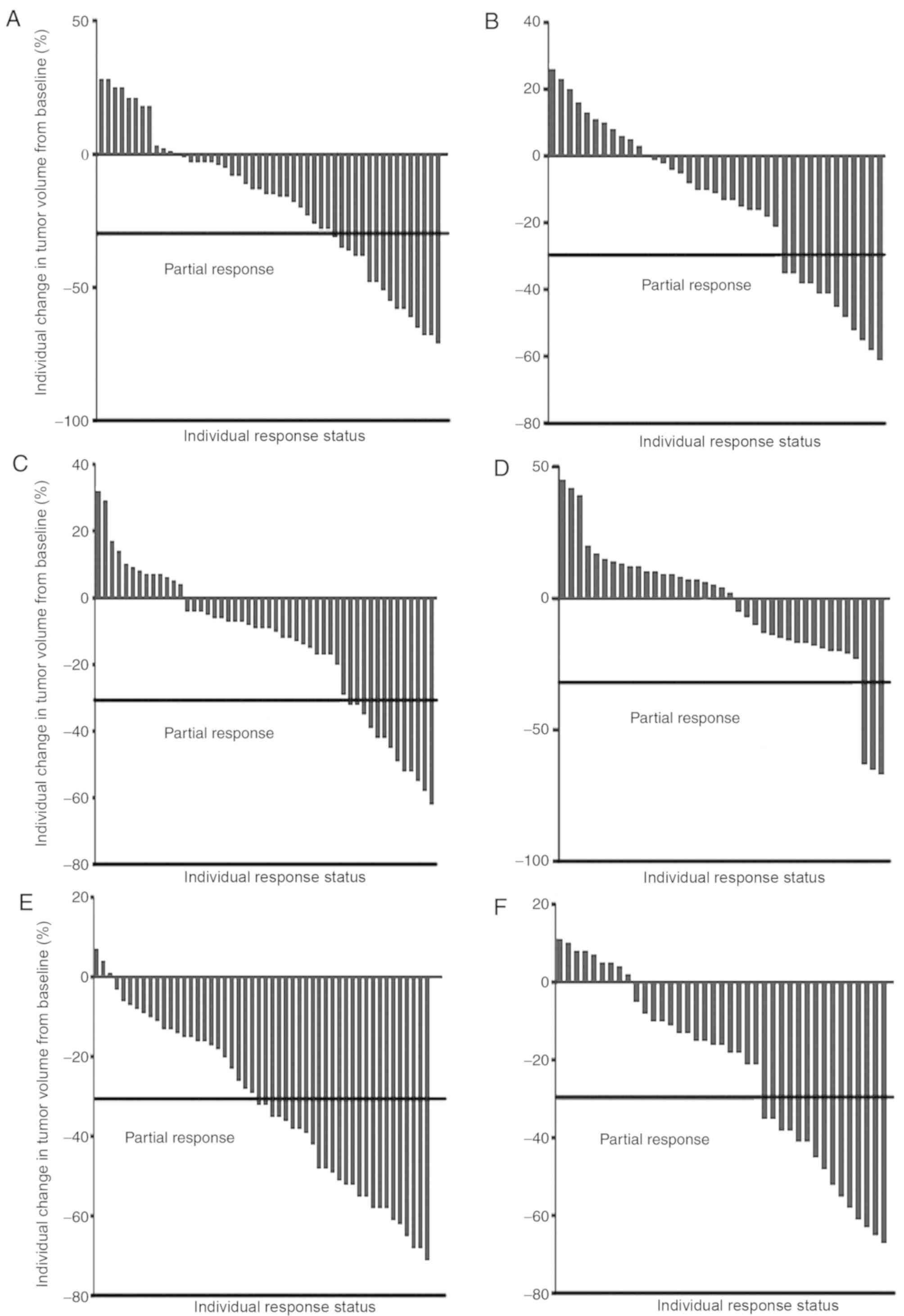
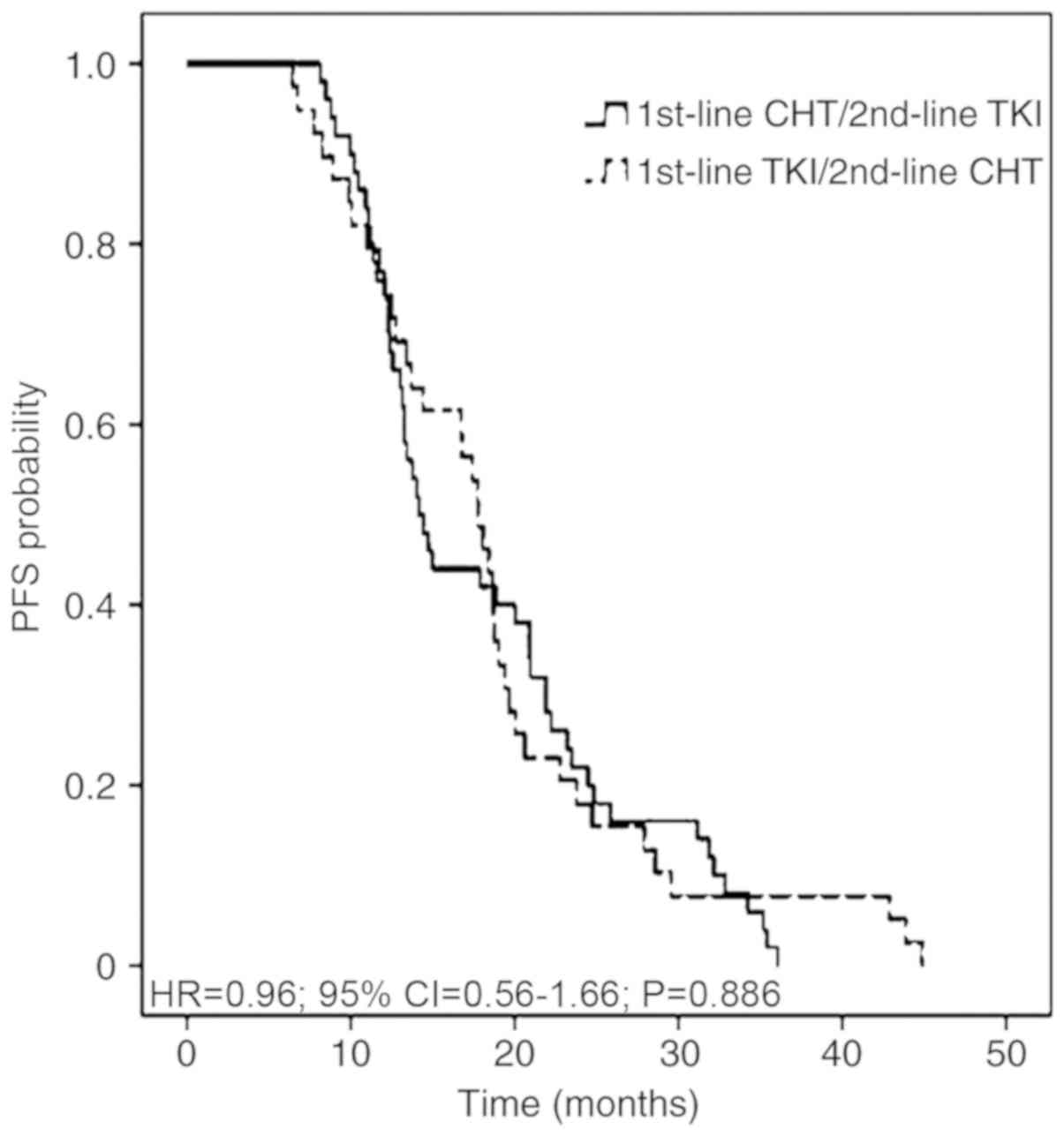
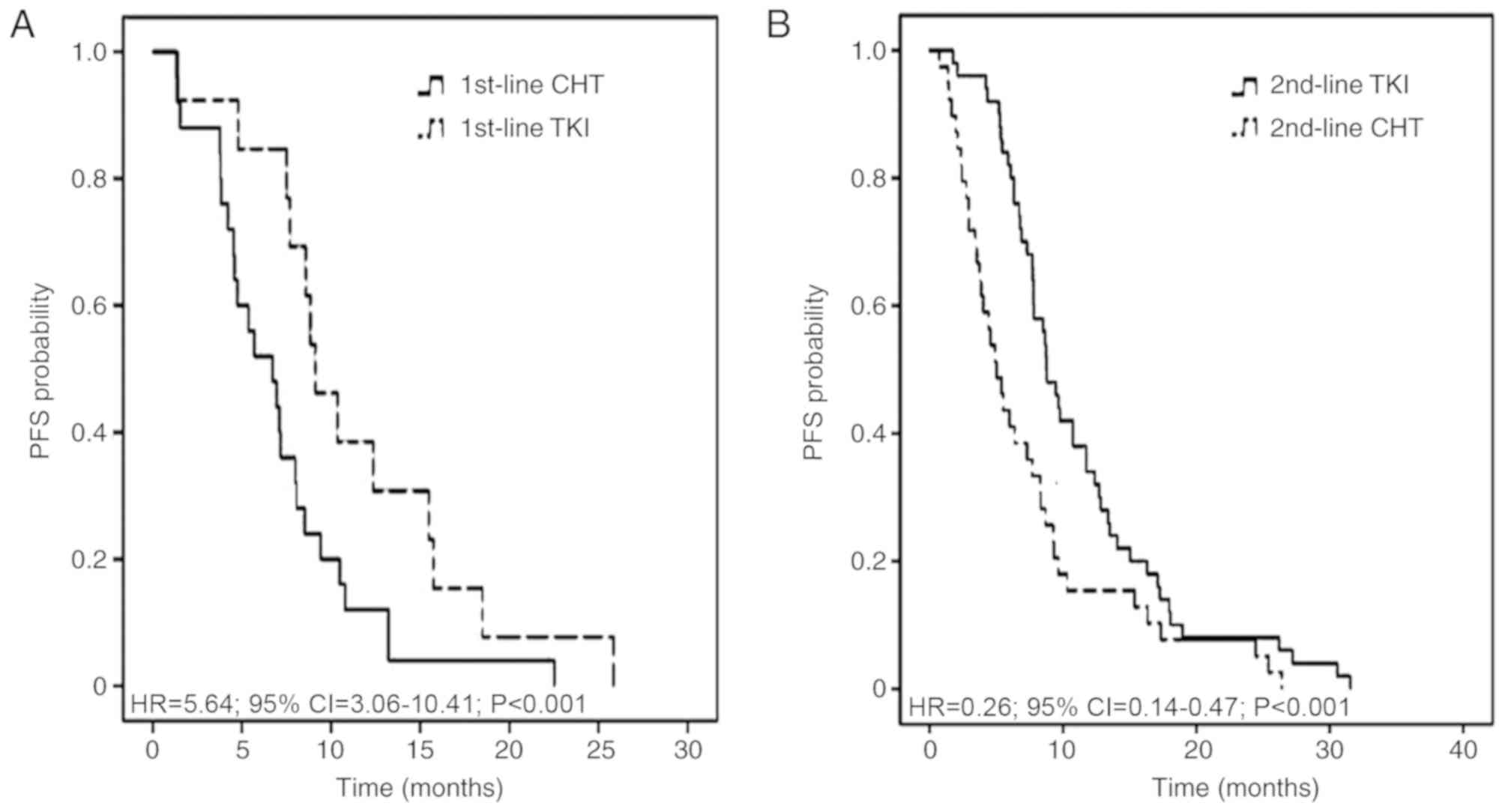
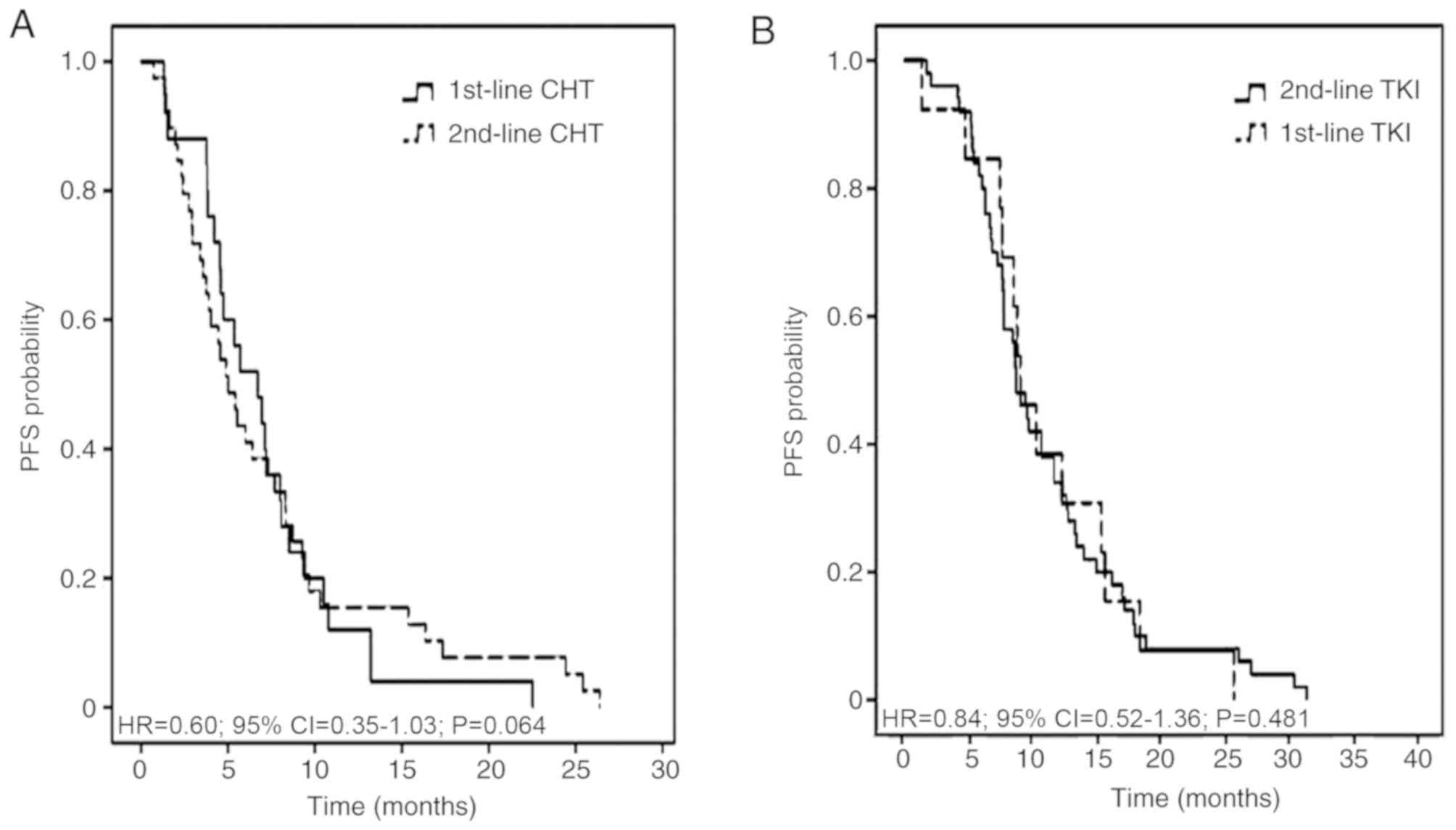
|
1
|
Kobayashi Y and Mitsudomi T: Not all
epidermal growth factor receptor mutations in lung cancer are
created equal: Perspectives for individualized treatment strategy.
Cancer Sci. 107:1179–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kimura S, Tanaka K, Harada T, Liu R,
Shibahara D, Kawano Y, Nakanishi Y and Okamoto I: Sensitivity of
epidermal growth factor receptor with single or double uncommon
mutations to afatinib confirmed by a visual assay. Cancer Sci.
109:3657–3661. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Costa C, Molina MA, Drozdowskyj A,
Giménez-Capitán A, Bertran-Alamillo J, Karachaliou N, Gervais R,
Massuti B, Wei J, Moran T, et al: The impact of EGFR T790M
mutations and BIM mRNA expression on outcome in patients with
EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the
randomized phase III EURTAC trial. Clin Cancer Res. 20:2001–2010.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Zhao X, He D, Wang J, Li W, Liu Y,
Ma L, Jiang M, Teng Y, Wang Z, et al: Loss of EGFR confers acquired
resistance to AZD9291 in an EGFR-mutant non-small cell lung cancer
cell line with an epithelial–mesenchymal transition phenotype. J
Cancer Res Clin Oncol. 144:1413–1422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oxnard GR, Arcila ME, Chmielecki J,
Ladanyi M, Miller VA and Pao W: New strategies in overcoming
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors in lung cancer. Clin Cancer Res. 17:5530–5537.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Y, Kang S, Fang W, Hong S, Liang W,
Yan Y, Qin T, Tang Y, Sheng J and Zhang L: Impact of Smoking Status
on EGFR-TKI Efficacy for Advanced Non-Small-Cell Lung Cancer in
EGFR Mutants: A Meta-analysis. Clin Lung Cancer. 16:144–151.e1.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-Small Cell Lung Cancer, Version 5.2017,
NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JC, Wu YL, Chan V, Kurnianda J,
Nakagawa K, Saijo N, Fukuoka M, McWalter G, McCormack R and Mok TS:
Epidermal growth factor receptor mutation analysis in previously
unanalyzed histology samples and cytology samples from the phase
III Iressa Pan-ASia Study (IPASS). Lung Cancer. 83:174–181. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) Edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Jin B, Chu T, Dong X, Yang H, Zhang
Y, Wu D, Lou Y, Zhang X, Wang H and Han B: EGFR tyrosine kinase
inhibitor (TKI) in patients with advanced non-small cell lung
cancer (NSCLC) harboring uncommon EGFR mutations: A real-world
study in China. Lung Cancer. 96:87–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida T, Kuroda H, Oya Y, Shimizu J,
Horio Y, Sakao Y, Hida T and Yatabe Y: Clinical outcomes of
platinum-based chemotherapy according to T790M mutation status in
EGFR-positive non-small cell lung cancer patients after initial
EGFR-TKI failure. Lung Cancer. 109:89–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arcila ME, Oxnard GR, Nafa K, Riely GJ,
Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA and Ladanyi M:
Rebiopsy of lung cancer patients with acquired resistance to EGFR
inhibitors and enhanced detection of the T790M mutation using a
locked nucleic acid-based assay. Clin Cancer Res. 17:1169–1180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neeman E, Gresham G, Ovasapians N,
Hendifar A, Tuli R, Figlin R and Shinde A: Comparing physician and
nurse eastern cooperative oncology group performance status
(ECOG-PS) ratings as predictors of clinical outcomes in patients
with cancer. Oncologist. 24:e1460–e1466. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen C, Kehl KL, Zhao B, Simon GR, Zhou S
and Giordano SH: Utilization patterns and trends in epidermal
growth factor receptor (EGFR) mutation testing among patients with
newly diagnosed metastatic lung cancer. Clin Lung Cancer.
18:e233–e241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dias-Santagata D, Akhavanfard S, David SS,
Vernovsky K, Kuhlmann G, Boisvert SL, Stubbs H, McDermott U,
Settleman J, Kwak EL, et al: Rapid targeted mutational analysis of
human tumours: A clinical platform to guide personalized cancer
medicine. EMBO Mol Med. 2:146–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanoue LT: Gefitinib or
Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. Yearbook Pulmon
Dis. 2010:149–151. 2010. View Article : Google Scholar
|
|
22
|
Cesare G, Fortunato C, Ciro G, Feld R,
Butts C, Gebbia V, Maione P, Morgillo F, Genestreti G, Favaretto A,
et al: First-line erlotinib followed by second-line
cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung
cancer: The TORCH randomized trial. J Clin Oncol. 30:3002–3011.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jänne PA, Wang X, Socinski MA, Crawford J,
Stinchcombe TE, Gu L, Capelletti M, Edelman MJ, Villalona-Calero
MA, Kratzke R, et al: Randomized Phase II trial of erlotinib alone
or with carboplatin and paclitaxel in patients who were never or
light former smokers with advanced lung adenocarcinoma: CALGB 30406
Trial. J Clin Oncol. 30:2063–2069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gatzemeier U, Pluzanska A, Szczesna A,
Kaukel E, Roubec J, De Rosa F, Milanowski J, Karnicka-Mlodkowski H,
Pesek M, Serwatowski P, et al: Phase III study of erlotinib in
combination with cisplatin and gemcitabine in advanced
non-small-cell lung cancer: the Tarceva Lung Cancer Investigation
Trial. J Clin Oncol. 25:1545–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herbst RS, Giuseppe G, Schiller JH, Natale
RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves
JA, et al: Gefitinib in combination with paclitaxel and carboplatin
in advanced non-small-cell lung cancer: A phase III trial--INTACT
2. J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herbst RS, Prager D, Hermann R,
Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P,
Li X, et al: TRIBUTE: A Phase III Trial of Erlotinib Hydrochloride
(OSI-774) Combined With Carboplatin and Paclitaxel Chemotherapy in
Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 23:5892–5899.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao L, Jin W, Long G and Jiang Y:
Sequential treatment of tyrosine kinase inhibitor and
platinum-based doublet chemotherapy on EGFR mutant non-small cell
lung cancer: A meta-analysis of randomized controlled clinical
trials. Onco Targets Ther. 10:1279–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cardona AF, Arrieta O, Zapata MI, Rojas L,
Wills B, Reguart N, Karachaliou N, Carranza H, Vargas C, Otero J,
et al: Acquired resistance to erlotinib in EGFR mutation-positive
lung adenocarcinoma among hispanics (CLICaP). Target Oncol.
12:513–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang JJ, Zhou Q, Yan HH, Zhang XC, Chen
HJ, Tu HY, Wang Z, Xu CR, Su J, Wang BC, et al: A phase III
randomised controlled trial of erlotinib vs gefitinib in advanced
non-small cell lung cancer with EGFR mutations. Br J Cancer.
116:568–574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen YC, Tseng GC, Tu CY, Chen WC, Liao
WC, Chen WC, Li CH, Chen HJ and Hsia TC: Comparing the effects of
afatinib with gefitinib or Erlotinib in patients with
advanced-stage lung adenocarcinoma harboring non-classical
epidermal growth factor receptor mutations. Lung Cancer. 110:56–62.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang CH, Lee CH and Wang JY: Gefitinib or
Erlotinib for Previously Treated Lung Adenocarcinoma: Which Is
Superior? J Clin Oncol. 35:1374–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sequist LV, Soria JC, Goldman JW, Wakelee
HA, Gadgeel SM, Varga A, Papadimitrakopoulou V, Solomon BJ, Oxnard
GR, Dziadziuszko R, et al: Rociletinib in EGFR-mutated
non-small-cell lung cancer. N Engl J Med. 372:1700–1709. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masters GA, Temin S, Azzoli CG, Giaccone
G, Baker S Jr, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller
JH, Smith TJ, et al: Systemic Therapy for Stage IV Non-Small-Cell
Lung Cancer: American Society of Clinical Oncology Clinical
Practice Guideline Update. J Oncol Pract. 33:2488–3515. 2015.
|
|
35
|
Shinno Y, Goto Y, Watanabe S, Sato J,
Morita R, Matsumoto Y, Murakami S, Kanda S, Horinouchi H, Fujiwara
Y, et al: Evaluation of time to failure of strategy as an
alternative surrogate endpoint in patients with lung cancer with
EGFR mutations. ESMO Open. 3:e0003992018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee S, Jeon H and Shim B: Prognostic value
of ferritin-to-hemoglobin ratio in patients with advanced
non-small-cell lung cancer. J Cancer. 10:1717–1725. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noronha V, Patil VM, Joshi A, Menon N,
Chougule A, Mahajan A, Janu A, Purandare N, Kumar R, More S, et al:
Gefitinib versus gefitinib plus pemetrexed and carboplatin
chemotherapy in -mutated lung cancer. J Clin Oncol. 18:124–136.
2020. View Article : Google Scholar
|