Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) is an
aggressive form of head and neck squamous cell carcinoma (HNSCC)
that has a poor prognosis (1). Due
to a lack of early clinical symptoms, HSCC is often diagnosed at
advanced stages, which makes treatment challenging; in addition,
the pathogenesis of the disease is not completely understood
(2).
An increasing number of studies have focused on the
involvement of non-coding RNAs in HSCC with the aim of identifying
possible therapeutic strategies (3,4).
Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) is
a long non-coding (lnc)RNA that has been reported to be involved in
neurogenesis (5), metabolic
(6) and vascular (7,8)
diseases, as well as certain types of cancer (9). MALAT1 affects neuronal differentiation
by activating the ERK and mitogen-activated protein kinase (MAPK)
signaling pathway in neuro-2a cells (5). MALAT1 has also been reported to be
upregulated in a rat model of diabetic gastroparesis, which
increases the expression of α-smooth muscle actin and smooth muscle
myosin heavy chains, and promotes the apoptosis of human gastric
smooth muscle cells (6). A previous
study also revealed that MALAT1 knockdown inhibited ischemic injury
and autophagy by targeting miR-30a in vitro and in
vivo (7). Additionally, MALAT1
is upregulated and exhibits oncogenic roles in different types of
cancer, including non-small cell lung (10) and pancreatic cancer (11), osteosarcoma (12), hepatocellular (13) and renal cell carcinoma (14), and glioma (15); however, the mechanism underlying
MALAT1 activity in HSCC is not completely understood.
microRNAs (miRNAs/miRs) are a type of short
non-coding RNAs (18–25 nucleotides in length) that regulate
post-transcriptional gene expression by affecting the stability and
translation of target mRNAs (15).
miR-194 serves roles in metabolic diseases (16,17),
dermatosis (8), the inflammatory
response (18) and neoplasms
(19,20). miR-194 is also involved in multiple
aspects of skeletal muscle glucose metabolism by regulating AKT,
glycogen synthase kinase 3 and oxidative phosphorylation (16). In a study of psoriasis, transfection
with miR-194 mimics suppressed the proliferation and promoted the
differentiation of keratinocytes by targeting Grainyhead-like 2
in vitro (8). miR-194 is not
only a serum marker of several types of cancer (21), but also functions as a tumor
suppressor by regulating malignant behavior of cancer cells
(22,23). Insulin-like growth factor 1 receptor
(IGF1R) and Yes-associated protein 1 (YAP1) have been identified as
targets of miR-194 by bioinformatics studies (24,25).
IGF1R, a transmembrane receptor tyrosine kinase, participates in
the regulation of tumor cell malignancy by activating PI3K and MAPK
(26,27). By contrast, YAP1 is an essential
regulatory component of the Hippo pathway; YAP1 is involved in
various types of malignant behavior, including
epithelial-mesenchymal transition, migration, metastasis, and
anticancer drug resistance (28,29).
The aim of the present study was to identify the
roles of lncRNA MALAT1 and miR-194 in HSCC, as well as the
potential underlying mechanism. By studying MALAT1, miR-194, IGF1R
and YAP1, the present study aimed to identify novel therapeutic
targets for HSCC.
Materials and methods
Clinical specimens
A total of 25 pairs of human HSCC tissues were
obtained from the patients admitted to the Department of
Otolaryngology, The First Affiliated Hospital of China Medical
University (Shenyang, China) between March 2016 and July 2016. The
patients (23 males and 2 females; age, 43–82 years; mean age, 57.8
years) did not receive radiotherapy or chemotherapy prior to
surgical resection. HSCC and adjacent non-tumor tissue (ANTT;
normal mucosa tissue ≥1.5 cm away from the tumor) specimens were
collected from each patient. The present study was approved by the
Ethical Committee and Institutional Review Board at The First
Affiliated Hospital of China Medical University. Informed consent
was obtained from all participants.
Cell culture
FaDu and 293T cells were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
Cells were cultured in DMEM (HyClone; GE Healthcare Life Sciences)
containing 10% FBS (Gibco,; Thermo Fisher Scientific, Inc.) at 37°C
with 5% CO2.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the specimens and cells
using the TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Total RNA was
reverse transcribed into cDNA using the PrimeScript RT reagent kit
with gDNA Eraser (Takara Biotechnology Co., Ltd.) or the TaqMan
miRNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following conditions were used for reverse
transcription: 37°C for 15 min; 85°C for 5 sec; and maintained at
4°C. Subsequently, MALAT1 and GAPDH expression was quantified by
qPCR, using 7500 Fast Real-time PCR (Invitrogen; Thermo Fisher
Scientific, Inc.) and SYBR® Premix Ex Taq II (Takara
Biotechnology Co., Ltd.). To quantify miR-194 expression levels,
qPCR was performed using TaqMan microRNA assays (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and TaqMan Universal
Master Mix II (Applied Biosystems; Thermo Fisher Scientific, Inc.),
as previously described (25). The
primer pairs and assays used for qPCR are listed in Tables SI and SII. According to the manufacturer's
protocol and referring to previous literature (30), the following thermocycling conditions
were used for qPCR: 16°C for 30 min, 42°C for 30 min; 85°C for 5
min; and maintained at 4°C. mRNA and miRNA expression levels were
quantified using the 2−∆∆Cq method (31), and normalized to the internal
reference genes GAPDH and U6, respectively.
Cell transfection
The short hairpin (sh)RNA targeting human MALAT1 was
reconstructed in the pGPU6/GFP/Neo vector (sh-MALAT1; Shanghai
GenePharma Co., Ltd.). A total of three different sh-MALAT1s were
used, each targeting a different sequence. A non-targeting vector
was used as the negative control (NC; sh-NC). The sequences of the
shRNAs are listed in Table
SIII.
Once the cells reached 70% confluence, they were
transfected in 24-well plate using Lipofectamine® 3000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The concentration of shRNA was 1
mg/ml. After 48 h, Geneticin (G418; Sigma-Aldrich; Merck KGaA) was
chosen to select the stable cell line for ~4 weeks.
miR-194 agomir (agomir-194) and miR-194 antagomir
(antagomir-194; Shanghai GenePharma Co., Ltd.) were used to
upregulate and downregulate miR-194 expression, respectively. The
sequences were as follows: Agomir-194 forward,
5′-UGUAACAGCAACUCCAUGUGGA-3′ and reverse,
5′-CACAUGGAGUUGCUGUUACAUU-3′; and antagomir,
5′-UCCACAUGGAGUUGCUGUUACA-3′; agomir-NC forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′; and antagomir-NC forward,
5′-UUGUACUACACAAAAGUACUG-3′. The reverse strand of agomir-194 had
two phosphorothioates at the 5′ end, four phosphorothioates and one
cholesterol group at the 3′ end, and one full-length nucleotide
2′-methoxy modification. The antagomir sequence had two
phosphorothioates at the 5′ end, four phosphorothioates and one
cholesterol group at the 3′ end, and one full-length nucleotide
2′-methoxy modification. Lipofectamine® 3000 was used
for transfection according to the manufacturer's instructions with
the confluency of cells at 50–70%. The final concentration of
agomir or antagomir was 30 pmol/ml. After 48 h, cells were
collected for subsequent experimentation.
Cell proliferation assay
The Cell Counting Kit-8 assay (CCK-8; Beyotime
Institute of Biotechnology) was used to analyze cell proliferation,
according to the manufacturer's instruction. Transfected cells were
seeded into 96-well plates (3×103 cells/well) and
incubated (37°C, 5% CO2) for 48 h. Subsequently, 10 µl
CCK-8 reagent was added to each well and incubated for another 2 h
at 37°C. The absorbance of each well was measured at 450 nm using a
microplate reader (Bio-Rad Laboratories, Inc).
Apoptosis analysis
Different groups of cells, including transfected
cells and controls were washed with PBS and stained with Annexin
V-phycoerythrin/7-aminoactinomycin D (Annexin V-PE/7-AAD Apoptosis
Detection kit; Nanjing KeyGen Biotech Co., Ltd.), according to the
manufacturer's instructions. Early and late apoptotic cells were
analyzed using a FACScan flow cytometer (BD Biosciences). BD
CSampler software (version: 1.0.264.21, Becton, Dickinson and
Company) was used to analyze the data.
Cell migration and invasion
assays
Transwell plates (diameter, 6.5 mm; pore size, 8 µm;
Costar; Corning, Inc.) were used to assess cell migration and
invasion. For the invasion assay, prior to plating the cells into
the chambers, the upper chambers were coated with
Matrigel® and incubated at 37°C for 4 h. FaDu cells were
seeded (4×105 cells/ml) into the upper chamber with 200
µl serum-free DMEM. DMEM medium (600 µl) containing 10% FBS was
plated into the lower chambers. Following incubation (37°C, 5%
CO2) for 48 h, the residual cells in the upper chambers
were removed using a cotton swab. Cells on the lower surface of the
Transwell membrane were stained with 10% Giemsa (Tiangen Biotech
Co., Ltd.) for 2 h at room temperature. The numbers of stained
cells were observed using an Olympus CX43 light microscope
(magnification, ×400; Olympus Corporation) in five randomly
selected fields of view, and a mean average was calculated.
Luciferase assay
The putative binding sites of miR-194 and MALAT1,
IGF1R 3′ untranslated region (UTR) or YAP1-3′UTR were obtained
using the StarBase (24) and
TargetScan (25) databases. The
wild-type (WT) or mutant (MUT) binding sequences were cloned into
pmirGLO dual-luciferase vectors (Shanghai GenePharma Co., Ltd.).
According to the manufacturer's instructions, 293T cells were
co-transfected with pmirGLO vector constructed with WT or MUT
fragments and agomir-194 or agomir-NC using
Lipofectamine® 3000 at 50–70% confluency. After 48-h
incubation, the Dual-Luciferase Reporter system (Promega
Corporation) was used to analyze luciferase activity, according to
the manufacturer's instruction. Firefly luciferase activity was
normalized to Renilla luciferase activity.
Western blotting
Total protein was extracted from the cell lysates
using RIPA Lysis Buffer with 1% PMSF (Beyotime Institute of
Biotechnology). The protein was determined using a BCA assay
(Beyotime Institute of Biotechnology). Equal amounts of protein (40
µg) were separated by 10% SDS-PAGE and transferred to PVDF
membranes. Membranes were blocked in a TTBS solution containing 5%
non-fat milk and 0.05% Tween 20 for 1 h at room temperature. Then
the membranes were incubated at 4°C for 12 h with primary
antibodies against IGF1R (cat. no. 20254-1-AP; 1:1,000; ProteinTech
Group, Inc.), YAP1 (cat. no. 13584-1-AP; 1:1,000; ProteinTech
Group, Inc.) and GAPDH (cat. no. 5174; 1:2,000; Cell Signaling
Technology, Inc.). Following primary antibody incubation, the
membranes were incubated with a goat anti-rabbit IgG (H+L), HRP
conjugate secondary antibody (cat. no. SA00001-2; 1:1,000;
ProteinTech Group, Inc.) at room temperature for 2 h. Protein bands
were visualized using an ECL kit (New Cell & Molecular Biotech
Co., Ltd.; http://www.ncmbio.com), and a DNR
bio-imaging microchemi system (Neven Yamin, Israel; http://dnr-is.com/products/microchemi).
Protein expression levels were quantified using ChemImager 5500
software (version 2.03; ProteinSimple) with GAPDH as the loading
control.
Tumor xenograft in nude mice
BALB/C athymic nude mice (age, 4 weeks; weight,
13–15 g) were purchased from the Cancer Institute of Chinese
Academy of Medical Science. All animal experiments were approved by
the Administrative Panel on Laboratory Animal Care of China Medical
University. The mice were injected with 5×105 different
groups of FaDu cells into the right armpit. The mice were divided
into five groups (n=5 per group): i) Control group receiving the
injection of FaDu cells without transfection; ii) sh-NC group; iii)
sh-MALAT1 group; iv) agomir-194 group; and v) sh-MALAT1 +
agomir-194 group. Tumor volumes were calculated and recorded every
four days until 40 days. Tumor volume (mm3) was
calculated according to the following formula: Volume=(length ×
width2)/2. The humane endpoints were set as follows: i)
Tumor diameter >2.0 cm; ii) ulcerated or necrotic tumor; iii)
tumor interfered with normal functions, including eating,
ambulating and eliminating; iv) a Body Condition Score (BCS) of
2/5, which indicated that the mouse was underconditioned, with
evident segmentation of the vertebral column and readily palpable
dorsal pelvic bones; and v) poor overall condition, including
displaying signs of pain, lethargy, labored breathing or lack of
responsiveness. Mice were euthanized using CO2 with a
flow rate of 3 l/min in a 10-liter cage. Animal death was verified
by the absence of heartbeat, response to firm toe pinch and
respiratory pattern.
Statistical analysis
Data are presented as the mean ± SD from at least
three independent experiments. Statistical analyses were performed
using GraphPad Prism software version 5.0 (GraphPad Software,
Inc.). Comparisons between two groups were analyzed using the
Student's t-test. Comparisons among multiple groups were analyzed
using one-way ANOVA, followed Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
MALAT1 knockdown inhibits HSCC cell
proliferation, migration and invasion, and promotes apoptosis
The expression of MALAT1 in ANTT and HSCC tissues
was analyzed using RT-qPCR. MALAT1 was significantly upregulated in
HSCC tissues compared with ANTT tissues (P<0.05; Fig. 1A). The efficiency of the three
different sh-MALAT1s as assessed, and sh-MALAT1#1 displayed the
highest efficiency among the three shRNAs; therefore, sh-MALAT1#1
was used for subsequent experiments (Fig. S1A). Following MALAT1 knockdown in
the FaDu cell line, HSCC cell proliferation was decreased compared
with the control group (P<0.05; Fig.
1B). Compared with the sh-NC group, cell migration and invasion
were significantly decreased in the sh-MALAT1 group (P<0.05;
Fig. 1C and D). In addition, the
MALAT1 knockdown group displayed increased apoptotic rates compared
with those in the sh-NC group (P<0.05; Fig. 1E).
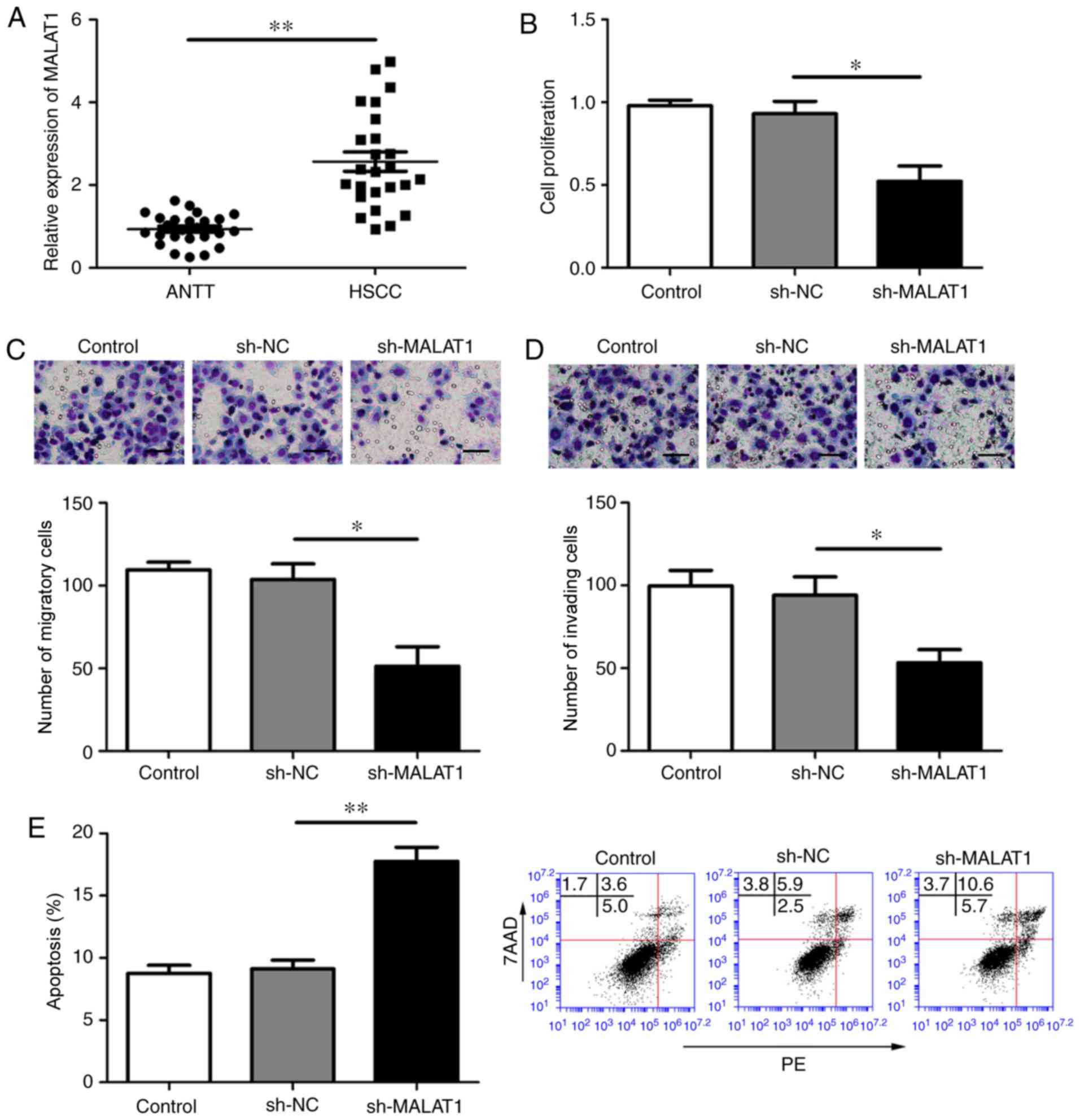 | Figure 1.MALAT1 knockdown inhibits HSCC cell
proliferation, migration and invasion, and promotes apoptosis. (A)
MALAT1 expression in ANTT and HSCC. (B-E) Effects of MALAT1 on FaDu
cell (B) proliferation, (C) migration, (D) invasion and (E)
apoptosis. Scale bar, 50 µm. *P<0.05 and **P<0.01 as
indicated. MALAT1, metastasis-associated lung adenocarcinoma
transcript 1; HSCC, hypopharyngeal squamous cell carcinoma; ANTT,
adjacent non-tumor tissue; sh, short hairpin RNA; NC, negative
control; PE, phycoerythrin; 7AAD, 7-Aminoactinomycin D. |
miR-194 targets MALAT1 and functions
as a tumor suppressor
The putative binding sites between MALAT1 and
miR-194 were predicted using StarBase and TargetScan (Fig. 2A). To verify the interaction between
MALAT1 and miR-194, a dual-luciferase reporter assay was performed.
Following co-transfection with agomir-194 and pmirGLO-MALAT1-WT,
the luciferase activity was decreased compared with that in the
agomir-NC and pmirGLO-MALAT1-WT group. Furthermore, the luciferase
activity of the agomir-194 and pmirGLO-MALAT1-MUT group was not
altered compared with that in the agomir-NC and pmirGLO-MALAT1-MUT
group (Fig. 2B). miR-194 expression
was subsequently detected by RT-qPCR. The level of miR-194
expression was downregulated in HSCC compared with ANTT (Fig. 2C).
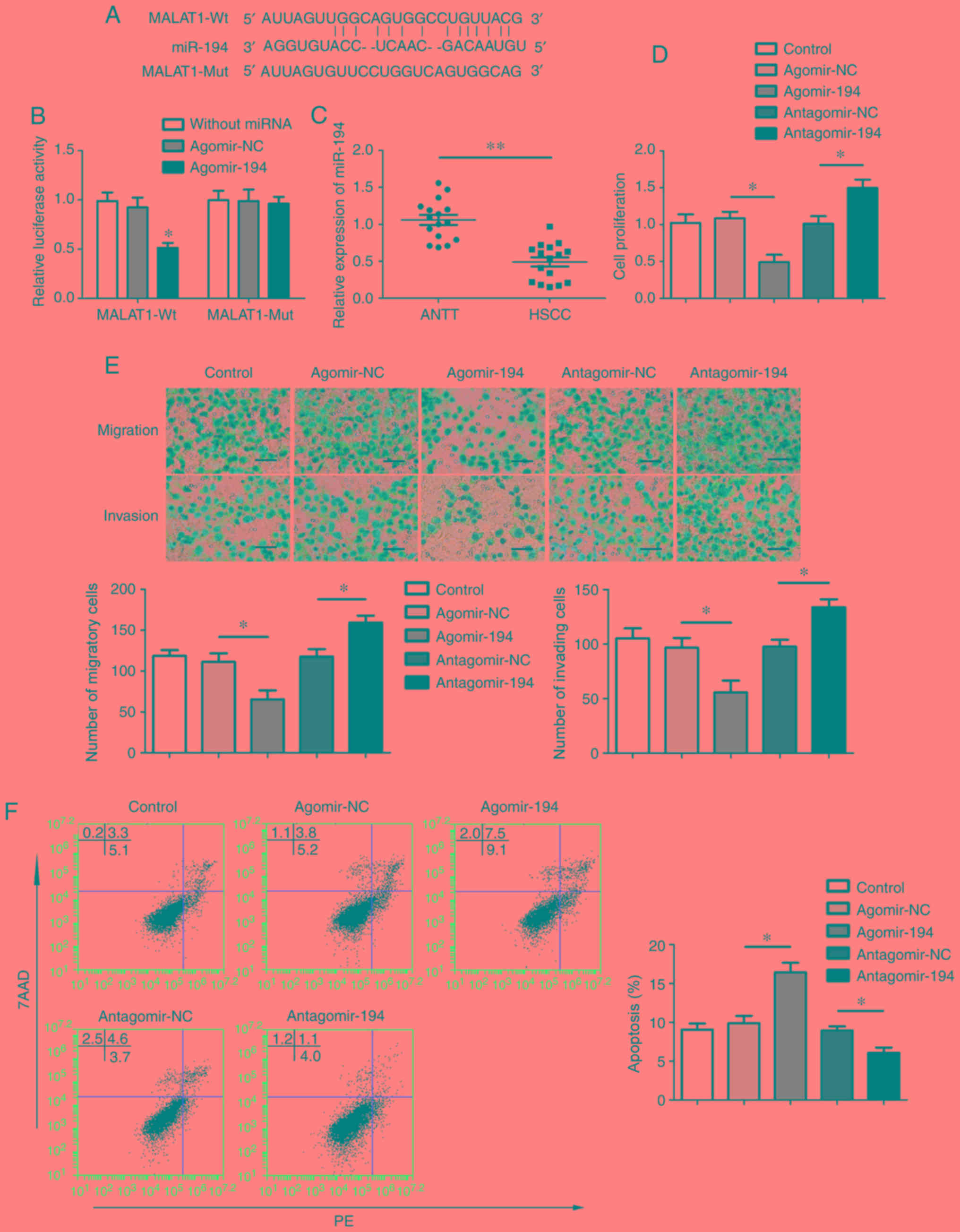 | Figure 2.miR-194 targets MALAT1 and functions
as a tumor suppressor. (A) The putative binding sites between
miR-194 and MALAT1 were predicted by StarBase and TargetScan. (B)
Relative luciferase activity in 293T cells was measured using a
dual-luciferase reporter assay. (C) miR-194 expression in ANTT and
HSCC. **P<0.01 vs. ANTT. (D-F) Effects of miR-194 on FaDu cell
(D) proliferation, (E) migration, invasion and (F) apoptosis. Scale
bar, 50 µm. *P<0.05 and **P<0.01 vs. MALAT-WT and agomir-NC
or as indicated miR, microRNA; MALAT1, metastasis-associated lung
adenocarcinoma transcript 1; ANTT, adjacent non-tumor tissue; HSCC,
hypopharyngeal squamous cell carcinoma; WT, wild-type; NC, negative
control; MUT, mutant; PE, phycoerythrin; 7AAD, 7-Aminoactinomycin
D. |
To evaluate the function of miR-194 in HSCC,
agomir-194, antagomir-194 and their respective NCs were transfected
into FaDu cells. miR-194 overexpression induced a significant
decrease in cell proliferation, migration and invasion, whereas
miR-194 inhibition resulted in increased cell proliferation,
migration and invasion compared with the corresponding control
groups (Fig. 2D and E). In addition,
miR-194 overexpression increased the percentage of apoptotic FaDu
cells, whereas miR-194 inhibition decreased apoptosis compared with
the corresponding control groups (Fig.
2F).
miR-194 mediates the suppressive
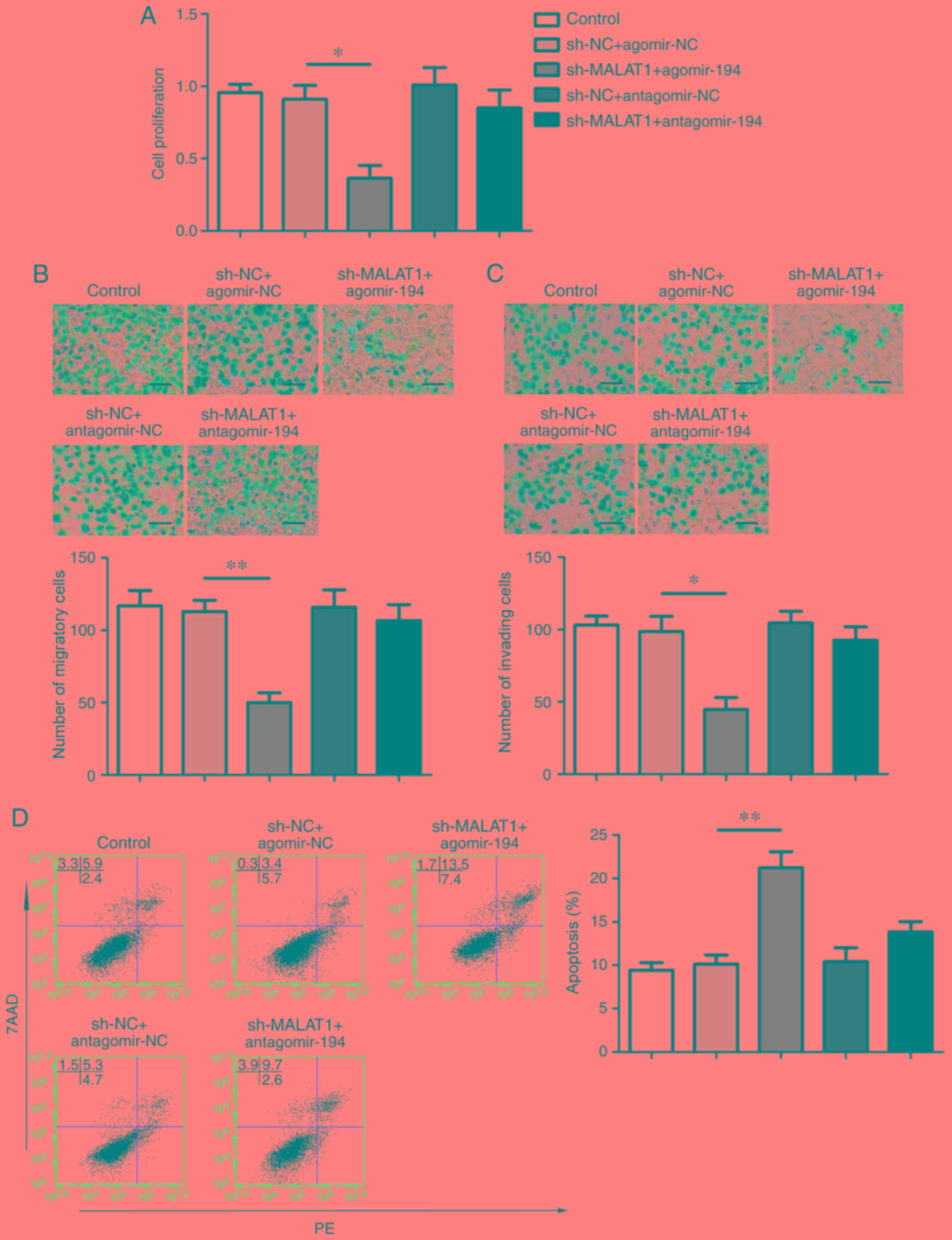
effects of MALAT1 knockdown
MALAT1 was identified as a target of miR-194;
therefore, the association between miR-194 and the suppressive
effects mediated by MALAT1 knockdown was investigated.
sh-MALAT1-transfeced FaDu cells were subsequently co-transfected
with agomir-194 or antagomir-194. The result indicated that MALAT1
knockdown decreased cell proliferation, whilst miR-194
overexpression aggravated these effects, compared with the
respective controls. As presented in Fig. 3, miR-194 downregulation in the
antagomir-194+sh-MALAT1 group partially reversed the aforementioned
effects. Similarly, cell migration and invasion were significantly
decreased in the sh-MALAT1 + agomir-194 group compared with the
sh-NC + agomir-NC group, whereas the inhibitory effects of the
sh-MALAT1 group were partially reversed by miR-194 inhibition.
Apoptosis was significantly enhanced in the sh-MALAT1 + agomir-194
group compared with the sh-NC + agomir-NC group, and miR-194
inhibition partially reversed the MALAT1 knockdown-mediated effects
(Fig. 3A-D).
MALAT1 knockdown decreases the
expression of IGF1R and YAP1
The aforementioned results suggested that miR-194
mediated the tumor-suppressive effects of MALAT1 knockdown;
therefore, it was hypothesized that the downstream targets of
miR-194 may be involved in the suppression of malignant
behavior.
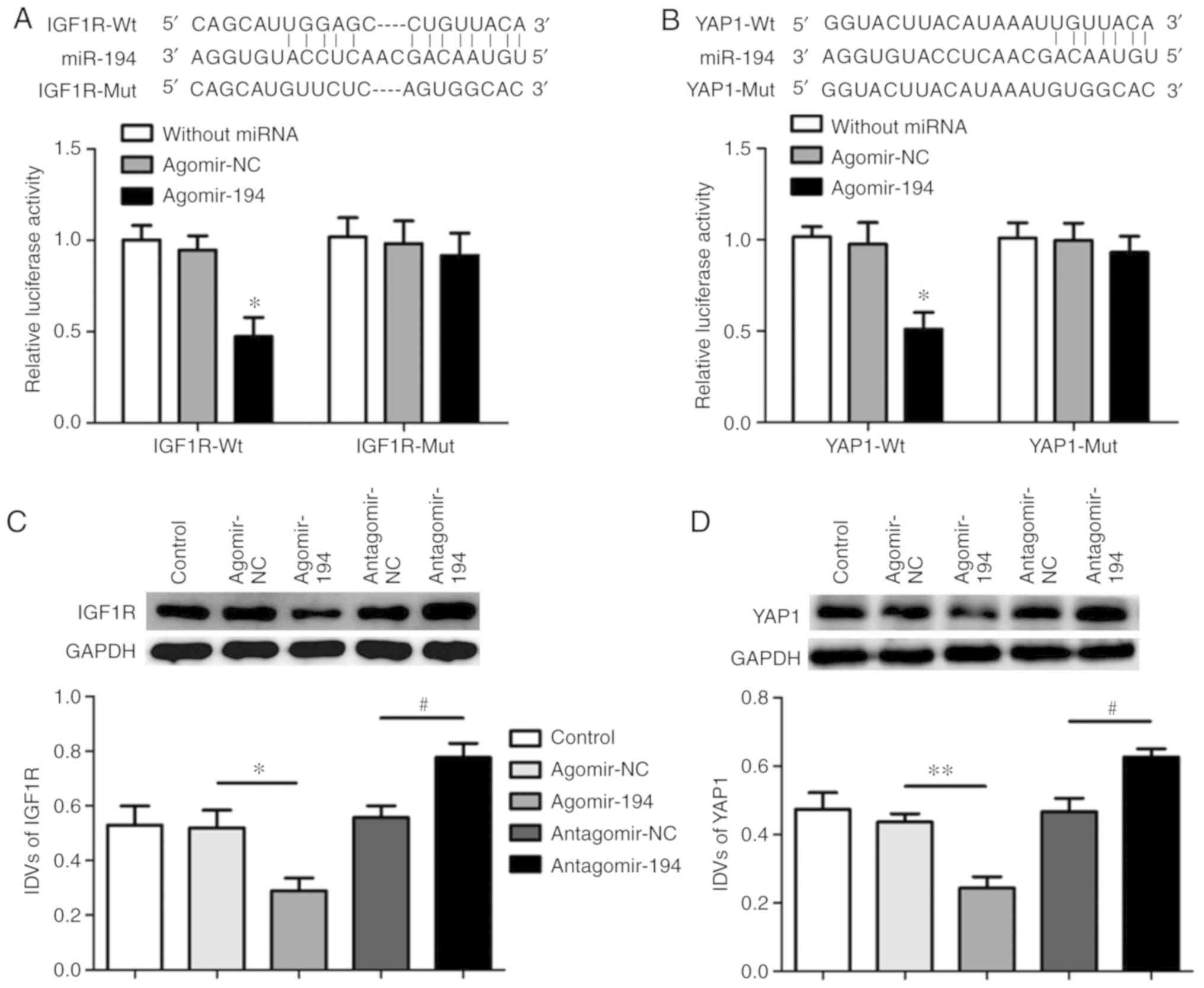
The binding sites of miR-194 with IGF1R or YAP1 were
predicted using the StarBase database and confirmed using a
dual-luciferase reporter assay (Fig. 4A
and B). The luciferase activity of the
agomir-194+IGF1R-3′UTR-WT group decreased compared with the
agomir-194+IGF1R-3′UTR-MUT and the agomir-NC+IGF1R-3′UTR-WT groups
(Fig. 4A). The binding effects and
sites between miR-194 and YAP1-3′UTR were also verified; the
results revealed that luciferase activity decreased in the
agomir-194+YAP1-3′UTR-WT group compared with the
agomir-194+YAP1-3′UTR-MUT and the agomir-NC + YAP1-3′UTR-WT groups
(Fig. 4B).
The protein expression levels of IGF1R and YAP1 were
assessed using western blotting. miR-194 overexpression
significantly decreased the expression levels of IGF1R and YAP1
compared with the agomir-NC group, whereas miR-194 inhibition
significantly increased the expression levels of IGF1R and YAP1
compared with the antagomir-NC group (Fig. 4C and D). The sh-MALAT1 + agomir-194
group exhibited reduced expression levels of IGF1R and YAP1 than
controls. IGF1R and YAP1 expression in the sh-MALAT1 + agomir-194
group were lower compared with the controls (Fig. 4E and F).
MALAT1 knockdown combined with miR-194
overexpression inhibits tumor growth
In the xenograft experiments, the tumor volume in
the right armpit of each nude mice was calculated every four days.
During the study, three mice reached the humane endpoints; two
displayed a BCS of 2/5 and one displayed an ulcerated tumor. The
largest tumor was observed in the control group, with following
dimensions: (1.65×1.452)/2=1,734 mm3. No mice
displayed multiple tumors. The tumor volume of the sh-NC group was
not significantly different compared with that of the control
group. The tumor volumes of the sh-MALAT1 and agomir-194 groups
were decreased compared with the sh-NC group. The lowest tumor
volume was observed in the sh-MALAT1 + agomir-194 group (Fig. 4G-I). The tumor volume in the
sh-MALAT1 + agomir-194 group significantly decreased compared with
the sh-NC, sh-MALAT1 and agomir-194 groups.
Discussion
In the present study, MALAT1 was upregulated in HSCC
tissues compared with ANTT, and MALAT1 knockdown inhibited HSCC
cell proliferation, migration and invasion, and promoted apoptosis.
miR-194 was identified as a target of MALAT1 and was expressed at
low levels in HSCC compared with ANTT specimens. By contrast,
downregulation of miR-194 promoted various cellular malignant
behaviors, whereas miR-194 overexpression inhibited malignant
behaviors. Furthermore, the results suggested that MALAT1 inhibited
the malignant behaviors of HSCC cells by binding miR-194, and
miR-194 inhibition reversed the MALAT1 knockdown-induced inhibitory
effects. Although a wound healing assay was not performed in the
present study to verify the migratory ability of HSCC cells, the
results of the Transwell assays suggested that MALAT1 and miR-194
altered the biological behaviors of HSCC cells. Binding sites
between IGF1R or YAP1 3′-UTRs and miR-194 were also identified, and
the expression levels of IGF1R and YAP1 were downregulated
following miR-194 overexpression or MALAT1 knockdown compared with
the respective control groups. In addition, MALAT1 knockdown
combined with miR-194 overexpression resulted in the smallest tumor
volume in vivo among all tested groups.
Previously, lncRNAs were considered to serve no
functions; however, increasing evidence has demonstrated that
lncRNAs are involved in the tumorigenesis and progression of
various neoplasms (32,33). The results of the present study
indicated that MALAT1 was highly expressed in HSCC specimens. In
addition, MALAT1 knockdown in FaDu cells inhibited malignancy in
vitro, indicating that MALAT1 may display an oncogenic role
during the development of HSCC. Consistent with the present study,
MALAT1 is upregulated and functions as oncogene in various types of
neoplasm, including non-small cell lung (34), gastric (35) and ovarian cancer (36), as well as oral squamous cell
carcinoma (37) and multiple myeloma
(38). Different mechanisms
underlying MALAT1 activity in the aforementioned types of cancer
have been demonstrated. MALAT1 is regulated by transcriptional
factor forkhead box O1 and promotes the malignancy of osteosarcoma
cells by inhibiting miR-26a-5p (12). In addition, MALAT1 facilitates
vasculogenic mimicry and angiogenesis in gastric cancer via the
vascular E-cadherin/β-catenin complex, ERK/matrix metallopeptidase
and focal adhesion kinase/paxillin signaling pathways (34). MALAT1 knockdown affects the distant
metastasis of tongue squamous cell carcinoma by upregulating
specific small proline-rich proteins (39). In addition, MALAT1 knockdown inhibits
ELAV-like RNA binding protein 1 (HuR)-TIA1 cytotoxic granule
associated RNA binding protein (TIA-1)-mediated autophagic
activation by interacting with HuR and TIA-1 in pancreatic cancer
(40). MALAT1 has been identified as
a biomarker for the progression and prognosis of esophageal cancer
(41), breast cancer (42) and colorectal cancer (43); therefore, MALAT1 may serve as a
potential target for cancer or adjunctive therapy. However, MALAT1
overexpression enhances temozolomide resistance of glioma by
upregulating zinc finger E-box binding homeobox (44).
Recently, miR-194 was reported to be a tumor
suppressor gene in the majority of cancer types, and low-level
miR-194 expression has been associated with high degrees of
malignancy (45). miR-194
overexpression inhibits colorectal cancer cell proliferation and
invasion by regulating the downstream target transforming growth
factor (46). Additionally, miR-194
overexpression negatively regulates AKT serine/threonine kinase 2,
which promotes gastric cancer cell proliferation and invasion
(47). Following miR-194
suppression, the proliferation of breast cancer cell lines was
significantly increased (48). The
results of the present study were consistent with the
aforementioned studies, suggesting that miR-194 was downregulated
in HSCC and served as a tumor suppressor.
Reciprocal inhibition mechanisms between lncRNAs and
miRNAs have been demonstrated in previous studies, indicating that
lncRNAs sponge miRNAs to prevent downstream binding to mRNAs
(49). Therefore, lncRNAs
participate in the physiology and pathology of cells by regulating
miRNAs levels (50). In the present
study, miR-194 was predicted as a target of MALAT1, and a
dual-luciferase reporter assay was performed to verify the
interaction between miR-194 and MALAT1. The results revealed that
altered expression levels of miR-194 involved MALAT1
knockdown-mediated malignant behaviors. miR-194 upregulation
inhibited MALAT1 knockdown-mediated malignant behaviors. MALAT1
knockdown combined with miR-194 overexpression significantly
suppressed HSCC cell proliferation and promoted HSCC cell apoptosis
compared with MALAT1 knockdown alone. Consistent with the results
of the present study, it has been reported that MALAT1 sponges
miR-503 to modulate epithelial-mesenchymal transition during
silica-induced pulmonary fibrosis (51). In ovarian cancer, MALAT1 serves an
oncogenic role by sponging miR-200c to promote malignant behavior
(36).
miRNAs target genes by binding to their 3′UTRs
(52). In the present study, IGF1R
and YAP1 were predicted to be the downstream targets of miR-194
using bioinformatics tools. The binding effects and sites were
investigated using a dual-luciferase reporter assay. The results
revealed that miR-194 overexpression reduced the expression of
IGF1R and YAP1, whereas miR-194 inhibition increased IGF1R and YAP1
expression compared with the corresponding control groups. IGF1R
exerts oncogenic roles in HNSCC (53,54), and
IGF1R knockdown suppresses prostate cancer cell proliferation,
migration and invasion (55). IGF1R
is also associated with a poor prognosis in patients with gastric
and breast cancer (56,57). Additionally, it has been revealed
that IGF1R is overexpressed in laryngeal squamous cell carcinoma
tissues (58), and IGF1R knockdown
enhances radiation sensitivity in human esophageal squamous cell
carcinoma (59). YAP1 activation is
also associated with a poor prognosis in patients with HNSCC
(60), and in head and neck cancer,
YAP1 upregulation is associated with cetuximab resistance (61).
In conclusion, the present study investigated the
role and effects of MALAT1 in HSCC. MALAT1 knockdown suppressed
malignant behaviors in vitro by targeting miR-194.
Therefore, MALAT1 and miR-194 may serve as novel therapeutic
targets for HSCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The Natural Science
Foundation of Liaoning (grant nos. 20180540097 and 201602881).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HW, XJ and WL designed the present study and
performed the experiments. FW and WO collected the data, while HW,
WO and FW analyzed the data. HW and XJ drafted the initial
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Frist Affiliated Hospital of China Medical
University (Shenyang, China; approval no. 2016-129) and the Animal
Ethics Committee of China Medical University Animal Center
(Shenyang, China; approval no. 2017111). Written informed consent
was provided by all patients and/or guardians prior to the study
start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Newman JR, Connolly TM, Illing EA, Kilgore
ML, Locher JL and Carroll WR: Survival trends in hypopharyngeal
cancer: A population-based review. Laryngoscope. 125:624–629. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan JY and Wei WI: Current management
strategy of hypopharyngeal carcinoma. Auris Nasus Larynx. 40:2–6.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian Y, Liu D, Cao S, Tao Y, Wei D, Li W,
Li G, Pan X and Lei D: Upregulation of the long noncoding RNA UCA1
affects the proliferation, invasion, and survival of hypopharyngeal
carcinoma. Mol Cancer. 16:682017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu S, Hui L, Yang N, Wang Y, Zhao N and
Jiang XJ: Upregulation of microRNA-194-5p inhibits hypopharyngeal
carcinoma cell proliferation, migration and invasion by targeting
SMURF1 via the mTOR signaling pathway. Int J Oncol. 54:1245–1255.
2019.PubMed/NCBI
|
|
5
|
Chen L, Feng PM, Zhu X, He SX, Duan JL and
Zhou D: Long non-coding RNA Malat1 promotes neurite outgrowth
through activation of ERK/MAPK signalling pathway in N2a cells. J
Cell Mol Med. 20:2102–2110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong Y, Zhu Y, Zhu B, Si X, Heng D, Tang
Y, Sun X and Lin L: LncRNA MALAT1 is up-regulated in diabetic
gastroparesis and involved in high-glucose-induced cellular
processes in human gastric smooth muscle cells. Biochem Biophys Res
Commun. 496:401–406. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo D, Ma J, Yan L, Li T, Li Z, Han X and
Shui S: Down-regulation of Lncrna MALAT1 attenuates neuronal cell
death through suppressing Beclin1-dependent autophagy by regulating
Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem.
43:182–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu X, An J, Hua Y, Li Z, Yan N, Fan W and
Su C: MicroRNA-194 regulates keratinocyte proliferation and
differentiation by targeting Grainyhead-like 2 in psoriasis. Pathol
Res Pract. 213:89–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Peng WX, Mo YY and Luo D:
MALAT1-mediated tumorigenesis. Front Biosci (Landmark Ed).
22:66–80. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma J, Wu K, Liu K and Miao R: Effects of
MALAT1 on proliferation and apo- ptosis of human non-small cell
lung cancer A549 cells in vitro and tumor xenograft growth in vivo
by modulating autophagy. Cancer Biomark. 22:63–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang CJ, Shi SB, Tian J, Xu J and Niu ZX:
lncRNA MALAT1, HOTTIP and PVT1 as predictors for predicting the
efficacy of GEM based chemotherapy in first-line treatment of
pancreatic cancer patients. Oncotarget. 8:95108–95115.
2017.PubMed/NCBI
|
|
12
|
Wang J and Sun G: FOXO1-MALAT1-miR-26a-5p
feedback loop mediates proliferation and migration in osteosarcoma
cells. Oncol Res. 25:1517–1527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Yao H, Wang K and Liu X: Long
non-coding RNA MALAT1 regulates ZEB1 expression by sponging
miR-143-3p and promotes hepatocellular carcinoma progression. J
Cell Biochem. 118:4836–4843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang HM, Yang FQ, Chen SJ, Che J and
Zheng JH: Upregulation of long non-coding RNA MALAT1 correlates
with tumor progression and poor prognosis in clear cell renal cell
carcinoma. Tumour Biol. 36:2947–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong Z, Wang L, Wang Q and Yuan Y: LncRNA
MALAT1/miR-129 axis promotes glioma tumorigenesis by targeting
SOX2. J Cell Mol Med. May 29–2018.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Latouche C, Natoli A, Reddy-Luthmoodoo M,
Heywood SE, Armitage JA and Kingwell BA: MicroRNA-194 modulates
glucose metabolism and its skeletal muscle expression is reduced in
diabetes. PLoS One. 11:e01551082016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jia Y, Guan M, Zheng Z, Zhang Q, Tang C,
Xu W, Xiao Z, Wang L and Xue Y: miRNAs in urine extracellular
vesicles as predictors of early-stage diabetic nephropathy. J
Diabetes Res. 2016:79327652016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong L, Sun M, Jiang Z, Li L and Lu B:
MicroRNA-194 inhibits lipopolysaccharide-induced inflammatory
response in nucleus pulposus cells of the intervertebral disc by
targeting TNF receptor-associated factor 6 (TRAF6). Med Sci Monit.
24:3056–3067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Yu Y, Fan S and Luo L: Knockdown
of long non-coding RNA NEAT1 inhibits proliferation and invasion
and induces apoptosis of osteosarcoma by inhibiting miR-194
expression. Yonsei Med J. 58:1092–1100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Wei C, Li J, Liu J and Qu J:
MicroRNA-194 represses glioma cell epithelialtomesenchymal
transition by targeting Bmi1. Oncol Rep. 37:1593–1600. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang ZH, Ren LL, Zheng P, Zheng HM, Yu YN,
Wang JL, Lin YW, Chen YX, Ge ZZ, Chen XY, et al: miR-194 as a
predictor for adenoma recurrence in patients with advanced
colorectal adenoma after polypectomy. Cancer Prev Res (Phila).
7:607–616. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mi J, Zou Y, Lin X, Lu J, Liu X, Zhao H,
Ye X, Hu H, Jiang B, Han B, et al: Dysregulation of the
miR-194-CUL4B negative feedback loop drives tumorigenesis in
non-small-cell lung carcinoma. Mol Oncol. 11:305–319. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M, Zhuang Q and Cui L: MiR-194
inhibits cell proliferation and invasion via repression of RAP2B in
bladder cancer. Biomed Pharmacother. 80:268–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:(Database Issue). D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
26
|
Zhang M, Liu J, Li M, Zhang S, Lu Y, Liang
Y, Zhao K and Li Y: Insulin-like growth factor 1/insulin-like
growth factor 1 receptor signaling protects against cell apoptosis
through the PI3K/AKT pathway in glioblastoma cells. Exp Ther Med.
16:1477–1482. 2018.PubMed/NCBI
|
|
27
|
Guan J, Zhou Y, Mao F, Lin Y, Shen S,
Zhang Y and Sun Q: MicroRNA-320a suppresses tumor cell growth and
invasion of human breast cancer by targeting insulin-like growth
factor 1 receptor. Oncol Rep. 40:849–858. 2018.PubMed/NCBI
|
|
28
|
Moroishi T, Hansen CG and Guan KL: The
emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 15:73–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song S, Honjo S, Jin J, Chang SS, Scott
AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et
al: The hippo coactivator YAP1 mediates EGFR overexpression and
confers chemoresistance in esophageal cancer. Clin Cancer Res.
21:2580–2590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gu Y, Cai R, Zhang C, Xue Y, Pan Y, Wang J
and Zhang Z: miR-132-3p boosts caveolae-mediated transcellular
transport in glioma endothelial cells by targeting
PTEN/PI3K/PKB/Src/Cav-1 signaling pathway. FASEB J. 33:441–454.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xue W, Chen J, Liu X, Gong W, Zheng J, Guo
X, Liu Y, Liu L, Ma J, Wang P, et al: PVT1 regulates the malignant
behaviors of human glioma cells by targeting miR-190a-5p and
miR-488-3p. Biochim Biophys Acta Mol Basis Dis. 1864:1783–1794.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Sun J and Yang F: The role of long
non-coding RNA H19 in breast cancer. Oncol Lett. 19:7–16.
2020.PubMed/NCBI
|
|
34
|
Zhang R, Xia Y, Wang Z, Zheng J, Chen Y,
Li X, Wang Y and Ming H: Serum long non coding RNA MALAT-1
protected by exosomes is up-regulated and promotes cell
proliferation and migration in non-small cell lung cancer. Biochem
Biophys Res Commun. 490:406–414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee NK, Lee JH, Ivan C, Ling H, Zhang X,
Park CH, Calin GA and Lee SK: MALAT1 promoted invasiveness of
gastric adenocarcinoma. BMC Cancer. 17:462017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pa M, Naizaer G, Seyiti A and Kuerbang G:
Long noncoding RNA MALAT1 functions as a sponge of MiR-200c in
ovarian cancer. Oncol Res. Sep 11–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang SM and Hu WW: Long non-coding RNA
MALAT1 promotes oral squamous cell carcinoma development via
microRNA-125b/STAT3 axis. J Cell Physiol. 233:3384–3396. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Handa H, Kuroda Y, Kimura K, Masuda Y,
Hattori H, Alkebsi L, Matsumoto M, Kasamatsu T, Kobayashi N, Tahara
KI, et al: Long non-coding RNA MALAT1 is an inducible stress
response gene associated with extramedullary spread and poor
prognosis of multiple myeloma. Br J Haematol. 179:449–460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fang Z, Zhang S, Wang Y, Shen S, Wang F,
Hao Y, Li Y, Zhang B, Zhou Y and Yang H: Long non-coding RNA
MALAT-1 modulates metastatic potential of tongue squamous cell
carcinomas partially through the regulation of small proline rich
proteins. BMC Cancer. 16:7062016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Chen H, Gao Y, Wang YW, Zhang GQ,
Pan SH, Ji L, Kong R, Wang G, Jia YH, et al: Long noncoding RNA
MALAT1 promotes aggressive pancreatic cancer proliferation and
metastasis via the stimulation of autophagy. Mol Cancer Ther.
15:2232–2243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang C, Yu Z, Yang H and Lin Y: Increased
MALAT1 expression predicts poor prognosis in esophageal cancer
patients. Biomed Pharmacother. 83:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miao Y, Fan R, Chen L and Qian H: Clinical
significance of long non-coding RNA MALAT1 expression in tissue and
serum of breast cancer. Ann Clin Lab Sci. 46:418–424.
2016.PubMed/NCBI
|
|
43
|
Zheng HT, Shi DB, Wang YW, Li XX, Xu Y,
Tripathi P, Gu WL, Cai GX and Cai SJ: High expression of lncRNA
MALAT1 suggests a biomarker of poor prognosis in colorectal cancer.
Int J Clin Exp Pathol. 7:3174–3181. 2014.PubMed/NCBI
|
|
44
|
Li H, Yuan X, Yan D, Li D, Guan F, Dong Y,
Wang H, Liu X and Yang B: Long non-coding RNA MALAT1 decreases the
sensitivity of resistant glioblastoma cell lines to temozolomide.
Cell Physiol Biochem. 42:1192–1201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao X, Hou Y, Tuo Z and Wei F:
Application values of miR-194 and miR-29 in the diagnosis and
prognosis of gastric cancer. Exp Ther Med. 15:4179–4184.
2018.PubMed/NCBI
|
|
46
|
Cai Y, Yan P, Zhang G, Yang W, Wang H and
Cheng X: Long non-coding RNA TP73-AS1 sponges miR-194 to promote
colorectal cancer cell proliferation, migration and invasion via
up-regulating TGFα. Cancer Biomark. 23:145–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qu F and Cao P: Long noncoding RNA SOX2OT
contributes to gastric cancer progression by sponging miR-194-5p
from AKT2. Exp Cell Res. 369:187–196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Y, Wei H, Liu Y and Zheng S:
Promotional effect of microRNA-194 on breast cancer cells via
targeting F-box/WD repeat-containing protein 7. Oncol Lett.
15:4439–4444. 2018.PubMed/NCBI
|
|
49
|
Jiang Y, Chen J and Chen G: Long noncoding
RNA IRAIN acts as tumor suppressor via miR-125b in multiple
myeloma. Oncol Lett. 18:6787–6794. 2019.PubMed/NCBI
|
|
50
|
Sun B, Liu C, Li H, Zhang L, Luo G, Liang
S and Lü M: Research progress on the interactions between long
non-coding RNAs and microRNAs in human cancer. Oncol Lett.
19:595–605. 2020.PubMed/NCBI
|
|
51
|
Yan W, Wu Q, Yao W, Li Y, Liu Y, Yuan J,
Han R, Yang J, Ji X and Ni C: MiR-503 modulates
epithelial-mesenchymal transition in silica-induced pulmonary
fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci
Rep. 7:113132017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stavast CJ and Erkeland SJ: The
non-canonical aspects of MicroRNAs: Many roads to gene regulation.
Cells. 8(pii): E14652019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang B, Li Y, Hou D, Shi Q, Yang S and Li
Q: MicroRNA-375 inhibits growth and enhances radiosensitivity in
oral squamous cell carcinoma by targeting insulin like growth
factor 1 receptor. Cell Physiol Biochem. 42:2105–2117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dale OT, Aleksic T, Shah KA, Han C,
Mehanna H, Rapozo DC, Sheard JD, Goodyear P, Upile NS, Robinson M,
et al: IGF-1R expression is associated with HPV-negative status and
adverse survival in head and neck squamous cell cancer.
Carcinogenesis. 36:648–655. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kato H, Sekine Y, Furuya Y, Miyazawa Y,
Koike H and Suzuki K: Metformin inhibits the proliferation of human
prostate cancer PC-3 cells via the downregulation of insulin-like
growth factor 1 receptor. Biochem Biophys Res Commun. 461:115–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Deng WY, Li N, Wan XB, Luo SX and Zhang
YW: Phosphorylated insulin-like growth factor-1 receptor expression
predicts poor prognosis of Chinese patients with gastric cancer.
Med Oncol. 31:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen
L, Ren X, Yao L, Sheng H, Li L, et al: Elevated HMGA2 expression is
associated with cancer aggressiveness and predicts poor outcome in
breast cancer. Cancer Lett. 376:284–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Luo J, Wu J, Li Z, Qin H, Wang B, Wong TS,
Yang W, Fu QL and Lei W: miR-375 suppresses IGF1R expression and
contributes to inhibition of cell progression in laryngeal squamous
cell carcinoma. Biomed Res Int. 2014:3745982014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhao H and Gu X: Silencing of insulin-like
growth factor-1 receptor enhances the radiation sensitivity of
human esophageal squamous cell carcinoma in vitro and in vivo.
World J Surg Oncol. 12:3252014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Eun YG, Lee D, Lee YC, Sohn BH, Kim EH,
Yim SY, Kwon KH and Lee JS: Clinical significance of YAP1
activation in head and neck squamous cell carcinoma. Oncotarget.
8:111130–111143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jerhammar F, Johansson AC, Ceder R,
Welander J, Jansson A, Grafström RC, Söderkvist P and Roberg K:
YAP1 is a potential biomarker for cetuximab resistance in head and
neck cancer. Oral Oncol. 50:832–839. 2014. View Article : Google Scholar : PubMed/NCBI
|