Introduction
Nutrition deprivation (ND) is a common feature of
the microenvironment of tumor cells. Under the stress of ND,
biosynthesis is suppressed, preserving energy to enable the
survival of the cancer cells (1).
AMP-activated protein kinase (AMPK) is a key energy sensor, which
functions by regulating the intracellular metabolism for the
maintenance of energy homeostasis, and serves an important role in
the survival of cancer cells under ND (2). AMPK activation maintains energy by
inhibiting biosynthesis via the suppression of mammalian target of
rapamycin (mTOR) complex 1 (3). It
has been reported that AMPK activation promotes cell survival by
phosphorylating eukaryotic elongation factor 2 kinase (eEF2K) and
blocking protein translation elongation under chronic ND conditions
(4,5).
Mitogen-activated protein kinase (ERK1/2) serves a
key role in mediating cell growth and the G1/S transition during
the cell cycle (6,7). ERK1/2 signaling is closely associated
with the induction of cyclin D1, which regulates the functions of
cyclin D-dependent kinases (CDKs) and the phosphorylation of the
retinoblastoma (Rb) protein. Rb phosphorylation disrupts its
association with transcription factor E2F, allowing the coordinated
transcription of genes required for DNA replication (8,9).
Furthermore, ERK1/2 drives the development of some types of cancer
by activating CDKs and the mTOR pathway, leading to cell cycle
progression and protein synthesis (10,11).
Conversely, overactivation of ERK1/2 may lead to proliferation
arrest, apoptosis, autophagy and senescence (12). Therefore, it is conceivable that
ERK1/2-induced G1/S transition, and the ensuing protein synthesis
may be suppressed in order to support the survival of cancer cells
under ND. However, the underlying mechanisms for ND-induced
suppression of G1/S transition and protein synthesis remain
unclear.
The results of the present study indicated the
blocking effect of ND-induced AMPK activation on the interaction
between eEF2K and dual-specificity mitogen-activated protein kinase
kinase (MEK)1/2, leading to the disruption of the
MEK1/2-ERK1/2-ribosomal protein S6 kinase α-1 (p90RSK)-eEF2K-MEK1/2
signaling loop, and thus to the deactivation of ERK1/2. The
findings uncover a mechanism that uses AMPK activation to
deactivate ERK1/2 by suppressing the interaction between eEF2K and
MEK1/2, thus promoting the survival of cancer cells under ND
conditions.
Materials and methods
Materials
Cell lines MKN45 and MG-63 were purchased from the
Shanghai Institute of Biochemistry and Cell Biology. Gibco
RPMI-1640 and Dulbecco's modified Eagle's medium (DMEM)were from
Thermo Fisher Scientific, Inc., and fetal bovine serum (FBS) was
from Hangzhou Sijiqin Biological Engineering Materials Co., Ltd.
Radioimmunoprecipitation assay and NP-40 lysis buffers, PD98059
[2-(2-amino-3-methoxyphenyl)chromen-4-one, a specific inhibitor of
MEK1/2 for downregulating ERK1/2 activity], MTT,
phenylmethylsulfonylfluoride (PMSF), and BeyoECL Star detection
reagents were from Beyotime Institute of Biotechnology. Monoclonal
antibodies against p-ERK1/2 Thr202/Tyr204
(cat. no. 4370S), p-eEF2K Ser366 (cat. no. 3691S), and
AlexaFluor® 555-(cat. no. 4413S) and 488-(cat. no.
4408S) conjugated antibodies were purchased from Cell Signaling
Technology Inc., and polyclonal antibodies against ERK1/2 (cat. no.
16443-1-AP), MEK1/2 (cat. no. 20348-1-AP), eEF2K (cat. no.
13510-1-AP) and α-actin (cat. no. 23660-1-AP) were obtained from
Wuhan Sanying Biotechnology. All primary antibodies were diluted in
PBS at 1:500. Horseradish peroxidase-conjugated mouse anti-rabbit
secondary antibodies (cat. no. D110059; 1:1,000 dilution) were
purchased from Shanghai Sangon Biotech Co., Ltd.).
Cell culture
Cells were cultured at 37°C in RPMI-1640, or DMEM
supplemented with 10% FBS. Experiments were performed on 70–80%
confluent cells. The cells were treated with acadesine (AICAR;
Beyotime Institute of Biotechnology) or ND. ND was established by
incubating cells in Hank's balanced salt solution
(HBSS)-4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)
(0.185 g/l CaCl2•2H2O, 0.2 g/l
MgSO4•7H2O, 0.4 g/l KCl, 0.06 g/l
KH2PO4, 0.35 g/l NaHCO3, 8 g/l
NaCl, 0.09 g/l Na2HPO4•7H2O, 20 mM
HEPES, pH 7.4) (11) containing
neither glucose nor amino acids.
Cell death assay
Cells were separated into 4 groups, CTRL (control),
PD98059 (20 µg/ml, the inhibitor of MEK1/2), siCTRL (siRNA
control), and sieEF2K (siRNA interference of eEF2K). All the groups
were cultured under ND. In order to assess cell death, cells were
diluted 1:10 in 0.4% Trypan blue (Sigma-Aldrich; Merck KGaA) and
counted in a hemocytometer. The mean percentage of dead (Trypan
blue-positive) cells was calculated from three independent samples
of each cell suspension.
Western blotting
Protein samples were collected by lysing cells with
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology) following the various treatments. Protein
concentrations were determined using the Bradford assay (Beyotime
Institute of Biotechnology). Proteins (20–30 µg) were separated by
SDS-PAGE (10% gels). The separated proteins were then transferred
to a nitrocellulose membrane. Membranes were then blocked with 5%
skimmed milk in PBS at room temperature for 50 min. Primary
antibodies (as aforementioned) were used in a 1:500 dilution and
secondary antibodies in 1:2,000. The protein bands on the membrane
were visualized using BeyoECL Star detection reagents (Beyotime
Institute of Biotechnology) and quantified with ImageJ v.1.48
(National Institutes of Health).
RNA interference of eEF2K and
ERK1/2
Short RNA sequences for RNA interference were
designed and synthesized by Shanghai GenePharma Co., Ltd. MKN45 and
MG-63 cells were placed in 96-well plates (for measurement of cell
growth), 24-well plates (for cell death assay) or six-well plates
(for western blotting or reverse transcription-quantitative
polymerase chain reaction) with a density of 1.5×104,
1×105 or 4×105 cells per well, respectively.
The cells were then transfected with 25 nM control siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′), a human eEF2K-targeting siRNA
(5′-GCUGGCCAUGAUGGUGAUUTT-3′) or a human ERK1/2-targeting siRNA
(5′-GCAAUGACCAUAUCUGCUATT-3′), using the siRNA transfection reagent
(Santa Cruz Biotechnology, Inc.) according to the manufacturer's
protocols. The transfection efficiency was evaluated by western
blotting.
Confocal fluorescence microscopy
Cells grown on glass coverslips were fixed with 4%
formalin for 20 min at room temperature, permeabilized with 0.5%
Triton X-100/PBS for 15 min and blocked with 5% bovine serum
albumin (Beyotime Institute of Biotechnology) at room temperature
for 1 h. The cells were incubated with primary
antibodies(anti-MEK1/2 and anti-p-eEF2k Ser366) at room
temperature for 2 h, washed and then incubated at room temperature
with AlexaFluor-conjugated secondary antibodies for 1 h, prior to
washing and mounting on glass slides. Finally, the cells were
observed using a confocal fluorescence microscope (Olympus I xSLA;
Olympus Corporation, Tokyo, Japan) and images were captured
(magnification, ×200).
Co-immunoprecipitation (co-IP)
assay
The native protein was prepared in NP-40 lysis
buffer or in PBS solution (100 mM PMSF). Antibodies against MEK1/2
(1:50 dilution) and ERK1/2 (1:50 dilution) were incubated with the
total protein lysate with rotation at 4°C for 6 h. Protein
A/G-agarose beads (cat. no. sc-2003; Santa Cruz Biotechnology,
Inc.) were added and rotated for another 2 h at 4°C. The agarose
beads were then washed 3 times with ice-cold PBS. Finally, the
proteins were eluted from the agarose beads using SDS and analyzed
against p-eEF2K Ser366 (1:500 dilution, cat. no. 3691S; Cell
Signaling Technology, Inc.) by western blotting as
aforementioned.
Statistical analysis
Cell death data are expressed as the mean ± standard
deviation and analyzed with one-way analysis of variance and
Bonferroni's post-hoc test. Statistical calculations were performed
using SPSS (v.16.0; SPSS Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
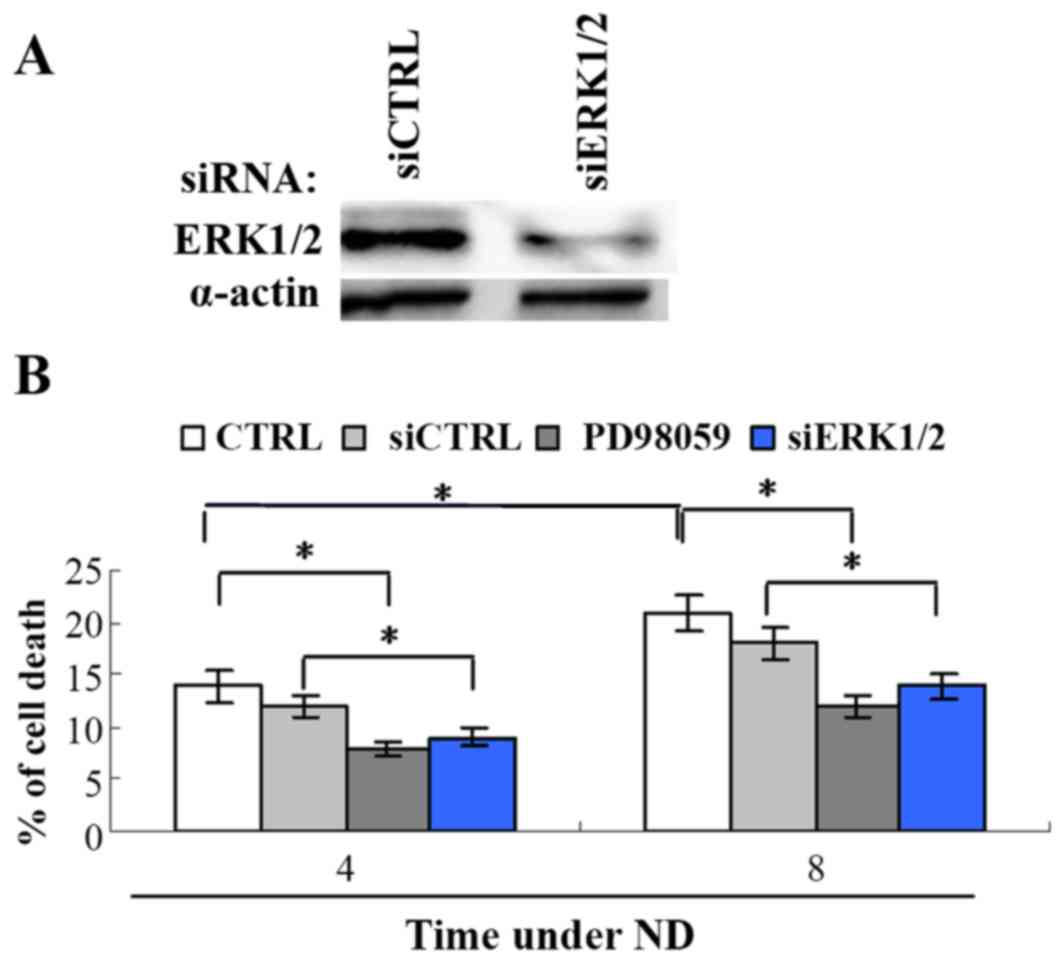
ERK1/2 activation promotes cell death
induced by ND
The signaling pathways for ND-induced cell death
remain unclear. It is known that the activation of ERK1/2 can
either promote cell survival and proliferation or induce apoptotic
cell death (13). To investigate the
role of ERK1/2 in ND-induced cell death, its activation was
inhibited by siRNA knockdown of ERK1/2 or the upstream signaling of
ERK1/2 was inhibited by the MEK1/2 inhibitor PD98059 in the gastric
cancer MKN45 cell line. The cell death assay revealed that MKN45
cell death significantly increased following ND treatment from 12
to 18%, at 4 and 8 h, respectively (Fig.
1), while ERK1/2 siRNA or PD98059 significantly attenuated cell
death comparing siRNA control or ND treatment only, respectively.
This result indicates that downregulating ERK1/2 activity promotes
the resistance of cells under ND.
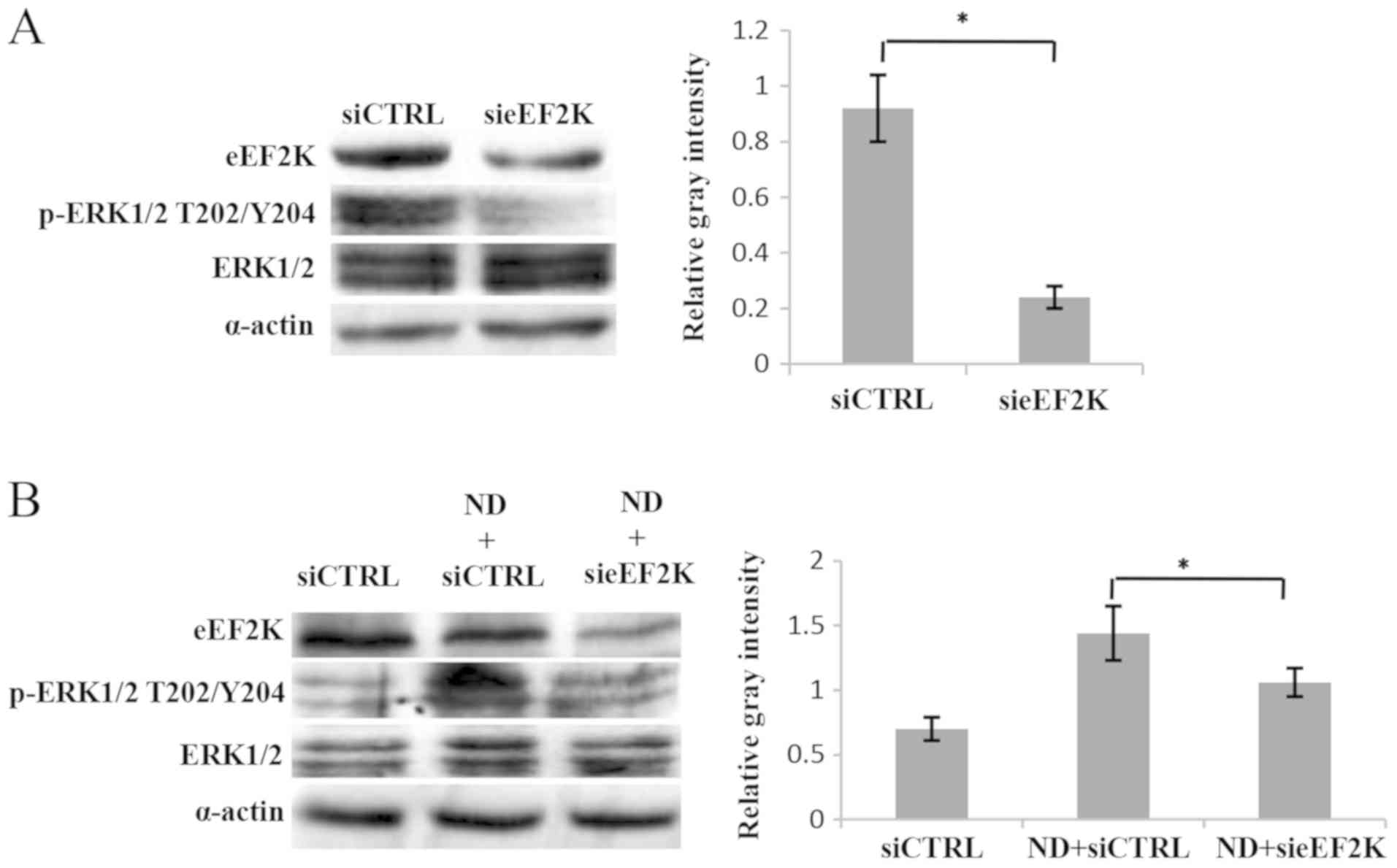
ERK1/2 activation is regulated by
eEF2K through a feedback mechanism
It is known that eEF2K is a downstream mediator in
the MEK-ERK-p90RSK-eEF2K signaling pathway (14). Notably, when the knockdown of eEF2K
was performed, the phosphorylation of ERK1/2 was significantly
reduced (Fig. 2A). Furthermore, ND
treatment led to an increase in ERK1/2 phosphorylation, which was
then decreased following eEF2K knockdown (Fig. 2B). These data indicate that ERK1/2 is
regulated by eEF2K in a feedback manner. To support this
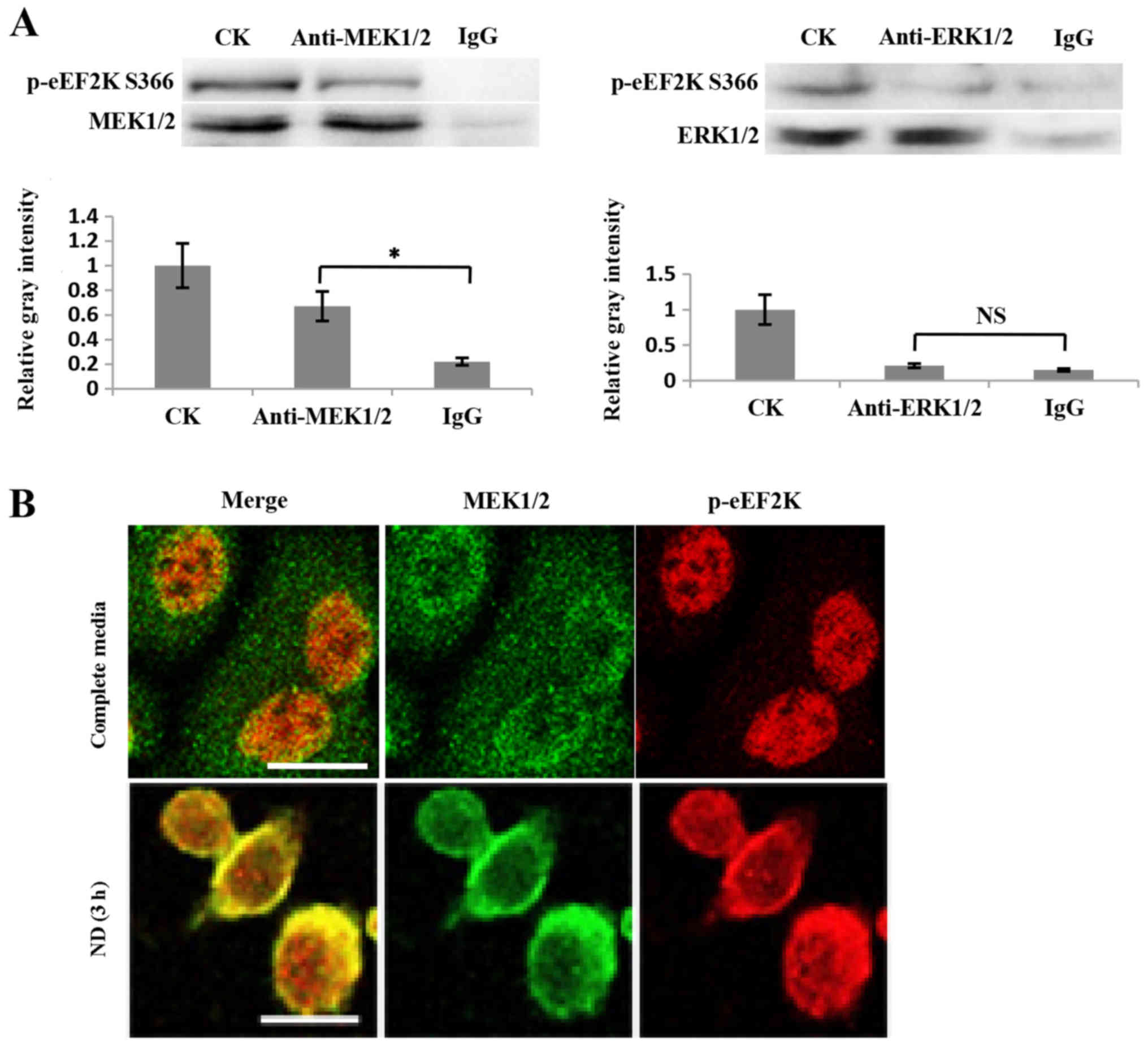
conclusion, a possible interaction between eEF2K and ERK1/2, or its
upstream factor MEK1/2, was investigated. The co-IP results
indicated that p-eEF2K Ser366 interacted with MEK1/2, but not
ERK1/2 (Fig. 3A). Furthermore, the
immunofluorescence results revealed that the colocalization of
MEK1/2 and eEF2K was enhanced when cells were treated with ND for 3
h (Fig. 3B). These data support the
conclusion that eEF2K interacts with MEK1/2 to form a feedback loop
regulating ERK1/2 activity. The ability of non-phosphorylated eEF2K
to physically bind to MEK1/2 was not investigated, however, it is
possible that EGF signaling enhances p-eEF2K Ser366 and its binding
to MEK1/2 based on the above results.
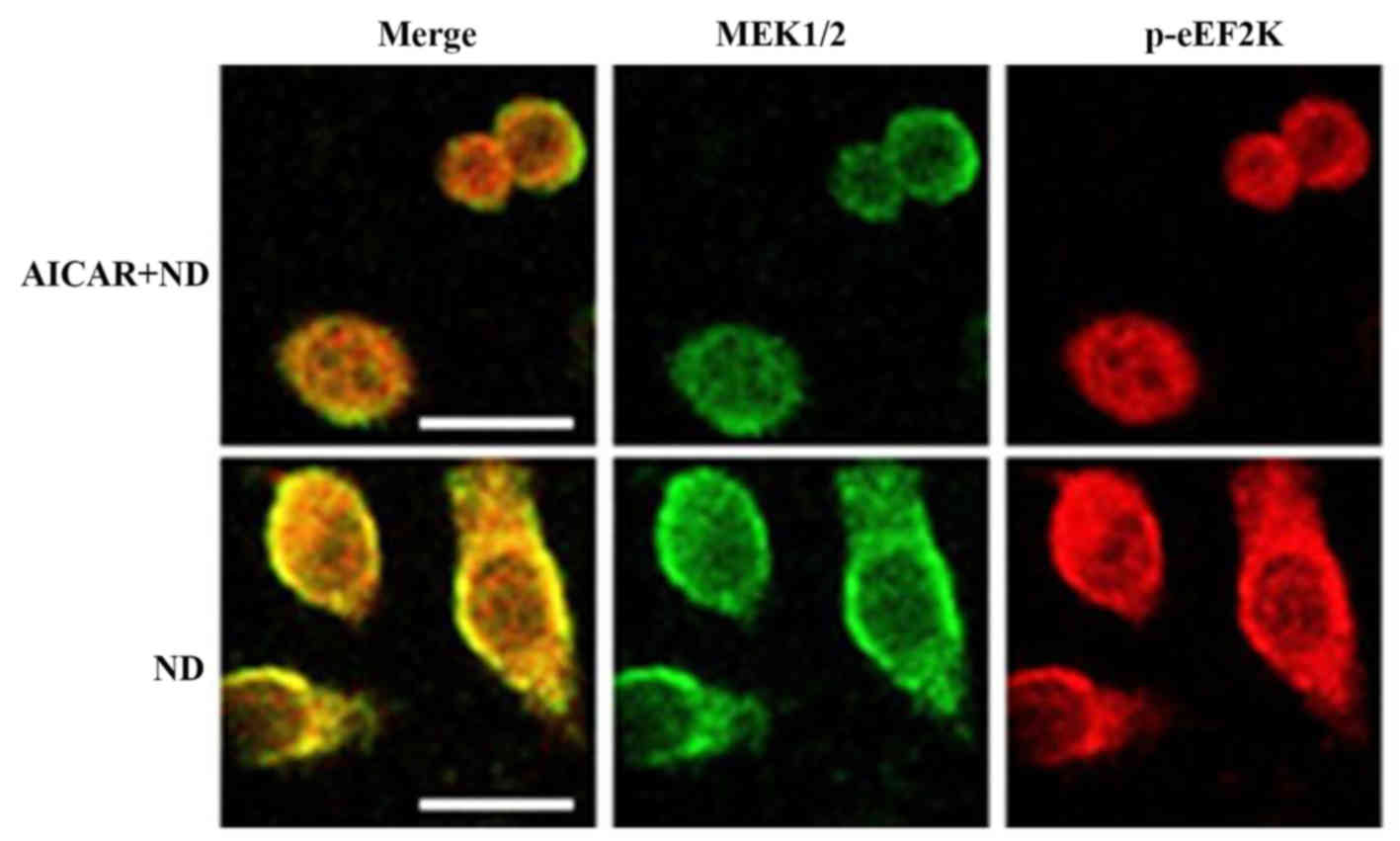
AMPK activation inhibits the
interaction between eEF2K and MEK1/2
It is known that AMPK is activated under ND to
promote cell survival (15). In
order to investigate the effect of AMPK activation on the feedback
regulation of eEF2K on MEK1/2-ERK1/2, the AMPK activator AICAR was
used on ND-treated cells. The immunofluorescence data indicated
that AICAR inhibited the interaction between eEF2K and MEK1/2
(Fig. 4). This supported the
conclusion that AMPK activation may inhibit the eEF2K-MEK1/2
interaction, leading to the deactivation of downstream ERK1/2,
which inhibits cell death under ND.
Discussion
AMPK is critical for cell survival under ND, as it
inhibits protein elongation to conserve energy, via phosphorylating
eEF2K. Although AMPK can physically interact with ERK1/2 (16,17), the
results of the present study indicated that AMPK can also inhibit
the interaction between eEF2K and MEK1/2 in the
MEK1/2-ERK1/2-p90RSK-eEF2K-MEK1/2 feedback loop. The present study
reveals a previously unknown signaling pathway in AMPK-induced cell
survival under the stress of ND.
ERK1/2 activity is another critical regulation point
for cell survival under ND, as siRNA ERK1/2 blocks the cell cycle
at the G1/S transition. However, ERK1/2 activity is
mediated not only by exogenous positive signals, but also by
endogenous negative signals, such as that of AMPK. The
sensitivities of AMPK to energy stresses are diverse among cell
lines. In certain cancer cell lines, such as HeLa and NIH 3T3 Ras
(15), one hypothesis could be that
the consistent AMPK activity under ND may be due to the immediate
suspension of cell proliferation and the alternative pathways
stimulated for ATP production. Therefore, the effect of anti-ND via
mediating ERK1/2 activity depends on the cell line.
eEF2K serves a positive role in regulating the
MEK1/2-ERK1/2 axis, as eEF2K siRNA led to a decrease in the
MEK1/2-ERK1/2 activity in MKN45 cells in the present study.
However, eEF2K activity can be regulated by a variety of upstream
factors, including calcium, AMPK, protein kinase A and ND (18–20).
Therefore, ND may cause diverse eEF2K responses in various cell
lines or cell culture conditions. Activated AMPK may directly
phosphorylate eEF2K (at Ser491 or Ser492)
(5,21) and negatively affect the
phosphorylation of eEF2K at Ser366. The positive
regulation loop of ERK1/2-p90RSK-eEF2K-MEK1/2 is critical for the
continuous proliferation of cancer cells, even following the
removal of growth factor stimulation.
The present study presents evidence that eEF2K
physically interacts with MEK1/2 and forms a positive feedback loop
consisting of MEK1/2-ERK1/2-p90RSK-eEF2K, and that the potential
positive loop is mediated by activated AMPK. However, the way in
which AMPK-modified eEF2K affects MEK1/2 activity is unclear. Any
other signaling input, other than AMPK, that may modify eEF2K and
affect its binding to MEK1/2 remains unknown. Furthermore, it is
unclear whether MEK1/2 undergoes direct phosphorylation by eEF2K
and whether the binding of both proteins is necessary for MEK1/2
activation by upstream stimuli. In order to provide answers to
these questions and explain the relevant mechanisms of the
regulation of MEK1/2, specific modifications of eEF2K should be
identified, and the occurrence of the potential phosphorylation
events must be examined.
Acknowledgements
Not applicable.
Funding
The present study was supported by a fund from the
Priority Academic Program for the Development of Jiangsu Higher
Education Institutions (Integration of Chinese and Western
Medicine).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and TZ conceived and designed the experiments.
ST, TZ, JC, YM and DX performed the experiments. JC analyzed the
data and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caro-Maldonado A and Muñoz-Pinedo C: Dying
for something to eat: How cells respond to starvation. Open Cell
Signal J. 3:42–51. 2011. View Article : Google Scholar
|
|
2
|
Jeon SM, Chandel NS and Hay N: AMPK
regulates NADPH homeostasis to promote tumour cell survival during
energy stress. Nature. 485:661–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hardie DG: Molecular pathways: Is AMPK a
friend or a foe in cancer? Clin Cancer Res. 21:3836–3840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu LL, Xie T, Zhang SY and Liu B:
Eukaryotic elongation factor-2 kinase (eEF2K): A potential
therapeutic target in cancer. Apoptosis. 19:1527–1531. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johanns M, PyrDitRuys S, Houddane A,
Vertommen D, Herinckx G, Hue L, Proud CG and Rider MH: Direct and
indirect activation of eukaryotic elongation factor 2 kinase by
AMP-activated protein kinase. Cell Signal. 36:212–221. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meloche S and Pouysségur J: The ERK1/2
mitogen-activated protein kinase pathway as a master regulator of
the G1- to S-phase transition. Oncogene. 26:3227–3239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lavoie JN, L'Allemain G, Brunet A, Muller
R and Pouyssegur J: Cyclin D1 expression is regulated positively by
the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol
Chem. 271:20608–20616. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weinberg RA: The retinoblastoma protein
and cell cycle control. Cell. 81:323–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bracken AP, Ciro M, Cocito A and Helin K:
E2F target genes: Unraveling the biology. Trends Biochem Sci.
29:409–417. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zi X and Agarwal R: Modulation of
mitogen-activated protein kinase activation and cell cycle
regulators by the potent skin cancer preventive agent silymarin.
Biochem Biophys Res Commun. 263:528–536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan HX, Xiong Y and Guan KL: Nutrient
sensing, metabolism, and cell growth control. Mol Cell. 49:379–387.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mebratu Y and Tesfaigzi Y: How ERK1/2
activation controls cell proliferation and cell death is
subcellular localization the answer? Cell Cycle. 8:1168–1175. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Li W, Williams M, Terada N, Alessi
DR and Proud CG: Regulation of elongation factor 2 kinase by
p90(RSK1) and p70 S6 kinase. EMBO J. 20:4370–4379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leprivier G, Remke M, Rotblat B, Dubuc A,
Mateo AR, Kool M, Agnihotri S, El-Naggar A, Yu B, Somasekharan SP,
et al: The eEF2 kinase confers resistance to nutrient deprivation
by blocking translation elongation. Cell. 153:1064–1079. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang Q, Zhao S, Wu J, Zheng F, Yang L, Hu
J and Hann SS: Inhibition of integrin-linked kinase expression by
emodin through crosstalk of AMPKα and ERK1/2 signaling and
reciprocal interplay of Sp1 and c-Jun. Cell Signal. 27:1469–1477.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng F, Wu J, Zhao S, Luo Q, Tang Q, Yang
L, Li L, Wu W and Hann SS: Baicalein increases the expression and
reciprocal interplay of RUNX3 and FOXO3a through crosstalk of AMPKα
and MEK/ERK1/2 signaling pathways in human non-small cell lung
cancer cells. J Exp Clin Cancer Res. 34:412015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Browne GJ and Proud CG: A novel
mTOR-regulated phosphorylation site in elongation factor 2 kinase
modulates the activity of the kinase and its binding to calmodulin.
Mol Cell Biol. 24:2986–2997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie J, Mikolajek H, Pigott CR, Hooper KJ,
Mellows T, Moore CE, Mohammed H, Werner JM, Thomas GJ and Proud CG:
Molecular mechanism for the control of eukaryotic elongation factor
2 kinase by pH: Role in cancer cell survival. Mol Cell Biol.
35:1805–1824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tavares CD, Ferguson SB, Giles DH, Wang Q,
Wellmann RM, O'Brien JP, Warthaka M, Brodbelt JS, Ren P and Dalby
KN: The molecular mechanism of eukaryotic elongation factor 2
kinase activation. J Biol Chem. 289:23901–23916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Browne GJ, Finn SG and Proud CG:
Stimulation of the AMP-activated protein kinase leads to activation
of eukaryotic elongation factor 2 kinase and to its phosphorylation
at a novel site, Serine 398. J Biol Chem. 279:12220–12231. 2004.
View Article : Google Scholar : PubMed/NCBI
|