Introduction
Bladder cancer (BC) is a common urological cancer,
and is recognized as an inflammatory and immunogenic disease
(1). Patients with advanced or
metastatic BC have a poor prognosis despite treatment with standard
chemotherapies including combination therapy with gemcitabine and
cisplatin (GC therapy) (2).
Furthermore, in patients with muscle-invasive BC, the 2-year
recurrence-free survival rate was reported to be 60.9% if pT0 was
not obtained by neo-adjuvant GC chemotherapy (3). Accordingly, information regarding the
pathological characteristics of BC cells is important to improve
outcomes for patients with BC. Specifically, understanding the
molecular mechanisms underlying cancer cell growth, invasion, and
metastasis is essential to formulate new treatment strategies for
these patients.
c-Met is a receptor tyrosine kinase that binds a
specific ligand, namely hepatocyte growth factor (HGF). In many
types of cancer, the HGF/c-Met system is important for malignant
potential, cancer cell invasion, metastasis, and determining
clinical outcomes (4,5). In fact, cancer cell proliferation, cell
cycle, migration, and angiogenesis were previously suggested as
HGF/c-Met-related pathological mechanisms, based on in vivo
and in vitro studies (6–8).
Furthermore, c-Met is closely associated with the regulation of
various cancer-related molecules such as cyclooxygenase (COX)-2,
heme oxygenase (HO)-1, and vascular endothelial growth factor
(VEGF)-A in various types of malignancies (9–12). In
recent years, the HGF/c-Met system has also been reported to
promote carcinogenesis and cancer cell progression by regulating
the immune system in various types of cancers (10,13).
Specifically, programmed cell death ligand 1 (PD-L1) is a
representative immune checkpoint inhibitor expressed on various
types of cancer cells that has been reported to downregulate the
immune response (14,15). Interestingly, a study has reported
that c-Met promotes cancer cell survival though the regulation of
PD-L1 expression in renal cell carcinoma (RCC) cells (10); however, several other reports have
supported the positive correlation between c-Met and PD-L1
expression in cancer tissues (12,16).
Thus, c-Met is recognized as a key modulator of various malignant
behaviors that functions by regulating cancer-related molecules and
the immune system via PD-L1.
As it relates to BC, c-Met has been shown to be
positively associated with malignant cell behavior and poor
prognosis (5,17). Furthermore, COX-2, HO-1, and VEGF-A
were reported to be closely associated with carcinogenesis,
malignant potential, and prognosis for BC (7,18,19).
Recent studies have also reported that PD-L1 expression in BC cells
has important roles in malignancy, progression, chemo-resistance,
and disease outcome in patients with BC (20,21).
However, little information is available regarding the
relationships between c-Met and COX-2, HO-1, VEGF-A, or PD-L1 in
human BC tissues.
Further, when the pathological significance of c-Met
in BC is discussed, we should note that its phosphorylation is
essential for its biological effects (17). Briefly, under various physiological
and pathological conditions, the phosphorylation of major
phosphorylation sites, specifically the kinase domain (Y1234/1235)
and the multifunctional docking domain (Y1349/1356), leads to an
increase in intrinsic activities and biological functions such as
cell motility and transformation (22,23).
With respect to the pathological significance of c-Met
phosphorylation in cancers, a previous report demonstrated that the
expression of phospho-c-Met (Y1349), termed pY1349 c-Met, is
positively associated with cancer growth, progression, and poor
survival in patients with RCC (18).
Likewise, one report indicated that high pY1235 c-Met expression is
associated with an increased risk of recurrence for ovarian cancer
patients (24); meanwhile, in
patients with BC, several reports have shown that phosphorylated
c-Met leads to highly malignant disease and poor survival (25,26).
However, the precise pathological significance of phosphorylated
c-Met in BC is not fully understood. In fact, the relationship
between phosphorylated c-Met expression and metastasis in these
patients has not yet been characterized. Furthermore, no study has
reported the relationships between phosphorylated c-Met and COX-2,
HO-1, VEGF-A, and PD-L1 in human BC tissues. Based on these
previous findings, herein, we focused on the relationships between
c-Met, pY1349 c-Met, and, pY1234/1235 c-Met expression and grade,
TNM classification, and the expression of COX-2, HO-1, VEGF-A, and
PD-L1 in patients with BC.
Materials and methods
Patients
We investigated 185 formalin-fixed paraffin-embedded
BC specimens from patients diagnosed with urothelial cancer via
histopathological examination. Patients who received neoadjuvant
therapy were excluded. In this study, T stage was also divided into
non-muscle invasive BC (Ta and T1) and muscle invasive BC (MIBC;
T2-4), as well as into absence of metastasis (N0 and M0) and
presence of metastasis (N1-3 and/or M1) groups for multivariate
analyses. This study protocol was approved by the Institutional
Review Board of Nagasaki University Hospital (12052899), and
written informed consent was provided by all patients.
Immunohistochemistry
The expression of all proteins was evaluated by
immunohistochemical techniques. An anti-c-Met antibody (Zymed
Laboratories Inc.) and two phospho-specific antibodies against
human c-Met antibodies (pY1234/1235 and pY1349; Cell Signaling
Technology) were used, the specificities of which were previously
confirmed to detect the immunoreactivity of phosphorylated c-Met in
several malignant tissues (25,27,28).
Other primary antibodies included anti-VEGF-A (Santa Cruz
Biotechnology), anti-COX-2 (Immuno-Biological Laboratories Co.),
anti-HO-1 (Enzo Life Sciences Inc.), and anti-PD-L1 (clone E1L3N,
Cell Signaling Technology, Inc.). Immunohistochemical staining and
evaluation were performed according to previous reports (19,25,29,30). In
short, five-micrometer-thick sections were deparaffinized in xylene
and rehydrated in solutions of ethanol. Antigen retrieval was
performed at 95°C for 40 min in 0.01 mol/l sodium citrate buffer
(pH 6.0) and then immersed in 3% hydrogen peroxide for 30 min.
Sections were incubated overnight with the primary antibodies at
4°C. The sections were then incubated with peroxidase using the
labeled polymer method with Dako EnVision+ Peroxidase (Dako;
Agilent Technologies, Inc.), and the peroxidase reaction was
visualized with the liquid 3,3-diaminobenzidine tetrahydrochloride
substrate. Sections were counterstained with hematoxylin. As a
positive control, RCC tissue was stained for HGFR/c-Met,
phosphorylated HGFR/c-Met, and COX-2, and a spleen section was
stained for HO-1 and PD-L1. A consecutive section from each sample,
processed without the primary antibody, was used as a negative
control. Further, save for that of PD-L1, the expression of all
molecules was evaluated semi-quantitatively based on staining
intensity and the percentage of stained cancer cells, as previously
described (19,25). For PD-L1 expression, the percentage
of PD-L1-positive cancer cells was above the threshold of 1%
according to a previous report (30). Such evaluations were performed using
a Nikon E-400 microscope and a digital imaging system (DU100;
Nikon). In addition, a computer-aided image analysis system (Win
ROOF, version 5.0; Mitani Corp.) was utilized to support this
evaluation.
Statistical analyses
The Mann-Whitney U test was used to compare
continuous variables. The χ2 test was used for
categorical comparisons of data. The crude and adjusted effects
were estimated by logistic regression analysis [described as odds
ratios (ORs) with 95% confidence intervals (95% CIs), together with
P-values]. All statistical analyses were performed with the
statistical package StatView for Windows (version 5.0; Abacus
Concept Inc.), and statistical significance of differences was
defined as P<0.05.
Results
Immunohistopathological
examinations
We previously showed examples of positively stained
tissues of c-Met, pY1234/1235 c-Met, pY1349 c-Met, COX-2, HO-1, and
VEGF-A in patients with urothelial cancer including BC (18,19,25,31).
Their staining patterns in this study were similar to those in
these previous reports. Therefore, in this study, we showed
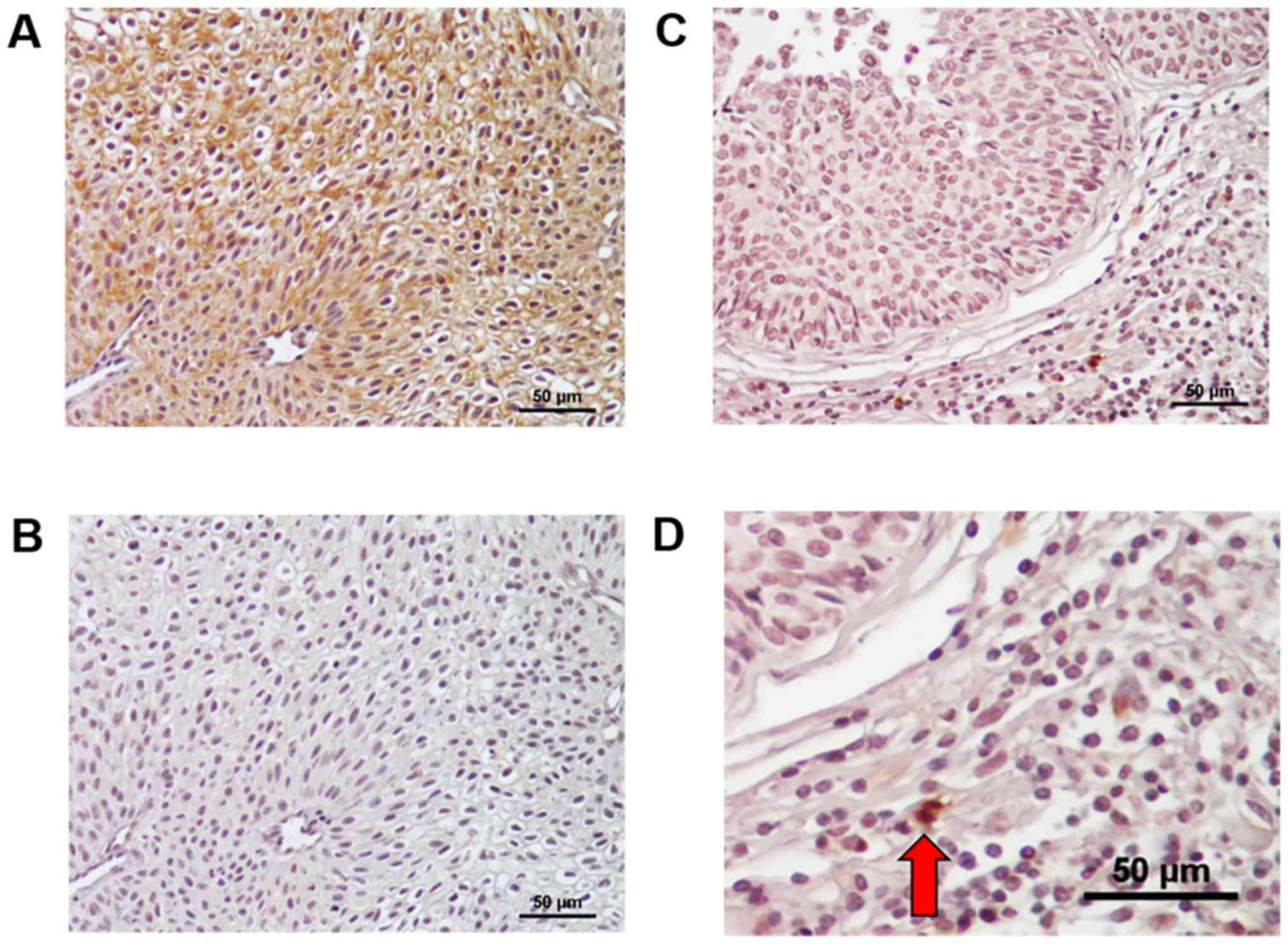
representative figures of PD-L1 expression in BC tissues in
Fig. 1. PD-L1 was mainly detected at
the cell membrane and partially in the cytoplasm of BC cells
(Fig. 1A), and such positive
staining was not detected in negative control of a consecutive
section (Fig. 1B). Further,
expression was found in infiltrating cells of stromal tissues from
some MIBC tissues; however, there were few cancer stromal cells in
NMIBC tissues (Fig. 1C and D).
Although representative examples of other cancer-related molecules
including c-Met, pY1234/1235 c-Met, and pY1349 c-Met were shown in
our previous reports (19,25,27),
similar staining patterns were confirmed in this study. Among 185
BC specimens, positive c-Met, pY1234/1235 c-Met, and pY1349 c-Met
expression was detected in 109 (58.9%), 59 (31.9%), and 82 (44.3%)
cases, respectively. Further, 120 (64.9%), 101 (54.6%), 104
(56.2%), and 80 (43.2%) specimens were judged positive for the
expression of COX-2, HO-1, VEGF-A, and PD-L1, respectively.
Correlations between
clinicopathological features and c-Met, pY1234/1235 c-Met, or
pY1349 c-Met expression
As shown in Table I,
positive expression of c-Met, pY1234/1235 c-Met, and pY1349 c-Met
was significantly associated with high grade (P=0.004, P=0.042,
P<0.001, respectively) and T stage (P=0.013, P=0.002, and
P<0.001, respectively). Moreover, the expression of pY1234/1235
c-Met and pY1349 c-Met was associated with N stage (P=0.003 and
0.006, respectively) and M stage (P=0.008 and 0.027, respectively);
however, such significant associations were not found for c-Met
expression. In addition, as shown in Table I, similar relationships were detected
between c-Met, pY1234/1235 c-Met, or pY1349 c-Met and muscle
invasion (T2-4) or metastasis (N1-3 and/or M1). The expression of
c-Met tended to be positively correlated with metastasis; however,
this did not reach statistical significance (P=0.083).
 | Table I.Associations with clinicopathological
features. |
Table I.
Associations with clinicopathological
features.
|
| c-Met, n | pY1234/1235 c-Met,
n | pY1349 c-Met,
n |
|---|
|
|
|
|
|
|---|
| Variable | Negative | Positive | Negative | Positive | Negative | Positive |
|---|
| Grade |
|
|
|
|
|
|
|
Low | 45 | 41 | 65 | 21 | 60 | 26 |
|
High | 31 | 68 | 61 | 38 | 43 | 56 |
|
P-value |
| 0.004 |
| 0.042 |
| <0.001 |
| T stage |
|
|
|
|
|
|
| Ta | 29 | 24 | 41 | 12 | 39 | 14 |
| T1 | 37 | 47 | 62 | 22 | 50 | 34 |
| T2 | 6 | 20 | 14 | 12 | 8 | 18 |
| T3 | 3 | 12 | 8 | 7 | 5 | 10 |
| T4 | 1 | 6 | 1 | 6 | 1 | 6 |
|
P-value |
| 0.013 |
| 0.002 |
| <0.001 |
| MIBC |
|
|
|
|
|
|
|
Absence | 66 | 71 | 103 | 34 | 89 | 48 |
|
Presence | 10 | 38 | 23 | 25 | 14 | 34 |
|
P-value |
| 0.001 |
| 0.001 |
| <0.001 |
| N stage |
|
|
|
|
|
|
| N0 | 74 | 102 | 124 | 52 | 102 | 74 |
|
N1-3 | 2 | 7 | 2 | 7 | 1 | 8 |
|
P-value |
| 0.238 |
| 0.003 |
| 0.006 |
| M stage |
|
|
|
|
|
|
| M0 | 74 | 99 | 122 | 51 | 100 | 73 |
| M1 | 2 | 10 | 4 | 8 | 3 | 9 |
|
P-value |
| 0.076 |
| 0.008 |
| 0.027 |
| Metastasis |
|
|
|
|
|
|
|
Absence | 73 | 97 | 121 | 49 | 100 | 70 |
|
Presence | 3 | 12 | 5 | 10 | 3 | 12 |
|
P-value |
| 0.083 |
| 0.003 |
| 0.004 |
Independent roles of c-Met,
pY1234/1235 c-Met, or pY1349 c-Met in muscle invasion or
metastasis
We next analyzed the independent relationships
between c-Met, pY1234/1235 c-Met, or pY1349 c-Met and muscle
invasion or metastatic status using multivariate logistic
regression analysis models. Similar to the results of univariate
analyses, c-Met, pY1234/1235 c-Met, and pY1349 c-Met levels were
all independently associated with muscle invasion, whereas only the
expression of pY1234/1235 c-Met and pY1349 c-Met was associated
with metastasis (Table II).
Regarding the relationship between c-Met and metastasis,
multivariate analysis showed no significant correlation
(P=0.190).
 | Table II.Multivariate analyses for muscle
invasion and metastasis. |
Table II.
Multivariate analyses for muscle
invasion and metastasis.
| Clinicopathological
features | OR | 95% CI | P-value |
|---|
| For muscle
invasiona |
|
|
|
|
c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 2.70 | 1.16–6.28 | 0.021 |
|
pY1234/1235 c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 2.98 | 1.37–6.46 | 0.006 |
| pY1349
c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 3.27 | 1.49–7.14 | 0.003 |
| For
metastasisb |
|
|
|
|
c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 2.43 | 0.65–9.12 | 0.190 |
|
pY1234/1235 c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 4.33 | 1.31–13.50 | 0.012 |
| pY1349
c-Met |
|
|
|
|
Negative | 1.00 | – | – |
|
Positive | 4.59 | 1.22–17.33 | 0.025 |
Association between cancer-related
molecules and c-Met, pY1234/1235 c-Met, and pY1349 c-Met
Univariate analyses showed that COX-2 expression was
significantly associated with pY1234/1235 c-Met (OR=2.49; P=0.012)
and pY1349 c-Met (OR=2.98; P=0.010); however, multivariate logistic
regression analysis, adjusted by grade, muscle invasion, and
metastasis, demonstrated that COX-2 expression was only
independently associated with pY1349 c-Met (OR=2.30; P=0.017;
Table III). Further, univariate
and multivariate analyses showed that both HO-1 and PD-L1 were
significantly associated with c-Met and pY1349 c-Met expression,
but not with pY1234/1235 c-Met expression (Table III). In contrast, VEGF-A expression
was not associated with the expression of c-Met, pY1234/1235 c-Met,
or pY1349 c-Met, even by univariate analysis (Table III).
 | Table III.Association between c-Met-related
parameters and cancer-related molecules. |
Table III.
Association between c-Met-related
parameters and cancer-related molecules.
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| For COX-2 |
|
|
|
|
|
|
| c-Met;
positive | 1.68 | 0.91–3.09 | 0.985 | 1.31 | 0.69–2.48 | 0.418 |
|
pY1234/1235 c-Met;
positive | 2.49 | 1.22–5.06 | 0.012 | 1.94 | 0.92–4.08 | 0.080 |
| pY1349
c-Met; positive | 2.98 | 1.56–5.72 | 0.010 | 2.30 | 1.16–4.58 | 0.017 |
| For HO-1 |
|
|
|
|
|
|
| c-Met;
positive | 2.37 | 1.30–4.32 | 0.005 | 2.02 | 1.08–3.78 | 0.028 |
|
pY1234/1235 c-Met;
positive | 1.33 | 0.71–2.48 | 0.378 | 0.99 | 0.51–1.95 | 0.984 |
| pY1349
c-Met; positive | 2.52 | 1.38–4.61 | 0.003 | 2.02 | 1.07–3.84 | 0.031 |
| For VEGF-A |
|
|
|
|
|
|
| c-Met;
positive | 1.28 | 0.71–2.31 | 0.412 | 0.99 | 0.53–1.85 | 0.972 |
|
pY1234/1235 c-Met;
positive | 1.21 | 0.64–2.26 | 0.560 | 0.95 | 0.48–1.86 | 0.870 |
| pY1349
c-Met; positive | 1.08 | 0.60–1.95 | 0.788 | 0.75 | 0.39–1.43 | 0.375 |
| For PD-L1 |
|
|
|
|
|
|
| c-Met;
positive | 4.25 | 2.22–8.15 | <-0.001 | 3.53 | 1.78–7.00 | <0.001 |
|
pY1234/1235 c-Met;
positive | 1.74 | 0.93–3.25 | 0.082 | 1.18 | 0.59–2.34 | 0.643 |
| pY1349
c-Met; positive | 4.20 | 2.26–7.80 | <0.001 | 3.17 | 1.64–6.12 | 0.001 |
Correlation between pathological
characteristics and COX-2, HO-1, VEGF-A, and PD-L1
As shown in Table
IV, univariate analyses showed that COX-2 expression was
positively correlated with VEGF-A (OR=2.28; P=0.009) and PD-L1
(OR=3.12; P=0.001), and multivariate analysis adjusted by grade,
muscle invasion, and metastasis confirmed these significant
correlations (OR=2.08; 95% CI=1.08–4.04; P=0.030 and OR=2.61; 95%
CI=1.29–5.27; P=0.008, respectively). In contrast, HO-1 expression
tended to be positively associated with PD-L1 expression (OR=1.77,
P=0.060); however, an independent correlation was not found by
multivariate analysis (Table IV).
Furthermore, there was no significant correlation between COX-2
expression and HO-1, HO-1 expression and VEGF-A, or VEGF-A
expression and PD-L1. Thus, HO-1 is not significantly correlated
with the expression of COX-2, VEGF-A, and PD-L1 (Table IV).
 | Table IV.Associations among cancer-related
molecules. |
Table IV.
Associations among cancer-related
molecules.
|
| Univariate
analysis | Multivariate
analysisa |
|---|
|
|
|
|
|---|
| Cancer-related
molecules | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Cox-2;
positive |
|
|
|
|
|
|
| HO-1;
positive | 1.54 | 0.84–2.82 | 0.166 | 1.26 | 0.67–2.38 | 0.471 |
| VEGF-A;
positive | 2.28 | 1.23–4.22 | 0.009 | 2.08 | 1.08–4.04 | 0.030 |
| PD-L1;
positive | 3.12 | 1.61–6.03 | 0.001 | 2.61 | 1.29–5.27 | 0.008 |
| HO-1; positive |
|
|
|
|
|
|
| COX-2;
positive | 1.54 | 0.84–2.82 | 0.166 | 1.21 | 0.63–2.32 | 0.576 |
| VEGF-A;
positive | 1.22 | 0.68–2.18 | 0.509 | 1.01 | 0.54–1.89 | 0.972 |
| PD-L1;
positive | 1.77 | 0.98–3.12 | 0.060 | 1.31 | 0.69–2.49 | 0.406 |
| VEGF-A;
positive |
|
|
|
|
|
|
| COX-2;
positive | 2.28 | 1.23–4.22 | 0.009 | 2.08 | 1.07–4.02 | 0.030 |
| HO-1;
positive | 1.22 | 0.68–2.18 | 0.509 | 1.00 | 0.54–1.88 | 0.995 |
| PD-L1;
positive | 0.72 | 0.61–1.97 | 0.759 | 0.72 | 0.37–1.39 | 0.323 |
| PD-L1;
positive |
|
|
|
|
|
|
| COX-2;
positive | 3.12 | 1.61–6.03 | 0.001 | 2.61 | 1.29–5.27 | 0.007 |
| HO-1;
positive | 1.76 | 0.98–3.19 | 0.060 | 1.30 | 0.69–2.48 | 0.419 |
| VEGF-A;
positive | 1.10 | 0.61–1.97 | 0.759 | 0.72 | 0.37–1.40 | 0.331 |
Correlation between cancer-related
molecules and muscle invasion or metastasis
The pathological roles of these cancer-related
molecules with respect to muscle invasion and metastasis are shown
in Table V. Univariate analyses
showed that levels of COX-2, HO-1, VEGF-A, and PD-L1 were all
associated with muscle invasive disease (OR=3.56; P=0.003, OR=2.23;
P=0.024, OR=2.65; P=0.008, and OR=3.71; P<0.001, respectively);
however, multivariate analyses demonstrated that only COX-2
expression was independently associated with muscle invasion
(OR=2.64; 95% CI=1.02–6.81; P=0.045; Table V). A similar analysis showed that
HO-1 and PD-L1 were associated with metastasis (OR=6.06; P=0.020
and OR=9.99; P=0.003, respectively) by univariate analysis;
however, only PD-L1 expression was identified as an independent
factor by multivariate analysis (OR=5.51; 95% CI=1.12–27.2;
P=0.036; Table V).
 | Table V.Association between cancer-related
molecules and malignant behaviour. |
Table V.
Association between cancer-related
molecules and malignant behaviour.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Cancer-related
molecules | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| For muscle
invasiona |
|
|
|
|
|
|
| COX-2;
positive | 3.56 | 1.55–8.18 | 0.003 | 2.64 | 1.02–6.81 | 0.045 |
| HO-1;
positive | 2.23 | 1.11–4.48 | 0.024 | 1.36 | 0.59–3.14 | 0.465 |
| VEGF-A;
positive | 2.65 | 1.29–5.45 | 0.008 | 2.10 | 0.87–5.04 | 0.098 |
| PD-L1;
positive | 3.71 | 1.86–7.43 | <0.001 | 2.20 | 0.97–4.96 | 0.058 |
| For
metastasisb |
| COX-2;
positive | 3.83 | 0.84–17.5 | 0.084 | 1.80 | 0.35–9.30 | 0.485 |
| HO-1;
positive | 6.06 | 1.33–27.7 | 0.020 | 4.24 | 0.86–20.9 | 0.076 |
| VEGF-A;
positive | 1.62 | 0.53–4.93 | 0.398 | 0.83 | 0.23–2.92 | 0.766 |
| PD-L1;
positive | 9.99 | 2.19–45.7 | 0.003 | 5.51 | 1.12–27.2 | 0.036 |
Pathological roles of c-Met, pY1234/1235 c-Met, and
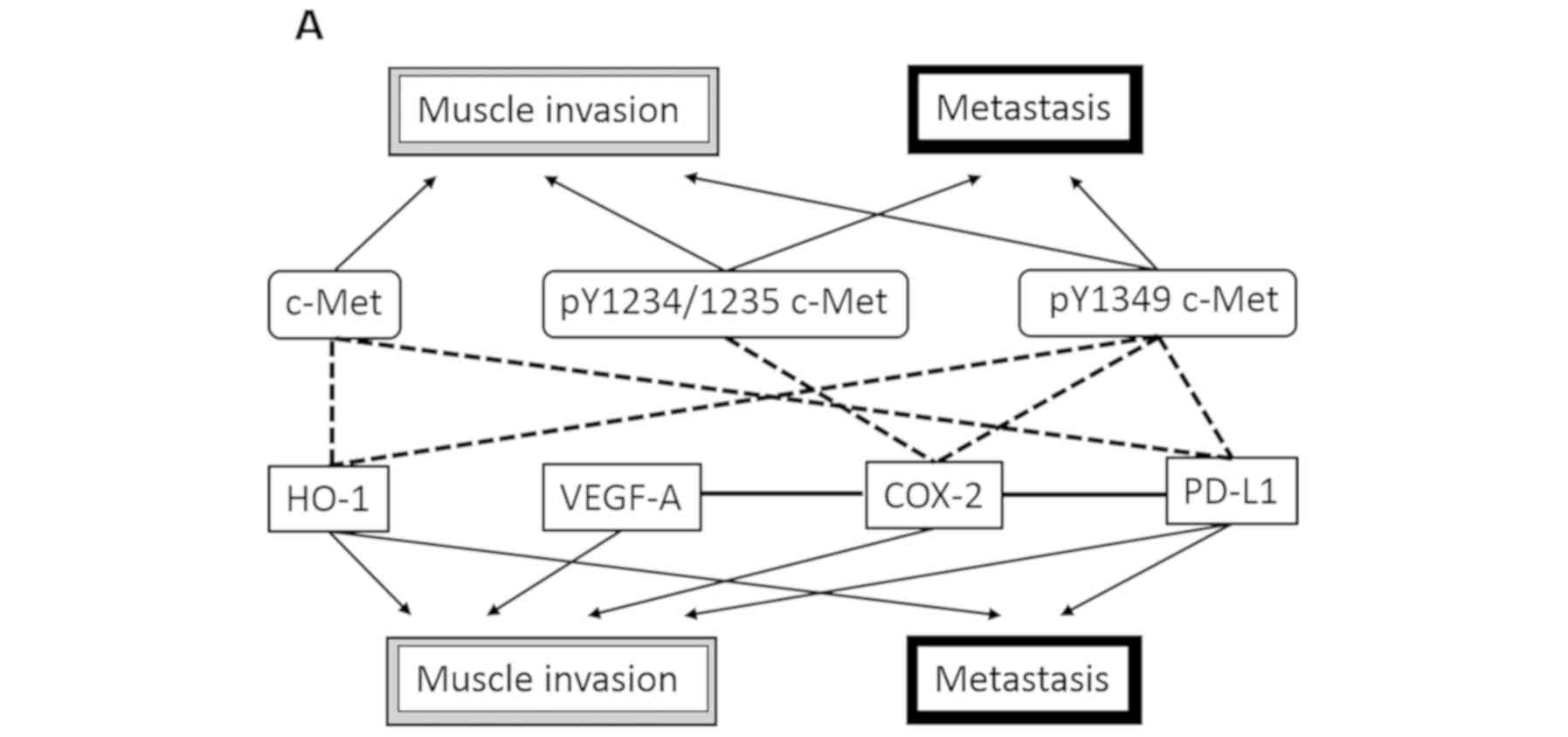
pY1349 c-Met. Finally, we present a schema of the pathological
roles of c-Met, pY1234/1235 c-Met, and pY1349 c-Met in Fig. 2. Univariate analyses showed that
pY1234/1235 c-Met and pY1349 c-Met were associated with both muscle
invasion and metastasis and that c-Met was significantly correlated
with BC muscle invasion only. Furthermore, complex mechanisms
comprising COX-2, HO-1, VEGF-A, and PD-L1 were speculated to be
linked to these pathological effects of c-Met signaling. In
contrast, multivariate analyses showed similar results regarding
the relationship between c-Met pathways and pathological
characteristics such as muscle invasion and metastasis (Fig. 2). However, although COX-2 and PD-L1
were thought to play significant roles in muscle invasion and
metastasis, respectively, HO-1 and VEGF-A was not associated with
either process (Fig. 2). In
addition, as shown in Fig. 2,
pY1234/1235 c-Met expression was not significantly correlated with
any of COX-2, HO-1, VEGF-A, and PD-L1.
Discussion
The present study showed that the levels of
pY1234/1235 c-Met and pY1349 c-Met are closely associated with
muscle invasion and metastasis in patients with BC. c-Met
expression was significantly associated with muscle invasion but
not with metastasis based on similar analyses. In contrast, our
previous report showed that levels of c-Met, pY1234/1235 c-Met, and
pY1349 c-Met were positively associated with pT stage by univariate
analyses; however, only pY1349 c-Met expression was independently
associated with pT stage based on a multivariate analysis model
(25). Thus, there was a difference
regarding the relationship between the expression of c-Met or
pY1234/1235 and cancer cell invasion between these two reports. We
speculated that this discrepancy was due to differences in
clinicopathological features and methodology caused by the study
population. In short, the previous study was performed based on 133
patients without metastatic BC, and the multivariate analysis model
did not include metastasis. The present study demonstrated, for the
first time, that the expression of both pY1234/1235 c-Met and
pY1349 c-Met was significantly associated with metastasis, whereas
c-Met expression was not, in patients with BC. These results
suggest that c-Met phosphorylation is a key process that stimulates
cancer cell invasion and metastasis in BC cells and that the kinase
domain (Y1234/1235) and multifunctional docking domain (Y1349) are
important phosphorylation sites that regulate such malignant
behaviors.
One of the most interesting results of the present
study was that c-Met was positively associated with the expression
of HO-1 and PD-L1. There have been several reports demonstrating
positive correlations between c-Met and HO-1 or PD-L1 in a variety
of cancers (10,12,16).
However, a significant association between c-Met expression and
HO-1 and PD-L1 based on multivariate analyses has not been
previously reported for patients with BC. Likewise, here, we
demonstrated that pY1349 c-Met was closely associated with the
expression of COX-2, HO-1, and PD-L1 in BC specimens, for the first
time. Unfortunately, besides BC, there are few reports on the
relationships between phosphorylated c-Met and cancer-related
molecules in human cancer tissues. Therefore, we believe that our
results are important to discuss the pathological mechanisms
associated with phosphorylated c-Met in malignancies.
In addition to correlations between c-Met pathways
and the expression of COX-2, HO-1, VEGF-A, or PD-L1, we clarified
the pathological significance of and interrelations among these
markers by multivariate analyses. Results showed that COX-2
expression was positively correlated with the expression of VEGF-A
and PD-L1. In contrast, HO-1 expression was not significantly
correlated with the expression of COX-2, VEGF-A, and PD-L1 in BC
tissues. In regard to the relationship between COX-2 and VEGF-A, a
positive correlation has been reported for a variety of
malignancies such as liposarcoma and gastric cancer (32,33).
Furthermore, one report indicated that COX-2 expression is
positively correlated with PD-L1 expression in human melanoma
tissues (34). However, there have
been no studies on such interrelations among COX-2, VEGF-A, and
PD-L1 in human BC tissues, but previous results support our
findings on the correlations among these cancer-related molecules.
Moreover, our univariate analyses suggested that muscle invasion
and metastasis in BC are regulated by complex mechanisms comprising
COX-2, HO-1, VEGF-A, and PD-L1, and this opinion is supported by
many previous reports (7,19,35–37).
However, interestingly, our multivariate analyses demonstrated that
muscle invasion was independently associated with COX-2 expression
and that metastasis was associated with PD-L1 expression, whereas
HO-1 and VEGF-A were not associated with either muscle invasion or
metastasis. Specifically, we were surprised that VEGF-A expression
appeared to play a minimal role in BC invasion and metastasis.
Unfortunately, we cannot describe the reasons for such findings.
However, we believe that this result is logical as nearly all
molecular agents with anti-VEGF-A activity have not been found to
improve outcomes for patients with BC (38). Furthermore, there is a possibility
that VEGF-A might modulate cancer cell invasion and/or metastasis
though COX-2 or PD-L1 in BC. Finally, as shown in our schematic, we
speculate that c-Met and phosphorylation of the multifunctional
docking domain (Y1349) play important roles in cancer cell invasion
and metastasis by regulating COX-2 and PD-L1 in patients with BC.
Therefore, it is possible that these molecules might serve as
useful targets to treat these patients.
An additional key finding in our study is the
determination that pY1234/1235 c-Met expression was closely
associated with both muscle invasion and metastasis in BC,
regardless of the expression of COX-2, HO-1, VEGF-A, and PD-L1.
Hence, inhibiting pathways that originate from pY1234/1235 c-Met
might lead to the inhibition of malignant behavior and progression
via independent mechanisms derived from COX-2, HO-1, VEGF-A, and
PD-L1 in BC. Presently, treatment strategies including immune
check-point inhibitors such as PD-L1-targeting agents comprise a
hot topic in the field of urological oncology (39–41).
Importantly, many investigators are interested in pursuing the
development of treatments that exploit COX-2, HO-1, and VEGF-A
inhibitors for BC (42–44). Further, the anti-cancer effects of
inhibiting kinase domain (Y1234/1235) phosphorylation and/or
suppressing its activities are speculated to be different from
those of COX-2, HO-1, VEGF-A, and/ or PD-L1 inhibitors in patients
with BC. Therefore, we hypothesize that pY1234/1235 is a potential
therapeutic target for BCs that are resistant to COX-2, HO-1,
VEGF-A, and/ or PD-L1 inhibitors.
Our study had several limitations based on
methodology. First, the staining patterns and pathological
significance of PD-L1 expression in cancer tissues were previously
reported to be dependent on the types of antibodies used, such as
VENTANA SP142, VENTANA SP263, DAKO 22C3, and DAKO 28-8 (16,44). In
this study, we used clone E1L3N of an anti-PD-L1 antibody that was
previously used for another study (30). Therefore, we should note that
differences in antibody specificities could exist if other
anti-PD-L1 antibodies were used. The next limitation is that we
evaluated PD-L1 expression in BC cells but not in infiltrating
immune cells of stromal tissues despite the fact that this marker
was expressed in both cancer cells and infiltrating immune cells in
tissues (45). In this study, we
focused on PD-L1 expression in BC cells because we wanted to
clarify the pathological networks associated with c-Met in BC cells
and various cancer-related factors including PD-L1 in patients with
BC. It is difficult to evaluate the expression of each molecule in
stromal cells that have infiltrated into NMIBC tissues because
infiltrating cells within NMIBC tissues were relatively rare. In
contrast, it is thought that PD-L1 expression in tumor-infiltrating
immune cells has no significant pathological role in tumor growth,
metastasis, and prognosis after cystectomy in patients with BC
(20). Furthermore, in
hepatocellular carcinoma, c-Met expression was found to be
positively associated with PD-L1 expression in cancer cells but not
in infiltrating cells (16). Based
on these facts, we investigated the relationships between c-Met
expression and various cancer-related molecules. We also emphasize
the importance of clarifying the pathological roles of PD-L1
expression in infiltrating cells within stromal tissues of BC.
However, to accomplish these goals, we only performed correlation
analysis, rather than a series of in vitro and in
vivo experiments. Hence, due to our study design, we are unable
to provide definitive conclusions regarding the pathological roles
of phosphorylated c-Met expression in BC. Nonetheless, we believe
that our results will prove useful in advancing future research
including the design of in vitro and in vivo
experiments, as c-Met can modulate numerous cancer-related factors
via complex mechanisms. In short, our results obtained via
multivariate analysis, including that of clinicopathological
features, are useful to discuss the pathological roles of the c-Met
pathway at the molecular level in patients with BC.
In conclusion, the present study demonstrated that
c-Met is positively associated with muscle invasion by regulating
HO-1 and PD-L1 and that pY1349 c-Met is associated with muscle
invasion and metastasis via the regulation of COX-2, HO-1, and
PD-L1 in patients with BC. Alternatively, pY1234/1235 was also
found to be associated with muscle invasion and metastasis;
however, no correlation was observed with various other
cancer-related molecules that were examined. From these results, we
postulated that pY1234/1235 might serve as a potential therapeutic
target for patients with BC and other diseases that are resistant
to inhibitors of COX-2, HO-1, VEGF-A, and/ or PD-L1. However,
unfortunately, since our results are solely based on correlation
analyses of immunohistochemical expression patterns, further in
vitro and in vivo studies are required to definitively
identify the detailed pathological roles of c-Met, pY1234/1235
c-Met, and pY1349 c-Met in BC.
Acknowledgements
The authors would like to thank Ms. Mitsuko Yoneda
(Department of Urology, Nagasaki University Hospital, Nagasaki,
Japan) for her excellent technical support.
Funding
The present study was supported by a grant from JSPS
KAKENHI (grant no. 18K09197).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YMu performed the experiments and contributed to
sample collection and writing of the manuscript. YMi conceived and
designed the experiments, generated and analyzed data, and
contributed to the writing of the manuscript. KA, YN, TY and AO
performed the experiments and analyzed the data. KM, TM and KO
contributed to clinical data collection and analyzed the data. HS
designed the experiment and was involved in revising the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The purpose of the present study was explained to
the participants, all of whom provided written consent prior to the
study. This study design was approved by the Institutional Review
Board of Nagasaki University Hospital (Nagasaki, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gakis G: The role of inflammation in
bladder cancer. Adv Exp Med Biol. 816:183–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okabe K, Shindo T, Maehana T, Nishiyama N,
Hashimoto K, Itoh N, Takahashi A, Taguchi K, Tachiki H, Tanaka T
and Masumori N: Neoadjuvant chemotherapy with gemcitabine and
cisplatin for muscle-invasive bladder cancer: Multicenter
retrospective study. Jpn J Clin Oncol. 48:934–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold L, Enders J and Thomas SM:
Activated HGF-c-Met axis in head and neck cancer. Cancers (Basel).
9:E1692017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu X, Zhang G, He L and Zhu Y:
Clinicopathological impacts of c-Met overexpression in bladder
cancer: Evidence from 1,336 cases. Onco Targets Ther. 12:2695–2702.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Birchmeier C, Birchmeier W, Gherardi E and
Vande Woude GF: Met, metastasis, mortality and more. Nat Rev Mol
Cell Biol. 4:915–925. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyata Y, Asai A, Mitsunari K, Matsuo T,
Ohba K, Mochizuki Y and Sakai H: Met in urological cancers. Cancers
(Basel). 6:2387–2403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noriega-Guerra H and Freitas VM:
Extracellular matrix influencing HGF/c-MET signaling pathway:
Impact on cancer progression. Int J Mol Sci. 19:E33002018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyata Y, Ashida S, Nakamura T, Mochizuki
Y, Koga S, Kanetake H, Shuin T and Kanda S: Overexpression of
hepatocyte growth factor receptor in renal carcinoma cells
indirectly stimulates tumor growth in vivo. Biochem Biophys Res
Commun. 302:892–897. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balan M, Mier y Teran E, Waaga-Gasser AM,
Gasser M, Choueiri TK, Freeman G and Pal S: Novel roles of c-Met in
the survival of renal cancer cells through the regulation of HO-1
and PD-L1 expression. J Biol Chem. 290:8110–8120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Sun Y, Zhang H, Liu X, Du W, Li Y,
Zhang J, Chen L and Jiang C: HGF/MET signaling promotes glioma
growth via up-regulation of Cox-2 expression and PGE2 production.
Int J Clin Exp Pathol. 8:3719–3726. 2015.PubMed/NCBI
|
|
12
|
Kammerer-Jacquet SF, Medane S, Bensalah K,
Bernhard JC, Yacoub M, Dupuis F, Ravaud A, Verhoest G, Mathieu R,
Peyronnet B, et al: Correlation of c-MET expression with PD-L1
expression in metastatic clear cell renal cell carcinoma treated by
sunitinib first-line therapy. Target Oncol. 12:487–494. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Papaccio F, Della Corte CM, Viscardi G, Di
Liello R, Esposito G, Sparano F, Ciardiello F and Morgillo F:
HGF/MET and the immune system: Relevance for cancer immunotherapy.
Int J Mol Sci. 19:E35952018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu-Monette ZY, Zhang M, Li J and Young KH:
PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor
immune response? Front Immunol. 8:15972017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lenouvel D, González-Moles MÁ, Talbaoui A,
Ramos-García P, González-Ruiz L, Ruiz-Ávila I and Gil-Montoya JA:
An update of knowledge on PD-L1 in head and neck cancers:
Physiologic, prognostic and therapeutic perspectives. Oral Dis.
26:511–526. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chun HW and Hong R: Significance of PD-L1
clones and C-MET expression in hepatocellular carcinoma. Oncol
Lett. 17:5487–5498. 2019.PubMed/NCBI
|
|
17
|
Hass R, Jennek S, Yang Y and Friedrich K:
c-Met expression and activity in urogenital cancers-novel aspects
of signal transduction and medical implications. Cell Commun
Signal. 15:102017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyata Y, Kanda S, Ohba K, Nomata K,
Hayashida Y, Eguchi J, Hayashi T and Kanetake H: Lymphangiogenesis
and angiogenesis in bladder cancer: Prognostic implications and
regulation by vascular endothelial growth factors-A, -C, and -D.
Clin Cancer Res. 12:800–806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuo T, Miyata Y, Mitsunari K, Yasuda T,
Ohba K and Sakai H: Pathological significance and prognostic
implications of heme oxygenase 1 expression in non-muscle-invasive
bladder cancer: Correlation with cell proliferation, angiogenesis,
lymphangiogenesis and expression of VEGFs and COX-2. Oncol Lett.
13:275–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pichler R, Heidegger I, Fritz J, Danzl M,
Sprung S, Zelger B, Brunner A and Pircher A: PD-L1 expression in
bladder cancer and metastasis and its influence on oncologic
outcome after cystectomy. Oncotarget. 8:66849–66864. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davick JJ, Frierson HF, Smolkin M and Gru
AA: PD-L1 expression in tumor cells and the immunologic milieu of
bladder carcinomas: A pathologic review of 165 cases. Hum Pathol.
81:184–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ferracini R, Longati P, Naldini L, Vigna E
and Comoglio PM: Identification of the major autophosphorylation
site of the Met/hepatocyte growth factor receptor tyrosine kinase.
J Biol Chem. 266:19558–19564. 1991.PubMed/NCBI
|
|
23
|
Bertotti A and Comoglio PM: Tyrosine
kinase signal specificity: Lessons from the HGF receptor. Trends
Biochem Sci. 28:527–533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raghav KP, Wang W, Liu S, Chavez-MacGregor
M, Meng X, Hortobagyi GN, Mills GB, Meric-Bernstam F, Blumenschein
GR Jr and Gonzalez-Angulo AM: cMET and phospho-cMET protein levels
in breast cancers and survival outcomes. Clin Cancer Res.
18:2269–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyata Y, Sagara Y, Kanda S, Hayashi T and
Kanetake H: Phosphorylated hepatocyte growth factor receptor/c-Met
is associated with tumor growth and prognosis in patients with
bladder cancer: Correlation with matrix metalloproteinase-2 and −7
and E-cadherin. Hum Pathol. 40:496–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamasaki K, Mukai S, Nagai T, Nakahara K,
Fujii M, Terada N, Ohno A, Sato Y, Toda Y, Kataoka H and Kamoto T:
Matriptase-induced phosphorylation of MET is significantly
associated with poor prognosis in invasive bladder cancer; an
immunohistochemical analysis. Int J Mol Sci. 19:E37082018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyata Y, Kanetake H and Kanda S: Presence
of phosphorylated hepatocyte growth factor receptor/c-Met is
associated with tumor progression and survival in patients with
conventional renal cell carcinoma. Clin Cancer Res. 12:4876–4881.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morena D, Maestro N, Bersani F, Forni PE,
Lingua MF, Foglizzo V, Šćepanović P, Miretti S, Morotti A, Shern
JF, et al: Hepatocyte growth factor-mediated satellite cells niche
perturbation promotes development of distinct sarcoma subtypes.
Elife. 5:e121162016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mitsunari K, Miyata Y, Asai A, Matsuo T,
Shida Y, Hakariya T and Sakai H: Human antigen R is positively
associated with malignant aggressiveness via upregulation of cell
proliferation, migration, and vascular endothelial growth factors
and cyclooxygenase-2 in prostate cancer. Transl Res. 175:116–128.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Le Goux C, Damotte D, Vacher S, Sibony M,
Delongchamps NB, Schnitzler A, Terris B, Zerbib M, Bieche I and
Pignot G: Correlation between messenger RNA expression and protein
expression of immune checkpoint-associated molecules in bladder
urothelial carcinoma: A retrospective study. Urol Oncol.
35:257–263. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyata Y, Kanda S, Nomata K, Eguchi J and
Kanetake H: Expression of cyclooxygenase-2 and EP4 receptor in
transitional cell carcinoma of the upper urinary tract. J Urol.
173:56–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung I, Gurzu S, Turdean S, Ciortea D,
Sahlean DI, Golea M and Bela T: Relationship of endothelial area
with VEGF-A, COX-2, maspin, c-KIT, and DOG-1 immunoreactivity in
liposarcomas versus non-lipomatous soft tissue tumors. Int J Clin
Exp Pathol. 8:1776–1782. 2015.PubMed/NCBI
|
|
33
|
Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu
Q and Li Q: Helicobacter pylori promotes angiogenesis depending on
Wnt/beta-catenin-mediated vascular endothelial growth factor via
the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer.
16:3212016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Botti G, Fratangelo F, Cerrone M, Liguori
G, Cantile M, Anniciello AM, Scala S, D'Alterio C, Trimarco C,
Ianaro A, et al: COX-2 expression positively correlates with PD-L1
expression in human melanoma cells. J Transl Med. 15:462017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shirahama T, Arima J, Akiba S and Sakakura
C: Relation between cyclooxygenase-2 expression and tumor
invasiveness and patient survival in transitional cell carcinoma of
the urinary bladder. Cancer. 92:188–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JH and Park J: Prognostic significance
of heme oxygenase-1, S100 calcium-binding protein A4, and
syndecan-1 expression in primary non-muscle-invasive bladder
cancer. Hum Pathol. 45:1830–1838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding X, Chen Q, Yang Z, Li J, Zhan H, Lu
N, Chen M, Yang Y, Wang J and Yang D: Clinicopathological and
prognostic value of PD-L1 in urothelial carcinoma: A meta-analysis.
Cancer Manag Res. 11:4171–4184. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mazzola CR and Chin J: Targeting the VEGF
pathway in metastatic bladder cancer. Expert Opin Investig Drugs.
24:913–927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bellmunt J, Powles T and Vogelzang NJ: A
review on the evolution of PD-1/PD-L1 immunotherapy for bladder
cancer: The future is now. Cancer Treat Rev. 54:58–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hori S, Miyake M, Tatsumi Y, Onishi S,
Morizawa Y, Nakai Y, Tanaka N and Fujimoto K: Topical and systemic
immunoreaction triggered by intravesical chemotherapy in an
N-butyl-N-(4-hydroxybutyl) nitorosamine induced bladder cancer
mouse model. PLoS One. 12:e01754942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel KR, Taylor BL, Khani F, Guzzo TJ,
Scherr DS, Ravishankar R, Lal P and Malkowicz SB: Impact of
neoadjuvant chemotherapy on concordance of PD-L1 staining fidelity
between the primary tumor and lymph node metastases in bladder
cancer. Urology. 131:150–156. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cesário JM, Brito RB, Malta CS, Silva CS,
Matos YS, Kunz TC, Urbano JJ, Oliveira LV, Dalboni MA and Dellê H:
A simple method to induce hypoxia-induced vascular endothelial
growth factor-A (VEGF-A) expression in T24 human bladder cancer
cells. Vitro Cell Dev Biol Anim. 53:272–276. 2017. View Article : Google Scholar
|
|
43
|
Patricia Moreno-Londoño A, Bello-Alvarez C
and Pedraza-Chaverri J: Isoliquiritigenin pretreatment attenuates
cisplatin induced proximal tubular cells (LLC-PK1) death and
enhances the toxicity induced by this drug in bladder cancer T24
cell line. Food Chem Toxicol. 109:143–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hurst EA, Pang LY and Argyle DJ: The
selective cyclooxygenase-2 inhibitor mavacoxib (Trocoxil) exerts
anti-tumour effects in vitro independent of cyclooxygenase-2
expression levels. Vet Comp Oncol. 17:194–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Schwamborn K, Ammann JU, Knüchel R,
Hartmann A, Baretton G, Lasitschka F, Schirmacher P, Braunschweig
T, Tauber R, Erlmeier F, et al: Multicentric analytical
comparability study of programmed death-ligand 1 expression on
tumor-infiltrating immune cells and tumor cells in urothelial
bladder cancer using four clinically developed immunohistochemistry
assays. Virchows Arch. 475:599–608. 2019. View Article : Google Scholar : PubMed/NCBI
|