Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor
that has a high incidence in southern China, with an annual
incidence rate of nearly 30/100,000 (1). A total of >70% of newly diagnosed
NPC patients are classified as having locally advanced disease
(2). With the advent of concurrent
chemotherapy, intensity-modulated radiation therapy (IMRT) and
imaging techniques, local control has been significantly improved,
and distant metastasis is the main cause of treatment failure in
NPC (3). Although some biomarkers
were found for evaluating the prognosis of recurrent NPC, the
overall survival rate of patients has not improved and the 5-year
survival rate is only 30% (4,5), This
makes the treatment of recurrent NPC a major clinical challenge
(6,7). Therefore, there is an urgent need to
find reliable prognostic markers and effective treatments.
As distant metastasis is a major obstacle for NPC
treatment, identifying NPC specific metastasis biomarkers is
important for NPC prognosis and predictive treatment. A serum
biomarker is the most convenient biomarker for detecting cancer and
provides prognostic value for cancer diagnosis, treatment and
management. Compared with imaging techniques, serum biomarkers are
easier and cheaper for patients (8,9). In the
past two decades, neutrophil gelatinase-associated lipocalin (NGAL)
has received widespread clinical attention as a biomarker for
kidney damage, cardiovascular damage and cancer (10–12).
NGAL, also known as lipocalin-2 (lcn2), is a 24 kDa glycoprotein in
humans encoded by the lcn2 gene located at position 3P11 of
chromosome 9. In recent years, it has become a biomarker for some
benign and malignant diseases (13–17). The
effect of NGAL in carcinogenesis is dependent on cancer type.
Upregulation of NGAL increases cell infiltration in breast,
bladder, stomach, gynecological, thyroid, lung, esophageal, colon
and chronic myeloid leukemia; but in pancreatic and oral cancer, it
reduces cell infiltration (18,19). In
addition, upregulation of NGAL can increase the proliferation of
cervical cancer and lung cancer cells (20,21).
NGAL is a well-known regulatory factor controlling epithelial
mesenchymal transition (EMT), invasion and migration.
Overexpression of NGAL activates snails, neural-cadherin,
fibronectin, matrix metalloproteinase (MMP)-9, nuclear factor-κB
and other pathways, which in turn upregulates genes involved in
stem cells, adhesion, and drug outflow (22–24).
Similarly, NGAL silencing reduced migration and invasion by
vimentin, MMP-2, and MMP-9, and increased epithelial (E)-cadherin
expression (25). These findings
suggest that NGAL plays a key role in the development and
progression of cancer. Recent studies (26–29) have
indicated that NGAL may have pro-oncogenic or anti-oncogenic
functions. In fact, its oncogenic effect is related to the complex
NGAL/MMP-9; while its anti-tumor effect is related to the
inhibition of the pro-neoplastic factor hypoxia inducible factor
(HIF)-1a, the HIF-1a-dependent vascular endothelial growth factor
and FAK (20). Its role in each
cancer type is dependent on the different tumor microenvironment
and different signaling pathway activation in cancer types.
However, the role of NGAL in NPC has not been well confirmed and
its expression and role in different stages of development of NPC
have not been studied in detail (30,31).
Therefore, studying the relationship between the expression of NGAL
and the clinical parameters of NPC aids understanding of whether
NGAL can be used as a biomarker for the diagnosis and prognosis of
NPC.
In this study, the expression of NGAL at different
stages of NPC was examined. In addition, through exogenous NGAL
transfection, the role of NGAL in the development, proliferation,
invasion, migration, EMT and other developmental processes of NPC
was investigated.
Materials and methods
Patients
The present study was approved by the Independent
Ethics Committee of the General Hospital of Tianjin Medical
University. Before analysis, consent from each patient was
received.
In this study, 209 NPC patients were sampled from
March 2012 to May 2016 at the Tianjin Medical University General
Hospital. Patients were selected according to the following
criteria: i) Histologically proven locally advanced NPC with biopsy
specimens; ii) Karnofsky score (>70); iii) concurrent
chemotherapy based on basic IMRT and cisplatin at the time of
initial diagnosis; iv) no malignant tumors or other complications
in the past; and v) NGAL staining could be detected in tumor
tissues. As a result, 158 patients qualified for this study. All
patients were staged using the American Joint Cancer Commission
2010 staging system. The clinical features are listed in Table I. All registered patients received a
similar treatment strategy, i.e., IMRT combined with
cisplatin-based chemotherapy. Biopsy specimens were obtained by
nasal endoscopy for pathological analysis.
 | Table I.Patient characteristics and
significance of neutrophil gelatinase-associated lipocalin
expression in clinical parameters. |
Table I.
Patient characteristics and
significance of neutrophil gelatinase-associated lipocalin
expression in clinical parameters.
|
Characteristics | High group
(n=54) | Low group
(n=104) | P-value |
|---|
| Sex |
|
| 0.738 |
|
Male | 29 | 59 |
|
| Female | 25 | 45 |
|
| Age |
|
| 0.727 |
|
≥50 | 21 | 38 |
|
|
<50 | 33 | 66 |
|
| BMI
(kg/m2) |
|
| 0.735 |
|
≥23 | 23 | 41 |
|
|
<23 | 31 | 63 |
|
| WHO histological
type |
|
| 0.241 |
|
Differentiated | 34 | 55 |
|
|
Undifferentiated | 20 | 49 |
|
| EBV infection |
|
| 0.388 |
|
Positive | 32 | 69 |
|
|
Negative | 22 | 35 |
|
| T
classification |
|
| 0.006 |
|
T1+T2 | 30 | 33 |
|
|
T3+T4 | 24 | 71 |
|
| Lymph node
metastasis |
|
| <0.001 |
|
Absent | 35 | 35 |
|
|
Present | 19 | 69 |
|
| Distant
metastasis |
|
| 0.01 |
|
Absent | 23 | 73 |
|
|
Present | 31 | 31 |
|
| Overall stage |
|
| 0.007 |
|
I+II | 39 | 29 |
|
|
III+IV | 15 | 75 |
|
Immunohistochemistry (IHC)
The expression of NGAL was determined by
immunohistochemical analysis. IHC kits (Cell Signaling
Technologies, Inc.) were used according to the manufacturer's
protocol. Monoclonal antibodies against hNGAL were purchased from
Abcam (cat. no. ab23477). Tissue sections were paraffinized and
rehydrated with xylene and ethanol, and sealed with 3% hydrogen
peroxide methanol solution for 30 min. After antigen repair, the
sections were incubated in a closed solution for 30 min at 4°C and
then incubated overnight with the first antibody (1:100 dilution)
at 4°C. The next day, the sections were incubated with the second
antibody at room temperature for 1 h and then stained with DAB and
hematoxylin at room temperature for 10 min (32).
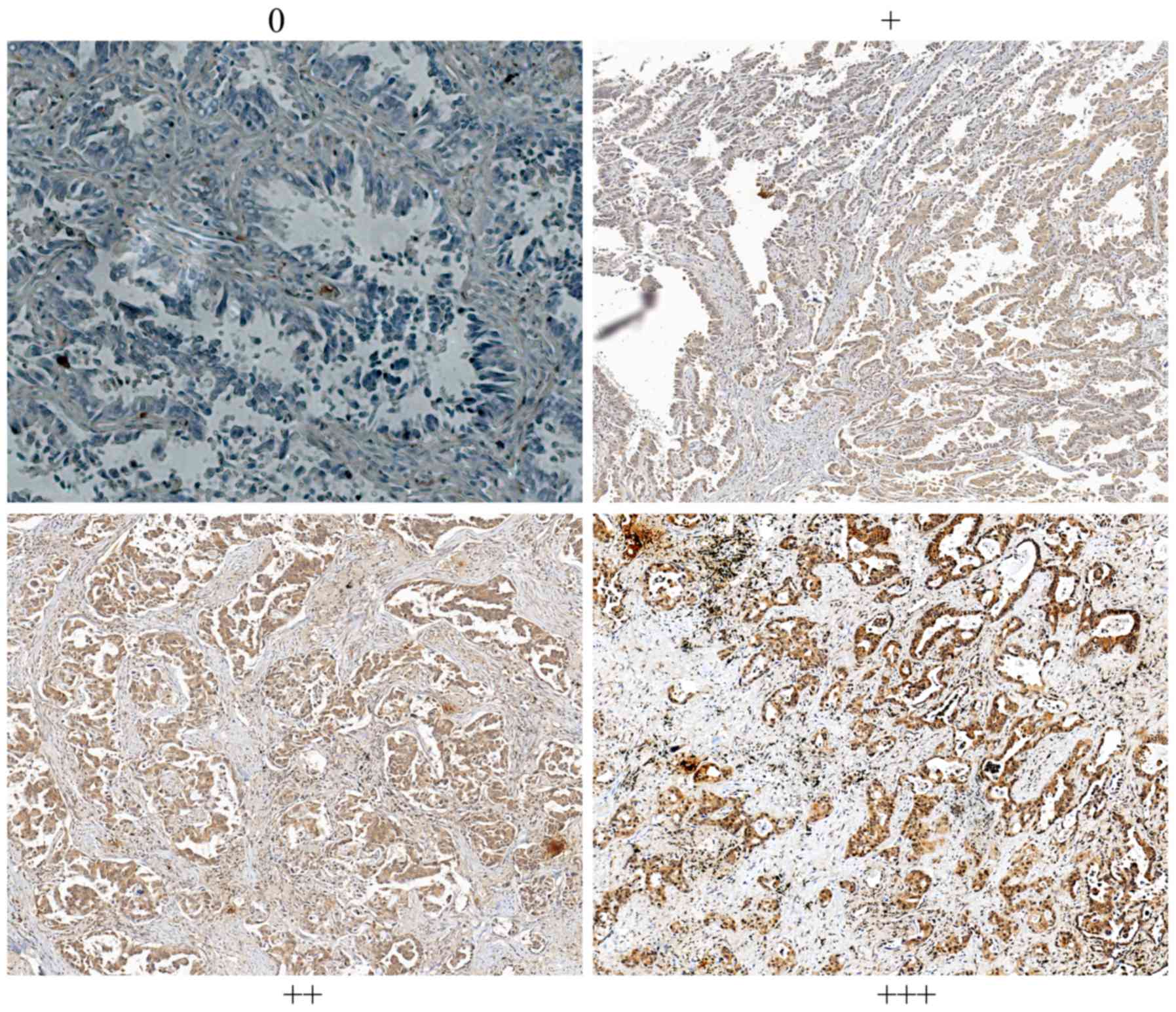
Scoring. All slides were observed under Nikon
Eclipse Ti-E automatic inverted light microscope and the
immunoreactivity of NGAL was examined. The staining intensity was
graded from 0 to 3+ (0 for non-staining; 1+ for weak
immunoreactivity; 2+ for moderate immunoreactivity; and 3+ for
strong immunoreactivity). Scale numbers 0 and 1 were considered to
indicate low expression, while 2+ and 3+ were considered to
indicate high expression. All the images were captured under ×200
magnification.
Cell culture and chemical agents
NPC cancer cell lines C666, HNE-3, CG1 and C666-1
were obtained from the Type Culture Collection of the Chinese
Academy of Sciences. All the cells were cultured in RPMI-1640
(Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin and 100 µg/ml streptomycin, all cells were cultured in
37°C incubator containing 5% CO2. When growth in
logarithmic phase, cells were seeded on 96-well plates for further
study.
The mRNA level of NGAL in each cell line was tested
by reverse transcription-quantitative (RT-q) PCR. In short,
according to the manufacturer's protocol, total RNA was extracted
from cells using TRIzol (Thermo Fisher Scientific, Inc.). ReverTra
Ace PCR RT kit (Toyobo Life Science) was used to retrieve the RNA
(10 µg) from each group and obtain the corresponding cDNA at 4°C.
THUNDER RBIRD q-PCR Mix (Toyobo Life Science) was used to carry out
RT-PCR on the ABI Prism 7900 Sequence Detection System and Cq was
standardized to maintain the signal of GAPDH gene. The fold-change
of expression was calculated as 2ΔCq (Treated-Untreated) (33). The primer for GAPDH was
5-AAACAGAAGGCAGCTTTACGATG-3 and 5-AAATGTTCTGATCCAGTAGCG-3. For
NGAL, the sense primer was 5-TCCCAGAGCTGAACGG-3 and anti-sense
primer was 5-GAAGTCGCGGAGACA-3. The qPCR cycle conditions were: One
cycle of 95°C for 30 sec, 40 cycles of 95°C for 15 sec, 58°C for 30
sec and 72°C for 30 sec.
Exogenous expression of NGAL
Exogenous expression using PC-DNA3.1-NGAL plasmid
vector (cat. no. V79020; Invitrogen; Thermo Fisher Scientific,
Inc.) was carried out in the C666 and HNE-3 cell line. Human full
length NGAL was cloned and empty vector PCDNA3.1 was also
transfected as a control. Briefly, cells were seeded at a
concentration of 2.5×104 cells /well in 1 ml medium in a
24-well plate. The next day, cells were transfected with PC-DNA3.1
control and PC-DNA3.1-NGAL plasmids (2 µg DNA) using Lipofectamine
3000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). When
fresh DMEM (Gibco; Thermo Fisher Scientific, Inc.) was replaced
with medium containing transfection reagent, the cells were allowed
to recover for 24 h. Then puromycin (1 µg/ml) was used to select
cells and establish a stable NGAL transfected clone. Stable
transfected cells were used for a proliferation assay, wound
healing assay and migration assay. Transient transfected cells were
used to analyze apoptosis and EMT.
Cell viability
In short, 2×103 cells/well were
inoculated into 96-well plates, six replicate wells were incubated
for 72 h. After 72 h, 10 µl MTT (5 mg/m; cat. no. M2128,
Sigma-Aldrich; Merck KGaA) was added to the cells and further
cultured. This incubation lasted for 2 h at 37°C. The MTT solution
was removed and then 100 µl DMSO (Merck KGaA) was added to each
well. The Infinite M200 Pro (Tecan Group Ltd.) was used to measure
absorbance at 570 nm in 1 h later (34).
Apoptosis assay by Annexin V-fluorescein
isothiocyanate (FITC) and propidium iodide (PI) staining. The
apoptosis of C666 and HNE-3 cells was quantified by double staining
of Annexin V-PI with FACscan flow cytometry (FACSCanto™ II; Becton,
Dickinson and Company). After 48 h treatment with 0.5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) PBS solution, the harvested
cells were washed with cold PBS and then suspended in 200 ml
combined buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl and 2.5 mM
CaCl2) and incubated together. A total of 5 ml Annexin
V-FITC was shielded from light for 10 min at room temperature. The
samples were washed with a buffer solution and re-suspended in PBS.
The apoptotic cells were stained with 5 μg/ml PI. The apoptotic
cells were identified by flow cytometry using FlowJo 10.0 (FlowJo
LLC). The PI has excitation maximum at 535 nm and fluorescence
emission maximum at 617 nm. The vector-transfected cells were used
as negative controls. Cells showing Annexin V-/PI+ were considered
necrotic, showing that Annexin V+/PI+ was considered to be late
apoptotic or secondary apoptotic, while Annexin V+/PI-cells were
considered to be early or primary apoptotic cells (35).
In vitro wound closure assay
The control PCDNA 3.1 and PCDNA 3.1-NGAL cells were
inoculated into 6-well plates and fused, then serum starved for 8
h. the confluent monolayer cells were scraped with the tip of a
pipette. PBS was used to wash the plate to remove non-adherent
cells and images were captured of the wounds at 0 and 24 h. The
edge of the wound was marked and the wound area was measured. Then,
the ratio of wound recovery was calculated as follows: Wound
recover ratio = [(initial wound area - wound area) / initial wound
area] ×100%.
Cell invasion assay
The serum of PCDNA 3.1 and PCDNA 3.1-NGAL cells was
starved for 18 h and then inoculated into a Transwell insert (cat.
no. 3422, Corning, Inc.) coated with matrix gel. After starvation,
the cells were treated with trypsin and inoculated in the upper
chamber of Transwell insert at the concentration of
5×104 cells. In the lower chamber, the medium containing
10% FBS was added as a chemical attractant. Then the cells were
incubated at 37°C for another 24 h. Migrating cells at the bottom
of Transwell insert were fixed in 70% ethanol for 30 min at room
temperature and stained with crystal violet solution for 30 min at
room temperature. The stained cells were observed under an inverted
light microscope (Nikon Eclipse Ti-E) and images were captured with
a Nikon 500 camera. After the image was taken, the film was
dissolved in 1% SDS (Sigma-Aldrich; Merck KGaA) solution and the
absorbance at 595 nm was read in Tecan reader at 37°C for 1 h.
Western blotting
Lysis of NPC cells (HNE-3 and C666) for protein
extraction. Protein was extracted with RIPA buffer containing 1 mM
PMSF (Sigma-Aldrich; Merck KGaA). The concentration of protein was
determined by bicinchoninic acid kit (Pierce; Thermo Fisher
Scientific, Inc.). The same amount of protein (10 µg/ lane) was
carried out by SDS gel electrophoresis (10%) for 120 min. Protein
was transferred to a polyvinylidene fluoride membrane (EMD
Millipore). 5% skimmed milk in Tris buffer saline (TBS) was used to
block the membrane at room temperature for 1 h. After blocking, the
membrane was incubated overnight at 4°C with antibodies, including
anti-caspase 3, 8 and 9, anti-BCL2, anti-vimentin, anti-smad2/3 and
anti-phosphorylated (p)-smad2/3 and anti-β-actin. All antibodies
were purchased from Abcam. The next morning, the TBS buffer
containing 0.05% Tween-20 (TBST) was used to wash the membrane and
the secondary antibody (Abcam; cat. no. ab97040; 1:3,000) linked
with horseradish peroxidase was used to detect the membrane for 1 h
at 37°C. After washing with TBST, the film was developed by
enhanced chemiluminescence kit (Yeasen BioTech; cat. no. 36222ES60)
and visualized by LAS 4000 imaging system. The intensity of target
gene bands normalized relative to the internal control β-actin was
measured and quantified by Image J software (Version 1.51; National
Institute of Health). All reactions were carried out in duplicate
and repeated to ensure consistent results (36).
Statistical analysis
Statistical analysis was performed using Graphpad
prism 6.0 (GraphPad Software, Inc.). Data were expressed as the
mean ± standard deviation and differences were evaluated by
analysis of variance and the Bonferroni post hoc test. Overall
survival (OS) was estimated using the Kaplan-Meier method.
Univariate analysis was performed using a log-rank test. The exact
test of χ2 and Fisher was used to compare the difference
between the NGAL high group and the NGAL low group. Multivariate
analysis was performed using the Cox proportional hazard model. All
statistical tests were bilateral tests. All the experiments were
repeated ≥3 times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient information
Among the 158 selected patients, 101 were male and
57 were female, with a median age of 52 (ranging from 24 to 77
years). The median body mass index (BMI) was 23.1 kg/m2
(range, 16.8–32.7 kg/m2). A total of 101 patients were
infected with Epstein-Barr virus (EBV; infection rate), the
infection rate was 63.9%. All tumors were classified as having a
non-keratinized phenotype. After a median follow-up of 60 months,
16 patients (10.1%) died and 13 patients (8.2%), 9 patients (5.7%)
and 12 patients (7.6%) developed local failure, local failure and
distant metastasis, respectively. The 5-year DFS and OS rates were
49.5 and 54.2%, respectively. Detailed patient characteristics are
shown in Table I.
Downregulation of NGAL is correlated
with poor prognosis of NPC
Both cancer cells and lymphocytes can produce NGAL,
which is a secreted protein and widely distributed in the whole
field of the tumor tissue sections. The representative staining of
NGAL in NPC is shown in Fig. 1.
Immunohistochemical results showed that all patients were divided
into the NGAL low expression group (N=104) and NGAL high expression
group (N=54), as shown in Table I.
NGAL expression was diffuse in tumor tissues. In this study, the
expression of NGAL staining was not significantly correlated with
age, sex, BMI and EBV infection clinicopathological parameters, but
was significantly correlated with clinical stage, lymph metastasis
and distant metastasis at diagnosis. Table I summarizes the detailed data,
indicating that lower expression is associated with known advanced
tumor parameters and metastasis.
The values of various potential prognostic factors
were assessed, including age, sex, BMI, overall stage and NGAL
predicting OS and DFS. The results of univariate and multivariate
analysis are shown in Table II. The
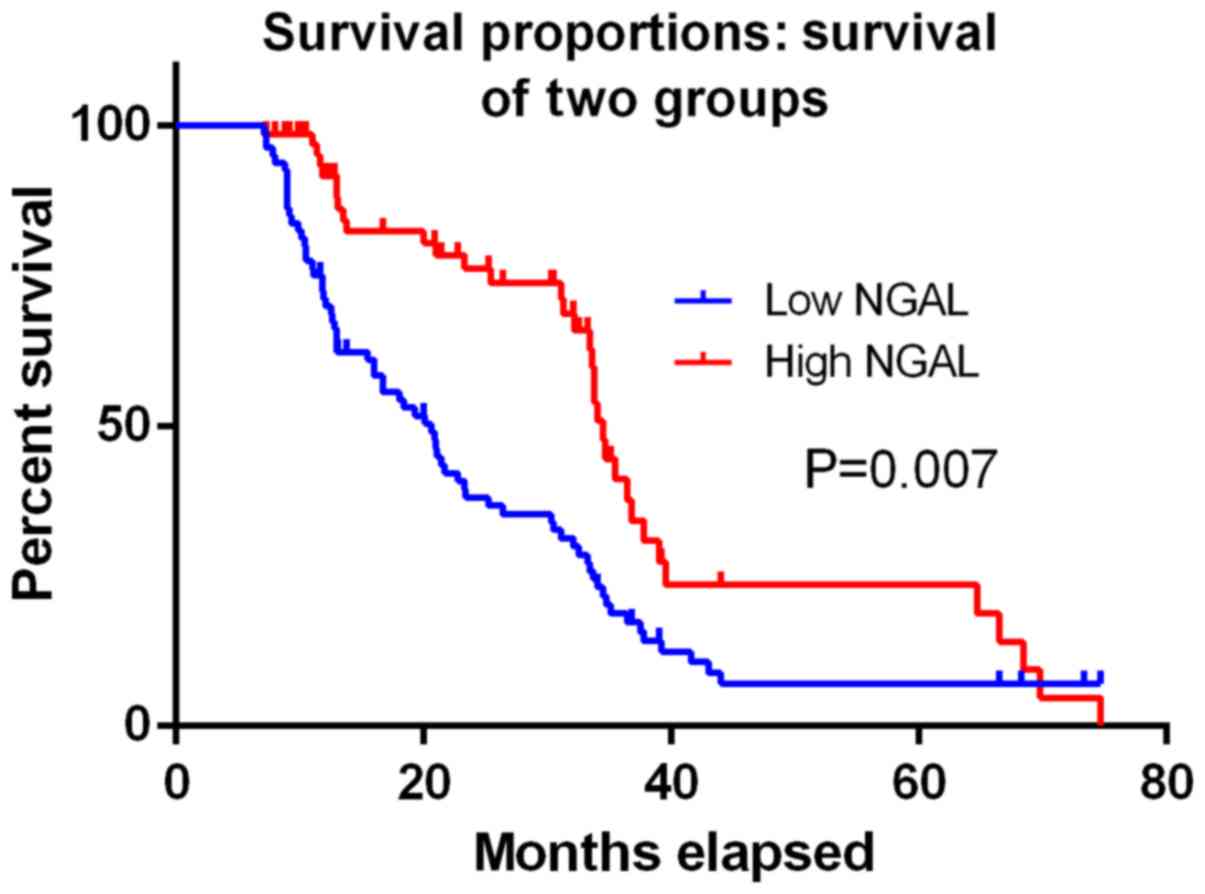
present results showed that high NGAL expression was associated
with improved OS (5y-OS: 72.4–45.6%, high to low expression,
P=0.007, Fig. 2). In univariate and
multivariate analysis, NGAL was considered an independent
prognostic factor for OS (the risk ratio of univariate analysis was
0.278, and that of multivariate analysis was 0.325, all P<0.01).
Detailed data are shown in Table
II.
 | Table II.Univariate and multivariate analyses
of prognostic parameters for survival in 158 nasopharyngeal
carcinoma patients. |
Table II.
Univariate and multivariate analyses
of prognostic parameters for survival in 158 nasopharyngeal
carcinoma patients.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Prognostic
parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Expression of NGAL
(low vs. high) | 0.278 | 0.184–0.601 | 0.001 | 0.325 | 0.121–0.601 | 0.001 |
| Age (years) | 1.587 | 0.872–1.745 | 0.065 | − | − | − |
| Sex (male vs.
female) | 1.255 | 0.723–1.641 | 0.147 | − | − | − |
| Tumor
differentiation | 1.247 | 0.835–1.645 | 0.122 | 1.356 | 0.656–1.775 | 0.125 |
| T
classification | 2.435 | 1.557–2.912 | 0.019 | 2.013 | 0.896–2.145 | 0.028 |
| Lymphatic
metastasis (absent vs. present) | 3.045 | 1.745–4.456 | 0.005 | 2.885 | 1.224–3.756 | 0.002 |
| Distant metastasis
(absent vs. present) | 4.877 | 2.204–7.011 | 0.001 | 4.132 | 2.254–5.624 | 0.001 |
Exogenous overexpression of NGAL
suppresses the proliferation, migration and invasion of NPC
cells
The significant downregulation of NGAL expression
predicted poor prognosis and survival was observed. The present
study therefore proposed to investigate the functional role of NGAL
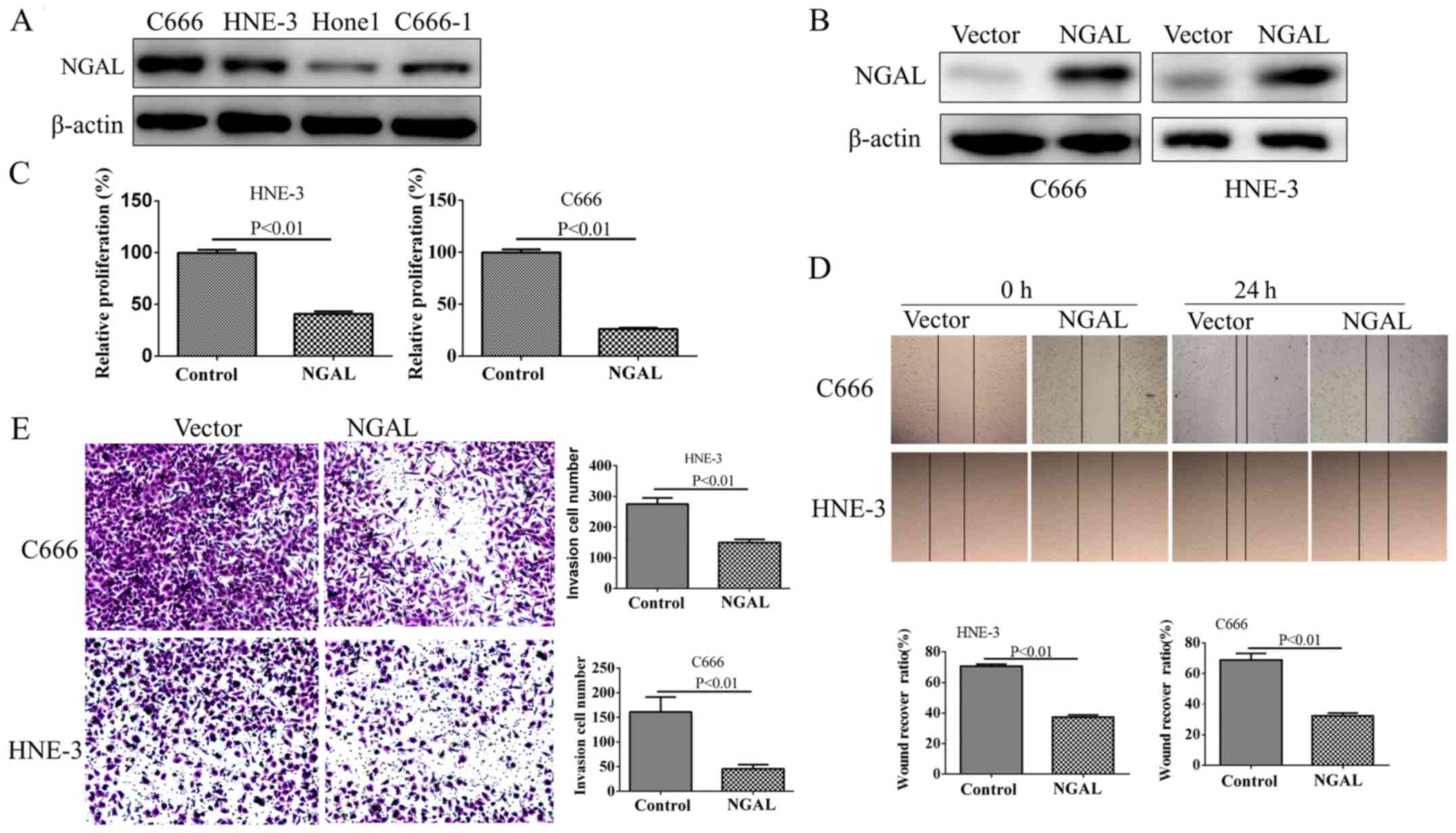
in NPC development and progression. First, the baseline expression
of NGAL from four NPC cell lines was tested by western blotting
(Fig. 3A). NGAL expression in C666
and HNE-3 was significantly increased compared with the other two
cell lines, thus C666 and HNE-3 were selected to for transfection
with PC-DNA3.1-NGAL. Stable-transfected clones were selected for
MTT, wound healing and transwell assays. Exogenous overexpression
of NGAL in C666 and HNE-3 was determined by western blotting
(Fig. 3B). From the MTT assay, after
growth for 72 h in contrast with the PCDNA3.1 control, C666-NGAL
and HNE-3-NGAL exhibited a significantly decreased proliferation
ability (Fig. 3C; P<0.01).
Furthermore, the migration ability and invasion ability of
C666-NGAL and HNE-3-NGAL measured by wound healing (Fig. 3D) and transwell assay (Fig. 3E) decreased significantly
(P<0.01), when compared with their corresponding cell lines.
These results suggest that NGAL plays a key role in NPC cell
growth. Meanwhile, wound healing and migration assays also suggest
that overexpression of NGAL could significantly decrease the
migration and invasive capability of NPC cells.
Exogenous overexpression of NGAL
induces apoptosis of NPC cells by activating caspase family
proteins
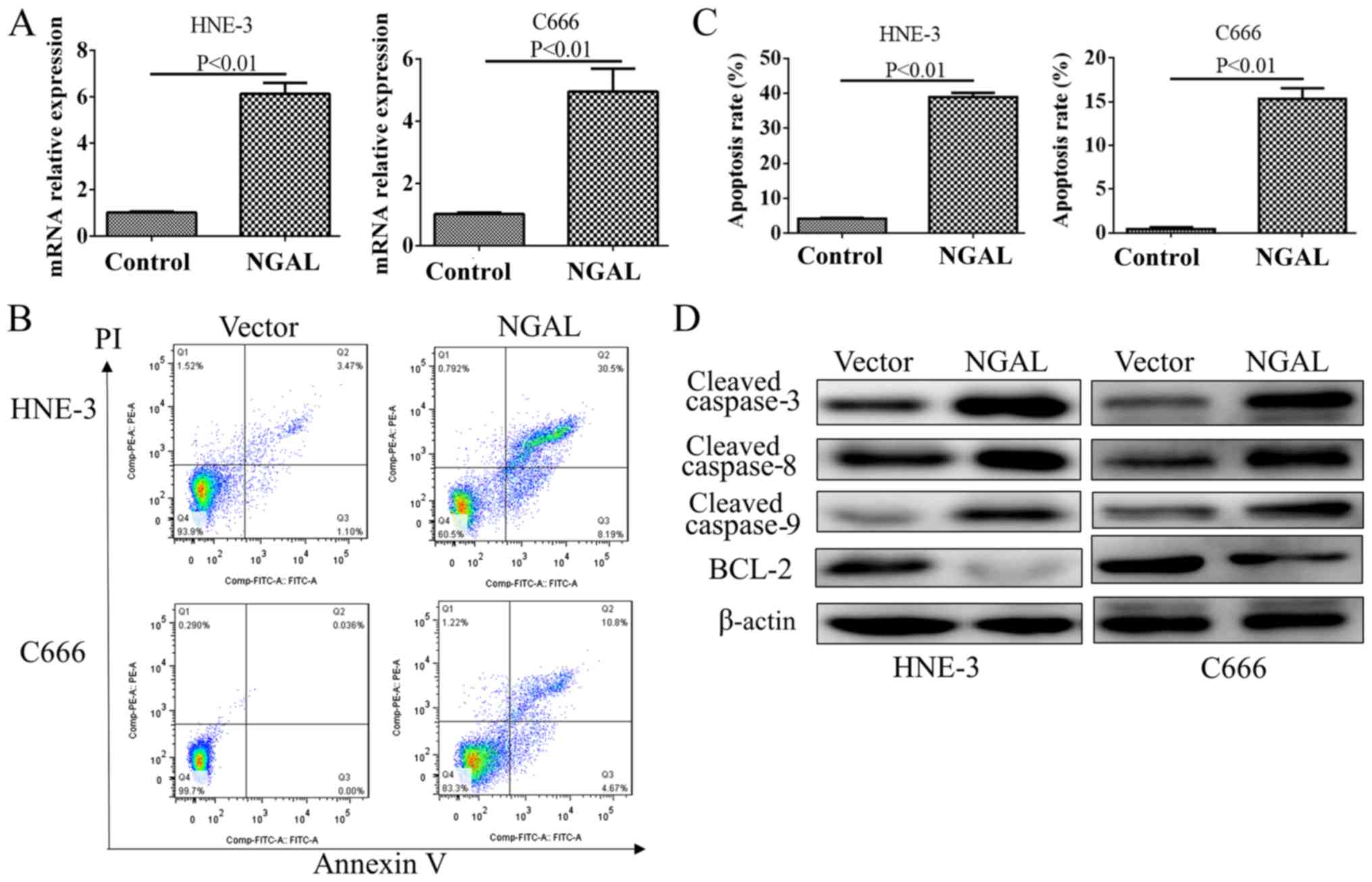
After a transient transfection for 48 h, the plasmid
transfection efficiency was significantly increased as demonstrated
by an RT-qPCR test for NGAL (Fig.
4A). Flow cytometry demonstrated that overexpression of NGAL
could produce moderate apoptosis in C666 and HNE-3, which is shown
in Fig. 4B and C. By testing caspase
family members, cleaved protein of caspase 3, 8 and 9 were all
found to be decreased in NGAL overexpression cells compared with
PC-DNA3.1 vector. However, BCL2 was upregulated by NGAL
overexpression in both cell lines (Fig.
4D).
Overexpression of NGAL inhibits the
EMT transition in NPC cells
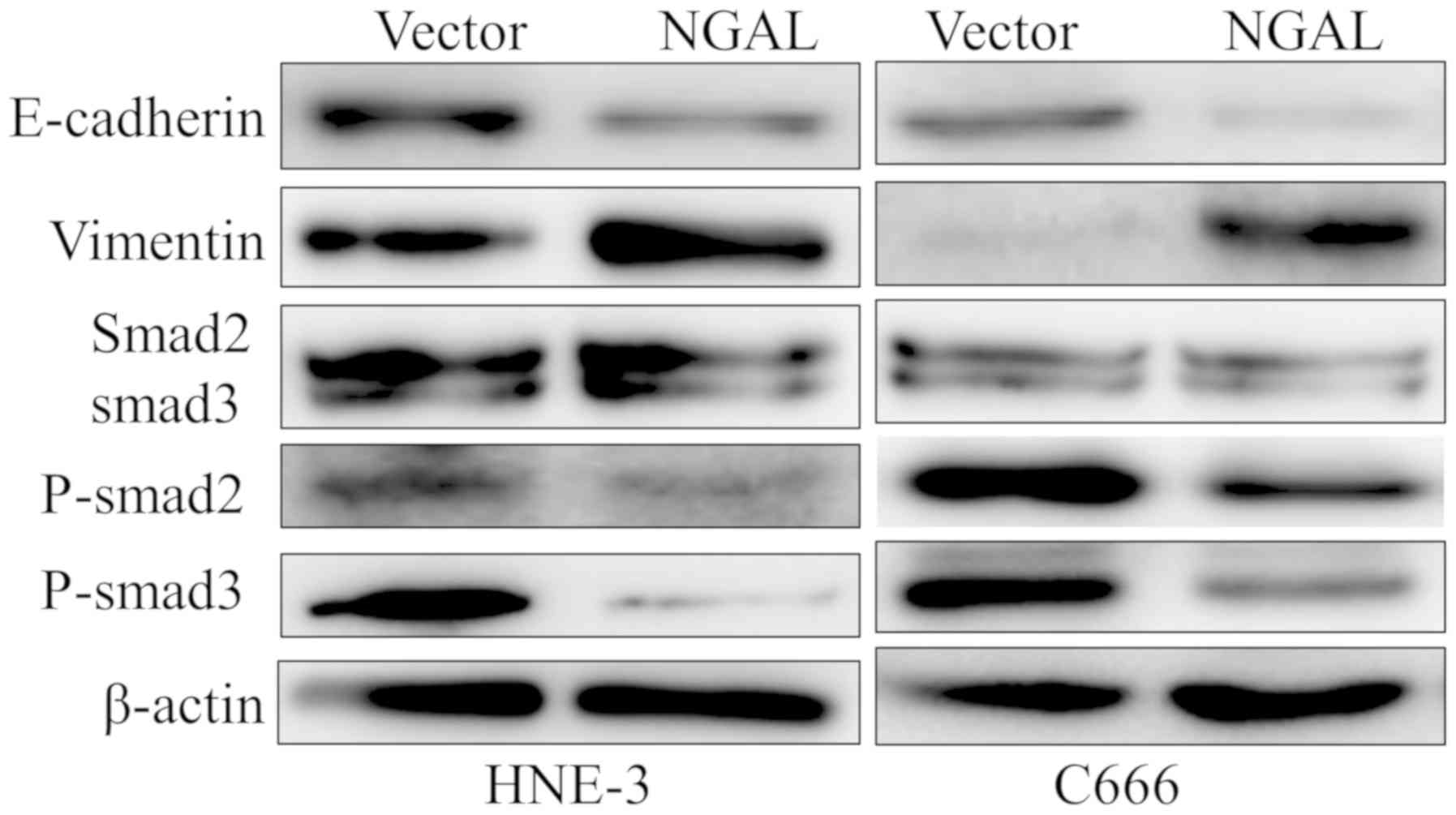
Moreover, the present study found that the cell EMT
marker E-cadherin expression was decreased, while vimentin
expression was increased when C666 and HNE-3 cells were transfected
with PCDNA3.1-NGAL compared with the blank vector groups (Fig. 5). Thus, these results indicated that
upregulation of NGAL could suppress the NPC cell EMT process in
vitro. Further investigation suggested that overexpression of NGAL
significantly inhibited the p-smad2/3 and total smad2/3, but
inhibition of p-smad2/3 is more effective than total smad2/3.
Discussion
There is evidence that NGAL may be a marker of
disease status in chronic and acute pathological conditions,
especially in inflammatory, metabolic, neurological and cancer
diseases (37–39). Multiple studies have explored the
possible role of NGAL in various cancer models and have shown that
NGAL has beneficial and harmful functions (37,40).
Although ongoing research is investigating the value of the
NGAL-proMMP-9 complex as an indicator of cancer disease status,
there is no detailed data on its full functional significance in
disease (41). As a result of
reports of the role of NGAL in cancer and the lack of information
about NPC, it is necessary to study its role in NPC.
In the current study, high NGAL was mainly expressed
in early stage NPC patients, ~72.2% among patients in stage 1 and
stage 2; while only 27.8% of patients from advanced tumors has high
expression of NGAL. According to our knowledge, the present study
is first time that NPC patients with high NGAL expression have been
revealed to have an increased survival outcome, which provides a
beneficial biomarker to predict the prognosis of NPC.
The current results also showed that the expression
of NGAL in NPC tumors was correlated with improved prognosis of OS
and disease-free survival. From the perspective of immunology,
previous study demonstrated that NGAL promotes recruitment of tumor
infiltrating leukocytes and tumor allografts using wild type
thyroid carcinoma cells are decreased compared with tumor
allografts from NGAL-depleted cell injected mice (29). In the current study, strong
NGAL-immune staining is positively correlated with improved
survival and the hypothesis is that NGAL might recruit cytotoxic
lymphocytes into NPC tumor tissues to elicit the tumor cells.
Exogenous overexpression of NGAL could inhibit the
proliferation, migration and invasiveness in vitro using NPC cells.
The NGAL-plasmid was transfected into C666 and HNE-3 successfully.
By MTT proliferation, wound healing and transwell assays, it was
found that overexpression NGAL in NPC cells inhibited the
proliferation and migration of NPC cells. These results suggest
NGAL plays a cancer suppressor role in NPC, which is different from
most solid tumors (12,15). Overexpression of NGAL also induces
moderate apoptosis and further study demonstrated that
overexpression of NGAL inhibited the caspase family activation. The
present study hypothesized that inducing apoptosis is one of the
tumor suppressor mechanisms of NGAL in NPC.
Both the clinical relevance study and the in vitro
study demonstrated that higher expression of NGAL could inhibit NPC
cell migration and metastasis, and EMT is one of important
mechanisms of cancer cell metastasis initiation. In the current
study, by western blotting, it was found that overexpression of
NGAL blocked EMT and possibly, this is one of mechanisms for NGAL
inhibiting the migration and invasiveness of NPC cells. As for the
mechanism of inhibiting EMT, the present study demonstrated that
NGAL overexpression reduced p-smad2/3. Solid evidence has suggested
that smad2/3 signaling enhancement could induce EMT and inhibiting
smad2/3 can reverse EMT (42,43).
There are also several limitations in the current
study. Firstly, the patient population is small in the present
study, so all the conclusions about NGAL with clinical
characteristics need to be further verified using a larger NPC
population. Secondly, in the mechanism study, only two cell lines
were used in the in vitro study and more NPC cell lines should be
used to test what role NGAL plays in different NPC cells, due to
different cell lines having various biological backgrounds.
In summary, despite the small population size, the
present study demonstrated the evaluation of NGAL expression in NPC
tumors for the first time in a Chinese population. Although the
population size is small, a significant correlation was observed. A
larger population size will be useful to further confirm the
prognostic significance of NGAL in NPC. However, lack of
investigation of the molecular mechanism is a limitation of this
study and the possible molecular regulated targets of NGAL needs
further investigation, along with the interfering signaling
pathways.
Acknowledgements
Not applicable.
Funding
The present study was funded by the project of the
Tianjin Health Bureau: Vestibular-auditory function in patients
with vestibular migraine (grant no. 2014KZ128).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG carried out the laboratory experiments. JHZ
conceived the study and participated in its design. HZ and JZ
analyzed data and images. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Independent
Ethics Committee of the General Hospital of Tianjin Medical
University (Tianjin, China). Prior to analysis, consent from each
patient was received.
Patient consent for publication
All patients agreed the use of their medical data
and publication. Informed written consent was provided by all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of Nasopharyngeal Carcinoma: Current Practice and Future
Perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang
LL, Liao XB, Xu HY, Chen L, Lai SZ, et al: Re-evaluation of 6th
edition of AJCC staging system for nasopharyngeal carcinoma and
proposed improvement based on magnetic resonance imaging. Int J
Radiat Oncol Biol Phys. 73:1326–1334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng L, Cao C, Cheng G, Hu Q and Chen X:
Cytomembranic PD-L1 expression in locoregionally advanced
nasopharyngeal carcinoma. OncoTargets Ther. 10:5483–5487. 2017.
View Article : Google Scholar
|
|
4
|
Hua YJ, Han F, Lu LX, Mai HQ, Guo X, Hong
MH, Lu TX and Zhao C: Long-term treatment outcome of recurrent
nasopharyngeal carcinoma treated with salvage intensity modulated
radiotherapy. Eur J Cancer. 48:3422–3428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong L, Wang L, Shen C, Hu C, Wang L and
Lu JJ: Salvage Intensity-Modulated Radiation Therapy (IMRT) for
Locally Recurrent Nasopharyngeal Cancer after Definitive IMRT: A
Novel Scenario of the Modern Era. Sci Rep. 6:328832016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen C, Fee W, Chen J, Chan C, Khong B,
Hara W, Goffinet D, Li D and Le QT: Salvage treatment for locally
recurrent nasopharyngeal carcinoma (NPC). Am J Clin Oncol.
37:327–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karam I, Huang SH, McNiven A, Su J, Xu W,
Waldron J, Bayley AJ, Kim J, Cho J, Ringash J, et al: Outcomes
after reirradiation for recurrent nasopharyngeal carcinoma: North
American experience. Head Neck. 38 (Suppl 1):E1102–E1109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wulfkuhle JD, Liotta LA and Petricoin EF:
Proteomic applications for the early detection of cancer. Nat Rev
Cancer. 3:267–275. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dawson S-J, Tsui DWY, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sadar S, Kaspate D and Vyawahare N:
Protective effect of L-glutamine against diabetes-induced
nephropathy in experimental animal: Role of KIM-1, NGAL, TGF-β1,
and collagen-1. Ren Fail. 38:1483–1495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papadopoulou-Marketou N, Margeli A,
Papassotiriou I, Chrousos GP, Kanaka-Gantenbein C and Wahlberg J:
NGAL as an Early Predictive Marker of Diabetic Nephropathy in
Children and Young Adults with Type 1 Diabetes Mellitus. J Diabetes
Res. 2017:75269192017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu F, Li N, Yang W, Wang R, Yu J and Wang
X: The expression analysis of NGAL and NGALR in clear cell renal
cell carcinoma. Gene. 676:269–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eilenberg W, Stojkovic S, Kaider A,
Piechota-Polanczyk A, Nanobachvili J, Domenig CM, Wojta J, Huk I,
Demyanets S and Neumayer C: Neutrophil Gelatinase Associated
Lipocalin (NGAL) for Identification of Unstable Plaques in Patients
with Asymptomatic Carotid Stenosis. Eur J Vasc Endovasc Surg.
57:768–777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sueud T, Hadi NR, Abdulameer R, Jamil DA
and Al-Aubaidy HA: Assessing urinary levels of IL-18, NGAL and
albumin creatinine ratio in patients with diabetic nephropathy.
Diabetes Metab Syndr. 13:564–568. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oikonomou E, Tsalamandris S, Karlis D,
Siasos G, Chrysohoou C, Vogiatzi G, Dimitropoulos S, Charalambous
G, Kouskouni E and Tousoulis D: The association among biomarkers of
renal and heart function in patients with heart failure: The role
of NGAL. Biomarkers Med. 12:1323–1330. 2018. View Article : Google Scholar
|
|
16
|
Forster CS, Jackson E, Ma Q, Bennett M,
Shah SS and Goldstein SL: Predictive ability of NGAL in identifying
urinary tract infection in children with neurogenic bladders.
Pediatr Nephrol. 33:1365–1374. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunsicker O, Feldheiser A, Weimann A,
Liehre D, Sehouli J, Wernecke KD and Spies C: Diagnostic value of
plasma NGAL and intraoperative diuresis for AKI after major
gynecological surgery in patients treated within an intraoperative
goal-directed hemodynamic algorithm: A substudy of a randomized
controlled trial. Medicine (Baltimore). 96:e73572017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang J, Li J, Li S, Li J, Yu C and Wei C:
Effect of Inhibiting NGAL Gene Expression on A549 Lung Cancer Cell
Migration and Invasion. Zhongguo Fei Ai Za Zhi. 18:187–192.
2015.(In Chinese). PubMed/NCBI
|
|
19
|
Wang PH, Ko JL, Yang SF and Lin LY:
Implication of human nonmetastatic clone 23 type 1 and its
downstream gene lipocalin 2 in metastasis and patients survival of
cancer of uterine cervix. Int J Cancer. 129:2380–2389. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Candido S, Maestro R, Polesel J, Catania
A, Maira F, Signorelli SS, McCubrey JA and Libra M: Roles of
neutrophil gelatinase-associated lipocalin (NGAL) in human cancer.
Oncotarget. 5:1576–1594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song B, Zhang H, Jiang L, Chi Y, Tian J,
Du W, Yu B and Han Z: Down-regulation of lipocalin 2 suppresses the
growth of human lung adenocarcinoma through oxidative stress
involving Nrf2/HO-1 signaling. Acta Biochim Biophys Sin (Shanghai).
47:805–814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chung IH, Wu TI, Liao CJ, Hu JY, Lin YH,
Tai PJ, Lai CH and Lin KH: Overexpression of lipocalin 2 in human
cervical cancer enhances tumor invasion. Oncotarget. 7:11113–11126.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mongre RK, Sodhi SS, Sharma N, Ghosh M,
Kim JH, Kim N, Park YH, Shin YG, Kim SJ, Jiao ZJ, et al: Epigenetic
induction of epithelial to mesenchymal transition by LCN2 mediates
metastasis and tumorigenesis, which is abrogated by NF-κB inhibitor
BRM270 in a xenograft model of lung adenocarcinoma. Int J Oncol.
48:84–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leung L, Radulovich N, Zhu CQ, Organ S,
Bandarchi B, Pintilie M, To C, Panchal D and Tsao MS: Lipocalin2
promotes invasion, tumorigenicity and gemcitabine resistance in
pancreatic ductal adenocarcinoma. PLoS One. 7:e466772012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monisha J, Roy NK, Padmavathi G, Banik K,
Bordoloi D, Khwairakpam AM, Arfuso F, Chinnathambi A, Alahmadi TA,
Alharbi SA, et al: NGAL is downregulated in oral squamous cell
carcinoma and leads to increased survival, proliferation, migration
and chemoresistance. Cancers (Basel). 10:2282018. View Article : Google Scholar
|
|
26
|
Dertli R, Biyik M, Yolacan R,
Karakarcayildiz A, Keskin M, Kayar Y and Asil M: May Neutrophil
Gelatinase-Associated Lipocalin (NGAL) Level Predict Mortality in
Patients with Hepatocellular Carcinoma (HCC)? J Gastrointest
Cancer. Nov 15–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Xu Q, Wang Y, Chen K and Li J: Serum
neutrophil gelatinase associated lipocalin (NGAL) as a biomarker
for predicting high dose methotrexate associated acute kidney
injury in children with acute lymphoblastic leukemia. Cancer
Chemother Pharmacol. 85:95–103. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han MY, Nie JW, Li YY, Zhu YZ and Wu G:
Downregulation of NGAL is required for the inhibition of
proliferation and the promotion of apoptosis of human gastric
cancer MGC-803 cells. Cell Physiol Biochem. 50:694–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pacifico F, Pisa L, Mellone S, Cillo M,
Lepore A and Leonardi A: NGAL promotes recruitment of tumor
infiltrating leukocytes. Oncotarget. 9:30761–30772. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiromoto T, Noguchi K, Yamamura M, Zushi
Y, Segawa E, Takaoka K, Moridera K, Kishimoto H and Urade M:
Up-regulation of neutrophil gelatinase-associated lipocalin in oral
squamous cell carcinoma: Relation to cell differentiation. Oncol
Rep. 26:1415–1421. 2011.PubMed/NCBI
|
|
31
|
Lin CW, Yang WE, Lee WJ, Hua KT, Hsieh FK,
Hsiao M, Chen CC, Chow JM, Chen MK, Yang SF, et al: Lipocalin 2
prevents oral cancer metastasis through carbonic anhydrase IX
inhibition and is associated with favourable prognosis.
Carcinogenesis. 37:712–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang S, Xin H, Li Y, Zhang D, Shi J, Yang
J and Chen X: Skimmin, a Coumarin from Hydrangea paniculata, Slows
down the Progression of Membranous Glomerulonephritis by
Anti-Inflammatory Effects and Inhibiting Immune Complex Deposition.
Evid Based Complement Alternat Med. 2013:8192962013.PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sen Z, Zhan XK, Jing J, Yi Z and Wanqi Z:
Chemosensitizing activities of cyclotides from Clitoria ternatea in
paclitaxel-resistant lung cancer cells. Oncol Lett. 5:641–644.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang S, Fu Y, Wang D and Wang J: Icotinib
enhances lung cancer cell radiosensitivity in vitro and in vivo by
inhibiting MAPK/ERK and AKT activation. Clin Exp Pharmacol Physiol.
45:969–977. 2018. View Article : Google Scholar
|
|
36
|
Zhang S, Yang J, Li H, Li Y, Liu Y, Zhang
D, Zhang F, Zhou W and Chen X: Skimmin, a coumarin, suppresses the
streptozotocin-induced diabetic nephropathy in wistar rats. Eur J
Pharmacol. 692:78–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xiao X, Yeoh BS and Vijay-Kumar M:
Lipocalin 2: An Emerging Player in Iron Homeostasis and
Inflammation. Annu Rev Nutr. 37:103–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lippi G, Meschi T, Nouvenne A, Mattiuzzi C
and Borghi L: Neutrophil gelatinase-associated lipocalin in cancer.
Adv Clin Chem. 64:179–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bauvois B and Susin SA: Revisiting
Neutrophil Gelatinase Associated Lipocalin (NGAL) in Cancer: Saint
or Sinner? Cancers (Basel). 102018.
|
|
40
|
Chakraborty S, Kaur S, Guha S and Batra
SK: The multifaceted roles of neutrophil gelatinase associated
lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta.
1826:129–169. 2012.PubMed/NCBI
|
|
41
|
Bouchet S and Bauvois B: Neutrophil
Gelatinase-Associated Lipocalin (NGAL), Pro-Matrix
Metalloproteinase-9 (pro-MMP-9) and Their Complex Pro-MMP-9/NGAL in
Leukaemias. Cancers (Basel). 6:796–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-β and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vincent T, Neve EP, Johnson JR, Kukalev A,
Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL,
et al: A SNAIL1-SMAD3/4 transcriptional repressor complex promotes
TGF-β mediated epithelial-mesenchymal transition. Nat Cell Biol.
11:943–950. 2009. View Article : Google Scholar : PubMed/NCBI
|