Introduction
Lung cancer notably contributes to tumor-associated
mortality worldwide which is accounting for 18.4% of all such
deaths and responsible for more than 1.8 million deaths each year
(1,2). Non-small cell lung cancer (NSCLC)
accounts for ~85% of histological subtypes among all types of lung
cancer (3). Chemotherapy is the most
commonly used therapeutic regimen for the majority of patients with
NSCLC, and significant progress has been achieved with targeted or
immunotherapeutic agents for the treatment of NSCLC (4). However, the 5-year survival rate of
patients with NSCLC still remains very low (5). The high mortality and low survival rate
of patients with NSCLC are largely attributed to the capability of
NSCLC cells to invade normal tissues, resulting in metastasis to
remote sites (6). Thus, the
development of novel strategies on anti-invasion and antimetastatic
treatment for NSCLC are urgently required. The complex pathogenesis
of NSCLC indicates that the combination of two or more antitumor
agents possessing different molecular mechanisms may be a
therapeutic option for efficient treatment (7).
Gemcitabine is a deoxycytidine analogue that has
been approved as a first-line therapy drug for NSCLC for almost a
decade (8). Gemcitabine has also
been approved for the treatment of ovarian, advanced lung and
pancreatic cancer due to its profound antitumor activity (9–11).
However, drug resistance and the adverse side effects (such as
anemia and leukopenia) associated with gemcitabine limit its
chemotherapeutic efficacy (12).
Previous studies have identified potential agents that may be used
in combination with gemcitabine as effective chemotherapeutic
regimes (13,14). For example, combination of
gemcitabine and carboplatin has been verified as an effective
adjuvant chemotherapeutic strategy for patients with completely
resected NSCLC in a phase II study (15). In addition, gemcitabine in
combination with nab-paclitaxel has been used in patients with
metastatic breast cancer (14).
Sorafenib is a non-selective multi-kinase inhibitor
that has the ability to suppress tumor cell proliferation and
angiogenesis, primarily by restraining the activities of targets in
tumor cells, such as the RAF/mitogen-activated protein kinase 1
(MEK)/ERK signaling pathway, vascular endothelial growth factor
receptor-3 and platelet-derived growth factor receptor β (16). Sorafenib has also been reported to
inhibit transforming growth factor-β1-induced
epithelial-to-mesenchymal transition (EMT) in A549 cells (17). The antitumor effect of sorafenib in
preclinical NSCLC models has been frequently reported (18,19).
Sorafenib treatment is associated with adverse side effects,
including hand-foot skin diseases, hypertension and diarrhea
(20), which results in
implementation of low doses, thus effecting efficacy. Thus,
development of novel strategies are required in order to relieve
the side effects or enhance the antitumor activity of sorafenib
(21,22).
Combination treatment with various agents is a
potential strategy to improve the synergistic therapeutic efficacy,
as different agents exhibit their therapeutic effects via different
molecular mechanisms, leading to a synergistic anticancer response
(23). In addition, combination
therapy has been demonstrated to overcome the harmful side effects
associated with high-dose drugs (24). For example, a randomized phase II
study revealed that combination treatment with gemcitabine and
sorafenib is well tolerated and was demonstrated to be efficient in
patients with advanced NSCLC compared with other combination
treatments (25). However, the
molecular mechanisms underlying the combination treatment with
gemcitabine and sorafenib in NSCLC remain largely unknown. The
present study aimed to investigate the effect of combination
therapy with gemcitabine and sorafenib for NSCLC in vitro
and in vivo.
Materials and methods
Materials and antibodies
Sorafenib was purchased from Dalian Meilun Biotech
Co., Ltd., and gemcitabine was purchased from Shanghai Yuanye
Biotech Co., Ltd. MTT was supplied by Sigma-Aldrich; Merck KGaA.
Primary antibodies against N-cadherin, E-cadherin and Twist-1 were
purchased from Cell Signaling Technology, Inc., whereas the
antibody against GAPDH and horseradish peroxidase-conjugated
secondary antibodies against rabbit or mouse IgG were purchased
from Weiao Biotechnology Co., Ltd.
Cell culture
A549, H1975 and H1650 cell lines were purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Cells were maintained in RPMI-1640 medium (Corning Inc.)
supplemented with 10% heat-inactivated FBS (Gibco; Thermo Fisher
Scientific, Inc.), and 100 µg/ml penicillin and 100 U/ml
streptomycin (Beyotime Institute of Biotechnology) at 37°C with 5%
CO2.
MTT assay
A549, H1975 and H1650 cells were seeded into 96-well
plates at a density of 4×103 cells/well and treated with
0–100 µg/ml gemcitabine or 0–50 µM sorafenib at 37°C for 48 h.
Subsequently, the cells were incubated with 0.5 mg/ml MTT at 37°C
for 4 h to determine cell viability. Following the MTT incubation,
the purple formazan crystals were dissolved in 150 µl DMSO, and
cell viability was subsequently analyzed using a microplate reader
(BioTek Instruments, Inc.) at a wavelength of 570 nm.
Morphological observation
A549 cells were incubated with 10 µg/ml gemcitabine
or 10 µM sorafenib at 37°C for 48 h. The morphology of the tumor
cells was observed using an inverted microscope (Nikon, Japan).
Magnification, ×400.
Flow cytometric analysis of the cell
cycle
Following treatment with 10 µg/ml gemcitabine and 10
µM sorafenib at 37°C for 48 h, A549 cells were collected and
subsequently fixed with 70% ethanol at 4°C for 12 h. The samples
were washed with pre-cooled PBS and incubated with 100 µg/ml of
RNaseA (Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 30 min. The
cells were subsequently stained with 50 µg/ml of propidium iodide
(Nanjing KeyGen Biotech Co., Ltd.) at 37°C for 15 min and cells
were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
The results were analyzed using Flow Jo software version 7.6.1
(TreeStar).
Flow cytometric analysis of
apoptosis
Following treatment with 10 µg/ml gemcitabine and 10
µM sorafenib at 37°C for 48 h, A549 cells were collected using
centrifugation at 800 × g at 4°C for 10 min. Then, cells were
stained with annexin V-fluorescein isothiocyanate (FITC) and PI
using the Annexin V-FITC Apoptosis Detection kit (Nanjing KeyGen
Biotech Co., Ltd.). Briefly, the cells were incubated with Annexin
V-FITC/PI at 37°C for 20 min, and apoptotic cells were subsequently
analyzed using a FACSCalibur flow cytometer (BD Biosciences);
Annexin V+/PI+ and Annexin
V+/PI− cells were considered to be apoptotic.
A549 cells treated with 100 nM docetaxel at 37°C for 48 h were used
as positive control. The data was analyzed using CellQuest software
version 3.1 (BD Biosciences).
Western blotting
Total protein was extracted from A549 cells and
tumor tissue homogenates using RIPA cell lysis buffer (Nanjing
KeyGen Biotech Co., Ltd.), and quantified using the BCA Protein
Quantitation kit (Nanjing KeyGen Biotech Co., Ltd.). Equal amounts
of total protein (20 µg) were separated by 12% SDS-PAGE and
subsequently transferred to polyvinylidene difluoride membranes.
Membrane blocking and incubation with the primary and secondary
antibodies were performed as previously described (26). Protein bands were visualized using
the Enhanced Chemiluminescent Detection kit (Pierce; Thermo Fisher
Scientific, Inc.). The intensity of resulting bands were quantified
using ImageJ version 1.47 (National Institutes of Health).
Reverse-transcription quantitative
(RT-qPCR)
Total RNA was extracted from A549 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), reverse transcribed into cDNA using the SuperScript First
Strand Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
at 42°C for 50 min according to the manufacturer's protocol. qPCR
was detected using the BeyoFast™ SYBR Green qPCR Mix (Beyotime
Institute of Biotechnology; cat. no. D7260) on Applied
Biosystems® 7500 Real-Time PCR Systems (Thermo Fisher
Scientific, Inc.). The thermocycling conditions were used as
follows: 95°C for 20 sec, followed by 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. The following primers sequences were used for
qPCR: E-cadherin forward, 5′-TGCGCGTGAAGGTTTGCCAGT-3′ and reverse,
5′-ACGTTGTCCCGGGTGTCATCCT-3′; N-cadherin forward,
5′-CATCATCATCCTGCTTATCCTTGT-3′ and reverse,
5′-GGTCTTCTTCTCCTCCACCTTCT-3′; Twist-1 forward,
5′-GCATGCATTCTCAAGAGGT-3′ and reverse, 5′-CAGTTTGATCCCAGCGTTTT-3′;
and GAPDH forward, 5′-GGACCTGACCTGCCGTCTAG-3′ and reverse,
5′-GTAGCCCAGGATGCCCTTGA-3′. Relative expression levels were
quantified using the 2−ΔΔCq method (27) and normalized to the internal
reference gene GAPDH.
Wound healing assay
Cell migration was assessed using the wound healing
assay. Briefly, A549 cells were cultured with 1640 medium
(containing 10% FBS) in a 12-well plates until they reached a
confluent monolayer, and the cell monolayers were subsequently
scratched using a 10 µl pipette tip. Culture medium was replaced
with fresh culture medium (no FBS) containing 10 µg/ml gemcitabine
or 10 µM sorafenib, followed by incubation for 0, 12, 24 and 48 h
at 37°C. Migratory cells were counted in five randomly selected
fields using an inverted light microscope at ×200 magnification
(Nikon).
Invasion assay
Chamber inserts (8-µm pore; Corning, Inc.) were
precoated with 300 µg/ml Matrigel for 30 min at 37°C according to
the manufacturer's instructions. A total of ~5×103 A549
cells were plated in the upper chambers of Transwell plates in
RPMI-1640 supplemented with 10% FBS and incubated in the presence
of 10 µg/ml gemcitabine or 10 µM sorafenib. A total of 500 µl
RPMI-1640 medium supplemented with 20% FBS was placed in the lower
chambers. Following incubation at 37°C for 48 h, invasive cells on
the lower chambers were stained with 0.1% crystal violet at 37°C
for 15 min. Stained cells were washed twice with PBS and counted in
randomly selected 5 fields using an inverted microscope (Nikon),
magnification, ×400. Subsequently, crystal violet was dissolved
using 33% acetic acid, and the invasive ability of the cells was
measured at a wavelength of 590 nm using a microplate reader
(BioTek Instruments, Inc.).
Tumor xenograft experiments
All experimental protocols involving animals were
approved by the Institutional Animal Committee of Shanghai Ninth
People's Hospital (Shanghai, China). A549 cells (1×106
cells/ml in 1 ml PBS) were subcutaneously injected into the right
thigh of BALB/c nude mice (4–6 weeks old; 18–22 g, Charles River
Laboratories, Inc.). The mice were housed at 24–26°C with 50–70%
humidity and 12-h day/night cycle and provided with free access to
food and water. The mice were randomized into four groups (6
mice/group) as follows: i) Vehicle (methylcellulose/Tween 80,
intraperitoneal injection); ii) sorafenib (40 mg/kg/day,
intraperitoneal injection); iii) gemcitabine (50 mg/kg/day,
intraperitoneal injection); and iv) sorafenib (40 mg/kg/day,
intraperitoneal injection) + gemcitabine (50 mg/kg/day,
intraperitoneal injection). The body weights of the mice was
measured twice/week, the tumor size was measured every other day
using calipers, and tumor volumes were determined using the
following formula: Volume=length × (width)2/2. The mice
were sacrificed before the end of the experiment if the maximum
tumor diameter reached 2 cm, and all mice were sacrificed after 28
days using CO2.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc.). Combination index (CI) of
gemcitabine and sorafenib was calculated by CalcuSyn 2.0 software
(Biosoft). CI values <1, =1 and >1 indicated synergism,
additive effect and antagonism, respectively. Data are presented as
the mean ± standard deviation. Differences between two groups were
analyzed using unpaired Student's t-test (2-tailed), whereas
one-way analysis of variance followed by Tukey's post hoc test was
used to compare differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Dose-dependent cytotoxicity of
gemcitabine and sorafenib to A549 cells
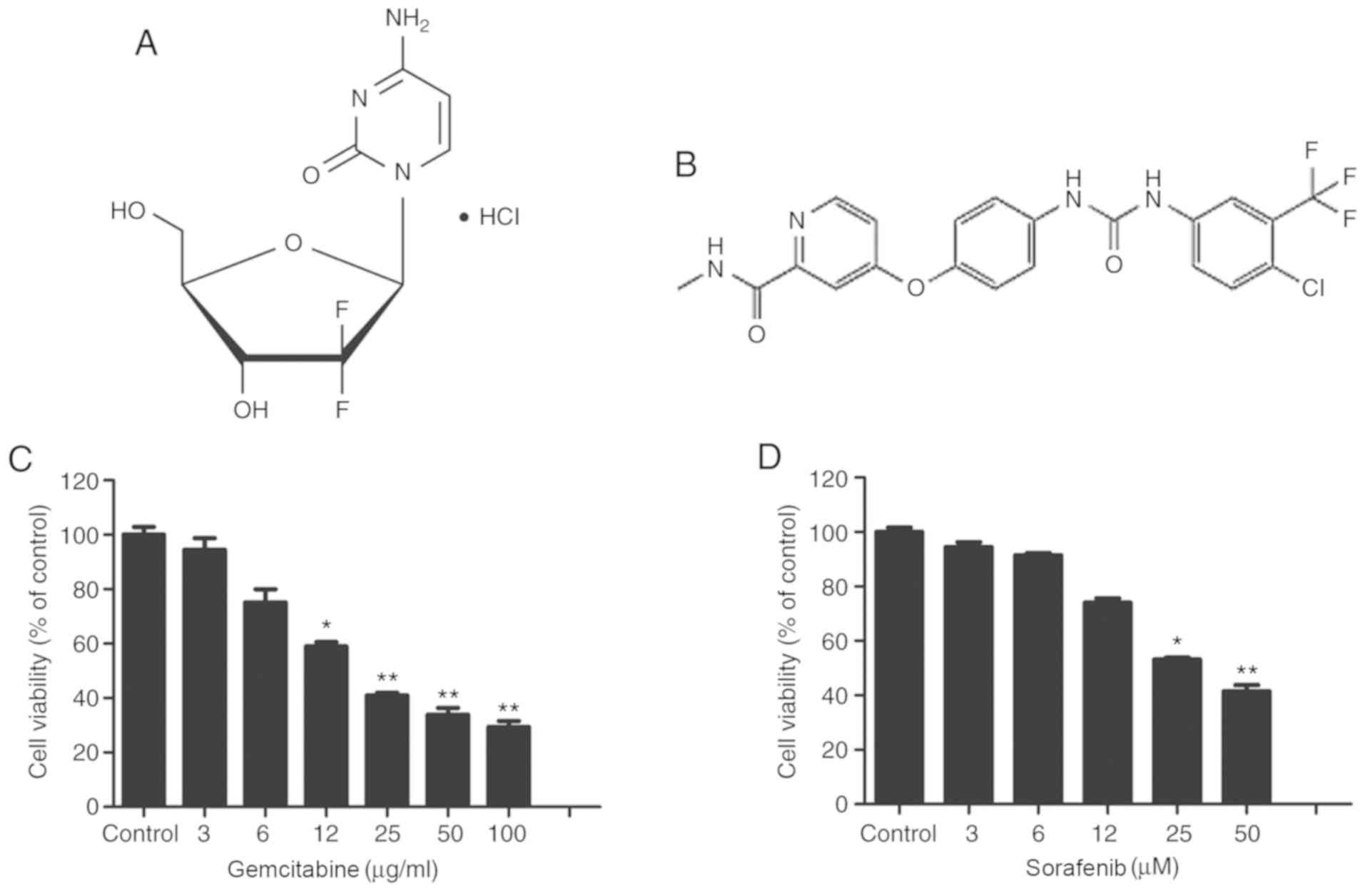
Fig. 1A and B
illustrate the molecular structures of gemcitabine and sorafenib,
respectively. Gemcitabine is a deoxycytidine analogue that has been
approved as a first-line therapy drug for NSCLC. Conversely,
sorafenib is a non-selective multi-kinase inhibitor, which has been
approved for the treatment of hepatocellular carcinoma. The present
study assessed the cytotoxic effects of gemcitabine and sorafenib
as single agents on A549 cells using the MTT assay. The results
demonstrated that treatment of A549 cells with varying
concentrations of gemcitabine (0–100 µg/ml) or sorafenib (0–100 µM)
for 48 h inhibited the viability of tumor cells in a dose-dependent
manner (P<0.05), and the half maximal inhibitory concentration
values of gemcitabine and sorafenib in A549 cells were 19.2 µg/ml
and 44.8 µM, respectively (Fig. 1C and
D).
Synergistic antitumor effects of
gemcitabine and sorafenib on A549 cells
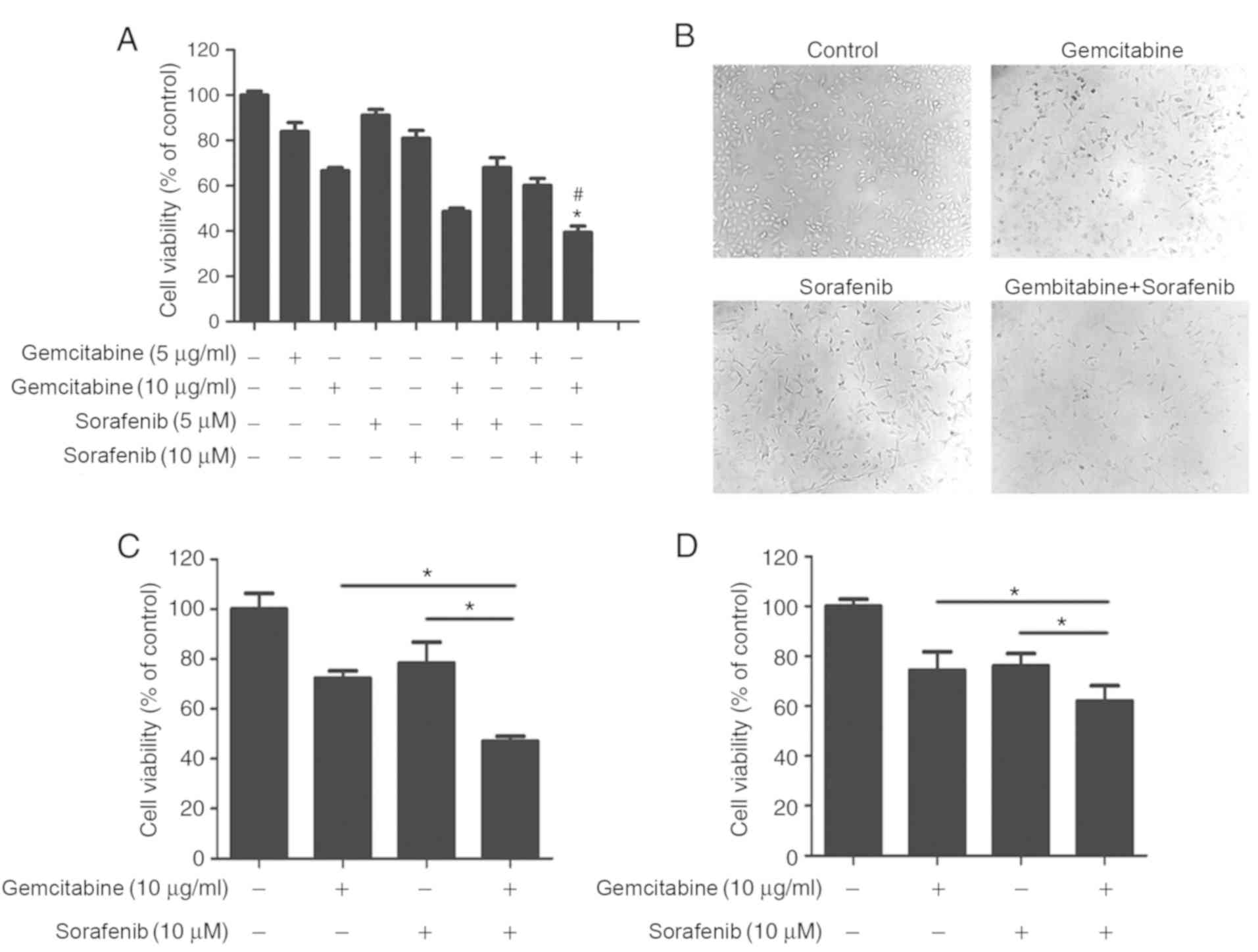
To determine whether a coadjutant antitumor effect
existed between gemcitabine and sorafenib, A549 cells were treated
with different concentrations of gemcitabine (5 µg/ml or 10 µg/ml)
and sorafenib (5 µM or 10 µM) either individually or in
combination. The results demonstrated that combination treatment
significantly inhibited A549 cell viability compared with
gemcitabine or sorafenib treatment alone (P<0.05, Fig. 2A), and the CI value calculated by
Calcusyn 2.0 software was 0.65 with 10 µg/ml gemcitabine and 10 µM
sorafenib. Subsequently, cell morphology was assessed, which
demonstrated regular spindle-shaped cells in the control group,
whereas irregular shapes were observed in cells treated with
gemcitabine or sorafenib alone. Cells in the combination treatment
group displayed multiple changes compared with the control group,
including decreased volume and irregular shapes (Fig. 2B). The effect of the combination
treatment was also assessed in NSCLC H1650 and H1975 cell lines.
The results demonstrated stronger cytotoxicity in H1650 and H1975
cells in the combination treatment group than sorafenib and
gemcitabine treated groups (P<0.05), Fig. 2C and D). Overall, cell viability
analysis indicated that gemcitabine and sorafenib exerted a
synergistic effect on NSCLC cells.
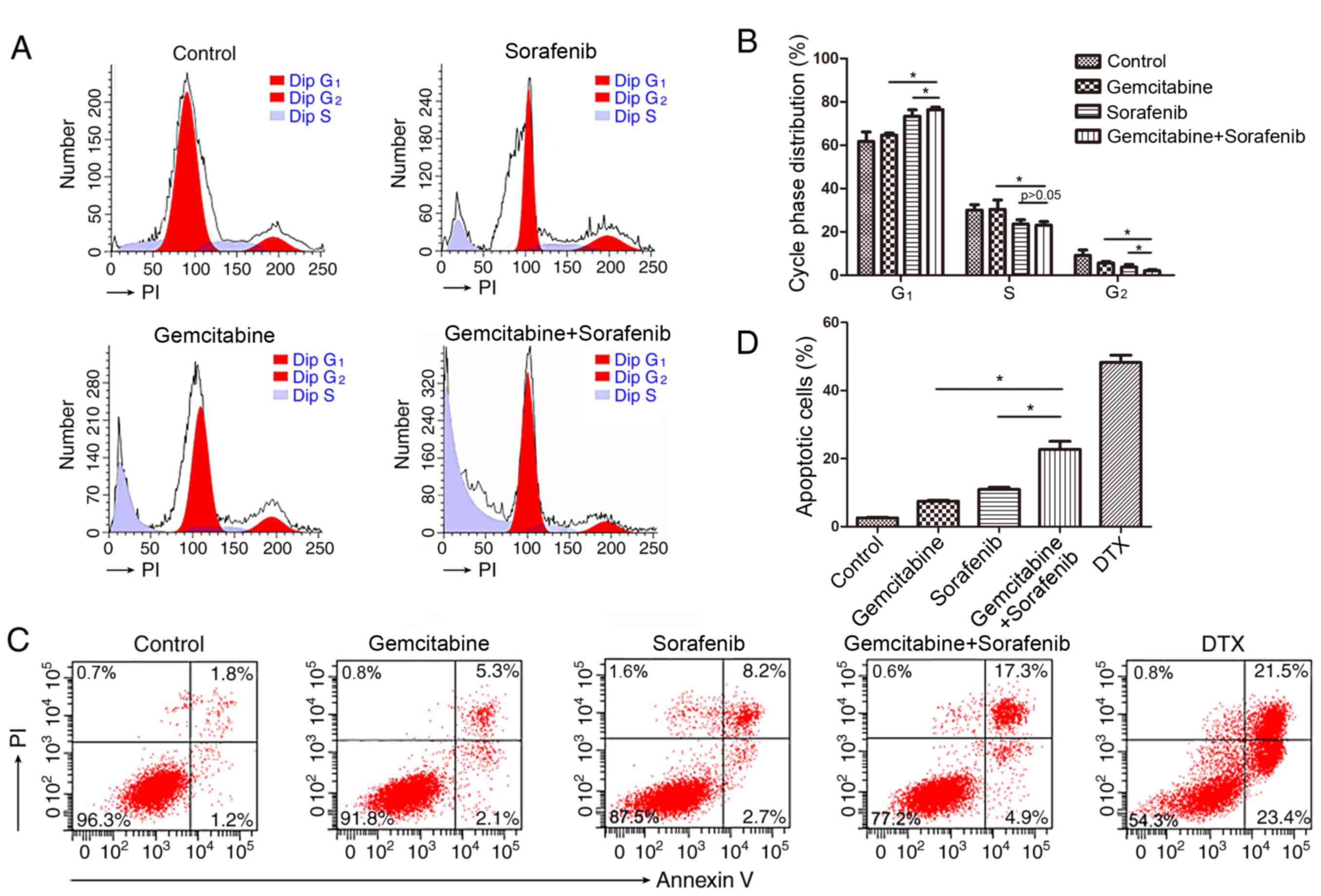
Combination of gemcitabine and sorafenib induces
cell cycle arrest and apoptosis in A549 cells. Previous studies
have reported that both gemcitabine and sorafenib disturb the cell
cycle of tumor cells (21,28). Thus, the present study investigated
the combined effects of gemcitabine and sorafenib on the cell
cycle. The results demonstrated that co-treatment induced a
significant increase in the number of cells in the G1
phase, while decreasing the number of cells in the S phase compared
with the control group, indicating that a G1-phase
arrest was induced in A549 cells by the combination treatment
(P<0.05), Fig. 3A and B).
Furthermore, a higher proportion of apoptotic cells (22.2%) was
detected in the combination treatment group compared with
gemcitabine (7.4%) or sorafenib (10.9%) monotherapy (P<0.05,
Fig. 3C-D).
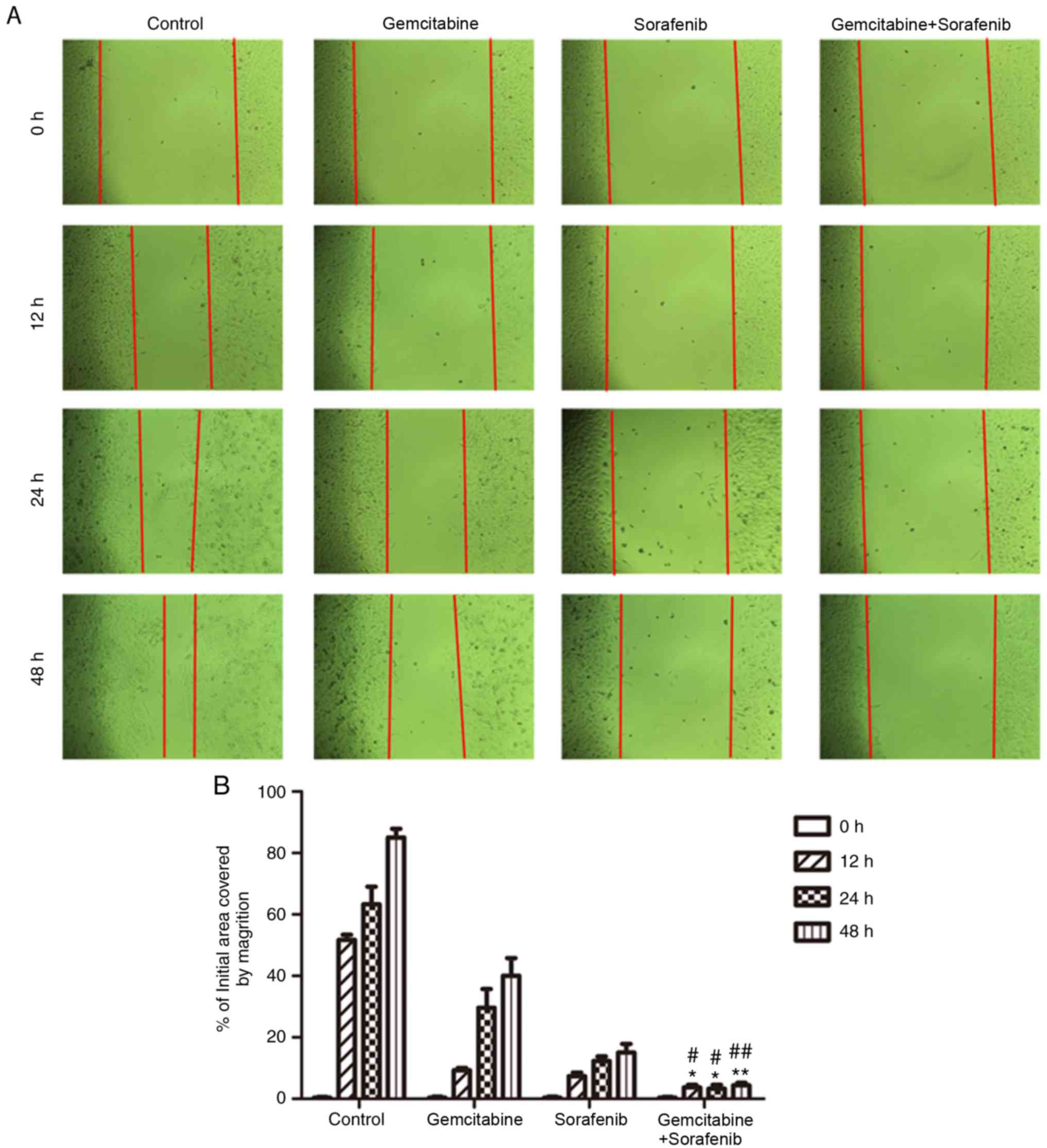
Effects of combination treatment on
A549 cell migration
The wound healing assay was performed to detect the
effects of combination treatment on the migratory ability of A549
cells. The results demonstrated that the scratch of cell monolayers
in the negative control group was almost healed, and that of
monotherapy groups was gradually closed following treatment for 48
h (Fig. 4A). However, the scratch of
the combination treatment group still remained wide apart at 48 h
(P<0.05, Fig. 4B). Taken
together, the results indicated that the migratory ability of A549
cells was notably inhibited following co-treatment with gemcitabine
and sorafenib.
Effects of combination treatment on
the invasive ability of A549 cells
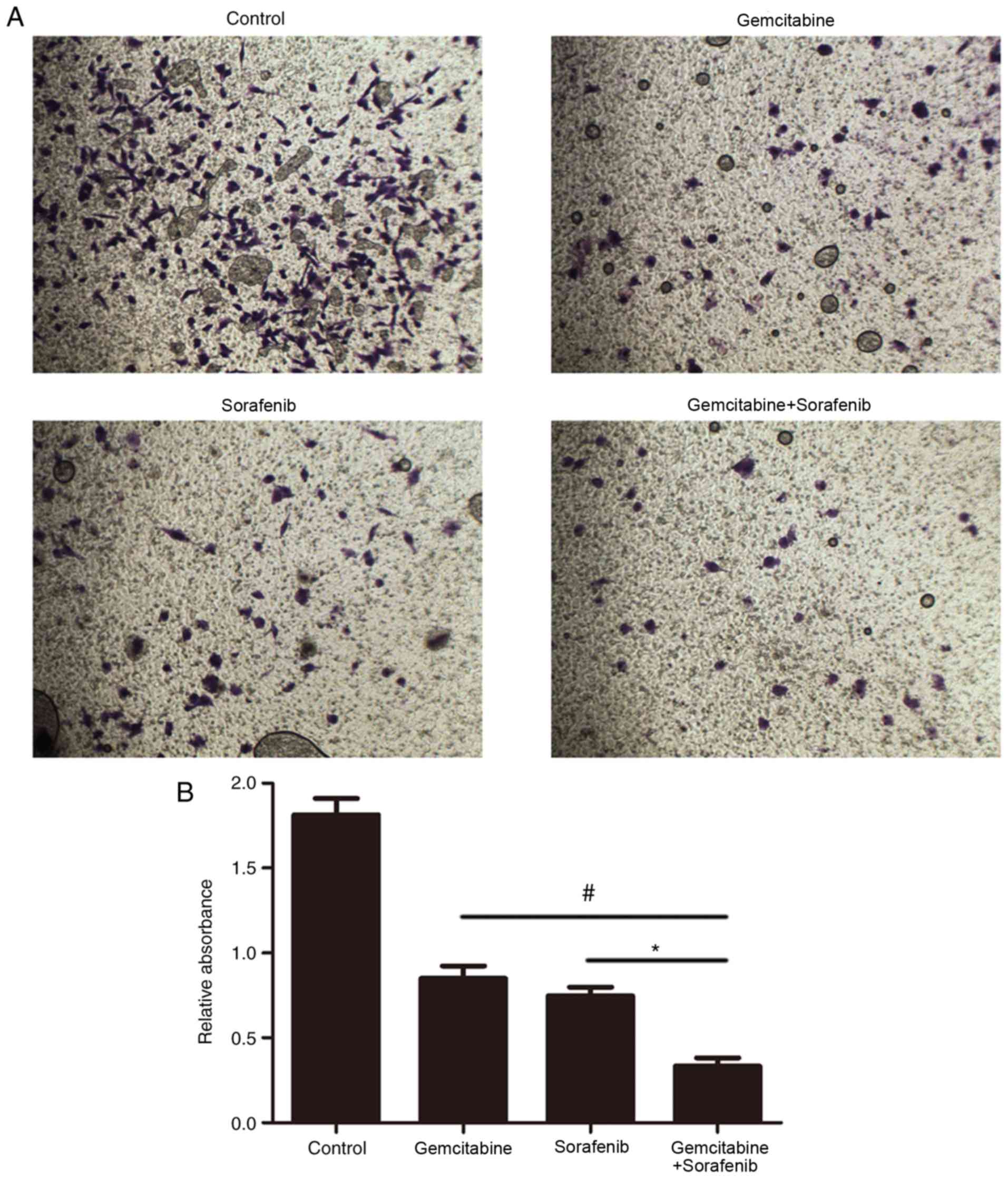
The effect of combination treatment on cell invasion
was subsequently investigated. The results demonstrated that the
number of invasive A549 cells in the combination treatment group
was significantly decreased compared with the control and
monotherapy groups (P<0.05, Fig. 5A
and B). These results suggested that combination treatment with
gemcitabine and sorafenib may inhibit the invasion of A549
cells.
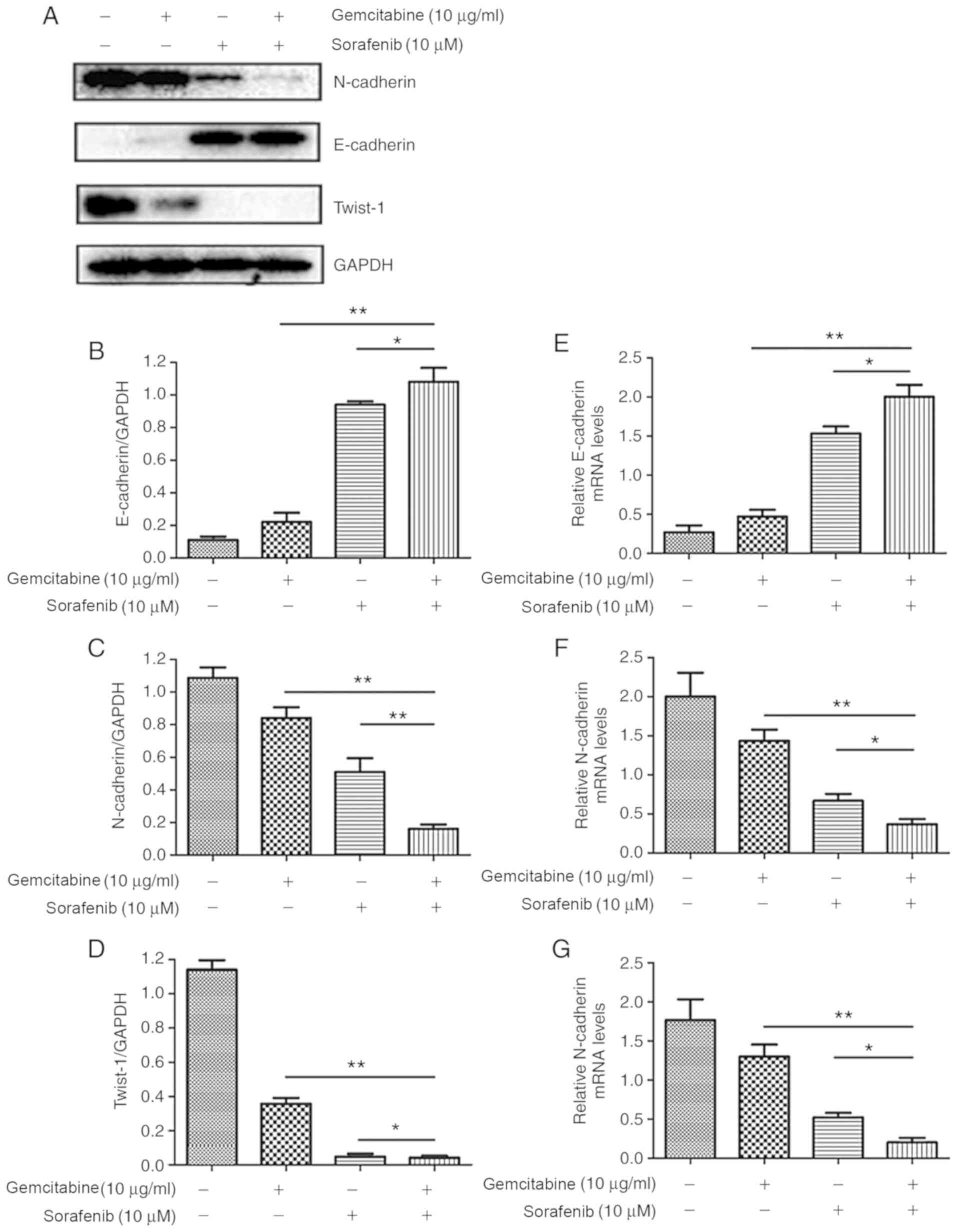
Combination treatment suppresses the
EMT of A549 cells
In order to further investigate the molecular
mechanisms underlying the effects of combination treatment on the
migration and invasion of A549 cells, expression levels of
EMT-associated proteins were assessed by western blotting. The
results demonstrated that co-treatment with gemcitabine and
sorafenib upregulated the expression of the epithelial marker
E-cadherin, whereas the expression levels of the mesenchymal
markers N-cadherin and Twist-1 were downregulated compared with the
basal level of the monotherapy and the negative control groups
(P<0.05, Fig. 6A-D). Furthermore,
RT-qPCR analysis indicated that N-cadherin and Twist-1 mRNA
expression levels were significantly lower in the combination
treatment group compared with the control and monotherapy groups,
whereas the expression of E-cadherin was increased (P<0.05,
Fig. 6E-G).
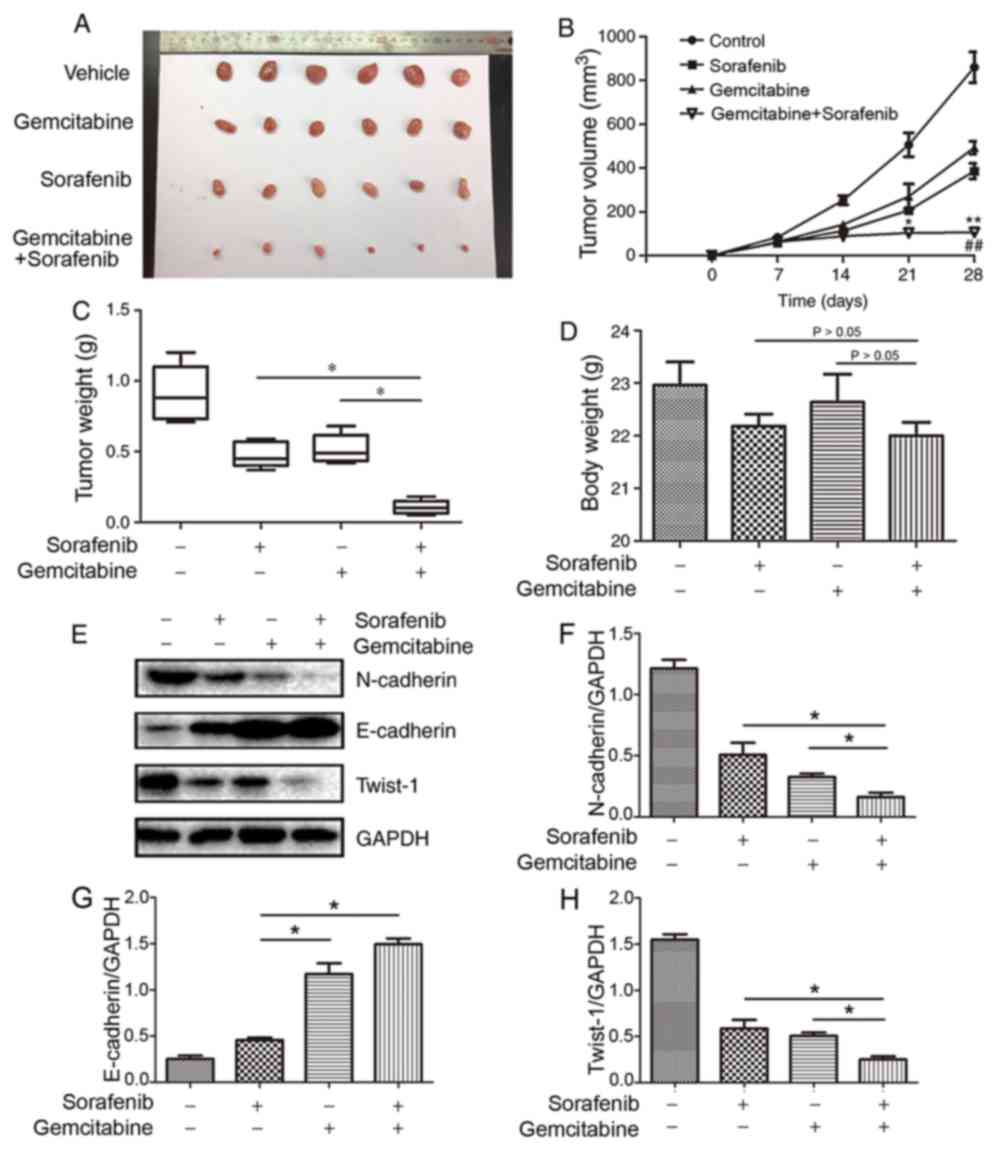
Synergistic antitumor effects of
combined treatment in NSCLC xenograft model
To further determine whether gemcitabine and
sorafenib exerted synergistic anti-NSCLC effects in vivo,
nude mice were subcutaneously injected with A549 cells to establish
a subcutaneous xenotransplanted tumor model. The results
demonstrated that co-treatment with gemcitabine and sorafenib
significantly inhibited tumor growth, as the average tumor volumes
were decreased by 54.1 and 41.8% compared with gemcitabine or
sorafenib alone, respectively (P<0.05, Fig. 7A and B). Furthermore, the combination
treatment group exhibited significantly decreased tumor weight
compared with the monotherapy groups (P<0.05, Fig. 7C), whereas no significant differences
were observed regarding the body weight (P>0.05, Fig. 7D). The protein expression levels of
N-cadherin and Twist-1 in the tumor tissues of the combination
treatment group were significantly decreased in the co-treatment
group compared with the monotherapy groups, whereas E-cadherin
expression increased (P<0.05, Fig.
7E-H). Taken together, these results suggested that combination
treatment with gemcitabine and sorafenib exerted antitumor effects
via the EMT process in vivo.
Discussion
Invasion and metastasis greatly contribute to the
poor prognosis of patients with NSCLC. The majority of cases of
relapsed NSCLC can be attributed to intractable late-stage
metastatic disease (29). Distant
metastases are confirmed in almost half of all clinically diagnosed
NSCLC cases (30). Multilevel
cross-reactions among the different targets in the metastatic
progression of lung cancer cells have been identified, and
suppression of one target allows others to act as immune escape
molecular mechanisms for tumor cells (31). Combination treatment consisting of
two or more anticancer agents is considered to be more effective in
inhibiting tumor progression compared with single-targeted agents
(32). A previous study has reported
that combination treatment of C2-ceramide and sorafenib has a
synergistic interaction in Bel-7402 cells via the PI3K/AKT/mTOR and
ERK signaling pathways (31).
Similarly, Li et al (33)
have demonstrated that the combination of gemcitabine and sorafenib
exhibits a synergistic effect in A549 cells by inhibiting epidermal
growth factor receptor (EGFR)-tyrosine kinase inhibitor
(TKI)-sensitive and EGFR-TKI-resistant cells. In addition,
gemcitabine inhibits micrometastasis of NSCLC by targeting
epithelial cellular adhesion molecule-positive circulating tumor
cells via the HGF/cMET signaling pathway (34). Previous studies have reported that
sorafenib suppresses cell migration and invasion by inhibiting the
MET and MEK/ERK signaling pathways (35,36). Li
et al (37) have confirmed
that sorafenib and gemcitabine or pemetrexed have synergistic
effects by inducing apoptosis and cell cycle arrest, and inhibiting
proliferation of lung cancer cells. However, the underlying
molecular mechanisms of gemcitabine in combination with sorafenib
on cell migration and invasion in NSCLC remain unclear. The present
study investigated whether gemcitabine and sorafenib exerted a
synergistic inhibitory effect on NSCLC in vitro and in
vivo, and aimed to determine the potential molecular mechanisms
underlying these interactions. The results demonstrated that
co-treatment with gemcitabine and sorafenib markedly induced the
cytotoxicity and cell cycle arrest in A549 cells. In addition, the
combination treatment was demonstrated to significantly inhibit the
invasion and migration of NSCLC cells by Transwell invasion and
wound healing assays. Taken together, the results of the present
study suggested that combination treatment with gemcitabine and
sorafenib inhibited the metastatic capability of NSCLC cells,
indicating its potential as a novel treatment option for NSCLC.
The EMT process serves an important role in cancer
metastasis and invasion (38).
During cancer progression, cells lose the cell-matrix contact,
cell-cell connections and normal epithelial polarity, while
simultaneously adopting mesenchymal characteristics that allow
migration into the surrounding matrices (39,40).
During EMT, the expression levels of mesenchymal markers such as
N-cadherin are upregulated, whereas the epithelial cell markers
such as E-cadherin are downregulated (41). Twist-1 is a transcriptional factor
that belongs to the basic helix-loop-helix family; previous studies
have reported that Twist-1 is an EMT inducer in the progression of
NSCLC (42,43). In the present study, inhibition of
EMT was observed in vitro and in vivo following
combination treatment, as demonstrated by the notably downregulated
expression of N-cadherin and Twist-1, as well the markedly
upregulated expression of E-cadherin, at both the mRNA and protein
levels.
In conclusion, the results of the present study
demonstrated that combination treatment with gemcitabine and
sorafenib exerted antimetastatic and anti-invasive effects by
inhibiting EMT in A549 cells. In addition, combination treatment
demonstrated synergistic cytotoxicity to NSCLC cells in
vitro and in vivo. Thus, combination treatment with
gemcitabine and sorafenib may provide valuable insights into the
development of synergetic anticancer agents.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81703779), the Fund of
Science and Technology Commission Shanghai Municipality (grant no.
17401900800) and the Fund of Shanghai Jiao Tong University School
of Medicine (grant no. JDYX2017QN007).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SSJ and YFY conceived and designed the present
study. SSJ, RW, FHW, XZ and SNL performed the experiments. SSJ
wrote the paper. RW, XZ, SNL, FHW and YFY reviewed and edited the
manuscript. All authors read and approved the final manuscript and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols involving animals were
approved by the Institutional Animal Committee of Shanghai Ninth
People's Hospital (Shanghai, China) (approval no.
SH9H-2019-A296-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
References
|
1
|
Kobayashi K, Seike M, Zou F, Noro R, Chiba
M, Ishikawa A, Kunugi S, Kubota K and Gemma A: Prognostic
significance of NSCLC and response to EGFR-TKIs of EGFR-mutated
NSCLC based on PD-L1 expression. Anticancer Res. 38:753–762.
2018.PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vasconcellos VF, Marta GN, da Silva EM,
Gois AF, de Castria TB and Riera R: Cisplatin versus carboplatin in
combination with third-generation drugs for advanced non-small cell
lung cancer. Cochrane Database Syst Rev. 1:CD0092562020.PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bremnes RM, Veve R, Hirsch FR and Franklin
WA: The E-cadherin cell-cell adhesion complex and lung cancer
invasion, metastasis, and prognosis. Lung Cancer. 36:115–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiang H, Chang Q, Xu J, Qian J, Zhang Y,
Lei Y, Han B and Chu T: New advances in antiangiogenic combination
therapeutic strategies for advanced non-small cell lung cancer. J
Cancer Res Clin Oncol. 146:631–645. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohutek F, Stratena M, Rosik A, Tamasova M
and Bystricky B: First-line treatment of nonsquamous NSCLC using
gemcitabine: A retrospective study of real-life practice. Lung
Cancer Manag. 5:123–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu BD, Guo J, Ye YZ, Du T, Cheng CS, Jiang
Q, Liu RN and Zhang YB: Specific inhibitor of Notch3 enhances the
sensitivity of NSCLC cells to gemcitabine. Onco Rep. 40:155–164.
2018.
|
|
10
|
Komiyama S, Kugimiya T, Takeya C,
Takahashi R and Kubushiro K: Platinum-resistant recurrent ovarian
cancer with long survival on bevacizumab and gemcitabine. J Obstet
Gynaecol Res. 44:1330–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Lee SC, Oh SY, Song SY, Lee N, Nam
EM, Lee S, Hwang IG, Lee HR, Lee KT, et al: Attenuated FOLFIRINOX
in the salvage treatment of gemcitabine-refractory advanced
pancreatic cancer: A phase II study. Cancer Commun (Lond).
38:322018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yalçin S, Erkan M, Ünsoy G, Parsian M,
Kleeff J and Gündüz U: Effect of gemcitabine and retinoic acid
loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic
cancer and stellate cell lines. Biomed Pharmacother. 68:737–743.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B and Tao ZZ: Piceatannol enhances the
antitumor efficacy of gemcitabine in human A549 non-small cell lung
cancer cells. Onco Res. 22:213–217. 2014. View Article : Google Scholar
|
|
14
|
Yoshitomi S, Taira N, Doihara H, Mizoo T,
Nogami T, Iwamoto T, Motoki T, Shien T, Ogasawara Y, Matsuoka J, et
al: A phase 1, dose-finding and pharmacokinetic study of
gemcitabine with nab-paclitaxel in patients with metastatic breast
cancer. Cancer Chemother Pharmacol. 78:289–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakurai R, Tomizawa Y, Yoshii A, Miura Y,
Tsurumaki H, Kaira K, Sunaga N, Kawashima O, Hisada T, Yamada M and
Saito R: A phase II study of biweekly gemcitabine and carboplatin
in completely resected stage IB-IIIA non-small cell lung cancer.
Cancer Chemother Pharmacol. 81:103–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Gold KA and Kim E: Sorafenib in
non-small cell lung cancer. Expert OpinInvestig Drugs.
21:1417–1426. 2012. View Article : Google Scholar
|
|
17
|
Zhang J, Chen YL, Ji G, Fang W, Gao Z, Liu
Y, Wang J, Ding X and Gao F: Sorafenib inhibits
epithelial-mesenchymal transition through an epigenetic-based
mechanism in human lung epithelial cells. PLoS One. 8:e649542013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takezawa K, Okamoto I, Yonesaka K,
Hatashita E, Yamada Y, Fukuoka M and Nakagawa K: Sorafenib inhibits
non-small cell lung cancer cell growth by targeting B-RAF in KRAS
wild-type cells and C-RAF in KRAS mutant cells. Cancer Res.
69:6515–6521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ngamphaiboon N, Dy GK, Ma WW, Zhao Y,
Reungwetwattana T, DePaolo D, Ding Y, Brady W, Fetterly G and Adjei
AA: A phase I study of the histone deacetylase (HDAC) inhibitor
entinostat, in combination with sorafenib in patients with advanced
solid tumors. Invest New Drugs. 33:225–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jean GW, Mani RM, Jaffry A and Khan SA:
Toxic effects of sorafenib in patients with differentiated thyroid
carcinoma compared with other cancers. JAMA Oncol. 2:529–534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pignochino Y, Dell'Aglio C, Basiricò M,
Capozzi F, Soster M, Marchiò S, Bruno S, Gammaitoni L, Sangiolo D,
Torchiaro E, et al: The combination of sorafenib and everolimus
abrogates mTORC1 and mTORC2 upregulation in osteosarcoma
preclinical models. Clin Cancer Res. 19:2117–2131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pal HC, Baxter RD, Hunt KM, Agarwal J,
Elmets CA, Athar M and Afaq F: Fisetin, a phytochemical,
potentiates sorafenib-induced apoptosis and abrogates tumor growth
in athymic nude mice implanted with BRAF-mutated melanoma cells.
Oncotarget. 6:28296–28311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Swarnakar NK, Thanki K and Jain S:
Enhanced antitumor efficacy and counterfeited cardiotoxicity of
combinatorial oral therapy using Doxorubicin- and Coenzyme
Q10-liquid crystalline nanoparticles in comparison with intravenous
Adriamycin. Nanomedicine. 10:1231–1241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Das M, Jain R, Agrawal AK, Thanki K and
Jain S: Macromolecular bipill of gemcitabine and methotrexate
facilitates tumor-specific dual drug therapy with higher
benefit-to-risk ratio. Bioconjug Chem. 25:501–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gridelli C, Morgillo F, Favaretto A, de
Marinis F, Chella A, Cerea G, Mattioli R, Tortora G, Rossi A,
Fasano M, et al: Sorafenib in combination with erlotinib or with
gemcitabine in elderly patients with advanced non-small-cell lung
cancer: A randomized phase II study. Ann Oncol. 22:1528–1534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang S, Fan J, Wang Q, Ju D, Feng M, Li
J, Guan ZB, An D, Wang X and Ye L: Diosgenin induces ROS-dependent
autophagy and cytotoxicity via mTOR signaling pathway in chronic
myeloid leukemia cells. Phytomedicine. 23:243–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamed SS, Straubinger RM and Jusko WJ:
Pharmacodynamic modeling of cell cycle and apoptotic effects of
gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother
Pharmacol. 72:553–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia Q, Zhang X, Chen Q, Chen X, Teng J,
Wang C, Li M and Fan L: Down-regulation of tissue factor inhibits
invasion and metastasis of non-small cell lung cancer. J Cancer.
11:1195–1202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morgensztern D, Ng SH, Gao F and Govindan
R: Trends in stage distribution for patients with non-small cell
lung cancer: A national cancer database survey. J Thorac Oncol.
5:29–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang S, Wang Q, Feng M, Li J, Guan Z, An
D, Dong M, Peng Y, Kuerban K and Ye L: C2-ceramide enhances
sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and
Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol.
101:1535–1546. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petrelli A and Giordano S: From single- to
multi-target drugs in cancer therapy: When aspecificity becomes an
advantage. Curr Med Chem. 15:422–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Pan YY and Zhang Y: Synergistic
interaction between sorafenib and gemcitabine in EGFR-TKI-sensitive
and EGFR-TKI-resistant human lung cancer cell lines. Oncol Lett.
5:440–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liao ZJ, Guo YH, Zhao Z, Yao JT, Xu R and
Nan KJ: Gemcitabine inhibits the micrometastasis of non-small cell
lung cancer by targeting the EpCAM-positive circulating tumor cells
via the HGF/cMET pathway. Int J Oncol. 45:651–658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cervello M, Bachvarov D, Lampiasi N,
Cusimano A, Azzolina A, McCubrey JA and Montalto G: Molecular
mechanisms of sorafenib action in liver cancer cells. Cell Cycle.
11:2843–2855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pasqualetti G, Ricciardi S, Mey V, Del
Tacca M and Danesi R: Synergistic cytotoxicity, inhibition of
signal transduction pathways and pharmacogenetics of sorafenib and
gemcitabine in human NSCLC cell lines. Lung Cancer. 74:197–205.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Wang S, Su ZF and Yuan Y:
Synergistic effects of sorafenib in combination with gemcitabine or
pemetrexed in lung cancer cell lines with K-ras mutations. Contemp
Oncol (Pozn). 20:33–38. 2016.PubMed/NCBI
|
|
38
|
Guo R, Hu T, Liu Y, He Y and Cao Y: Long
non-coding RNA PRNCR1 modulates non-small cell lung cancer cells
proliferation, apoptosis, migration, invasion and EMT through
PRNCR1/miR-126-5p/MTDH axis. Biosci Rep BSR20193153. 2020.
|
|
39
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial- mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garg M: Epithelial, mesenchymal and hybrid
epithelial/mesenchymal phenotypes and their clinical relevance in
cancer metastasis. Expert Rev Mol Med. 19:e32017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He R, Zhang FH and Shen N: LncRNA
FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through
suppressing E-cadherin and regulating WNT pathway in non-small cell
lung cancer (NSCLC). Biomed Pharmacother. 95:331–338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsai CH, Lin LT, Wang CY, Chiu YW, Chou
YT, Chiu SJ, Wang HE, Liu RS, Wu CY, Chan PC, et al:
Over-expression of cofilin-1 suppressed growth and invasion of
cancer cells is associated with up-regulation of let-7 microRNA.
Biochim Biophys Acta. 1852:851–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Li C, Yang Y, Liu X, Li R, Zhang M,
Yin Y and Qu Y: Synaptotagmin 7 in twist-related protein 1-mediated
epithelial-Mesenchymal transition of non-small cell lung cancer.
EBioMedicine. 46:42–53. 2019. View Article : Google Scholar : PubMed/NCBI
|