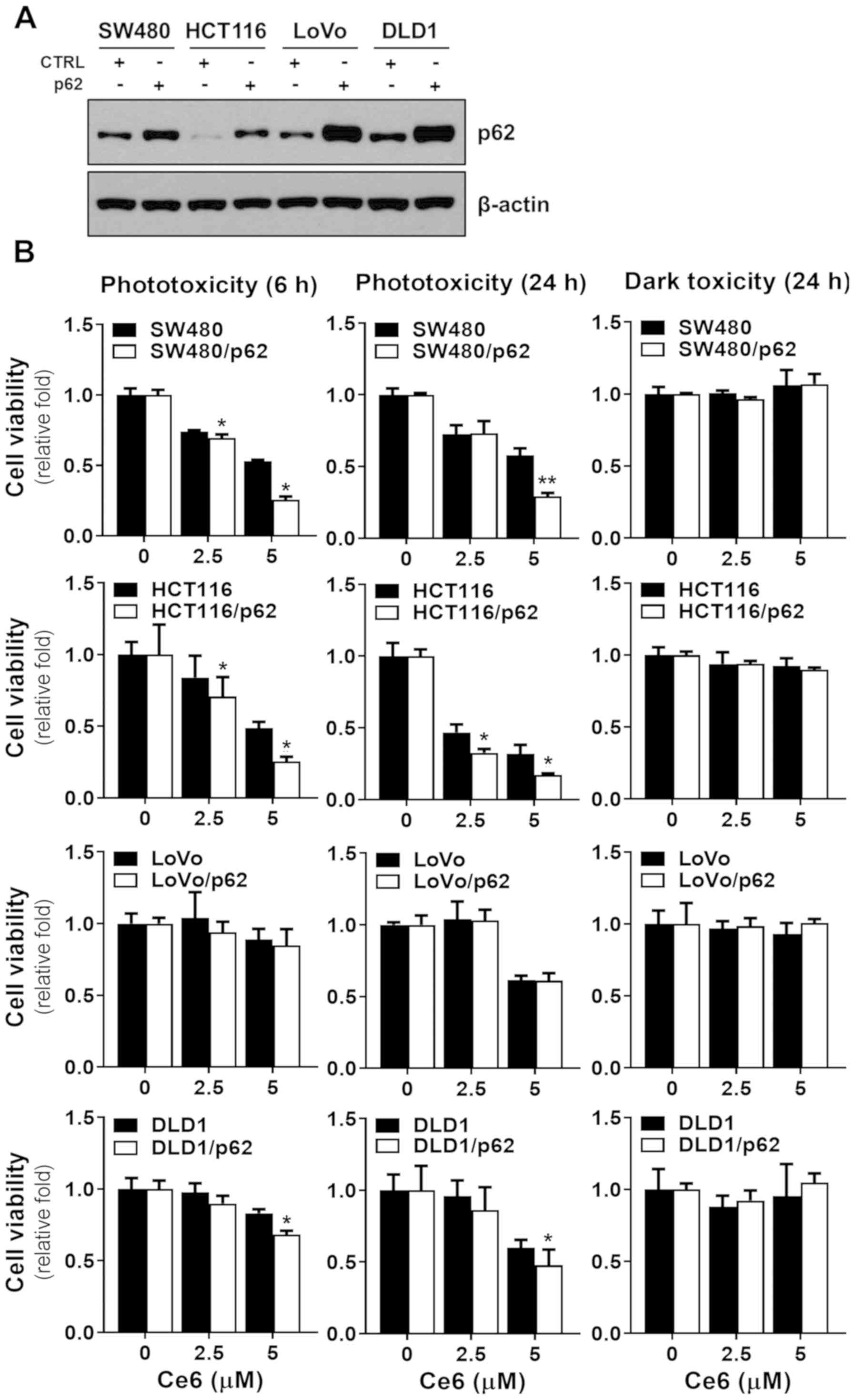
these observations, we overexpressed p62 in SW480, HCT116, LoVo,
and DLD1 cells. The expression levels of p62 in SW480, HCT116,
LoVo, and DLD1 cells were checked by immunoblot analysis along with
anti-p62 antibody (Fig. 4A). Next,
we checked whether the PDT effect depended on p62 overexpression.
Cells were incubated with Ce6 for 6 h and then irradiated with 4
J/cm2 red light (670 nm wavelength), and then cell
survival rate was measured after 6 h or 24 h. There was no dark
toxicity in which only Ce6 was treated without laser irradiation in
all colorectal cancer cell lines (Fig.
4B). The cell lines showed relatively high expression level of
p62. There was no difference in the PDT effect between the DLD1 and
LoVo cell lines overexpressing p62 (Fig.
4B). In contrast, overexpression of p62 in colorectal cancer
cells with normally low expression of p62 showed increased PDT
effect (Fig. 4B). There was no
significant difference in cell viability due to PDT between 6 h and
24 h after irradiation. To confirm the effect of p62 on PDT
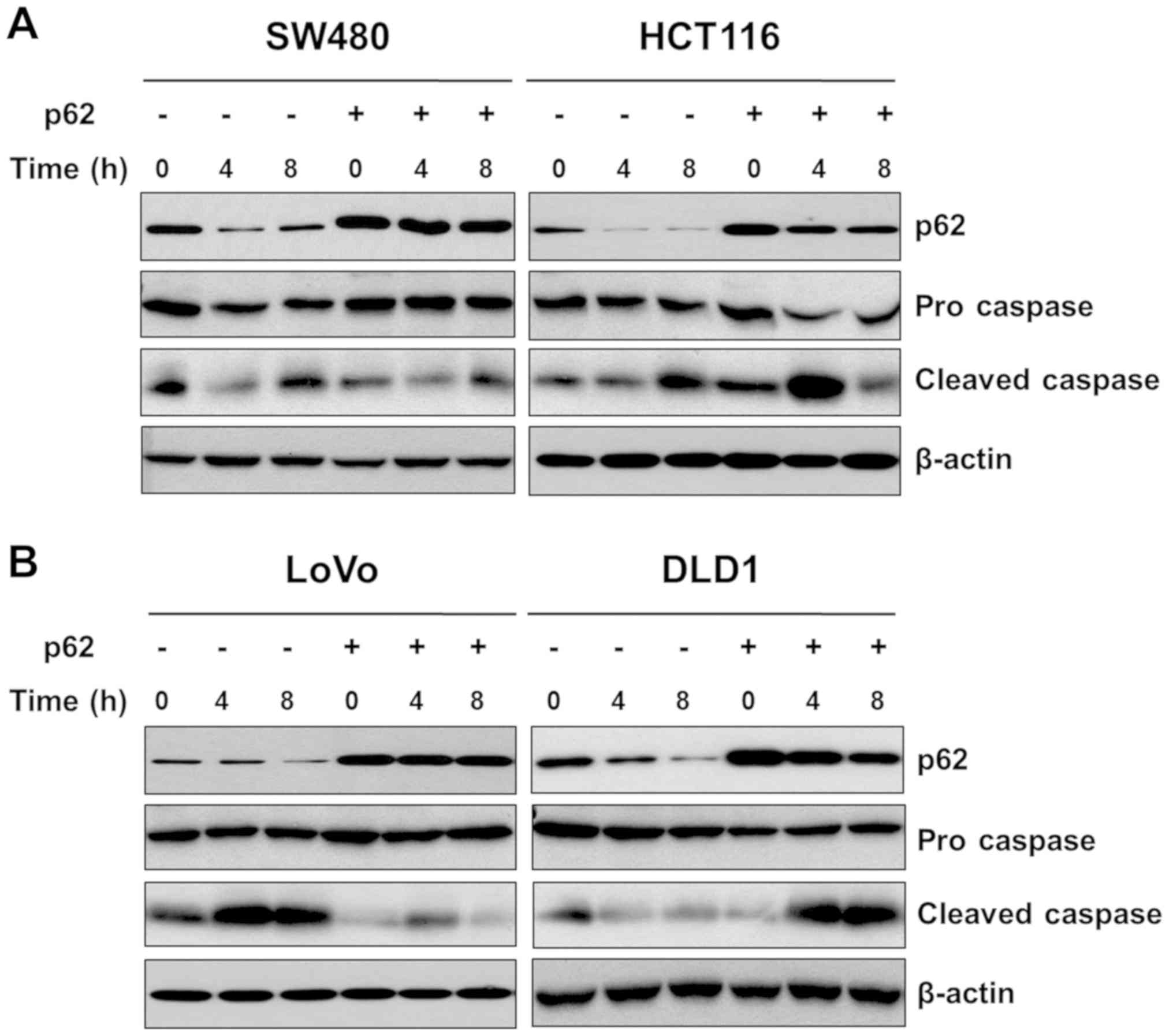
efficacy, we evaluated cleaved caspase-3, a marker of apoptosis.
SW480 and HCT116 cells with low p62 expression showed a higher rate
of apoptosis compared to cells overexpressing p62 (Fig. 5A). In the MTT assay, LoVo cells
showed the same PDT effect regardless of the level of p62
overexpression (Fig. 4B), and the
apoptosis marker had no effect on p62 overexpression (Fig. 5B). These data suggest that p62
overexpression in colorectal cancer cell is be directly related to
PDT efficacy, that p62 plays a role in inducing a higher rate of
cancer cell death.
Variation of other drug effects by p62
overexpression
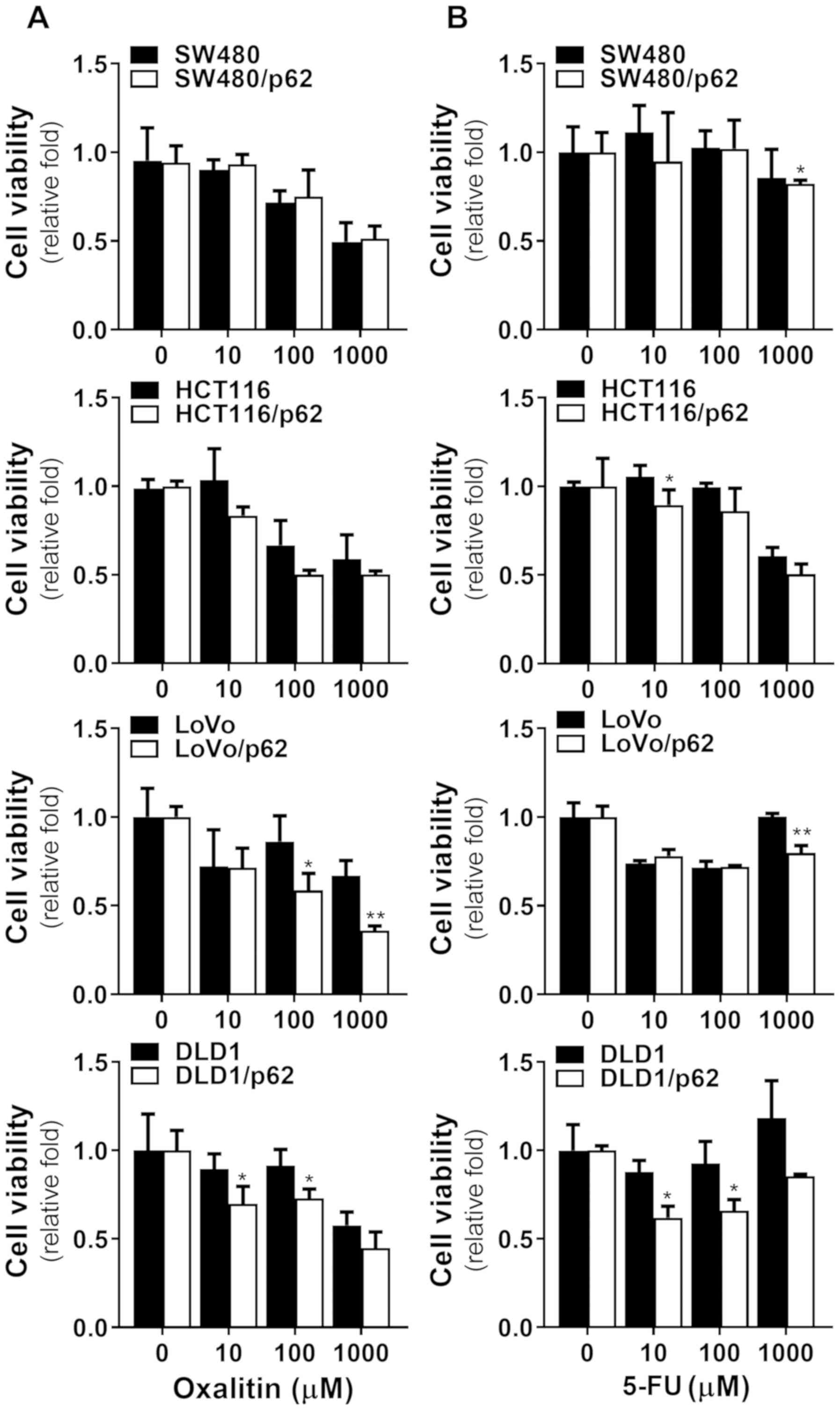
To confirm whether other therapeutic drugs were also
affected by p62 overexpression, we tested oxalitin (Fig. 6A) and 5-FU (Fig. 6B), representative drugs used in
colorectal cancer, on colorectal cancer cell lines overexpressing
p62. When treated with conventional compounds, most tested cell
lines showed no significantly increased toxicity associated with
p62 overexpression compared to PDT. However, the effects of
oxalitin and 5-FU were significantly different in the DLD1 and LoVo
cell lines. This suggests that PDT acts on different cell death
pathways and that p62 overexpression increases the effect of PDT,
but not those of other drugs.
Antitumor effect of p62 overexpression
in PDT
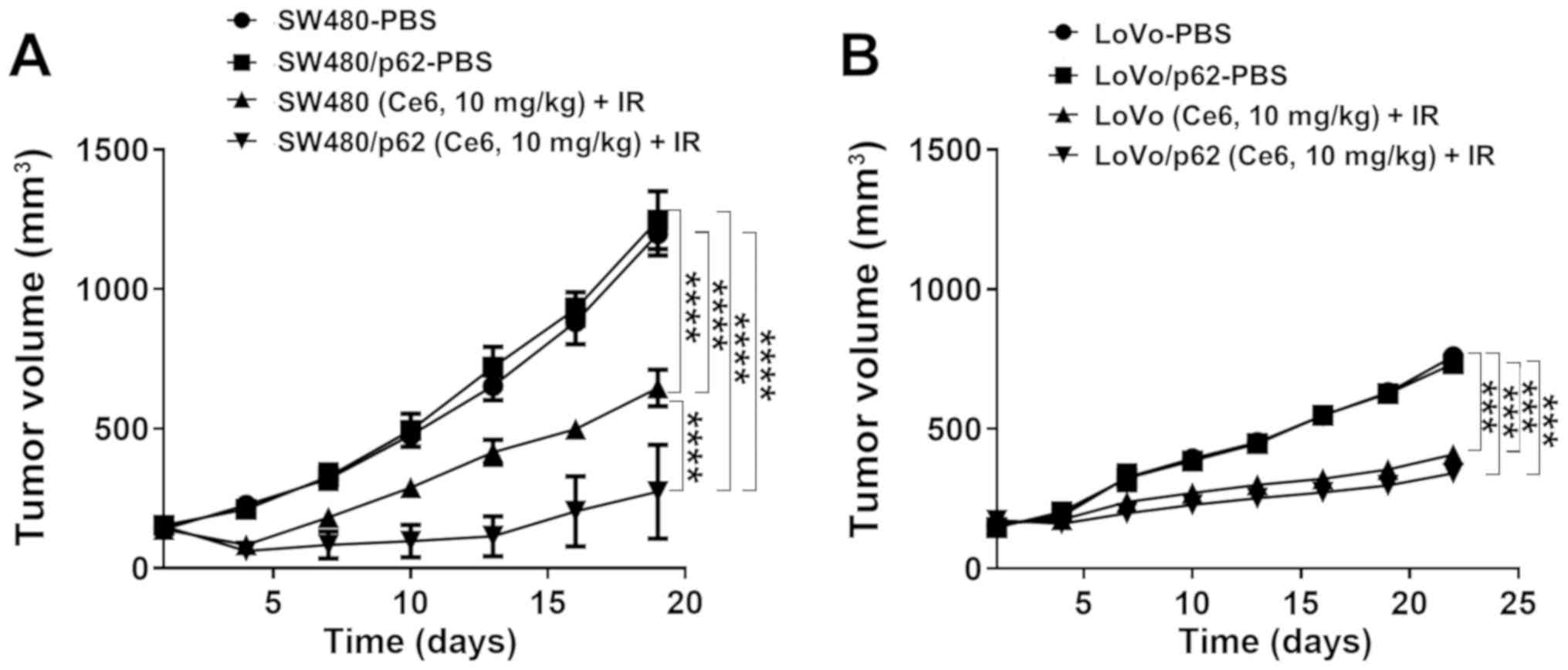
To test the effects of p62 overexpression-dependent
PDT on tumors in vivo, PDT was applied to xenograft tumor
models created by subcutaneous injection of colorectal cancer cells
(SW480/SW480-p62, LoVo/LoVo-p62). When the tumor size reached
100–200 mm3, 1.25 mg/kg Ce6 was administered to the
tumors, and Ce6 was applied 6 h later followed by irradiation with
a 670-nm diode light (150 J/cm2). There was no
significant difference in tumorigenesis between SW480-p62 and SW480
cells. However, when PDT was applied, SW480-p62 cells showed a
greater reduction in tumor size (Figs.
7A and S3). LoVo-injected
tumors showed no difference between p62 overexpression and
tumorigenesis. After PDT, the tumor size of LoVo-p62 cells was
greater than that of LoVo cells. However, the reduction rate was
lower than that of SW480 (Figs. 7B
and S3), suggesting that the level
of p62 overexpression affects PDT in vivo.
Discussion
We investigated whether elevated p62 expression
significantly affects PDT efficacy. We also found that other
chemotherapy treatments showed increased efficacy in cell lines
overexpressing p62.
Previous studies suggested that p62 plays a role in
tumor progression, with abnormal accumulation of p62 increasing the
rate of tumorigenesis (26).
Moreover, p62 has been identified as an effective substrate and
regulator of autophagy (20). In
PDT, simultaneously produced ROS induces autophagy and an apoptosis
pathways in cancer cells (8).
Combination therapy that includes an autophagy inhibitor increases
the anticancer effect of PDT (28).
p62 overexpression increased the therapeutic effect of PDT in in
vitro and in vivo models. Furthermore, p62-knockdown
cells, created by using shRNA, were slightly less susceptible to
PDT. These data demonstrate that sufficient p62 expression is
associated with the antitumor effect of PDT. Some previous studies
reported that p62-deficient cells showed reduced aggregate
formation with attenuated ROS levels, reduced apoptosis, and
improved survival after PDT (29,30). In
tumor promotion, p62 has been associated with cancer therapy
resistance, particularly, resistance to platinum-typed chemotherapy
reagents (31). However, the p62
expression level is related to cisplatin-resistance, and
insufficient p62 degradation leads to resistance to cell death in
ovarian carcinoma (26). Most
studies reported that autophagy increases tumor survival. PDT
studies with porphyrin IX in HCT116 colon cancer cells reported
that inhibition of autophagy was effective for PDT antitumor
effects (32). In a study of
sinoporphyrin sodium mediated PDT and photosan-II mediated PDT
applied to human colorectal cancer cells, autophagy inhibition
increased the efficacy of PDT (33,34). In
our study, overexpression of p62, which plays an important role in
autophagy, increased the effect of PDT, conflicting with previously
reported studies. However, it is unclear how p62 is associated with
autophagy in the tumor environment caused by PDT. As we observed
early effects after PDT, it is difficult to explain the association
with autophagy, which is a limitation of this study and requires
further analysis.
This study showed that the antitumor effect of
platinum-based colorectal cancer chemotherapy with oxalitin and
chemotherapy with 5-FU was improved when the expression of p62 was
increased. PDT affects cancer cells by generating excess ROS, which
is performed by a photosensitizer (29). In all cancer treatment methods, cell
resistance limits cancer therapy efficacy and effectiveness.
Resistance in PDT consists of inducing drug efflux on the cell
surface and creating an internal resistance system (35).
Overall, p62 is an important substrate for autophagy
and plays a role in tumor-genesis by maintaining homeostasis of the
cancer cell microenvironment (36).
We predicted that low expression of p62 would increase PDT
efficacy. However, the results indicated that overexpression of p62
increased the effect of PDT. In most studies, inhibition of p62 has
been reported to enhance antitumor effects; however, our findings
suggest that over-expression of p62 would be more effective.
Moreover, deletion of p62 in some colorectal cancer cells reduced
their PDT susceptibility. Further studies of the effect of p62
expression on antitumor mechanisms are needed. Our results suggest
that p62 is an important substance in antitumor effect processes
and is a suitable candidate as a therapeutic target.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by The Basic Science
Research Program through The National Research Foundation of Korea
(NRF) funded by The Ministry of Education, Science and Technology
(grant nos. NRF-2017R1A2B4011122 and NRF-2019R1C1C1006898).
Availability of data and materials
All datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
JHK and IWK conceived and designed the study, and
wrote the manuscript JHK and IWK performed the experiments. JHK
collected and analyzed the data. IWK interpreted the data and
reviewed the manuscript. Both authors have read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
The present animal care and use protocol was
reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) in College of Medicine, Hanyang University of
Korea (approval no. 2019-0043A).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mármol I, Sánchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal Carcinoma: A
General Overview and Future Perspectives in Colorectal Cancer. Int
J Mol Sci. 18:182017. View Article : Google Scholar
|
|
2
|
Kawczyk-Krupka A, Bugaj AM, Latos W,
Zaremba K, Wawrzyniec K and Sieroń A: Photodynamic therapy in
colorectal cancer treatment: The state of the art in clinical
trials. Photodiagn Photodyn Ther. 12:545–553. 2015. View Article : Google Scholar
|
|
3
|
Shishkova N, Kuznetsova O and Berezov T:
Photodynamic therapy in gastroenterology. J Gastrointest Cancer.
44:251–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barr H, MacRobert AJ, Tralau CJ, Boulos PB
and Bown SG: The significance of the nature of the photosensitizer
for photodynamic therapy: Quantitative and biological studies in
the colon. Br J Cancer. 62:730–735. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawczyk-Krupka A, Kwiatek B, Czuba ZP,
Mertas A, Latos W, Verwanger T, Krammer B and Sieroń A: Secretion
of the angiogenic factor VEGF after photodynamic therapy with ALA
under hypoxia-like conditions in colon cancer cells. Photodiagn
Photodyn Ther. 21:16–18. 2018. View Article : Google Scholar
|
|
6
|
Halaburková A, Jendželovský R, Kovaľ J,
Herceg Z, Fedoročko P and Ghantous A: Histone deacetylase
inhibitors potentiate photodynamic therapy in colon cancer cells
marked by chromatin-mediated epigenetic regulation of CDKN1A. Clin
Epigenetics. 9:622017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dolmans DE, Fukumura D and Jain RK:
Photodynamic therapy for cancer. Nat Rev Cancer. 3:380–387. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy - mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mroz P, Yaroslavsky A, Kharkwal GB and
Hamblin MR: Cell death pathways in photodynamic therapy of cancer.
Cancers (Basel). 3:2516–2539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Broekgaarden M, Weijer R, van Gulik TM,
Hamblin MR and Heger M: Tumor cell survival pathways activated by
photodynamic therapy: A molecular basis for pharmacological
inhibition strategies. Cancer Metastasis Rev. 34:643–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orenstein A, Kostenich G, Roitman L,
Shechtman Y, Kopolovic Y, Ehrenberg B and Malik Z: A comparative
study of tissue distribution and photodynamic therapy selectivity
of chlorin e6, Photofrin II and ALA-induced protoporphyrin IX in a
colon carcinoma model. Br J Cancer. 73:937–944. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodríguez ME, Catrinacio C, Ropolo A,
Rivarola VA and Vaccaro MI: A novel HIF-1α/VMP1-autophagic pathway
induces resistance to photodynamic therapy in colon cancer cells.
Photochem Photobiol Sci. 16:1631–1642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain A, Lamark T, Sjøttem E, Larsen KB,
Awuh JA, Øvervatn A, McMahon M, Hayes JD and Johansen T: p62/SQSTM1
is a target gene for transcription factor NRF2 and creates a
positive feedback loop by inducing antioxidant response
element-driven gene transcription. J Biol Chem. 285:22576–22591.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu
HL, Yang C and Liu HF: p62 links the autophagy pathway and the
ubiqutin-proteasome system upon ubiquitinated protein degradation.
Cell Mol Biol Lett. 21:292016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rusten TE and Stenmark H: p62, an
autophagy hero or culprit? Nat Cell Biol. 12:207–209. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moscat J and Diaz-Meco MT: p62 at the
crossroads of autophagy, apoptosis, and cancer. Cell.
137:1001–1004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Islam MA, Sooro MA and Zhang P: Autophagic
Regulation of p62 is Critical for Cancer Therapy. Int J Mol Sci.
19:192018. View Article : Google Scholar
|
|
23
|
Li SS, Xu LZ, Zhou W, Yao S, Wang CL, Xia
JL, Wang HF, Kamran M, Xue XY, Dong L, et al: p62/SQSTM1 interacts
with vimentin to enhance breast cancer metastasis. Carcinogenesis.
38:1092–1103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Battista RA, Resnati M, Facchi C, Ruggieri
E, Cremasco F, Paradiso F, Orfanelli U, Giordano L, Bussi M, Cenci
S, et al: Autophagy mediates epithelial cancer chemoresistance by
reducing p62/SQSTM1 accumulation. PLoS One. 13:e02016212018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuma A, Komatsu M and Mizushima N:
Autophagy-monitoring and autophagy-deficient mice. Autophagy.
13:1619–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwadate R, Inoue J, Tsuda H, Takano M,
Furuya K, Hirasawa A, Aoki D and Inazawa J: High Expression of
SQSTM1/p62 Protein Is Associated with Poor Prognosis in Epithelial
Ovarian Cancer. Acta Histochem Cytochem. 47:295–301. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The Cancer Cell Line Encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei MF, Chen MW, Chen KC, Lou PJ, Lin SY,
Hung SC, Hsiao M, Yao CJ and Shieh MJ: Autophagy promotes
resistance to photodynamic therapy-induced apoptosis selectively in
colorectal cancer stem-like cells. Autophagy. 10:1179–1192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Agostinis P, Berg K, Cengel KA, Foster TH,
Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel
D, et al: Photodynamic therapy of cancer: An update. CA Cancer J
Clin. 61:250–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubio N, Verrax J, Dewaele M, Verfaillie
T, Johansen T, Piette J and Agostinis P: p38(MAPK)-regulated
induction of p62 and NBR1 after photodynamic therapy promotes
autophagic clearance of ubiquitin aggregates and reduces reactive
oxygen species levels by supporting Nrf2-antioxidant signaling.
Free Radic Biol Med. 67:292–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan XY, Zhang Y, Zhang JJ, Zhang LC, Liu
YN, Wu Y, Xue YN, Lu SY, Su J and Sun LK: p62/SQSTM1 as an
oncotarget mediates cisplatin resistance through activating
RIP1-NF-κB pathway in human ovarian cancer cells. Cancer Sci.
108:1405–1413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ouyang G, Xiong L, Liu Z, Lam B, Bui B, Ma
L, Chen X, Zhou P, Wang K, Zhang Z, et al: Inhibition of autophagy
potentiates the apoptosis-inducing effects of photodynamic therapy
on human colon cancer cells. Photodiagn Photodyn Ther. 21:396–403.
2018. View Article : Google Scholar
|
|
33
|
Xiong L, Liu Z, Ouyang G, Lin L, Huang H,
Kang H, Chen W, Miao X and Wen Y: Autophagy inhibition enhances
photocytotoxicity of Photosan-II in human colorectal cancer cells.
Oncotarget. 8:6419–6432. 2017.PubMed/NCBI
|
|
34
|
Zhu B, Li S, Yu L, Hu W, Sheng D, Hou J,
Zhao N, Hou X, Wu Y, Han Z, et al: Inhibition of Autophagy with
Chloroquine Enhanced Sinoporphyrin Sodium Mediated Photodynamic
Therapy-induced Apoptosis in Human Colorectal Cancer Cells. Int J
Biol Sci. 15:12–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olsen CE, Weyergang A, Edwards VT, Berg K,
Brech A, Weisheit S, Høgset A and Selbo PK: Development of
resistance to photodynamic therapy (PDT) in human breast cancer
cells is photosensitizer-dependent: Possible mechanisms and
approaches for overcoming PDT-resistance. Biochem Pharmacol.
144:63–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reina-Campos M, Shelton PM, Diaz-Meco MT
and Moscat J: Metabolic reprogramming of the tumor microenvironment
by p62 and its partners. Biochim Biophys Acta Rev Cancer.
1870:88–95. 2018. View Article : Google Scholar : PubMed/NCBI
|