Introduction
Choriocarcinoma is the most aggressive type of
trophoblastic neoplasia (1). It is
prone to early distant metastases and has aggressive features,
leading to a generally poor prognosis (2,3). With
advancements in multidrug combination chemotherapy, the cure rate
of choriocarcinoma has significantly improved (4). However, some patients still fail to
respond to treatment due to the strong proliferative and invasive
abilities of choriocarcinoma, as well as the severe toxic side
effects of chemotherapy, and multidrug resistance to chemotherapy
(5). Thus, it is important to
inhibit the proliferation and invasion of choriocarcinoma cells,
and to explore new anticancer drugs with low toxicity and high
efficiency.
Dihydromyricetin (DMY) is a natural flavonoid
isolated from Ampelopsis grossedentata, which reportedly has
anti-inflammatory, antioxidative, anticancer, antimicrobial and
immune-enhancing effects (6). DMY
exerts a strong antitumor effect with low toxicity to normal cells
(7–9). Its mechanism of action primarily
involves the inhibition of proliferation, migration and invasion,
as well as the induction of apoptosis (10–12). In
a previous study, the effect of DMY-induced apoptosis on
choriocarcinoma cells was studied (13). The present study aimed to explore the
inhibitory effect of DMY on the proliferation of JAR cells and its
associated mechanisms of action, in order to provide data to
support the development of DMY as a novel therapy for
choriocarcinoma.
Cell proliferation is closely associated with the
cell cycle, which is an essential mechanism by which all living
organisms grow and proliferate. The eukaryotic cell cycle usually
comprises four phases. The cells grow continuously during
interphase, which comprises three phases: G1, S And
G2 (14). The
G1 phase is the gap between the M and S phases, and it
is an active period of metabolic activity, cell growth and repair.
It is an important phase wherein the cells decide whether to enter
the S phase. The S phase is flanked by the G1 and
G2 phases, during which the cells continue to grow.
During the S phase, the cells undergo DNA replication. The
G2 phase is the gap between the S and M phases, and DNA
synthesis occurs in anaphase, which is the preparatory phase of
mitosis. During this period, DNA synthesis is terminated;
furthermore, several RNAs and proteins. including tubulin and
maturation promoters. are produced. At specific points in the
G2 phase, cells encounter different checkpoints at which
the decision of proceeding to the next phase or pausing to allow
more time for preparation is made. The S, G1 and
G2 phases form part of the interphase, which is a
markedly active period for proliferating cells. The M phase is the
mitotic phase; during this phase, the nucleus divides via mitosis,
which is followed by cytoplasmic division via cytokinesis (14). Cyclins are important proteins in the
cell cycle control system. Detection of changes in the cell cycle
and cyclins can further clarify the mechanisms underlying cell
proliferation.
The transforming growth factor-β (TGF-β)/SMAD
signaling pathway is also closely associated with cell
proliferation. Previous studies have shown that this pathway can
regulate the proliferation of choriocarcinoma cells (15,16).
However, the inhibitory effect of DMY on proliferation, in
association with the TGF-β/SMAD signaling pathway, needs further
clarification.
Materials and methods
Reagents
The following commercial reagents were used: DMY
(>99% purity; Beijing Century Aoke Biotechnology Co., Ltd.),
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), DMEM
(Gibco; Thermo Fisher Scientific, Inc.), 0.25% trypsin with 0.02%
EDTA (Gibco; Thermo Fisher Scientific, Inc.), MTT reagent (Gibco;
Thermo Fisher Scientific, Inc.), antibodies against GAPDH (rabbit
anti-human polyclonal; cat. no. E12-052; EnoGene Biotech Co.,
Ltd.), SMAD3 (rabbit anti-human monoclonal; cat. no. ab40854;
Abcam), p-SMAD3 (rabbit anti-human monoclonal; cat. no. ab52903;
Abcam), SMAD4 (rabbit anti-human monoclonal; cat. no. ab40759;
Abcam), Cyclin A1 (rabbit anti-human polyclonal; cat. no. E1A5313;
EnoGene Biotech Co., Ltd.) and Cyclin D1 (rabbit anti-human
monoclonal; cat. no. ab134175; Abcam), and a secondary antibody
(peroxidase-conjugated AffiniPure goat anti-rabbit IgG; cat. no.
ab6721; Abcam).
Cell culture
The human JAR cell line (male fetus choriocarcinoma)
was purchased from Guangzhou Jennio Biotech Co., Ltd. JAR cells
were cultured in 10% DMEM at 37°C in the presence of 5%
CO2. Subsequently, the cells were subcultured with 0.25%
trypsin and 0.02% EDTA until confluence reached 70–80%.
MTT assay
MTT assays were performed as described previously
(13). Cells were seeded in 96-well
plates, cultured overnight until the cell confluence reached
30–40%, and incubated for 48 h with various concentrations of DMY:
0, 20, 40, 80, 100, 120, 160, 240 and 320 mg/l (0, 62, 125, 250,
312, 375, 500, 749 and 999 µM), in 200 µl 10% DMEM per well. There
were 6 wells for each group. MTT reagent was used to determine cell
proliferation, and absorbance was determined using a microplate
reader (Multiskan MK3; Thermo Fisher Scientific, Inc.) at a
wavelength of 492 nm. The experiment was repeated three times.
Live cell imaging
According to the MTT results, 0, 40, 60 and 100 mg/l
(0, 125, 187 and 312 µM) were selected as the appropriate
concentrations to perform further experiments. Cell proliferation
was observed under an inverted light microscope at ×100
magnification (Olympus IX73; Olympus Corporation), and cell
confluence was analyzed using the PAULA live cell imaging system
(Leica Microsystems GmbH).
Colony forming assay
Cells were seeded in culture plates and allowed to
adhere overnight. Once the confluence reached 30–40%, the cells
were incubated with different concentrations of DMY (0, 40, 60 and
100 mg/l) in 10% DMEM at 37°C of 5% CO2. Following a
48-h incubation, the cells were resuspended and subsequently
replated at a density of 400 cells/well in 6-well plates. After a
12-day culture, the cells were stained with crystal violet for 30
min at room temperature, colonies were counted using ImageJ version
1.46 (National Institutes of Health), and images of cell morphology
were captured using a camera (Nikon Corporation).
Cell cycle analysis
Cells were seeded in culture plates and allowed to
adhere overnight. Once the cell confluence had reached 30–40%, the
cells were incubated with different concentrations of DMY (0, 40,
60 and 100 mg/l). After a 48-h incubation period, the cell cycle
was analyzed using flow cytometry. The cells were processed with
the Cell Cycle kit [cat. no. CCS012; Multi Sciences (Lianke)
Biotech Co., Ltd.], according to the manufacturer's instructions.
The samples were analyzed using a FACSCalibur flow cytometer
(Becton, Dickinson and Company).
Reverse transcription-quantitative PCR
(RT-qPCR)
Cells were incubated with different concentrations
of DMY (0, 40, 60 and 100 mg/l) for 48 h. Total RNA was isolated
using TRIzol® reagent (Sigma-Aldrich; Merck KGaA),
according to the manufacturer's instructions. cDNA synthesis was
performed using the PrimeScript RT Reagent kit (cat. no. RR036A;
Takara Biotechnology Co., Ltd.) according to the manufacturer's
instructions. qPCR was performed using the SYBR Green qPCR Master
mix (cat. no. RR820A; Takara Biotechnology Co., Ltd.), according to
the manufacturer's instructions. PCR was performed under the
following conditions: Denaturation at 95°C for 30 sec, followed by
40 cycles at 95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec,
finally 1 cycle at 95°C for 60 sec, 55°C for 30 sec and 95°C for 30
sec. Three repeated wells were set up of each sample. The
expression intensity of each gene was normalized against the
expression of GAPDH. Differential gene expression was calculated as
the ratio of the expression levels of target genes in JAR cells
using the 2−ΔΔCq method (17). The PCR primer sequences are presented
in Table I.
 | Table I.Primer pairs used in quantitative
PCR. |
Table I.
Primer pairs used in quantitative
PCR.
| Gene | Sequence (5′ to
3′) |
|---|
| SMAD4 | F:
gacagcagcagaatggat |
|
| R:
caggagcaggatgattgg |
| SMAD3 | F:
ccagggctttgaggctgtcta |
|
| R:
gcaaaggcccattcaggtg |
| Cyclin A1 | F:
tgtcaccgttcctccttg |
|
| R:
gcatcttcacgctctattt |
| Cyclin D1 | F:
gcgaggaacagaagtgcg |
|
| R:
tggagttgtcggtgtagatgc |
| GAPDH | F:
gcaccgtcaaggctgagaac |
|
| R:
tggtgaagacgccagtgga |
Western blotting
Cells were incubated with different concentrations
of DMY (0, 40, 60 and 100 mg/l) for 48 h, collected and lysed on
ice with RIPA buffer. The cell lysates were centrifuged at 14,000 ×
g for 10 min at 4°C. Subsequently, the supernatant was extracted,
and the protein concentration was quantified using a bicinchoninic
acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). For
SDS-PAGE, 12% gel was used to separate 30 µg protein per sample,
which was then transferred onto a polyvinylidene difluoride
membrane. Nonfat milk (5%) was used to block the membranes for 2 h
at room temperature. Next, the membranes were incubated with
primary antibodies overnight at 4°C. The primary antibodies used
were against GAPDH (1:2,000), SMAD3 (1:5,000), SMAD4 (1:5,000),
cyclin A1 (1:1,000) and cyclin D1 (1:10,000). Subsequently, the
membranes were washed in 0.1% Tween 20-TBST buffer and incubated
with an appropriate secondary antibody (1:5,000) for 1 h at room
temperature. Immunoreactive bands were detected using an enhanced
chemiluminescent substrate (Pierce; Thermo Fisher Scientific, Inc.)
and imaged using the Tanon 6100 Chemiluminescent Imaging system
(Tanon Science and Technology Co., Ltd.). Band densities were
calculated using the Quantity One software version 4.6.2 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
All experiments were repeated ≥3 times, unless
otherwise indicated. Data were analyzed using SPSS 19.0 (IBM Corp.)
and presented as the mean ± standard deviation. Statistical
analysis was performed using one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
DMY inhibits the proliferation and
colony formation of JAR cells
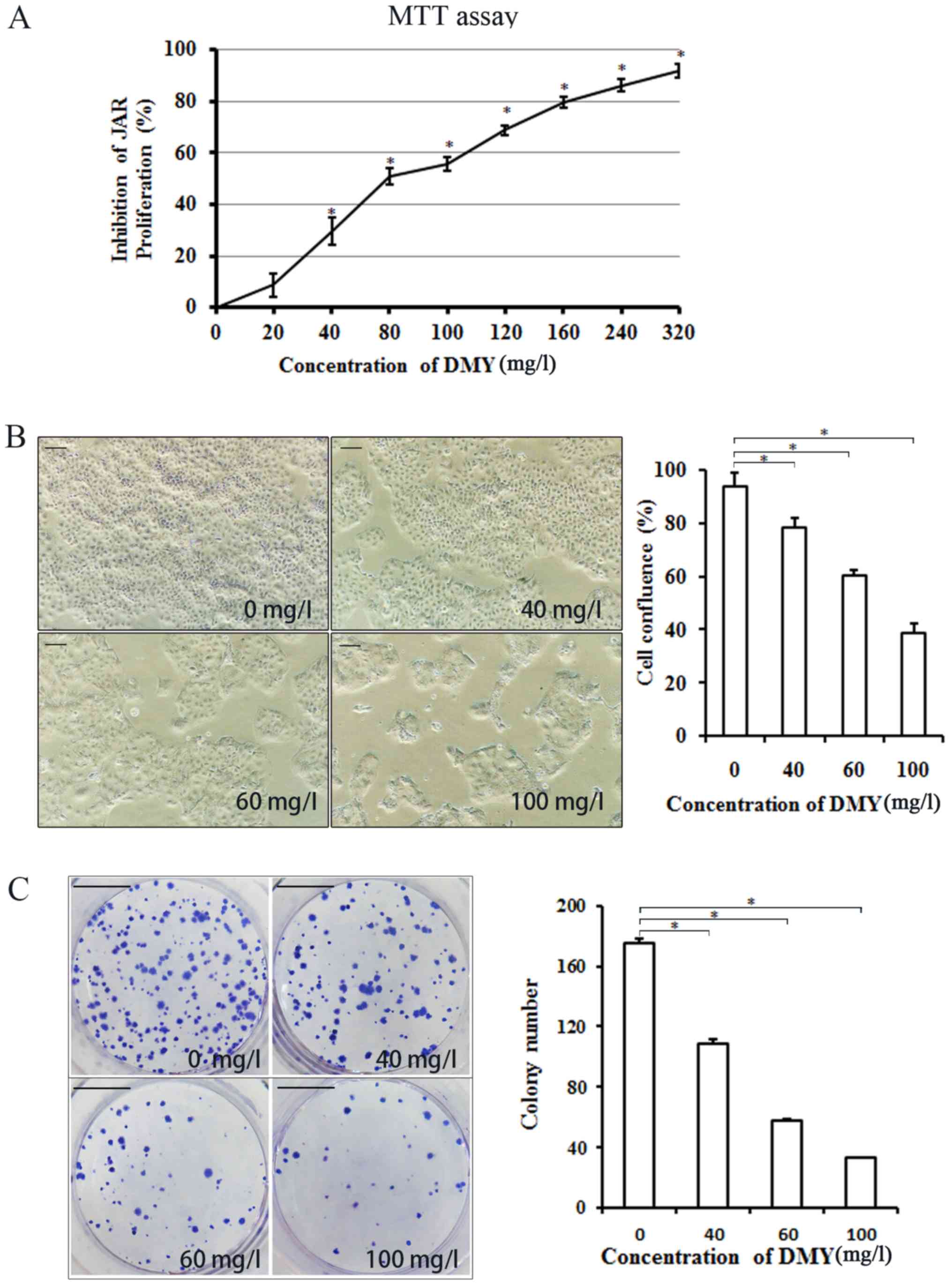
The MTT assay results showed that the proliferation
of JAR cells was significantly inhibited by DMY in a
concentration-dependent manner compared with that of the untreated
cells (P<0.05; Fig. 1A). Cell
confluence decreased significantly with increasing concentrations
of DMY, as assessed using an inverted light microscope (P<0.05;
Fig. 1B). The colony forming assay
demonstrated that the number of JAR cells clones decreased
significantly with increasing concentrations of DMY, compared with
the number of untreated cell clones (P<0.05; Fig. 1C).
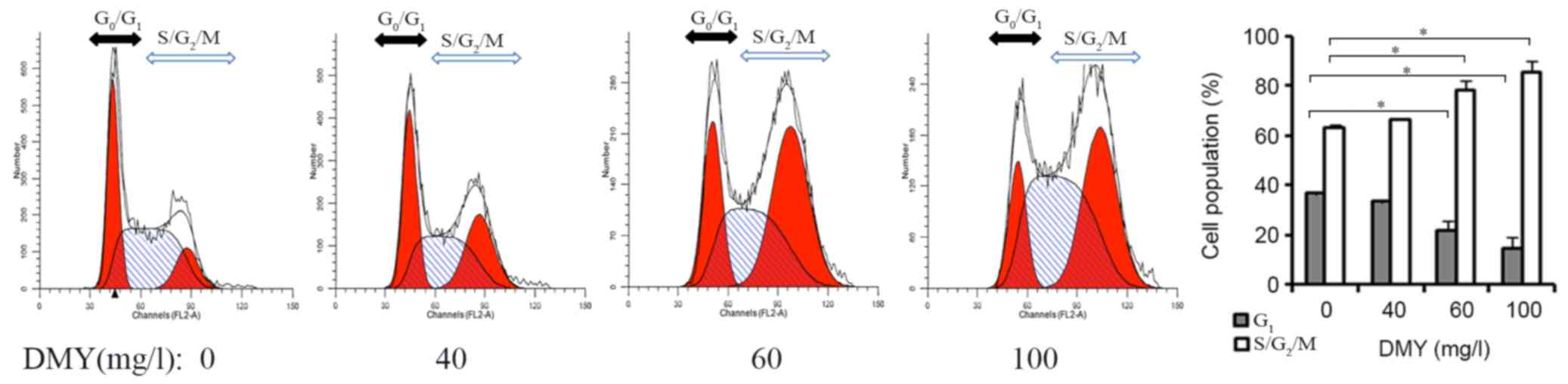
DMY affects cell cycle distribution in
JAR cells
The effects of the different concentrations of DMY
were evaluated on the cell cycle distribution in JAR cells using
flow cytometry. Compared with the number of untreated cells,
increasing concentrations of DMY (60 and 100 mg/l) significantly
decreased the number of JAR cells in the G1 phase,
whilst increasing the number of cells in the S/G2/M
phase (Fig. 2).
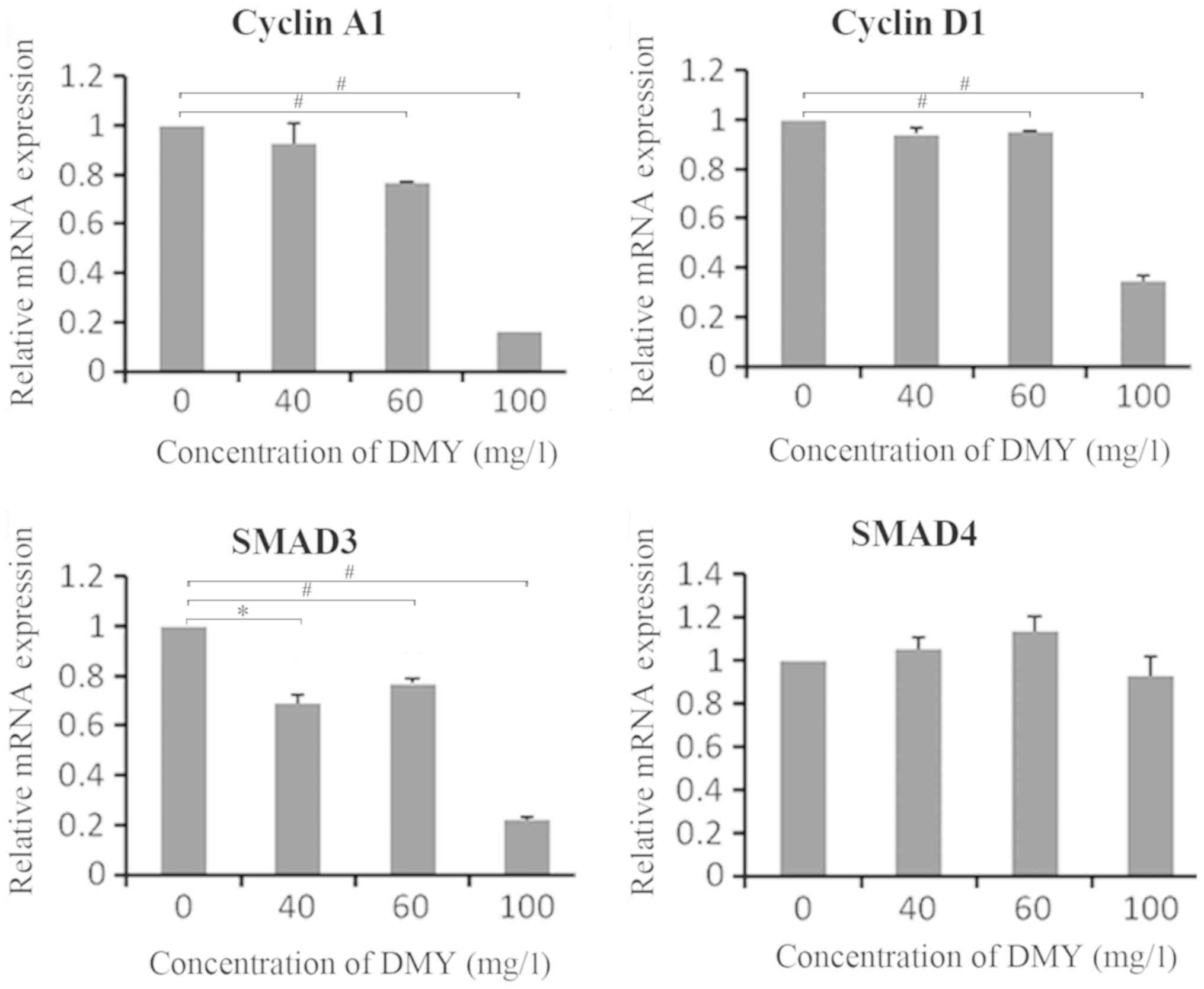
DMY regulates the mRNA expression
levels of SMAD3, cyclin A1 and cyclin D1 in JAR cells
After a 48-h incubation with different
concentrations of DMY, the mRNA expression levels of SMAD3, SMAD4,
cyclin A1 and cyclin D1 were quantified using RT-qPCR (Fig. 3). The results indicated that the mRNA
expression levels of cyclin A1 and cyclin D1 decreased with 60 and
100 mg/l DMY (P<0.01). The mRNA expression level of SMAD3
decreased with 40, 60 and 100 mg/l DMY (P<0.05, P<0.01).
However, no significant differences were observed in the mRNA
expression levels of SMAD4 (P>0.05; Fig. 3).
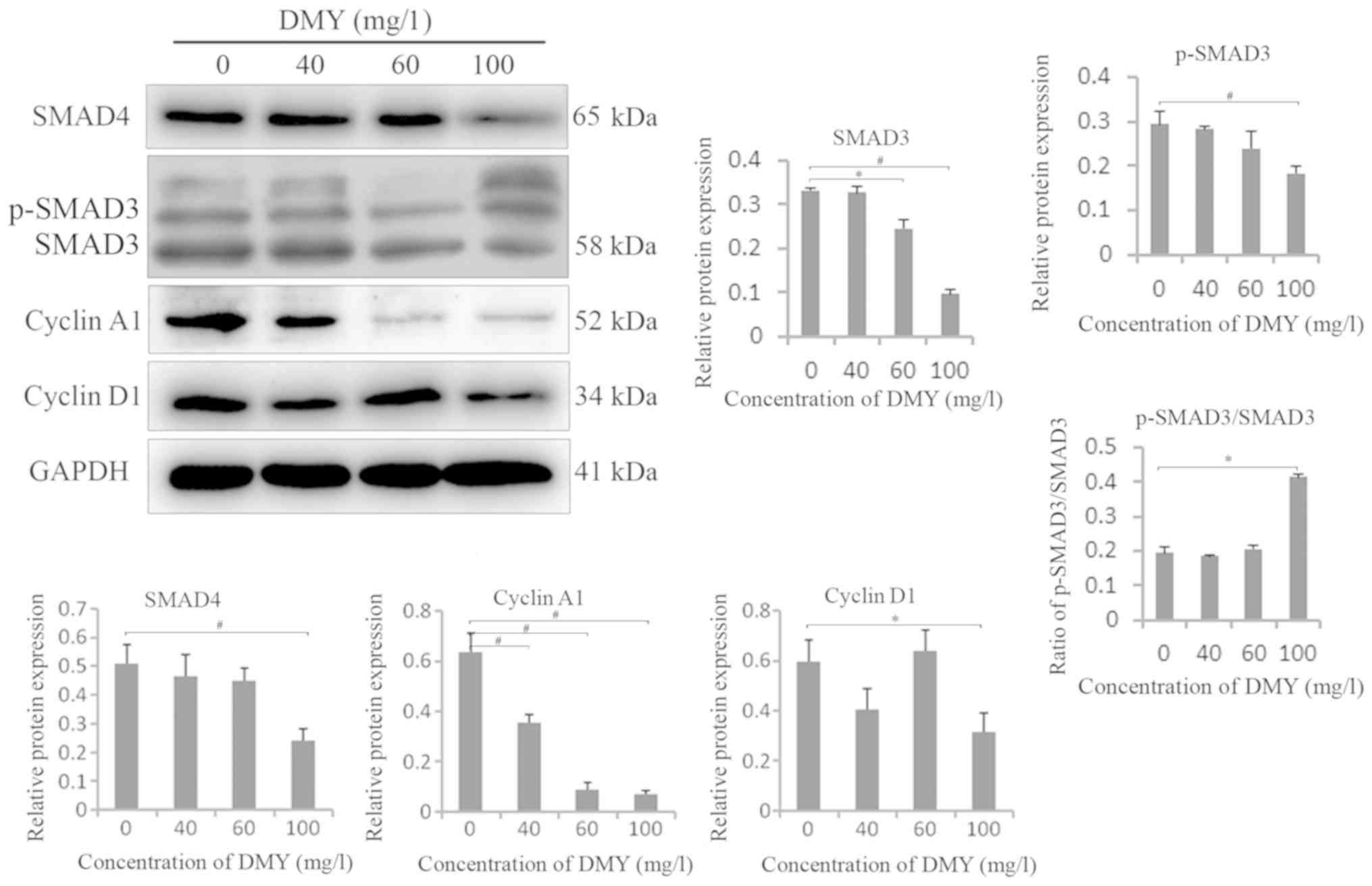
DMY regulates the protein expression
levels of SMAD3, p-SMAD3, SMAD4, cyclin A1 and cyclin D1 in JAR
cells
Western blotting revealed that the protein
expression levels of SMAD3, p-SMAD3, SMAD4, cyclin A1 and cyclin D1
were decreased in JAR cells treated with DMY compared with those in
the untreated cells particularly in the 100-mg/l group. The ratio
of p-SMAD3/SMA3 increased in 100 mg/l group (P<0.05; Fig. 4).
Discussion
In the present study, MTT and colony forming assays
were performed to determine the short and long-term inhibitory
effects of different concentrations of DMY on the proliferation of
JAR cells, respectively. It was found that DMY inhibited the short
and long-term proliferation of JAR cells in a
concentration-dependent manner. The cell cycle, apoptosis and some
signaling pathways are closely associated with cell proliferation.
In a previous study, DMY was demonstrated to inhibit cell
proliferation by inducing apoptosis in JAR cells (13). In the present study, the inhibitory
effect of DMY on the proliferation of JAR cells was explored, based
on the cell cycle and TGF-β/SMAD signaling pathway (15). The cell cycle was analyzed using flow
cytometry, and the results showed that DMY inhibited cell
proliferation in association with the regulation of the cell
cycle.
Flow cytometry revealed S/G2/M cell cycle
arrest in JAR cells treated with DMY. Cyclins are important in the
cell cycle control system; cyclins progressively accumulate
throughout interphase and abruptly disappear during mitosis during
each cell cycle. Escape from cell cycle arrest or from certain
nonproliferating states requires the accumulation of cyclins
(14). Since the discovery of
cyclins in sea urchins by Evans et al (18) in 1983, our understanding of the
functions of cyclins in the cell cycle has improved. In mammalian
cells, cyclin A accumulates during the late G1 phase and
is degraded before metaphase (19).
Cyclin D was first isolated in a study on parathyroid carcinoma
conducted by Motokura et al (20), and it is necessary in the
G1/S phase of the cell cycle. Cyclin D is generally
considered to play a regulatory role by combining with
cycle-dependent kinases (CDKs; CDK4 or CDK6) to promote transition
from the G1 to the S phase (21). The overexpression of cyclin D1 and
CDK4 can cause abnormal proliferation and uncontrolled
differentiation of cells; these processes are closely associated
with the occurrence and development of tumors (22,23).
Numerous studies have shown that cyclins are fundamental to the
cell cycle, which is important in cell proliferation and
differentiation, and is closely associated with the occurrence and
development of tumors. For example, alantolactone suggested to
inhibit cell proliferation, inducing G2/M phase arrest
by downregulating cyclin A1 in HepG2 cells (24). A naturally occurring sesquiterpene
lactone-Santonin causes SKBR-3 cell cycle arrest at the
G2/M phase by suppressing the expression of cyclins A
and B1 (25). MircoRNA-27a
inhibition reportedly triggers G2/M arrest in SKOV-3 and
OVACAR-3 cells, accompanied by the downregulation of cyclins A and
B1 (26). Fucosterol (a phytosterol
in marine algae and many other plant species) also triggers
G2/M cell cycle arrest in A549 and SKLU-1 cells, which
is associated with the downregulation of CDK1, cyclin A and cyclin
B1, as well as the upregulation of negative regulators of the cell
cycle (27). WDR5 induces
S/G2/M cell cycle arrest via cyclin D1 in a process that
is regulated by H3K4me3 (28). In
the present study, the results showed that the expression levels of
cyclin A1 and cyclin D1 were decreased; thus, DMY may inhibit the
proliferation of JAR cells by causing S/G2/M arrest via
a decrease in the expression levels of cyclin A1 and D1.
In addition to the association between cyclins and
cell proliferation, some studies have shown that the TGF-β/SMAD
signaling pathway is also associated with cell proliferation
(29,30). TGF-β activates TGF-β receptor (TβR)I
and TβRII, resulting in the phosphorylation of receptor-regulated
SMAD2/3, which is associated with the common mediator SMAD4. The
SMAD2/3/4 complex is translocated to the nucleus, where it binds to
DNA and regulates the transcription of several genes (31,32).
Increased mRNA and protein expression levels of SMAD3 and SMAD4
promote cell proliferation, migration and invasion (33). Previous studies have shown that the
TGF-β/SMAD signaling pathway can regulate the proliferation of
choriocarcinoma cells (15,16). In the present study, the mRNA and
protein expression levels of SMAD3 and SMAD4 were examined, and the
results revealed significantly decreased mRNA and protein
expression levels of SMAD3 in cells treated with 100 mg/l DMY. The
protein expression levels of p-SMAD3 also decreased in 100 mg/l
group. Of note, the ratio of p-SMAD3/SMA3 increased in 100 mg/l
group, and this may be due to an increase in the bands above p-SMAD
3 instead. No significant changes were detected in the mRNA
expression level of SMAD4; however, its protein expression was
significantly reduced in the 100-mg/l group. Therefore, it was
hypothesized that the treatment might increase protein degradation.
Overall, the results indicate that DMY may inhibit the
proliferation of JAR cells by decreasing the expression of SMAD3
and SMAD4.
However, one limitation of the present study is that
no rescue experiments were performed to determine whether the
overexpression of cyclin A1, cyclin D1, SMAD3 and SMAD4 prevents
the inhibitory effect of DMY on the proliferation of JAR cells.
Nevertheless, the present results provide a foundation for future
investigations into more detailed mechanisms.
In summary, the findings of the present study
suggest that DMY inhibits the proliferation of human
choriocarcinoma JAR cells, potentially by inducing cell cycle
arrest via the downregulation of cyclin A1, cyclin D1, SMAD3 and
SMAD4. However, the role of other mechanisms, and whether the
overexpression of cyclin A1, cyclin D1, SMAD3 and SMAD4 prevents
the inhibitory effect of DMY on the proliferation of JAR cells need
further clarification. Future studies on DMY will aid in developing
it as a potential novel drug for the treatment of
choriocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Subjects
in Universities and Colleges of Hebei Province of China [Pathology
and Pathophysiology; grant no. JiJiaoGao (2013) 4], the Excellent
Innovation Talent Support Plan of Hebei Education Department (grant
no. SLRC2017018), and the Project in Hebei Province Department of
Population and Family Planning Commission (grant no. 2013-A13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZZ designed the study, performed the experiments,
analyzed and interpreted the data, and prepared the first draft of
the manuscript. YJL, QX, DYS and XRL contributed to the design of
the study, data analysis and revision of the manuscript. XJL
performed the experiments. YHL developed the concept of the study
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pires LV, Yi Y, Cheng JC, Pizzolato LS,
Cordero E, Leung PCK and Brum IS: Lapatinib inhibits
amphiregulin-induced BeWo choriocarcinoma cell proliferation by
reducing ERK1/2 and AKT signaling pathways. Anticancer Res.
39:2377–2383. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu S, Wu C, Tan Q and Liu H: Long
noncoding RNA H19 promotes chemotherapy resistance in
choriocarcinoma cells. J Cell Biochem. 120:15131–15144. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pearce H, Edwards DC, Levy JA, McGreen BH,
Mackovick L, Brennan M, McHugh M, Mapow B, Schanne FJ and Belkoff
L: Acute pulmonary hemorrhage associated with metastatic testicular
choriocarcinoma in a 46-year-old incarcerated male. Urol Ann.
11:109–112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Breitbach GP, Sklavounos P, Veith C, Costa
SD, Kuhn W, Solomayer EF, Juhasz-Boess I and Tempfer C: Oral
etoposide for metastatic choriocarcinoma: A case report and review
of guidelines. Arch Gynecol Obstet. 299:1115–1119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu C, Yu S, Tan Q, Guo P and Liu H: Role
of AhR in regulating cancer stem cell-like characteristics in
choriocarcinoma. Cell Cycle. 17:2309–2320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Li Q, Liu Z, Yang K, Chen Z, Cheng Q
and Wu L: The versatile effects of dihydromyricetin in health. Evid
Based Complement Alternat Med. 2017:10536172017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang L, Zhang Q, Ren H, Ma S, Lu C, Liu
B, Liu J, Liang J, Li M and Zhu R: Dihydromyricetin enhances the
chemo-sensitivity of nedaplatin via regulation of the p53/Bcl-2
pathway in hepatocellular carcinoma cells. PLoS One.
10:e01249942015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong IL, Wang BC, Yuan J, Duan LX, Liu Z,
Liu T, Li XM, Hu X, Zhang XY, Jiang T, et al: Potent and nontoxic
chemosensitizer of P-glycoprotein-mediated multidrug resistance in
cancer: Synthesis and evaluation of methylated epigallocatechin,
gallocatechin, and dihydromyricetin derivatives. J Med Chem.
58:4529–4549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kao SJ, Lee WJ, Chang JH, Chow JM, Chung
CL, Hung WY and Chien MH: Suppression of reactive oxygen
species-mediated ERK and JNK activation sensitizes
dihydromyricetin-induced mitochondrial apoptosis in human non-small
cell lung cancer. Environ Toxicol. 32:1426–1438. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou DZ, Sun HY, Yue JQ, Peng Y, Chen YM
and Zhong ZJ: Dihydromyricetin induces apoptosis and cytoprotective
autophagy through ROS-NF-κB signalling in human melanoma cells.
Free Radic Res. 51:517–528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang X, Lian T, Guan X, Liu B, Hao S,
Zhang J, Bao S, Tan X and Zhu R: Dihydromyricetin reduces TGF-β via
P53 activation-dependent mechanism in hepatocellular carcinoma
HepG2 cells. Protein Pept Lett. 24:419–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang QY, Li R, Zeng GF, Liu B, Liu J, Shu
Y, Liu ZK, Qiu ZD, Wang DJ, Miao HL, et al: Dihydromyricetin
inhibits migration and invasion of hepatoma cells through
regulation of MMP-9 expression. World J Gastroenterol.
20:10082–10093. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo Y, Xu Q, Lu Y, Sun D, Wang K, Lei Y,
Liang X and Li Y: Dihydromyricetin induces apoptosis in a human
choriocarcinoma cell line. Oncol Lett. 16:4229–4234.
2018.PubMed/NCBI
|
|
14
|
Alberts B, Bray D, Hopkin K, Johnson A,
Lewis J, Raff M, Roberts K and Walter P: Essential cell biology.
(Fourth). Garland Science. Taylor & Francis Group, LLC. (USA).
2014.
|
|
15
|
Li Y, Xu Q, Zhang Z, Liu S, Shi C and Tan
Y: The impact of TGF-β1 on the mRNA expression of TβR I, TβR II,
Smad4 and the invasiveness of the JEG-3 placental choriocarcinoma
cell line. Oncol Lett. 4:1344–1348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan Y, Xu Q, Li Y, Mao X and Zhang K:
Crosstalk between the p38 and TGF-β signaling pathways through
TβRI, TβRII and Smad3 expression in plancental choriocarcinoma
JEG-3 cells. Oncol Lett. 8:1307–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giulietti A, Overbergh L, Valckx D,
Decallonne B, Bouillon R and Mathieu C: An overview of real-time
quantitative PCR: Applications to quantify cytokine gene
expression. Methods. 25:386–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Evans T, Rosenthal ET, Youngblom J, Distel
D and Hunt T: Cyclin: A protein specified by maternal mRNA in sea
urchin eggs that is destroyed at each cleavage division. Cell.
33:389–396. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fung TK and Poon RY: A roller coaster ride
with the mitotic cyclins. Semin Cell Dev Biol. 16:335–342. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motokura T, Bloom T, Kim HG, Jüppner H,
Ruderman JV, Kronenberg HM and Arnold A: A novel cyclin encoded by
a bcl1-linked candidate oncogene. Nature. 350:512–515. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masclef L, Dehennaut V, Mortuaire M,
Schulz C, Leturcq M, Lefebvre T and Vercoutter-Edouart AS: Cyclin
D1 stability is partly controlled by O-GlcNAcylation. Front
Endocrinol (Lausanne). 10:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan KX, Liu BC, Shi XL, You BR and Xu M:
Role of cyclinD1 and CDK4 in the carcinogenesis induced by silica.
Biomed Environ Sci. 18:286–296. 2005.PubMed/NCBI
|
|
23
|
Hsu CC, Chen CH, Hsu TI, Hung JJ, Ko JL,
Zhang B, Lee YC, Chen HK, Chang WC and Lin DY: The 58-kda
microspherule protein (MSP58) represses human telomerase reverse
transcriptase (hTERT) gene expression and cell proliferation by
interacting with telomerase transcriptional element-interacting
factor (TEIF). Biochim Biophys Acta. 1843:565–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang X, Wang H, Li Y, Xiao Y, Zhao L,
Zhang T, Zhou S, Zhou X, Li Y, Shou Z, et al: Alantolactone induces
apoptosis through ROS-mediated AKT pathway and inhibition of
PINK1-mediated mitophagy in human HepG2 cells. Artif Cells Nanomed
Biotechnol. 47:1961–1970. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Z, Wang C, Huang M, Tao Z, Yan W and Du
Y: Naturally occurring sesquiterpene lactone-santonin, exerts
anticancer effects in multi-drug resistant breast cancer cells by
inducing mitochondrial mediated apoptosis, caspase activation, cell
cycle arrest, and by targeting Ras/Raf/MEK/ERK signaling pathway.
Med Sci Monit. 25:3676–3682. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Si L, Jia Y, Lin R, Jian W, Yu Q and Yang
S: MicroRNA-27a regulates the proliferation, chemosensitivity and
invasion of human ovarian cancer cell lines by targeting Cullin 5.
Arch Biochem Biophys. 668:9–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao Z, Shen X, Dong P, Liu G, Pan S, Sun
X, Hu H, Pan L and Huang J: Fucosterol exerts antiproliferative
effects on human lung cancer cells by inducing apoptosis, cell
cycle arrest and targeting of Raf/MEK/ERK signalling pathway.
Phytomedicine. 61:1528092019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun W, Guo F and Liu M: Up-regulated WDR5
promotes gastric cancer formation by induced cyclin D1 expression.
J Cell Biochem. 119:3304–3316. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou J, Zhang C, Zhou B and Jiang D:
miR-183 modulated cell proliferation and apoptosis in ovarian
cancer through the TGF-β/Smad4 signaling pathway. Int J Mol Med.
43:1734–1746. 2019.PubMed/NCBI
|
|
30
|
Sun L, Dong Z, Gu H, Guo Z and Yu Z:
TINAGL1 promotes hepatocellular carcinogenesis through the
activation of TGF-β signaling-medicated VEGF expression. Cancer
Manag Res. 11:767–775. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo Y, Fu Z, Hu Y, Li Y, Xu Q, Sun D and
Tan Y: Effects of transforming growth factor-β1 on the
proliferation and invasion of the HTR-8/SVneo cell line. Oncol
Lett. 8:2187–2192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Liu X and Zhao C: Smad4 gene
silencing enhances the chemosensitivity of human lymphoma cells to
adriamycin via inhibition of the activation of transforming growth
factor β signaling pathway. J Cell Biochem. 120:15098–15105. 2019.
View Article : Google Scholar : PubMed/NCBI
|