Introduction
In recent years, lung cancer has become the most
commonly diagnosed cancer type (11.6% of the total cases) and the
leading cause of cancer-related death (18.4% of the total cancer
deaths), worldwide (1). According to
statistics, China accounted for one-third of the global lung cancer
deaths (2). In addition, with an
aging world population, the proportion of elderly patients with
lung cancer has increased concomitantly (2). Furthermore, the average risk of death
from lung cancer increases by 62% for every 5 years of age
(2). Non-small cell lung cancer
(NSCLC) accounts for 80–85% of lung cancer cases (3). Surgery, external beam radiotherapy
(EBRT) or chemotherapy used alone or in combination are the
standard treatments recommended for patients with NSCLC (4). 125I brachytherapy performed
by implantation of 125I radioactive seeds in the tumors
can induce extensive necrosis of tumors and improve the quality of
life of patients (5). Previous
studies have demonstrated that iodine-125 (125I) seed
implantation brachytherapy exhibits good clinical results as a
minimally invasive and effective treatment for a variety of tumors,
such as pancreatic or liver cancer and gynecologic malignancies
(6–8). Recently, 125I brachytherapy
has been used for the treatment of lung cancers; however, most of
these studies focused on assessing the effectiveness of
125I brachytherapy combined with chemotherapy or
radiotherapy (3,5,9,10).
Patients aged ≥60 years are considered to be elderly
patients according to the World Health Organization (11). Elderly patients with lung cancer
develop various chronic diseases and multiple organ dysfunctions
such as those of the heart, lung, kidney and liver, which may
increase the risk of treatment-related death (12). As such, these patients are more
likely to experience an intolerance to conventional antitumor
treatments such as surgery, chemotherapy and radiotherapy (12,13). The
treatment of elderly patients with lung cancer remains a
challenge.
125I seed brachytherapy may provide a
novel and effective treatment for elderly patients with lung
cancer. To the best of our knowledge, no studies have investigated
the effectiveness of CT-guided 125I brachytherapy alone
in elderly patients with NSCLC to date. Thus the present study
aimed to analyze the data of 26 elderly patients with NSCLC treated
with 125I seed implantation alone and determine the
feasibility, efficacy and complications of the technique.
Materials and methods
Patients and eligibility
This study was approved by the Ethics Committee of
Hebei General Hospital (Shijiazhuang, China). The ethics committee
waived informed patient consent due to the retrospective nature of
this study. All patient records were anonymized prior to
analysis.
The present study retrospectively analyzed the data
of 26 elderly (≥60 years as defined by the WHO) patients treated
with 125I seed brachytherapy between January 2015 and
August 2017. All patients were diagnosed histopathologically using
bronchoscopy or transthoracic needle biopsy and staged according to
the manual of the American Joint Committee for Cancer Staging, 7th
edition.
The inclusion criteria were as follows: i) Age ≥60
years; ii) histopathological diagnosis of NSCLC; iii) maximum
diameter of the lesion ≤8 cm; iv) Karnofsky performance status
score ≥50; v) expected survival ≥3 months; vi) patients in early
stages of the disease who were not candidates for surgery because
of co-morbidities; or in the advanced stages with stable metastases
and recurrence or progression of primary lung lesions after
chemotherapy or radiotherapy; or patients who refused the
aforementioned treatments; vii) patients who did not receive
radiotherapy or chemotherapy within 6 months prior to the
brachytherapy; viii) epidermal growth factor receptor/anaplastic
lymphoma kinase/ROS proto-oncogene 1 receptor tyrosine kinase/ROS
proto-oncogene mutations were not detected; and ix) patients
provided individual signed informed consent for 125I
brachytherapy and publication. The exclusion criteria were as
follows: i) Severe cardio-pulmonary dysfunction, such as higher
than grade III cardiac function (New York Heart Association
grading) (14), malignant arrhythmia
or active tuberculosis; ii) platelet count
<20.0×109/l; iii) abnormal coagulation function.
Materials
Radioactive 125I seeds (Jinan Xinke
Pharmaceutical Science and Technology Co., Ltd.) of type 6711–99,
with a length of 4.5 mm and a diameter of 0.8 mm were used. The
125I seeds produced gamma-rays (5% of 35 keV, 95% of 28
keV) with an incipient rate of 7 cGy/h, activities of 0.5–0.8 mCi,
half-life of 59.6 days, half-value thickness of 0.025 mm of lead
and penetration of 17 mm. Seed activities were verified using a
radioisotope dose calibrator CRC-25R (CAPINTEC, Inc.; http://www.lejiesh.com) prior to operation.
Anisotropic factor φ was used in the dose calculation equation of
the brachytherapy planning system to perform average anisotropy
correction. The treatment planning system (TPS) was Prowess Panther
Brachy v.5.0 (Prowess Inc.). Mick 200-TPV Needle Applicators
(implantation gun) and 18-gauge seed implantation needles were
purchased from Mick Radio-Nuclear Instruments, Inc. The 3D-printed
template was provided from Beijing Unicorn Science and Technology
Ltd. (http://www.bjunicorn.com). A vacuum
cushion was produced by Tianchang Hengsheng Medical Devices Co.,
Ltd.
Radiation dosimetry and implant
planning
A contrast-enhanced CT scan of the entire lung with
a 5-mm slice thickness was performed in all patients 1 week prior
to the seed implantation. Continuous axial images were transferred
to the TPS. The gross tumor volume (GTV) and the surrounding organs
at risk (OARs) including the spinal cord, heart and esophagus, were
carefully delineated in each CT slice. Dosimetric evaluation
parameters included the dose delivered to 90 (D90) or 100% (D100)
of the clinical target volume (CTV) and the percentage of CTV
receiving 90 (V90), 100 (V100) or 150% (V150) of the prescription
dose. The dosimetric goal was to achieve a prescription dose of
80–140 Gy, D90 ≥90% and V90 ≥90%. For patients who had received
prior radiotherapy, the prescription dose was appropriately reduced
according to the tolerated dose of OARs. The matched peripheral
dose of OARs was limited according to the recommendations of the
American Brachytherapy Society (15)
and the Radiation Therapy Oncology Group (RTOG)/European
Organization for Research and Treatment of Cancer (EORTC) criteria
(16) as follows: Large vessels,
<80 Gy; heart, <45 Gy; esophagus, <60 Gy; and spinal cord,
<45 Gy. Two patients were prescribed a dose of 80 Gy due to the
tumor reappearing within 6 months after radiotherapy adjacent to
the spinal cord. Seeds with low activity (0.5–0.6 mCi) were used in
the tumor target area adjacent to the OARs. The implanted seed
spacing was 0.5–1 cm, and the needle spacing was 1 cm. TPS was used
to calculate the direction and depth of needles, as well as the
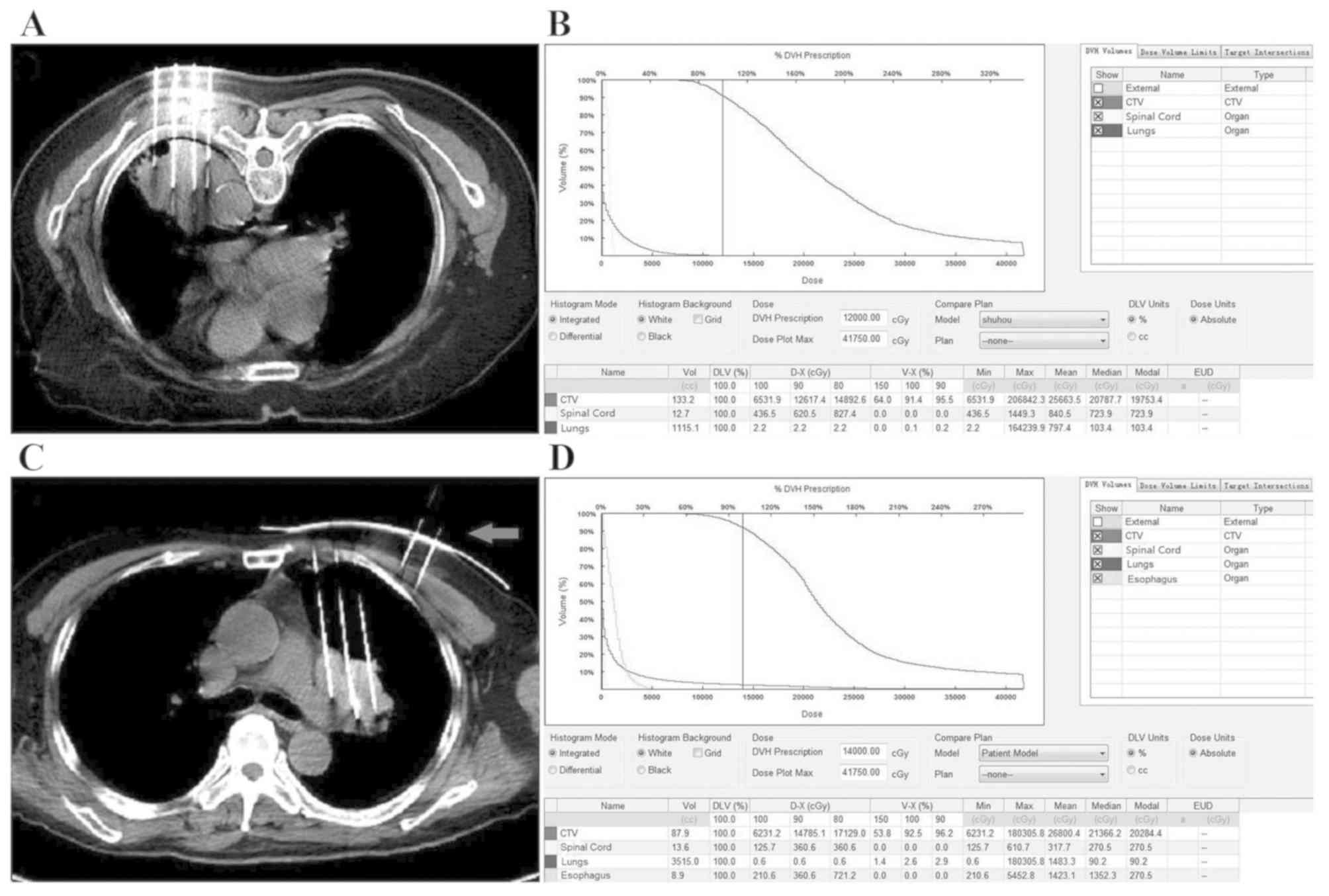
number and distribution of implanted seeds (Fig. 1A-C and E), and to generate
dose-volume histograms (DVH) and isodose curves of different
percentages (Fig. 1D and F).
Post-operative chest CT scans were obtained immediately after seed
implantation, and the images were transferred to TPS to calculate
postoperative DVH for the dosimetric verification (Fig. 2B and D). In three patients, treatment
was guided using a 3D-printed template. The skin contour of
patient, needle coordinates and puncture holes were reconstructed
in TPS, and a 3D printing output file was generated. A
stereolithography-600 3D printer (Beijing Unicorn Science and
Technology Ltd.) was used to print the 3D template.
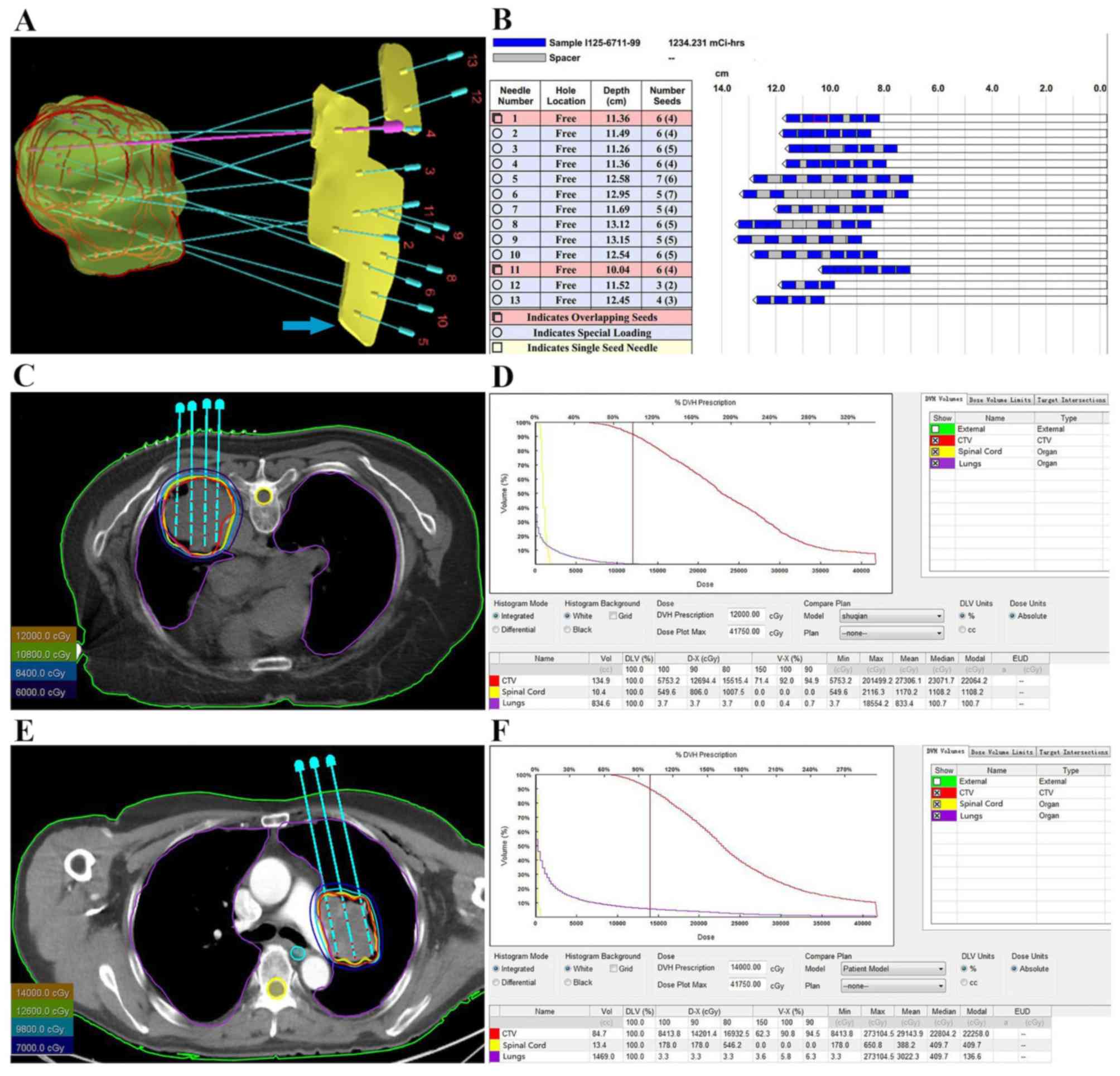 | Figure 1.Preoperative planning and DVH of two
patients with NSCLC. (A) TPS displaying 3D image of CTV and the
direction of needles. (B) TPS displaying the depth of needles and
the number and distribution of seeds. (C) Radiation oncologist
outlined external contour (outer contour of the body, green line),
CTV (red line) and OARs, including the spinal cord (yellow line)
and the lungs (purple line). TPS drew the isodose curve and
displayed needles and seeds in one of the patient. (D) Preoperative
DVH presenting dose distribution in CTV and OARs in one of the
patient. (E) The external contour (green line), CTV (red line),
spinal cord (yellow line), lungs (purple line), and the isodose
curve in another patient. (F) Preoperative DVH of another patient.
TPS, treatment planning system; DVH, dose-volume histogram; CTV,
clinical target volume; OARs, surrounding organs at risk; NSCLC,
non-small cell lung carcinoma. |
125I implantation. Following
intramuscular injection of 10 mg diazepam, patients were fixed on
the CT table with the vacuum cushion in the same position. CT scans
(SOMATOM Force, Siemens.; http://www.siemens-healthineers.com/computed-tomography/dual-source-ct/somatom-force)
were used to determine the tumor site and layers of seeds
implantation. Next, in most patients of freehand implantation
(without template-guided), the puncture points of the needles,
according to the preplan, were marked on the surface of the body of
the patient with the help of the CT laser. In the 3D-printed
template-guided patients, the 3D template was fixed according to
the marks on the body surface. Local infiltration anesthesia
combined with intercostal nerve block anesthesia with 1% lidocaine
was supplied to the puncture range following disinfection. Using
CT-imaging guidance, 18-gauge needles were inserted into the
farthest edge of tumors and were spaced at a distance of 1.0 cm in
a parallel array according to the preplan (Fig. 2A and C). Precautions were taken to
avoid puncturing the surrounding large blood vessels, bronchi,
ribs, scapula or spinal cord. The 125I seeds were
implanted into the tumor through each needle and were released
while drawing back the needles from deep to shallow. The space
between seeds was kept at 0.5–1.0 cm. Post-operative CT scans were
performed immediately after the implantation procedure to confirm
that the seed distribution was correct and to exclude any
implantation-related complications such as pneumothorax, pulmonary
hemorrhage or seed migration. The vital signs of all patients were
monitored during and after the procedure.
Follow-up
CT or positron-emission tomography/CT scans were
performed monthly to evaluate the therapeutic effects for the first
3 months, and then every 3 months following implantation.
Postoperative symptoms and treatment-related complications were
recorded during follow-up.
Endpoints
Efficacy and safety of CT-guided 125I
brachytherapy were the primary endpoints of the present study.
Efficacy was evaluated according to the Response Evaluation
Criteria in Solid Tumors v.1.1 (17)
and classified as complete response (CR), partial response (PR),
stable disease (SD) and progressive disease (PD). The response rate
(RR) was confirmed in cases with CR and PR. The local control rate
(LCR) was confirmed in cases with CR, PR and SD. Clinical
complications were assessed from the beginning of the implantation
procedure. The acute and late radiation toxicities were assessed in
accordance with the toxicity criteria of RTOG and EORTC (16). The secondary endpoint was the overall
survival (OS). OS time was defined as the interval between the date
of implantation and the date of death from any cause.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp.) software. The characteristics of patients were
expressed as continuous variables and/or categorical variables.
Continuous data were expressed as the mean ± SD, and categorical
data were expressed as the median and interquartile range. RR and
LCR were expressed based on the number and percentage of patients.
OS was analyzed using Kaplan-Meier curves. Deaths from any cause
were scored as events when calculating survival rate.
Results
Patient characteristics
Of the 26 patients who enrolled in the present
study, 14 (53.8%) were men and 12 (46.2%) were women. The median
age was 77 years (age range, 65–85 years). A toal of 14 patients
presented central type and 12 presented peripheral type tumors
(Table I). The median size of tumors
was 5.8 cm (range, 2.0–7.7 cm). In this group, 125I seed
brachytherapy was used as a radical treatment for 17 inoperable
stage I and II (T1-3, N0-1) patients, six of whom were received
salvage treatment for recurrence after radiotherapy. All patients
with stage III had received prior chemotherapy, and six different
patients had received prior radiotherapy. 125I seed
implantation was used as a salvage treatment for patients with
stage III progressive primary lesions following radiotherapy and
chemotherapy. Patient characteristics are presented in Table I.
 | Table I.Demographic and clinicopathological
features of patients with NSCLC (n=26). |
Table I.
Demographic and clinicopathological
features of patients with NSCLC (n=26).
|
Characteristics | Cases, n (%) |
|---|
| Sex |
|
|
Male | 14 (53.8) |
|
Female | 12 (46.2) |
| Age, median years
(range) | 77 (65–85) |
| KPS |
|
|
<80 | 14 (53.8) |
|
≥80 | 12 (46.2) |
| Histology |
|
| AC | 11 (42.3) |
|
SCC | 14 (53.8) |
|
ASC | 1 (3.8) |
| Clinical stage
(AJCC) |
|
| I | 5 (19.2) |
| II | 12 (46.2) |
|
III | 9 (34.6) |
| Tumor location |
|
|
Central | 14 (53.8) |
|
Peripheral | 12 (46.2) |
| Maximum tumor
diameter, cm |
|
| ≤3 | 5 (19.2) |
|
3-8 | 21 (80.8) |
| Concomitant
illness |
|
|
COPD | 3 (11.5) |
|
Emphysema or bulla | 8 (30.8) |
| Prior history of
chemotherapy | 10 (38.5) |
| Prior history of
EBRT | 11 (42.3) |
Efficacy of 125I seed
implantation
All patients successfully underwent 125I
seed implantation, among whom three patients received 3D-printed
template guided implantation (Figs.
1A and 2C) and the rest were
treated with freehand implantation. The median number of seeds
implanted was 57 (range, 14–100) and the median number of needles
was 5 (range, 2–18). Postoperative dosimetric measurements
demonstrated that the actual D90 ranged between 79 and 148 Gy (mean
D90, 113 Gy; Table SI). All 26
patients were able to provide follow-up data. The median follow-up
time was 9.4 months (range, 3–31 months). Following the 6-month
follow-up period, for all pulmonary lesions, CR was achieved in 11
(42.3%) cases, PR in 9 (34.6%) cases, SD in 4 (15.4%) cases and PD
in 2 (7.7%) cases. The 6-month RR and LCR were 76.9% (20/26) and
92.3% (24/26), respectively (Table
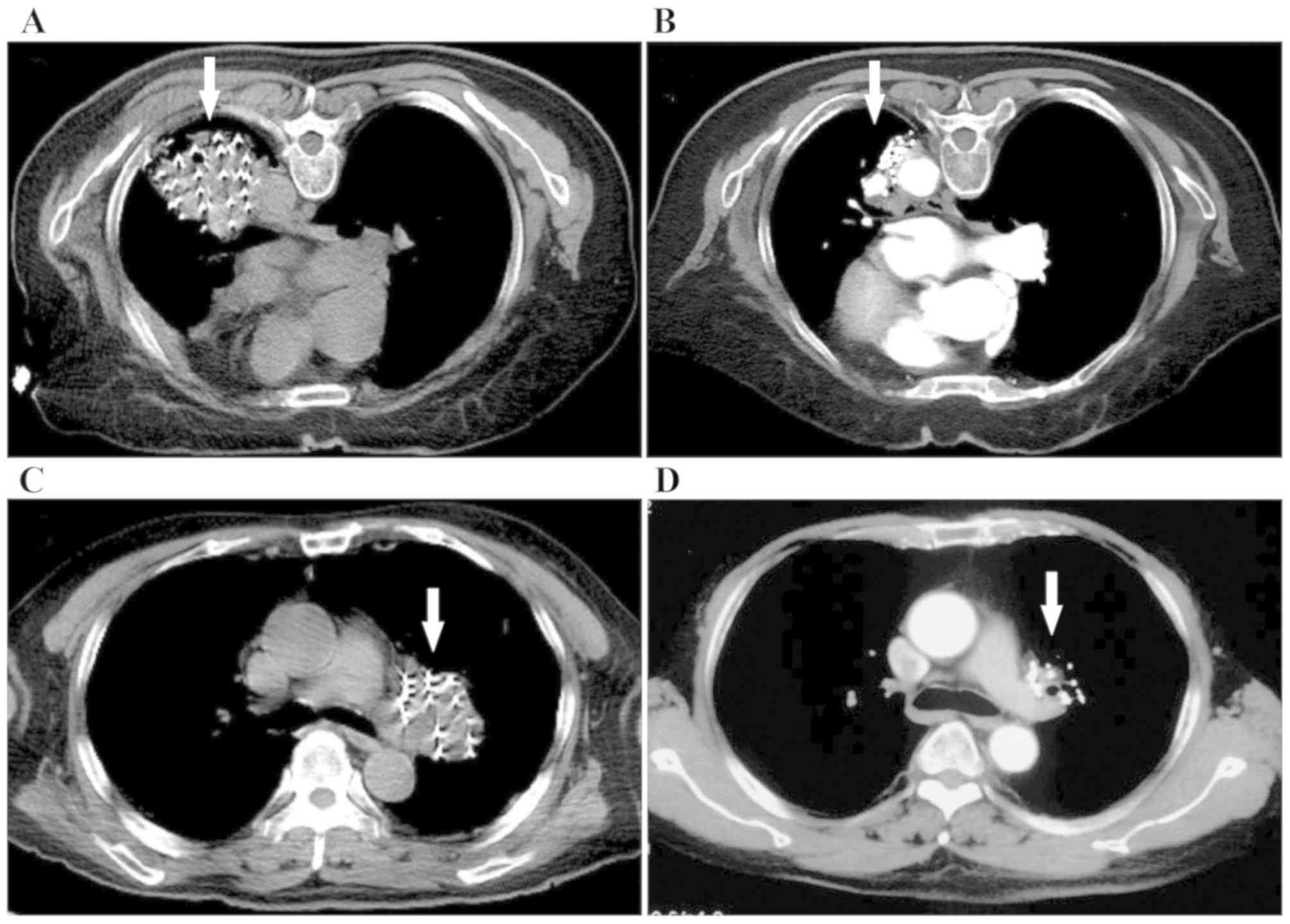
II). Furthermore, the results demonstrated that two patients
remained CR at 8 and 12 months of follow-up, respectively (Fig. 3A-D). The symptom of coughing was
significantly relieved in 16/19 (84.2%) cases, asthma was markedly
relieved in 6/10 (60%) cases, and chest pain was relieved in 7/7
(100%) cases within 4 weeks of implantation (Table II).
 | Table II.Clinical efficacy of 125I
brachytherapy and symptoms associated with non-small cell lung
cancer. |
Table II.
Clinical efficacy of 125I
brachytherapy and symptoms associated with non-small cell lung
cancer.
| Local control
efficacy | Cases, n (%) |
|---|
| CR | 11 (42.3) |
| PR | 9 (34.6) |
| SD | 4 (15.4) |
| PD | 2 (7.7) |
| RR = CR + PR | 20 (76.9) |
| LCR = CR + PR +
SD | 24 (92.3) |
| Symptom |
|
|
Cough | 16/19 (84.2) |
|
Asthma | 6/10 (60.0) |
| Chest
pain | 7/7 (100.0) |
Six patients died during the follow-up period: One
patient died of respiratory failure caused by bilateral lung
infection 4.2 months following implantation; two died of widespread
metastases at 6.8 and 17 months, respectively, following
implantation; two died of cardiovascular disease, one of which died
of arrhythmia at 6.5 months after implantation, and the other died
of acute myocardial infarction at 12.1 months after implantation;
and one died of upper gastrointestinal hemorrhage caused by
esophageal and gastric varices at 3.3 months after implantation.
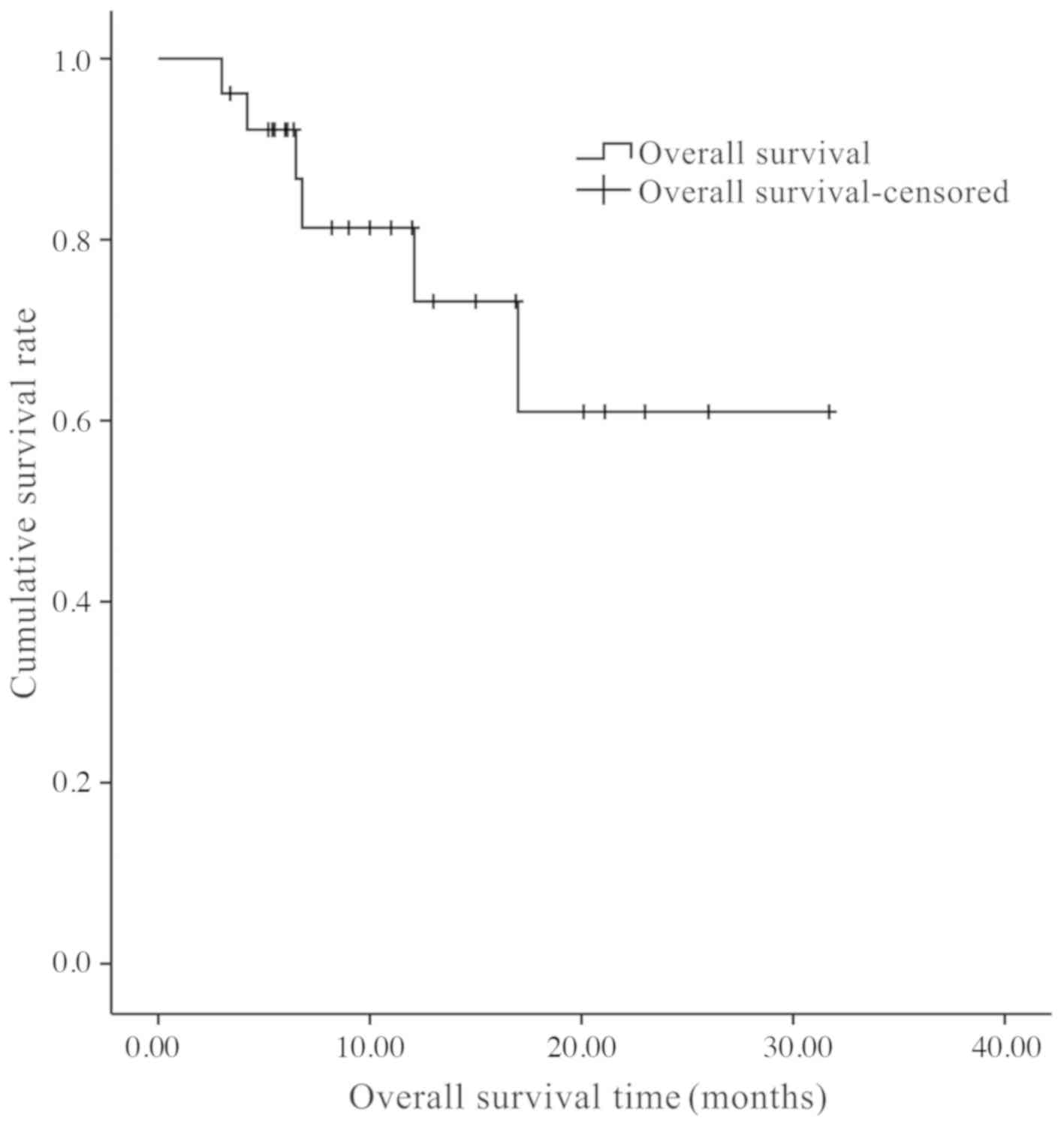
None of these deaths were related to the brachytherapy. The mean OS
time was 11.7±7.6 months, and the 0.5- and 1-year OS rates were
90.1 and 73.3%, respectively (Fig.
4).
Complications
No severe complications occurred during the
follow-up period (Table III).
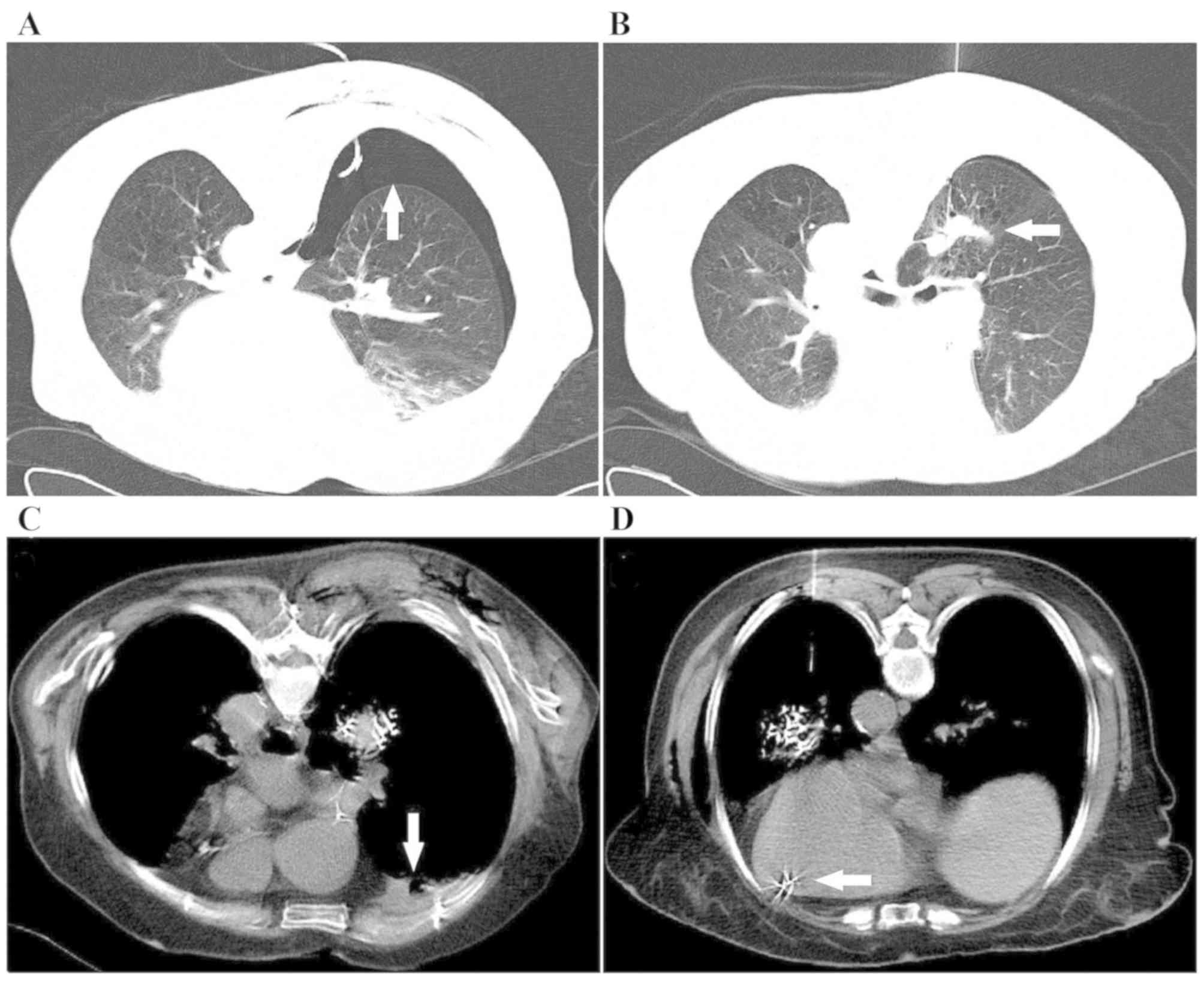
Pneumothorax occurred in six patients (23.1%) (Fig. 5A); five of these patients had
unilateral lung volume compression of <10% (lung surface
retraction ≤2 cm) without any symptoms, and the lung gradually
recovered without specific treatment. Unilateral lung volume
compression of ~30% (lung surface retraction between 2 and 4 cm)
with mild chest tightness was experienced by one patient, and the
pulmonary re-expansion was satisfactory within 72 h after immediate
treatment with closed thoracic drainage. One patient (3.8%)
developed subcutaneous emphysema and recovered 3 days after closed
drainage. Hemoptysis was developed by four patients (15.4%), who
recovered following conservative hemostasis treatment with a total
bleeding volume of 5–10 ml (Fig.
5B). Hemothorax was experienced by one patient (3.8%) during
the operation and was immediately treated with closed drainage and
hemostasis treatment (Fig. 5C). No
active bleeding was confirmed by chest CT examination on the 3rd
day after operation and a total of 150 ml of blood was drained.
Seed migration (n=2) occurred in one patient during the operation
by CT scan who survived without any symptoms at the end of the
20-month follow-up (Fig. 5D).
Radiation pneumonitis and myelosuppression were not observed.
 | Table III.Complications in elderly patients
with NSCLC following 125I implantation. |
Table III.
Complications in elderly patients
with NSCLC following 125I implantation.
| Complications | Cases, n (%) |
|---|
| Pneumothorax | 6 (23.1) |
| Mild | 5 (19.2) |
| Moderate | 1 (3.8) |
| Subcutaneous
emphysema | 1 (3.8) |
| Hemothorax | 1 (3.8) |
| Hemoptysis | 4 (15.4) |
| Seed migration | 1 (3.8) |
Discussion
Surgery, radiotherapy and chemotherapy are standard
treatments for lung cancer (4).
However, elderly patients with lung cancer are often limited by
their own pathological and physiological characteristics, such as
multiple other diseases (particularly cardiovascular and pulmonary
dysfunction) as well as organ and immune dysfunction, which
increase the risks of surgery and systemic chemotherapy (12). In addition, the survival benefits of
chemotherapy alone are low and are hampered by potential toxicity
such as nausea and vomiting, myelosuppression, liver and kidney
function damage, cardiotoxicity and hair loss (12,13).
This suggests that clinicians should not make treatment strategies
based only on the tumor stage. Therefore, it is important to choose
a suitable treatment for elderly patients.
Radiotherapy is an important local treatment for
lung cancer. Radiation dose must reach a certain level to destroy
malignant tumor cells (18–20). Numerous studies have confirmed that
increasing the radiation dose can significantly improve LCR. In a
phase I dose-escalation study by Rosenzweig et al (18), the 2-year OS rate for patients with
stage I–II disease who received <80 Gy was 60% compared with 66%
for patients who received >80 Gy (P<0.05), with a median
survival time of 25.0 months and 53.6 months, respectively. In a
randomized trial from China (19),
5-year LCR and 2-year OS rate improved significantly in patients
with stage III lung cancer treated with a total dose of 68–74 Gy
compared with those treated with 60–64 Gy (LCR, 51 vs. 36%;
P=0.032; OS, 39.4 vs. 25.6%, P=0.048). However, dose escalation in
the RTOG 0617 trial (20) failed to
improve survival, which may be related to the following reasons: i)
More than half of the patients used 3D conformal radiotherapy
(3DCRT), whereas those who used intensity-modulated radiotherapy
(IMRT) only accounted for ~45%. Physically, IMRT achieved improved
dose distribution compared with 3DCRT. A higher dose led to greater
side effects (especially cardiac and pulmonary), offsetting the
survival advantage of incremental radiotherapy (21). Underestimation of the severe toxicity
of high-dose radiotherapy may be the main reason behind the failed
improvements of survival rates (21,22). ii)
The RTOG 0617 study included locally advanced patients with stage
III. The treatment failure or death of locally advanced NSCLC was
mainly related to distant metastasis (20,22). The
increase in the LCR caused by incremental radiotherapy failed to
translate into an increase in survival (20,22). It
has been reported that the lowest biologically effective dose (BED)
to kill lung cancer cells may exceed 100 Gy (22,23).
However, it is difficult to administer a radical radiation dose to
tumors due to the poor tolerance to radiation for OARs, even with
advanced technology including image-guided radiotherapy and IMRT.
Using these modern techniques, current radiotherapy applying a
uniform prescription dose of 60 Gy or slightly higher generates
LCRs of <50% and a 5-year OS rate of 10–15% for locally advanced
NSCLC tumors (22,23). Stereotactic body radiation therapy
(SBRT) involving extreme hypofractionation with high-dose radiation
to the target volume and low doses to the surrounding normal
tissues is considered superior compared with EBRT. However, SBRT is
most suitable for small lesions (typically <5 cm), and the
accuracy and reproducibility of treatments are essential (24,25). In
addition, SBRT is contraindicated for lesions within 2 cm from the
bronchial tree, and a marked prevalence of complications such as
pulmonary toxicity and rib toxicity have been reported (24,25).
125I seed implantation is a minimally
invasive treatment for tumors that has been rapidly developed in
the past decade and has been listed as a treatment for prostate
cancer (26). 125I seeds
are implanted into tumors and continuously generate low-energy
gamma-rays to kill tumor cells in a similar manner to multiple
hyperfractionated radiotherapy, which can prevent the accelerated
repopulation of tumor cells (27,28). In
addition, low radiation dose rates can induce reoxygenation and
increase blood flow in hypoxic tumors, thus producing a
radiation-induced bystander effect to kill tumor cells, which can
overcome uneven distribution of radiation doses (27,28). Of
note, the dose distribution around 125I seeds obeys an
inverse-square law; the radiant energy decreases rapidly as the
distance from the seed increases (29). Therefore, 125I
brachytherapy can target the entire irradiation dose to the target
tumor volume, while providing a very low dose to adjacent normal
tissues, thus improving local efficacy and reducing the incidence
of side effects (27,28). Zhang et al (30) reported 125I seed
implantation in localized advanced pulmonary carcinomas and
demonstrated that the LCR was 78.1% at the 2-month follow-up, with
a 1-year survival rate of 65.0%. In the present study, the 6-month
RR and LCR were 76.9 and 92.3%, respectively. The mean OS was
11.7±7.6 months and the 0.5- and 1-year OS rates were 90.1 and
73.3%, respectively. The present study demonstrated an improved RR,
LCR and 1-year OS compared with that of Zhang et al
(30) due to the patients in the
present study having early-stage NSCLC. These studies have
demonstrated the good results of 125I seed implantation
for NSCLC (30–32). However, these studies all involved
combination therapies of 125I brachytherapy with
chemotherapy or EBRT. To the best of our knowledge, no previous
studies have investigated the efficacy of 125I
brachytherapy alone in elderly patients with NSCLC. The results of
the present study indicated that 125I brachytherapy may
be used alone to improve the LCR, relieve the symptoms and prolong
the OS time. Li et al (31)
performed CT fluoroscopy-guided 125I seed implantation
for patients with stage T1-3N0M0 NSCLC and reported that at the
median follow-up of 31.5 months, the LCR was 78.6% and the 1-, 2-
and 3-year OS rates were 95.8, 78.0 and 55.0%, respectively.
Another study reported that 125I brachytherapy combined
with chemotherapy was an effective treatment for inoperable stage
III/IV NCSLC. The RR was 88.5%, and the 1- and 2-year OS rates were
82.8 and 37.1%, respectively (32).
These studies demonstrated a more positive outcome compared with
the results of the present study. This was likely because a
combination of treatments was not performed in the present study,
and the patients were elderly with various other medical
conditions. In the present study, 60–100% of patients with symptoms
of cough, asthma and chest pain had a significant alleviation
within 4 weeks after implantation, which directly improved the
quality of life.
In addition, the present study used the BED equation
(33) to compare doses between
125I brachytherapy and EBRT. The prescription dose of
80–140 Gy used in the present study, which was equivalent to 68–118
Gy of EBRT, contributed to a satisfactory local control without
radiation damage to the surrounding normal tissues. In the present
study, during the follow-up period, two cases recurred at the edge
of the target area, and the possible causes were analyzed. It may
be due to the insufficient peripheral doses of target volume. Most
studies of 125I implantation only delineated GTV as
target areas (34). However, a
number of microtumor infiltrative lesions usually occur outside the
edge of the primary tumor and are difficult to observe by CT scan
(31). Therefore, it is recommended
to extend the GTV by 0.5 cm to form CTV as the target volume for
125I seed implantation (31). PTV involves organ movement and daily
placement error, which is a concept of EBRT and is rarely
considered in 125I seed implantation (31,34).
Furthermore, in order to avoid the insufficient peripheral dose,
the expert consensus requires D90 ≥90% prescription dose (34). Due to the short penetration distance
and rapid dose drop around the seed, dose cold spots are prone to
occur in and around the target area. It is important to perform
intraoperative TPS verification during implantation to provide
real-time guidance to replant the seeds in the cold zone (35).
The most common complications of 125I
brachytherapy are related to the implantation operation and include
pneumothorax, pulmonary hemorrhage and hemoptysis, which can be
relieved by timely treatment (36).
A previous study demonstrated that the incidence of pneumothorax
and hemorrhage in 125I seed implantation was 12.5–31.0
and 9.2–46.9%, respectively (31).
In the present study, the incidences of pneumothorax, hemoptysis
and hemothorax were 23.1, 15.4 and 3.8%, respectively. In addition,
the incidence of pneumothorax was slightly higher compared with the
previous aforementioned study, as elderly patients with lung cancer
usually suffer from chronic obstructive pulmonary disorder and
emphysema, especially bulla, which are more likely to contribute to
the development of pneumothorax during the puncture procedure.
Pneumothorax is more likely to occur in central compared with
peripheral type lung cancer, as more lung tissue is passed through
during implantation (36). In
addition, rib obstruction and respiratory movement may affect the
implantation, resulting in repeated punctures and increased risk of
lung injury (36). In the present
study, 3D-printed templates were used in three patients, which
improved the accuracy and safety of implantation, lowered the
difficulty of puncture, shortened the operation time and reduced
radiation exposure from repeated CT scans. However, respiratory
movements may cause changes in the relative tumor location,
rendering the 3D templates unusable. In addition, following seed
implantation, the relative position of the seeds changes with the
change of tumor volume, resulting in changes in the actual exposure
dose of the target volume and OARs (37). If the tumor volume shrinks too fast,
the dose parameters such as D90 and V90 may significantly increase,
potentially leading to complications (37). Our previous study revealed that when
the tumor volume shrank at a rate of 15–20% per month, it did not
cause a significant change in dose (37). The shrinkage rate of tumor depends on
its radiosensitivity (37). In the
current study, small cell lung cancer with high radiosensitivity
was not included; therefore, the effect of the dynamic dose on the
target area was not significant. In order to reduce the incidence
of complications, low activity seeds were used in the tumor target
areas adjacent to OARs such as the bronchi, esophagus, heart and
blood vessels. In addition, an increased risk of treatment-related
toxicity was observed in tumors directly abutting the proximal
bronchial tree, termed ‘ultra-central’ tumors. For these tumors,
there was no consensus regarding the 125I seed
implantation regimen. According to our experience, the distance
between the seeds and the bronchial tree should be >1 cm, and
low activity seeds are preferred (37). In order to reduce the risk of
hemorrhage and hemoptysis in the present study, firstly,
contrast-enhanced CT was performed prior to operation to determine
the location of blood vessels inside and around the tumor, and the
preplan of the current study was designed to avoid puncture injury
of blood vessels. Secondly, it was necessary to fully evaluate and
discontinue anticoagulant drug treatments, such as aspirin and
warfarin prior to operation. In the present study, two seeds were
observed to migrate through the blood vessels to the heart during
the operation in one patient. In addition, 125I seeds
have been reported to migrate to the lung and heart in the
brachytherapy for prostate cancer and hepatocellular carcinoma
without serious symptoms (8,38). No cardiac symptoms were observed in
this patient during the follow-up period of the present study,
although potential adverse effects were reported when seeds
migrated to the heart, such as acute myocardial infarction
(39). The long-term effects of seed
migration have not been monitored. Accurate placement of seeds and
care to avoid punctures into blood vessels may reduce seed
migration. There were also reports of small amounts of radiation
pneumonitis around the implanted seeds, which have no effect on
lung function (40). Additionally,
in the present study, no radioactive complications such as
radiation pneumonitis, radiation esophagitis, cardiotoxicity and
tracheal necrosis were observed after 125I
brachytherapy.
The present study has several limitations, such as
its retrospective design, the relatively small sample size from a
single institution, heterogeneous patients and a short follow-up
period. Therefore, large-scale prospective studies with long-term
follow-up are required to verify the findings of the present study.
Furthermore, future clinical trials need to focus on the efficacy
of 125I brachytherapy compared with other radiation
techniques such as SBRT in early stages of NSCLC or combined with
systemic treatment in advanced NSCLC. In the future, the
combination regimen and the dosage of brachytherapy and EBRT should
be investigated.
In conclusion, the present study preliminarily
demonstrated that CT-guided 125I seed brachytherapy was
an effective and safe treatment for elderly patients with NSCLC.
CT-guided 125I seed brachytherapy led to favorable local
control, fewer side effects, good symptomatic relief and an
improved quality of life. 125I seed brachytherapy may be
an alternative or a complementary treatment option to surgery and
radiotherapy for elderly patients with NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ and JW conceived and designed the present study.
JZ, HZ, JXZ, KX and ZL performed most of the experiments. JZ, ZZ,
YD, CM and AS drafted the initial manuscript and analyzed the data.
All authors have read and approved the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Hebei General Hospital (Shijiazhuang, China). The ethics committee
waived informed patient consent due to the retrospective nature of
this study. All patient records were anonymized prior to
analysis.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zuo TT, Zheng RS, Zeng HM, Zhang
SW and He J: Lung cancer incidence and mortality in China in 2013.
Zhonghua Zhong Liu Za Zhi. 39:795–800. 2017.(In Chinese; Abstract
Available In Chinese From The Publisher). PubMed/NCBI
|
|
3
|
Yu X, Li J, Zhong X and He J: Combination
of Iodine-125 brachytherapy and chemotherapy for locally recurrent
stage III non-small cell lung cancer after concurrent
chemoradiotherapy. BMC Cancer. 15:6562015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
5
|
Song J, Fan X, Zhao Z, Chen M, Chen W, Wu
F, Zhang D, Chen L, Tu J and Ji J: 125I brachytherapy of
locally advanced non-small-cell lung cancer after one cycle of
first-line chemotherapy: A comparison with best supportive care.
Onco Targets Ther. 10:1345–1352. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin Z, Du Y, Li Z, Jiang Y, Chen J and Liu
Y: Endoscopic ultrasonography-guided interstitial implantation of
iodine 125-seeds combined with chemotherapy in the treatment of
unresectable pancreatic carcinoma: A prospective pilot study.
Endoscopy. 40:314–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin J, Yang W, Jiang N, Zheng Q, Huang J,
Huang N, Li A and Jiang H: Incidence and prediction of seed
migration to the chest after iodine-125 brachytherapy for
hepatocellular carcinoma. Brachytherapy. 16:1252–1256. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tong L, Liu P, Huo B, Guo Z and Ni H:
CT-guided 125I interstitial brachytherapy for pelvic
recurrent cervical carcinoma after radiotherapy. Onco Targets Ther.
10:4081–4088. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Dan G, Jiang J, Zheng Y, Zheng X and
Deng D: Repeated iodine-125 seed implantations combined with
external beam radiotherapy for the treatment of locally recurrent
or metastatic stage III/IV non-small cell lung cancer: A
retrospective study. Radiat Oncol. 11:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Li J, Li R, Zhang Y, Han M and Ma
W: Efficacy and safety of iodine-125 radioactive seeds
brachytherapy for advanced non-small cell lung cancer-A
meta-analysis. Brachytherapy. 17:439–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Özcan MF, Altınova S and Atan A: Treatment
approaches to small renal masses in patients of advanced age (≥75
years). Turk J Urol. 44:281–286. 2018.PubMed/NCBI
|
|
12
|
Di Maio M and Perrone F: Quality of life
in elderly patients with cancer. Health Qual Life Outcomes.
1:442003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verma V, Simone CB II and Werner-Wasik M:
Acute and late toxicities of concurrent chemoradiotherapy for
locally-advanced non-small cell lung cancer. Cancers (Basel).
9:E1202017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goode KM, Nabb S, Cleland JG and Clark AL:
A comparison of patient and physician-rated New York Heart
Association class in a community-based heart failure clinic. J Card
Fail. 14:379–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stewart A, Parashar B, Patel M, O'Farrell
D, Biagioli M, Devlin P and Mutyala S: American brachytherapy
society consensus guidelines for thoracic brachytherapy for lung
cancer. Brachytherapy. 15:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aras M, Erdil TY, Dane F, Gungor S, Ones
T, Dede F, Inanir S and Turoglu HT: Comparison of WHO, RECIST 1.1,
EORTC, and PERCIST criteria in the evaluation of treatment response
in malignant solid tumors. Nucl Med Commun. 37:9–15.
2016.PubMed/NCBI
|
|
18
|
Rosenzweig KE, Fox JL, Yorke E, Amols H,
Jackson A, Rusch V, Kris MG, Ling CC and Leibel SA: Results of a
phase I dose-escalation study using three-dimensional conformal
radiotherapy in the treatment of inoperable nonsmall cell lung
carcinoma. Cancer. 103:2118–2127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan S, Sun X, Li M, Yu J, Ren R, Yu Y, Li
J, Liu X, Wang R, Li B, et al: A randomized study of involved-field
irradiation versus elective nodal irradiation in combination with
concurrent chemotherapy for inoperable stage III nonsmall cell lung
cancer. Am J Clin Oncol. 30:239–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bradley JD, Paulus R, Komaki R, Masters G,
Blumenschein G, Schild S, Bogart J, Hu C, Forster K, Magliocco A,
et al: Standard-dose versus high-dose conformal radiotherapy with
concurrent and consolidation carboplatin plus paclitaxel with or
without cetuximab for patients with stage IIIA or IIIB
non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two
factorial phase 3 study. Lancet Oncol. 16:187–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Zhou Z, Liang J, Feng Q, Xiao Z,
Hui Z, Wang X, Lv J, Chen D, Zhang H, et al: Intensity-Modulated
radiation therapy may improve local-regional tumor control for
locally advanced non-small cell lung cancer compared with
three-dimensional conformal radiation therapy. Oncologist.
21:1530–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Men Y, Feng L, Kang J, Sun X, Yuan
M, Jiang W and Hui Z: A current review of dose-escalated
radiotherapy in locally advanced non-small cell lung cancer. Radiol
Oncol. 53:6–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong FM, Zhao J, Wang J and Faivre-Finn C:
Radiation dose effect in locally advanced non-small cell lung
cancer. J Thorac Dis. 6:336–347. 2014.PubMed/NCBI
|
|
24
|
Zhao J, Yorke ED, Li L, Kavanagh BD, Li
XA, Das S, Miften M, Rimner A, Campbell J, Xue J, et al: Simple
factors associated with radiation-induced lung toxicity after
stereotactic body radiation therapy of the thorax: A pooled
analysis of 88 studies. Int J Radiat Oncol Biol Phys. 95:1357–1366.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma JT, Liu Y, Sun L, Milano MT, Zhang SL,
Huang LT, Jing W, Zhao JZ, Han CB and Kong FS: Chest wall toxicity
after stereotactic body radiation therapy: A pooled analysis of 57
studies. Int J Radiat Oncol Biol Phys. 103:843–850. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maki S, Itoh Y, Kubota S, Okada T,
Nakahara R, Ito J, Kawamura M, Naganawa S, Yoshino Y, Fujita T, et
al: Clinical outcomes of 125I brachytherapy with and without
external-beam radiation therapy for localized prostate cancer:
Results from 300 patients at a single institution in Japan. J
Radiat Res. 58:870–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ZM, Lu J, Liu T, Chen KM, Huang G and
Liu FJ: CT-guided interstitial brachytherapy of inoperable
non-small cell lung cancer. Lung Cancer. 74:253–257. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL
and Cheng WY: Bystander effects induced by continuous low-dose-rate
125I seeds potentiate the killing action of irradiation on human
lung cancer cells in vitro. Int J Radiat Oncol Biol Phys.
72:1560–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loft SM and Dale RG: The incorporation of
specific tissue/nuclide attenuation data into the Anderson method
for producing brachytherapy volume-dose histograms. Phys Med Biol.
35:1519–1531. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang FJ, Li CX, Wu PH, Wu YX, Jiao DC,
Liu J and Li YL: CT guided radioactive 125I seed implantation in
treating localized advanced pulmonary carcinoma. Zhonghua Yi Xue Za
Zhi. 87:3272–3275. 2007.(In Chinese). PubMed/NCBI
|
|
31
|
Li J, Yu M, Xiao Y, Yang L, Zhang J, Ray E
and Yang X: Computed tomography fluoroscopy-guided percutaneous
125I seed implantation for safe, effective and real-time
monitoring radiotherapy of inoperable stage T1-3N0M0 non-small-cell
lung cancer. Mol Clin Oncol. 1:1019–1024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Guan J, Yang L, Zheng X, Yu Y and
Jiang J: Iodine-125 brachytherapy improved overall survival of
patients with inoperable stage III/IV non-small cell lung cancer
versus the conventional radiotherapy. Med Oncol. 32:3952015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matzkin H, Chen J, German L and Mabjeesh
NJ: Comparison between preoperative and real-time intraoperative
planning 125I permanent prostate brachytherapy:
Long-term clinical biochemical outcome. Radiat Oncol. 8:2882013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Y, Jiang Y, Ji Z, Jiang P, Xu F,
Zhang Y, Guo F, Peng R, Li X, Sun H, et al: Efficacy and safety of
CT-guided 125I seed implantation as a salvage treatment
for locally recurrent head and neck soft tissue sarcoma after
surgery and external beam radiotherapy: A 12-year study at a single
institution. Brachytherapy. 19:81–89. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Zhang H, Wang Z, Zhao J, Yu H and
Wang J: The advantages of intraoperative TPS real-time planning in
treating retroperitoneal metastatic carcinoma with 125I seed
brachytherapy. J Intervent Radiol. 26:1011–1014. 2017.
|
|
36
|
Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW,
Cheung YC and Chou AS: Risk factors of pneumothorax and bleeding:
Multivariate analysis of 660 CT-guided coaxial cutting needle lung
biopsies. Chest. 126:748–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Zhang HT and Yu HM: The
significance of dynamic dose verification in radioactive (125)I
seeds implantation treatment technology. Zhonghua Yi Xue Za Zhi.
98:1810–1812. 2018.(In Chinese). PubMed/NCBI
|
|
38
|
Blair HF, Porter A and Chen QS: In vivo
detection of an 125I seed located in the intracardiac region after
prostate permanent brachytherapy. Int J Radiat Oncol Biol Phys.
58:888–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu AX, Wallner KE, Frivold GP, Ferry D,
Jutzy KR and Foster GP: Prostate brachytherapy seed migration to
the right coronary artery associated with an acute myocardial
infarction. Brachytherapy. 5:262–265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ji Z, Jiang Y, Guo F, Peng R, Sun H, Wang
P, Fan J and Wang J: Radiation-related adverse effects of CT-guided
implantation of 125I seeds for thoracic recurrent and/or
metastatic malignancy. Sci Rep. 9:148032019. View Article : Google Scholar : PubMed/NCBI
|