Introduction
Non-small cell lung carcinoma (NSCLC) accounts for
>80% of patients with lung cancer, and was a leading cause of
cancer-associated mortality, both worldwide and in China (2008)
(1,2). Over the past decade, increasing efforts
have been made to identify novel biomarkers and classify the
molecular mechanisms underlying NSCLC progression (3–5).
However, an urgent need to identify novel drivers and biomarkers
for NSCLC remains.
Currently, >150 RNA post-transcriptional
modifications had been identified, including N6-methyladenosine
(m6A) (6),
threonylcarbamoyladenosine (t6A) (7)
and pseudouridine (8,9) modifications of ribosomal RNA and
transfer RNA (tRNA). Emerging studies have demonstrated that RNA
modifications serve crucial roles in human cells (10–12). For
example, m6A modifications of mRNAs influenced RNA stability and
translation (11) and t6A
modification at position 37 of adenosine-starting codons decoding
tRNAs imparted a unique structure to the anticodon loop, which
enhanced its binding to ribosomes in vitro (7,12). yrdC
N6-threonylcarbamoltransferase domain containing protein (YRDC) has
been revealed to be involved in the formation of t6A in tRNA
(13). Previous studies have
indicated that dysregulation of YRDC expression was associated with
the progression of bladder cancer; YRDC expression was upregulated
in bladder cancer samples and knockdown of YRDC significantly
suppressed bladder cancer cell proliferation (14). However, the molecular mechanisms
underlying YRDC in NSCLC remain unclear.
The present study aimed to elucidate the prognostic
value and functional roles of YRDC in NSCLC, and to assess the
expression levels of YRDC in NSCLC samples compared with normal
samples. The association between YRDC expression and survival time
was evaluated using the Kaplan-Meier Plotter database. The present
study also knocked-down YRDC in NSCLC cells in order to evaluate
the influence of YRDC on proliferation and apoptosis. These
analyses will provide evidence to support the concept that YRDC
could act as a biomarker for the prognosis prediction and treatment
of NSCLC.
Materials and methods
Cell culture
The NSCLC cell lines A549, H1975 and H1299 were
purchased from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences and cultured in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences) supplemented with 10% bovine serum,
penicillin (100 U/ml) and streptomycin (100 U/ml; all Gibco; Thermo
Fisher Scientific, Inc.). A549 cells were incubated at 37°C in a
humidified atmosphere consisting of 95% air and 5%
CO2.
Construction of YRDC knockdown
lentivirus
The short hairpin (sh)RNA sequence targeting YRDC
(5′-CCGGCAGTTCTCTGAATGTCGAGGACTCGAGTCCTCGACATTCAGAGAACTGTTTTT-3′)
was obtained from Shanghai Genechem Co., Ltd. Recombinant
lentiviral vectors were constructed as previously described
(15). The empty GV115 lentiviral
vector (Shanghai Genechem Co., Ltd.) was used as the short hairpin
(sh)RNA control. A total of 6 µg SureSilencing shRNA plasmids
(Qiagen GmbH) and shRNA control were transfected to knockdown YRDC
expression levels, using standard molecular techniques. At 48 h
post-transfection, the transfection efficiency of shYRDC was
determined using reverse transcription-quantitative (RT-q)PCR and
western blotting.
Western blot analysis
The western blot analysis was performed as
previously described (16). The
antibodies used in the present study included anti-YRDC (1:1,000;
cat. no. ab70795; Abcam) and anti-GAPDH (1:1,000; cat. no.
sc-32233; Santa Cruz Biotechnology, Inc.). Secondary antibodies
(Goat anti-mouse and goat anti-rabbit IgG-horseradish peroxidase;
1:5,000; cat. no. A9044 and cat. no. A9169, respectively;
Sigma-Aldrich; Merck KGaA) were purchased from Sigma-Aldrich; Merck
KGaA.
RT-qPCR
RT-qPCR was performed as previously described
(17). Total RNA was extracted from
A549, H1975 and H1299 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized using a RevertAid First Strand cDNA Synthesis kit
(Promega Corporation) according to the manufacturers' protocol.
miScriptSYBR GreenPCR kit (Qiagen, Inc.) was used to perform the
qPCR. The qPCR primers used in the present study were: YRDC
forward, 5′-GGCGTCCAAGACCCACATC-3′ and reverse,
5′-ACAGGCCACTTTAAGCATTCC-3′; and GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The 2−ΔΔCq method was used
to calculate the relative expression levels of the target genes
(18).
Cell proliferation and viability
assays
The adherent cell cytometry system,
Celigo® (Celigo Inc.), was used to detect the cell
proliferation in A549 cells, as previously described (15). Plates were analyzed using an adherent
cell cytometer equipped with bright field and fluorescent channels.
Gating parameters were adjusted for each fluorescence channel to
exclude background and other non-specific signals. The
Celigo® system provided a gross quantitative analysis
for each fluorescence channel and individual well, including total
count and average integrated red fluorescence intensity of gated
events. Fluorescence images were detected using a fluorescence
microscope at ×200 magnification. An MTT assay was performed as
previously described (19). A total
of 2×103 A549 cells were seeded and cultured in 96-well
plates for 5 days at 37°C in 5% CO2. Cells were stained
with MTT dye (0.5 mg/ml; Sigma Aldrich; Merck KGaA) for 2 h at
37°C. The cell culture medium was removed and replaced with 150 ml
dimethyl sulfoxide (Sigma Aldrich; Merck KGaA) to dissolve the
purple formazan crystals. The absorbance was subsequently measured
at a wavelength of 570 nm, with 655 nm as the reference
wavelength.
Cell apoptosis assay
For the cell apoptosis assay, A549 cells
(3×105/well) were seeded into 6-well plates and assayed
with an Annexin V-APC Apoptosis Detection kit (eBioscience; Thermo
Fisher Scientific, Inc.) 48 h after transfection according to the
manufacturer's protocol. Apoptotic cells were subsequently detected
using a flow cytometer (FACScalibur; Becton, Dickinson and
Company). The results were obtained by analyzing the data with
FlowJo software (version 7.6.1; FlowJo, LLC, Ashland, OR, USA).
Colony formation assays
A549 cells were plated into 6-well plates at a
density of 500 cells/well. After 10 days, the colonies were stained
with 1% crystal violet (Sigma-Aldrich; Merck KGaA) for 30 sec at
room temperature following fixation with 4% paraformaldehyde for 5
min at room temperature. Images of the cell colonies were captured
using an inverted light microscope (magnification, ×200;
MicroPublisher 3.3RTV; Olympus Corporation).
Public datasets analysis
The Cancer Genome Atlas (TCGA) Lung Cancer Cohort
data, (http://tcga.cancer.gov/dataportal; accessed June
2016), which includes the lung adenocarcinoma (LUAD) cohort
(comprising 517 primary LUAD tissues and 59 adjacent non-cancerous
tissues) and the lung squamous cell carcinoma (LUSC) cohort
(comprising 501 primary LUSC tissues and 51 adjacent non-cancerous
tissues) were downloaded from the Firebrowse database (http://firebrowse.org/). The YRDC expression levels
were also compared between NSCLC and adjacent non-cancerous tissues
using public datasets including GSE18842 (20), GSE19804 (21) and GSE19188 (22). Moreover, the present study then
analyzed a public dataset Depmap (https://depmap.org/portal/gene/YRDC?tab=dependency) to
further validate the roles of YRDC. The gene-level differential
dependency scores are available on at https://depmap.org/rnai/index.
Kaplan-Meier Plotter analysis
Kaplan-Meier curves were created using the
Kaplan-Meier Plotter (www.kmplot.com) (17)
in order to analyze the prognostic value of YRDC expression. The
Kaplan-Meier Plotter is capable of assessing the effect of 54,675
genes on survival time using 10,293 cancer samples, including 5,143
breast cancer, 1,648 ovarian cancer, 2,437 lung cancer and 1,065
gastric cancer samples, with mean follow-up periods of 69, 40, 49
and 33 months, respectively. Patients with NSCLC were divided into
high- and low-expression groups based on the median expression
level of YRDC (Cutoff value for OS analysis, 717; Cutoff value for
DFS analysis, 796) in order to evaluate the overall survival (OS)
time and disease-free survival (DFS) time. The OS and DFS rate
estimates were calculated using the Kaplan-Meier method with the
log-rank test.
Protein-protein interaction (PPI)
network and module analysis
Correlations between the expression of YRDC and
target gene expression in lung cancer were analyzed using the
cBioPortal database (http://www.cbioportal.org/index.do). The top 1,000
co-expressing mRNAs were considered as potential downstream targets
of YRDC in NSCLC. The present study constructed a PPI network using
The Search Tool for the Retrieval of Interacting Genes (STRING;
version 10.5; http://string-db.org) (23). YRDC-mediated PPI networks were
constructed in NSCLC datasets using the STRING database (The
threshold was a combined score of >0.4). The PPI network was
presented using Cytoscape software (version 3.6.0; http://www.cytoscape.org) (24). The Mcode plugin (version 1.5.1; Bader
Lab; University of Toronto) (25)
was then used to identify key modules (degree cut-off ≥2 and the
nodes with edges ≥2-core) in this network. The ClueGO plug-in in
Cytoscape software (version 2.5.0; http://www.cytoscape.org/) was used to identify genes
associated with Gene Ontology (http://geneontology.org/) terms (26). P<0.05 indicated a statistically
significant difference.
Statistical analysis
SPSS v. 19.0 (IBM Corp.) was used for all
statistical analyses. Results are expressed as the mean ± standard
deviation. The unpaired Student's t-test was used to calculate
statistical significance of YRDC expression levels between normal
tissues and NSCLC samples. For more than two groups, one-way
analysis of variance followed by the Newman-Keuls post-hoc test was
used. Each experiment was performed in triplicate. P<0.05 was
considered to indicate a statistically significant difference.
Results
YRDC is upregulated in NSCLC
samples
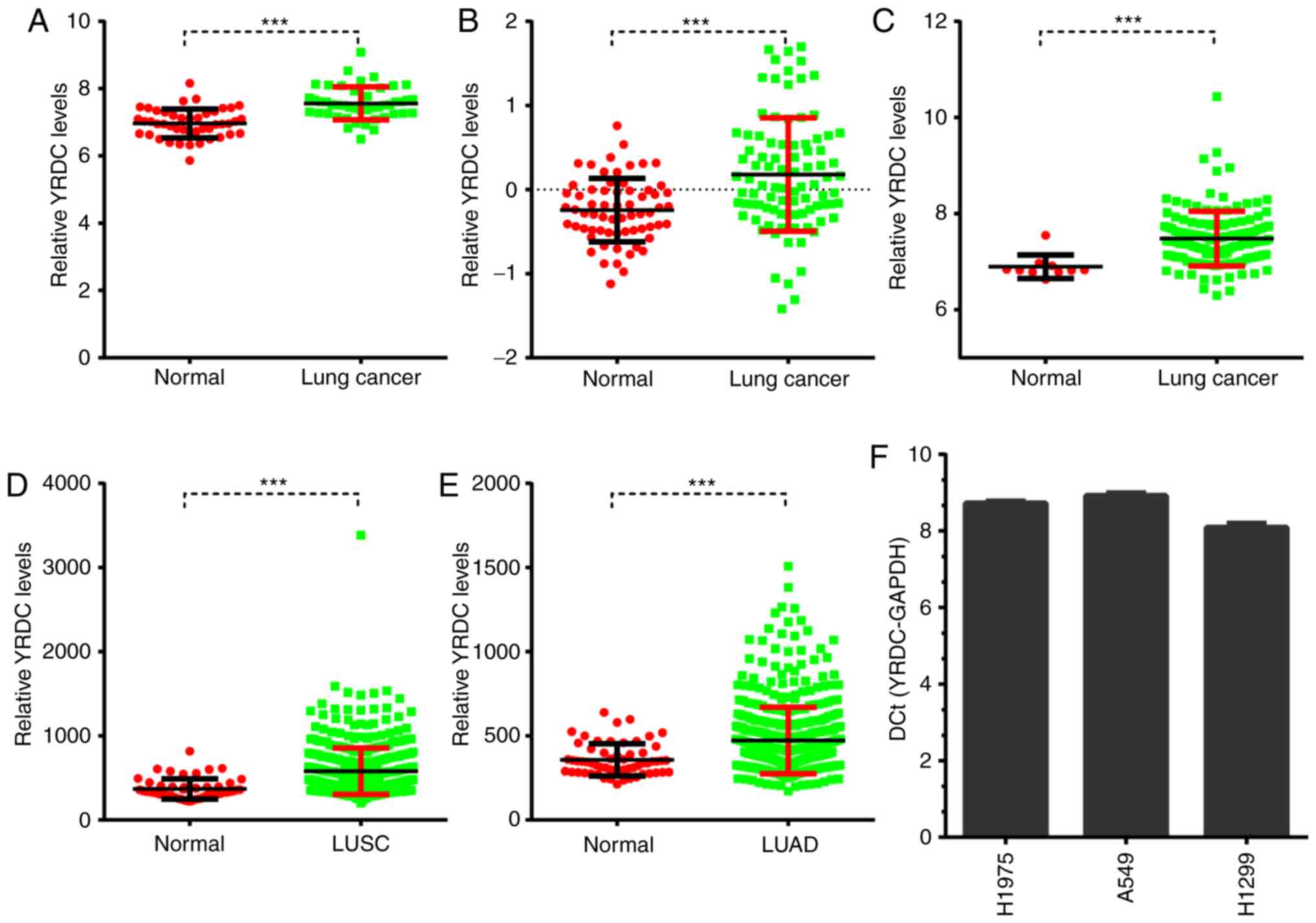
The present study compared the YRDC expression
levels between NSCLC and adjacent non-cancerous tissues using
public datasets. A total of three GEO datasets were screened in
order to investigate the expression pattern of YRDC in NSCLC. It
was observed that YRDC was significantly upregulated in NSCLC
compared with normal samples in GSE18842 (P<0.001), GSE19804
(P<0.001) and GSE19188 (P<0.001; Fig. 1A-C).
Furthermore, The Cancer Genome Atlas (TCGA) datasets
were analyzed to validate the aforementioned GEO dataset analyses.
TCGA lung adenocarcincoma (LUAD) dataset included 59 normal and 517
LUAD samples, and TCGA lung squamous cell carcinoma (LUSC) dataset
included 51 normal and 501 LUSC samples. As presented in Fig. 1, the present study demonstrated that
YRDC was upregulated in both LUAD (P<0.05) and LUSC (P<0.05)
samples compared with normal tissues (Fig. 1D and E). Of note, the absolute
expression of YRDC in 3 NSCLC cell lines (H1975, A549 and H1299
cells) was compared with the expression of GAPDH. It was observed
that YRDC was expressed in these NSCLC cell lines (Fig. 1F).
YRDC is associated with poor prognosis
in patients with NSCLC
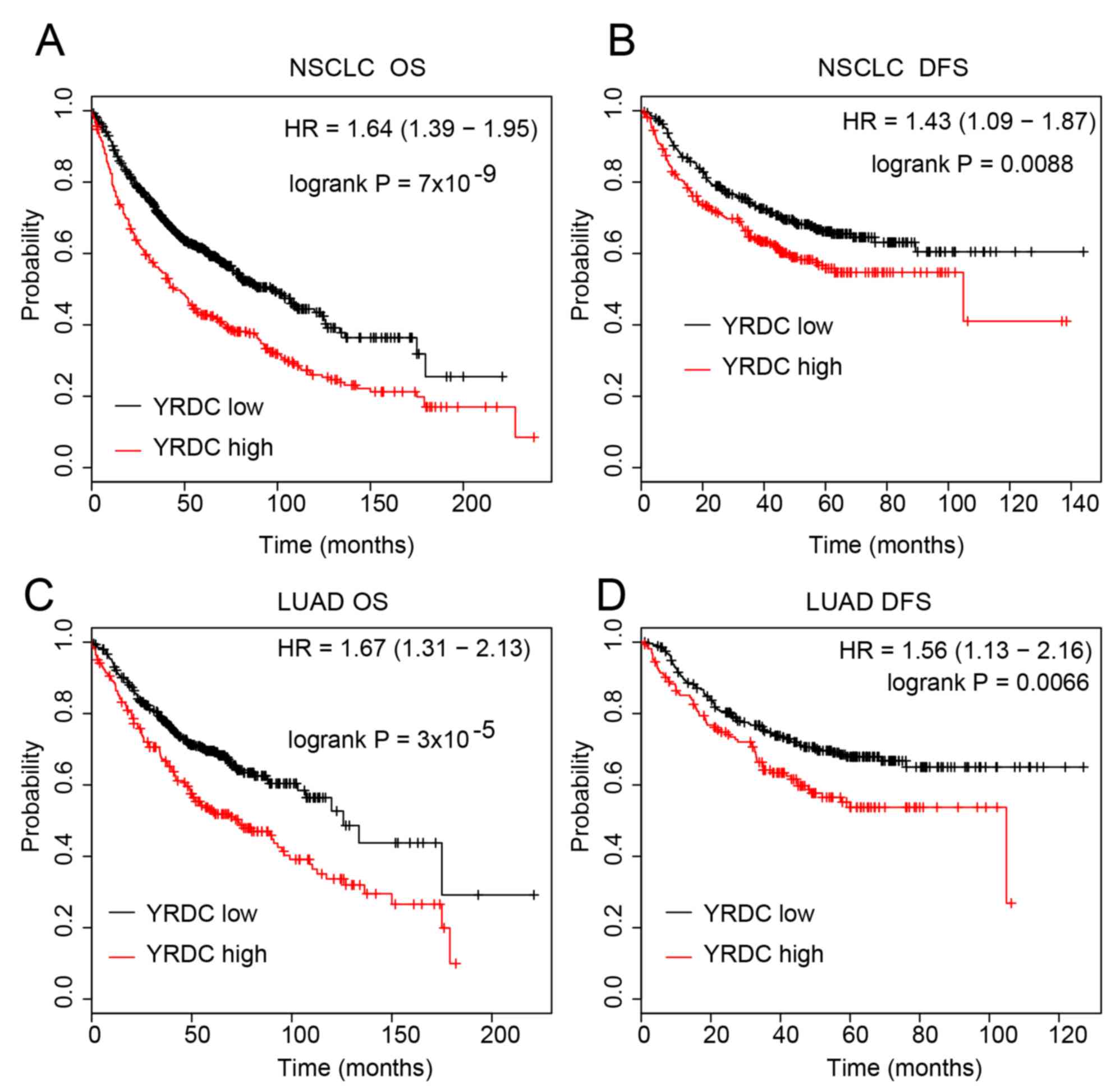
The present study performed a Kaplan Meier curve
analysis of YRDC using the Kaplan Meier plotter database in order
to further investigate the clinical importance of YRDC in NSCLC.
The results revealed that compared with low expression, high
expression of YRDC was associated with shorter OS (P<0.0001) and
DFS time (P<0.01) in lung cancer (Fig. 2A and B). The association between YRDC
expression and survival time in LUAD and LUSC was then assessed.
This further analysis revealed that higher expression of YRDC was
significantly associated with shorter OS (P<0.0001) and DFS
(P<0.01) time in LUAD (Fig. 2C and
D). These results suggested that YRDC may serve as a biomarker
for the prognosis prediction of LUAD.
Bioinformatics analysis reveals the
potential molecular mechanisms underlying YRDC in the progression
of NSCLC
The molecular mechanism underlying YRDC in NSCLC
previously remained largely unclear. In the present study, a
co-expression analysis of YRDC in NSCLC was performed using a
dataset downloaded from TCGA. The top 1,000 co-expressing mRNAs
were considered as the potential downstream targets of YRDC in
NSCLC.
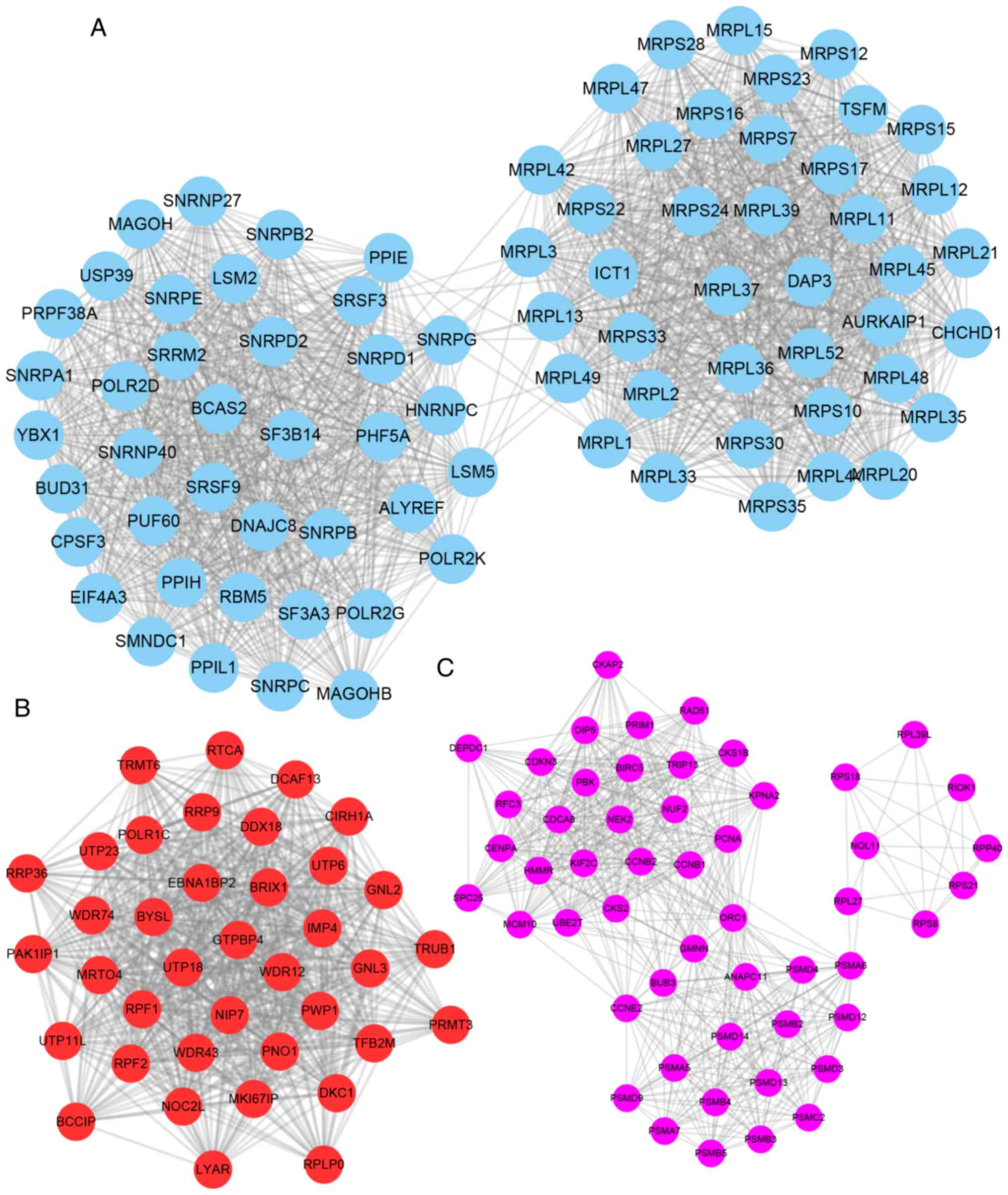
Furthermore, the present study constructed
YRDC-mediated PPI networks in NSCLC using the STRING database
(combined score >0.4). The Mcode plugin was used to identify key
modules (degree cut-off ≥2 and the nodes with edges ≥2-core) in
this network. The top three hub modules are presented in Fig. 3. Module 1 contained 79 nodes and
2,145 edges, module 2 contained 38 nodes and 987 edges and module 3
contained 52 nodes and 845 edges (Fig.
3A-C).
Functional annotation of YRDC-mediated
hub PPI networks in NSCLC
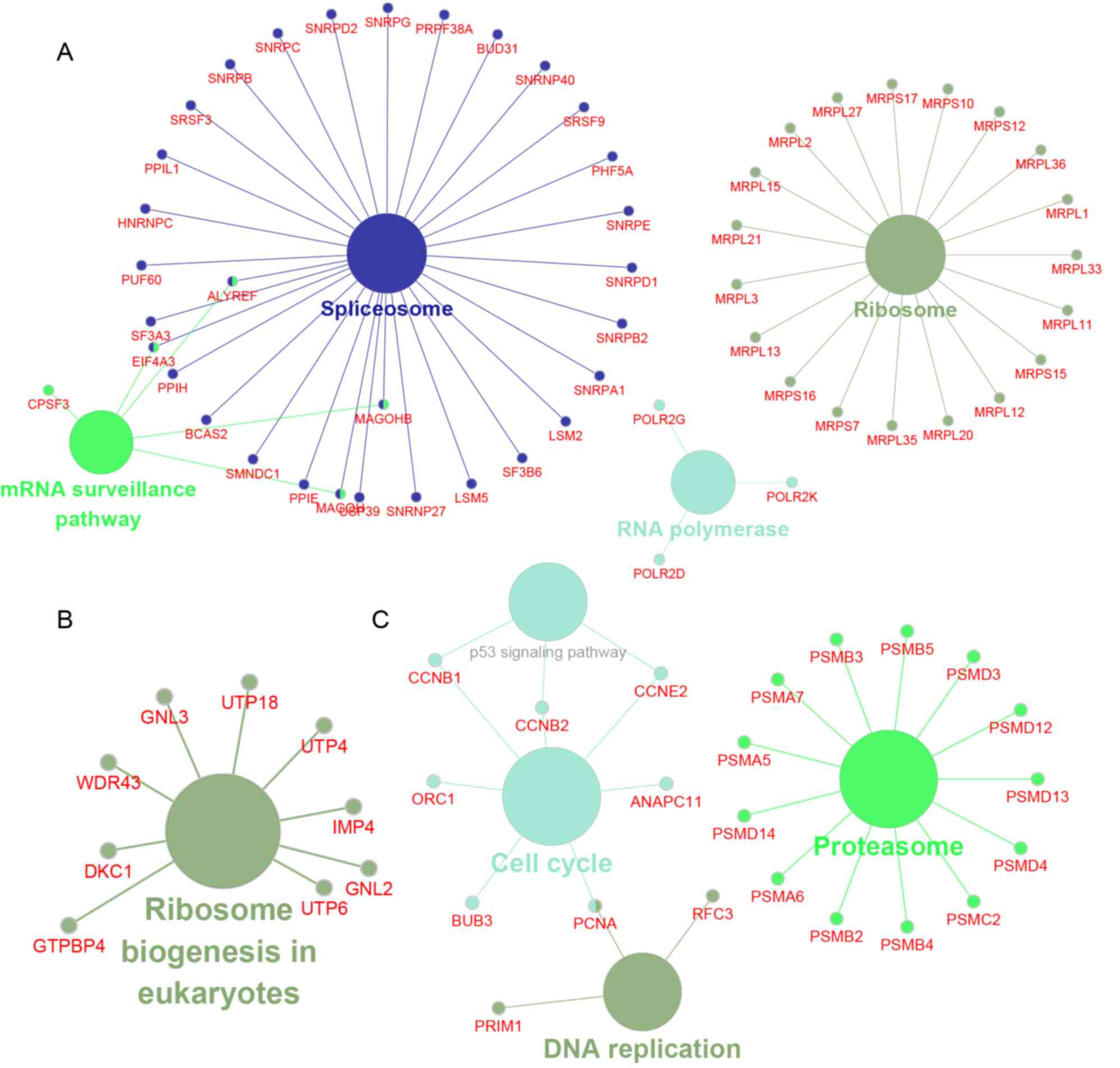
The present study next performed a bioinformatics
analysis of YRDC-mediated hub PPI networks in NSCLC using the
ClueGo plug-in in Cytoscape. The results revealed that
YRDC-associated module 1 was involved in regulating spliceosomes,
the mRNA surveillance pathway, ribosomes and RNA polymerase
(Fig. 4A); module 2 was involved in
regulating ribosome biogenesis in eukaryotes (Fig. 4B); and module 3 was involved in the
regulation of the p53 signaling pathway, proteasomes, the cell
cycle and DNA replication (Fig.
4C).
Silencing of YRDC inhibits A549 cell
proliferation and cell colony formation
The aforementioned bioinformatics analysis revealed
that YRDC was involved in regulating cell proliferation-associated
biological processes, such as p53 signaling, the cell cycle and DNA
replication. In order to validate these findings, the present study
performed loss-of-function assays using A549 cells. The present
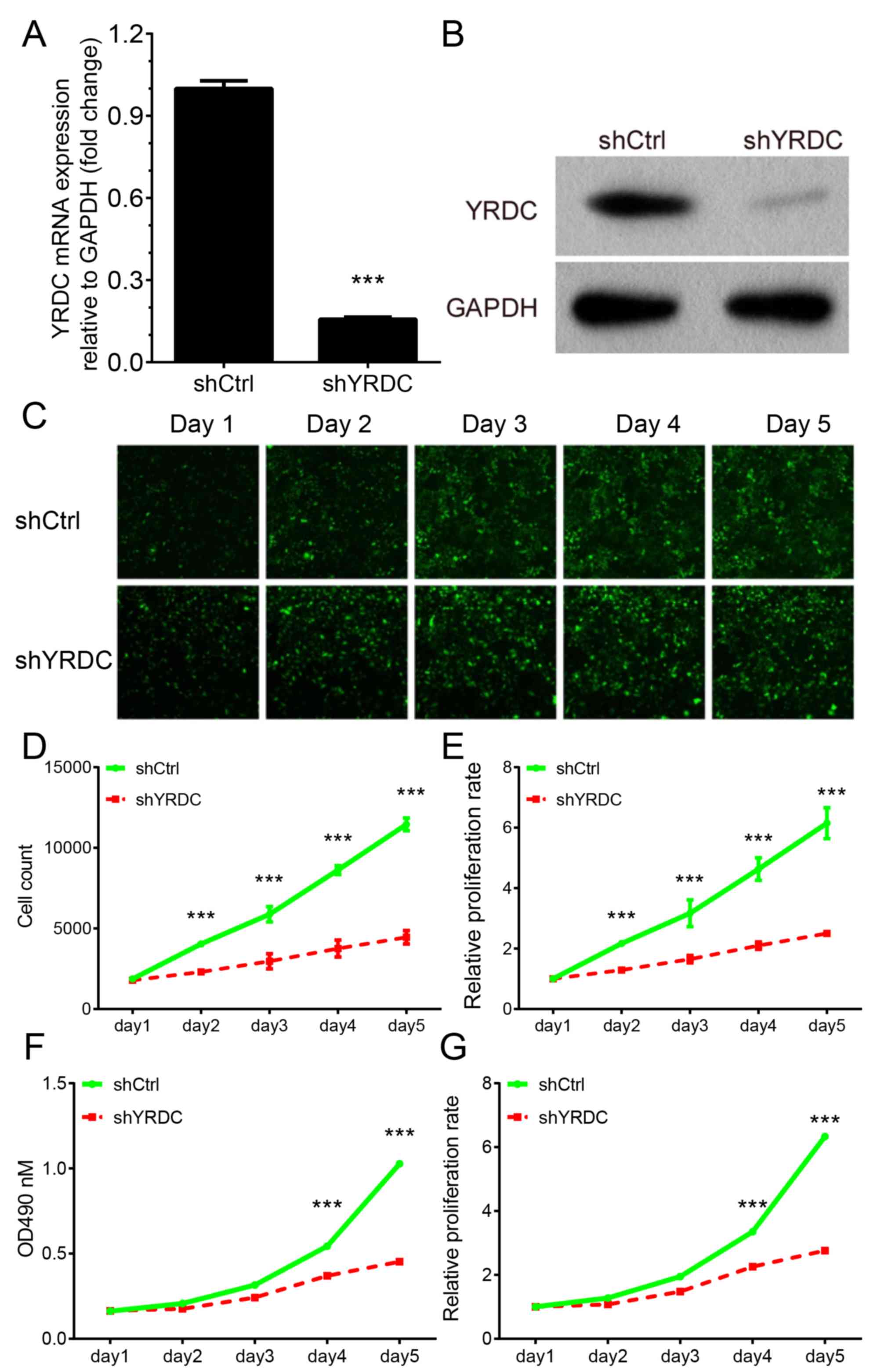
study successfully knocked-down the mRNA and protein expression
levels of YRDC in A549 cells using a shRNA against YRDC (Fig. 5A and B).
The present study then performed two different types
of assay in order to assess the influence of YRDC on tumor growth.
Celigo® cell counting assay revealed that silencing of
YRDC markedly suppressed A549 cell proliferation. The relative cell
number in YRDC knockdown group decreased by >80% in comparison
with the control group (P<0.001, Fig.
5C-E) 5 days after transfection. A similar effect on cell
viability was also observed using the MTT assay (P<0.001,
Fig. 5F-G). YRDC knockdown could
inhibit cell growth by >80% compared with the control. The
present study then analyzed a public dataset Depmap (https://depmap.org/portal/gene/YRDC?tab=dependency) to
further validate the roles of YRDC. The present results
demonstrated that knockdown of YRDC significantly suppressed the
growth of several NSCLC cell lines (Fig. S1).
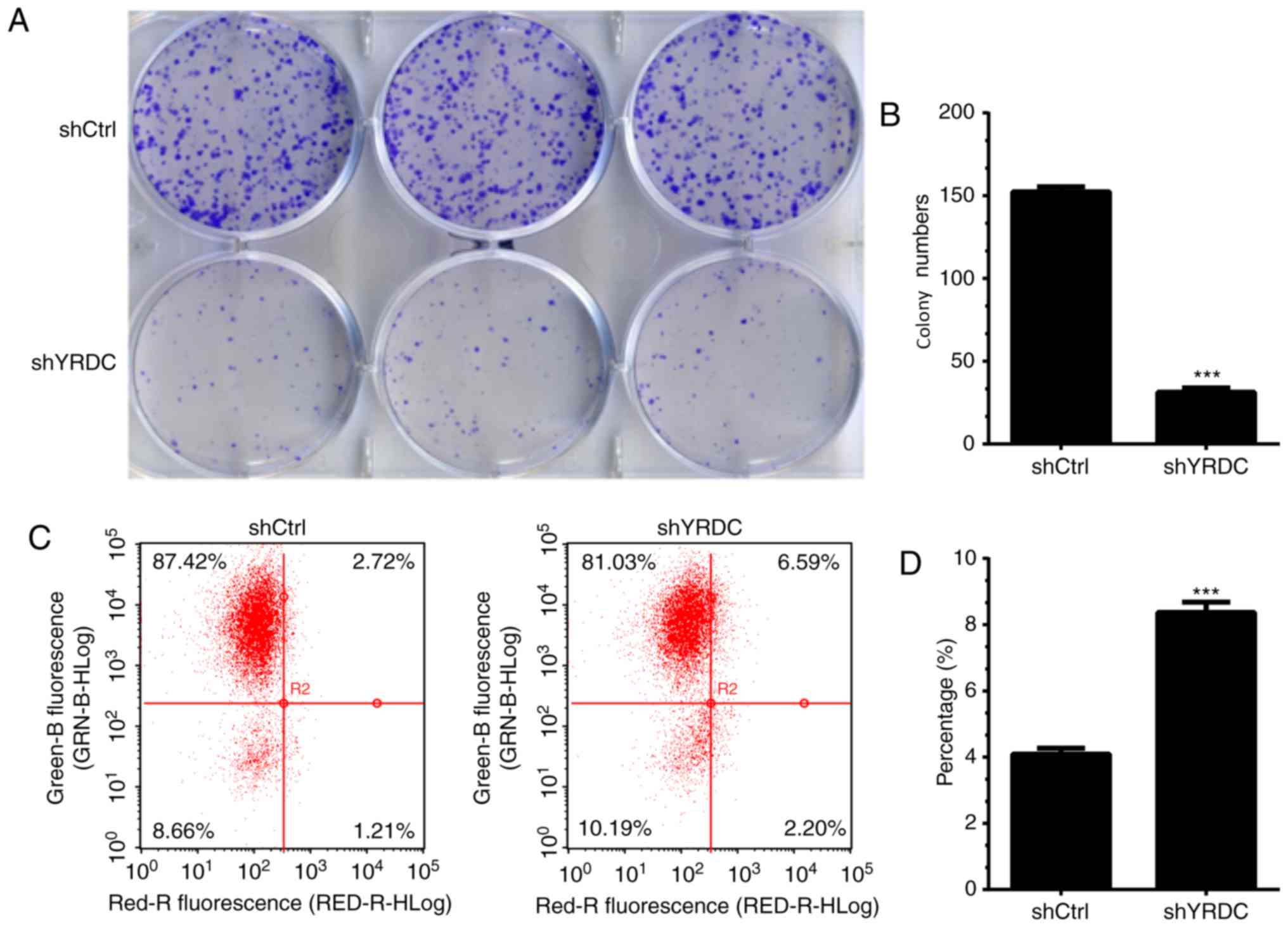
Furthermore, the cell colony formation assay was
conducted in order to determine the roles of YRDC in regulating
NSCLC growth. YRDC knockdown markedly inhibited the ability of A549
cells to form colonies compared with controls. The cell colonies in
the YRDC knockdown group decreased by 75% compared with the control
group (P<0.001, Fig. 6A and B).
The current results suggest that YRDC may serve as an oncogenic
factor in NSCLC.
Knockdown of YRDC induces cell
apoptosis in NSCLC cells
Dysregulation of the apoptotic pathways is
considered to be a hallmark of human cancer (27). The present study performed an annexin
V-APC staining assay in order to determine the influence of YRDC on
cell apoptosis in NSCLC. The percentage of apoptotic A549 cells
significantly increased by 50% in the YRDC knockdown group when
compared with the control group (P<0.001, Fig. 6C and D) 5 days after transfection,
suggesting that YRDC plays an oncogenic role in NSCLC, at least in
part, by suppressing cell apoptosis.
Discussion
Lung cancer is one of most common types of human
cancer in China (28). In the past
decade, an increasing amount of research has focused on the
identification of novel biomarkers and drivers of cancer in NSCLC
(29–31). A series of regulators associated with
NSCLC proliferation and metastasis have been identified. For
example, polycomb repressive complex 2 played crucial roles in
Kirsten rat sarcoma virus-driven NSCLC progression (30), NOVA alternative splicing regulator 1
promoted NSCLC cell growth by regulating RNA splicing of human
telomerase reverse transcriptase, and YEATS domain containing 2
regulated NSCLC tumorigenesis by regulating histone acetylation
(31). However, the molecular
mechanisms underlying NSCLC remained unclear. In the present study,
it was revealed that YRDC served as an oncogene in NSCLC. Knockdown
of YRDC in the NSCLC cell line A549 suppressed proliferation and
cell colony formation, but induced A549 cell apoptosis.
The functions of YRDC in human cancer remains
largely unclear. Previous studies have indicated that YRDC may
serve as a subunit for a tRNA threonylcarbamoyl transferase, and
was involved in the formation of t6A modification in tRNA (13,32). The
aberrant protein translation was revealed to be associated with the
progression of human cancer, such as glioma (33,34). A
recent study reported that YRDC was upregulated in tumor samples
compared with adjacent non-cancerous tissues, and promoted cancer
cell growth and metastasis in bladder cancer (14). In the present study, YRDC-mediated
PPI networks in NSCLC were constructed, and a bioinformatics
analysis of YRDC was performed using a co-expression analysis. The
results revealed that YRDC was involved in regulating spliceosomes,
ribosomes, the p53 signaling pathway, proteasomes, the cell cycle
and DNA replication.
The most widely used biomarkers in lung cancer
currently include cancer antigen (CA) 125 (35), carcinoembryonic antigen and CA153
(36); however, the accuracy of
these biomarkers remains limited. A number of novel biomarkers have
been identified in NSCLC. For example, B7-H3 expression was
upregulated in serum samples and served as a biomarker for the
prognosis of NSCLC (37), and
mutations in p53 could predict poor prognosis in NSCLC (38,39).
Notably, previous studies demonstrated the great prognostic
potential of long non-coding (lnc) RNAs in NSCLC (40–42).
Downregulation of lncRNA low expression in tumor (40), promoter of CDKN1A antisense DNA
damage activated RNA (41) and
cancer susceptibility 2 (42)
predicts poor prognosis in NSCLC. In the present study, it was
observed that YRDC was upregulated in NSCLC samples, and higher
expression of YRDC was associated with shorter OS and DFS time in
NSCLC. These results suggested that YRDC could serve as a novel
biomarker for NSCLC.
There were, however, a number of limitations in the
present study. The present study lacks investigations into the
molecular mechanisms underlying YRDC-associated regulation of NSCLC
progression, and these require further investigation. Of note, the
bioinformatics analysis in the present study revealed that YRDC was
involved in regulating multiple biological processes. Further
validation of the effects of YRDC on these biological processes is
required.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that YRDC was
upregulated in and associated with shorter OS and DFS time in
NSCLC. Furthermore, knockdown of YRDC suppressed NSCLC cell growth
and induced apoptosis in vitro. The results from the present
study provide evidence to support YRDC as a potential new biomarker
for the therapy and prognosis prediction of NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Effect of miR-30s
on the tumor microenvironment of non-small cell lung cance (grant
no. 2016HMKY05).
Availability of data and materials
The datasets analyzed in the present study are
available in The Cancer Genome Atlas repository, (https://cancergenome.nih.gov/).
Authors' contributions
HS and GZ conceived and designed the experiments,
and wrote the article. HS, EZ, ZY, MY and XX performed the
experiments. HS, YZ, JN and RL analyzed the data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qiao WL, Shi BW, Han YD, Tang HM, Lin J,
Hu HY and Lin Q: Testes-specific protease 50 as an independent risk
factor for poor prognosis in patients with non-small cell lung
cancer. Oncol Lett. 15:8796–8804. 2018.PubMed/NCBI
|
|
2
|
Su Q, Sun YP, Liu YH, Li Z, Yang HY, Sun
ZG, Cao BW and Jia JH: Prognostic factors in older patients with
advanced non-small cell lung cancer in China. Tumori. 100:69–74.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen S, Zhang R, Guo Y, Loehrer E, Wei Y,
Zhu Y, Yuan Q, Moran S, Fleischer T, Bjaanaes MM, et al: A
multi-omic study reveals BTG2 as a reliable prognostic marker for
early-stage non-small cell lung cancer. Mol Oncol. 12:913–924.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng X, Cheng M, Fu B, Fan X, Wang Q, Yu
X, Sun R, Tian Z and Wei H: Targeting LUNX inhibits non-small cell
lung cancer growth and metastasis. Cancer Res. 75:1080–1090. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCarroll JA, Gan PP, Erlich RB, Liu M,
Dwarte T, Sagnella SS, Akerfeldt MC, Yang L, Parker AL, Chang MH,
et al: TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis
to promote tumorigenesis and anoikis resistance in non-small cell
lung cancer. Cancer Res. 75:415–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yue Y, Liu J and He C: RNA
N6-methyladenosine methylation in post-transcriptional gene
expression regulation. Genes Dev. 29:1343–1355. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang BI, Miyauchi K, Matuszewski M,
D'Almeida GS, Rubio MAT, Alfonzo JD, Inoue K, Sakaguchi Y and
Suzuki T, Sochacka E and Suzuki T: Identification of 2-methylthio
cyclic N6-threonylcarbamoyladenosine (ms2ct6A) as a novel RNA
modification at position 37 of tRNAs. Nucleic Acids Res.
45:2124–2136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaidyanathan PP, AlSadhan I, Merriman DK,
Al-Hashimi HM and Herschlag D: Pseudouridine and
N6-methyladenosine modifications weaken PUF protein/RNA
interactions. RNA. 23:611–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao BS and He C: Pseudouridine in a new
era of RNA modifications. Cell Res. 25:153–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang CY, Lin MH and Su HT: A method for
measuring RNA N 6-methyladenosine modifications in cells and
tissues. J Vis Exp. 5:2016.
|
|
11
|
Yu J, Chen M, Huang H, Zhu J, Song H, Zhu
J, Park J and Ji SJ: Dynamic m6A modification regulates local
translation of mRNA in axons. Nucleic Acids Res. 46:1412–1423.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiaville PC, El Yacoubi B, Köhrer C,
Thiaville JJ, Deutsch C, Iwata-Reuyl D, Bacusmo JM, Armengaud J,
Bessho Y, Wetzel C, et al: Essentiality of
threonylcarbamoyladenosine (t(6)A), a universal tRNA modification,
in bacteria. Mol Microbiol. 98:1199–1221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
El Yacoubi B, Lyons B, Cruz Y, Reddy R,
Nordin B, Agnelli F, Williamson JR, Schimmel P, Swairjo MA and de
Crécy-Lagard V: The universal YrdC/Sua5 family is required for the
formation of threonylcarbamoyladenosine in tRNA. Nucleic Acids Res.
37:2894–2909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang B, Zhai W, Hu G, Huang C, Xie T,
Zhang J and Xu Y: MicroRNA-206 acts as a tumor suppressor in
bladder cancer via targeting YRDC. Am J Transl Res. 8:4705–4715.
2016.PubMed/NCBI
|
|
15
|
Cui F, Hu J, Ning S, Tan J and Tang H:
Overexpression of MCM10 promotes cell proliferation and predicts
poor prognosis in prostate cancer. Prostate. 78:1299–1310. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu T, An S, Choy MT, Zhou J, Wu S, Liu S,
Liu B, Yao Z, Zhu X, Wu J and He Z: LncRNA DUXAP9-206 directly
binds with Cbl-b to augment EGFR signaling and promotes non-small
cell lung cancer progression. J Cell Mol Med. 23:1852–1864. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanchez-Palencia A, Gomez-Morales M,
Gomez-Capilla JA, Pedraza V, Boyero L, Rosell R and Fárez-Vidal ME:
Gene expression profiling reveals novel biomarkers in nonsmall cell
lung cancer. Int J Cancer. 129:355–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu TP, Tsai MH, Lee JM, Hsu CP, Chen PC,
Lin CW, Shih JY, Yang PC, Hsiao CK, Lai LC and Chuang EY:
Identification of a novel biomarker, SEMA5A, for non-small cell
lung carcinoma in nonsmoking women. Cancer Epidemiol Biomarkers
Prev. 19:2590–2597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou J, Aerts J, den Hamer B, van Ijcken W,
den Bakker M, Riegman P, van der Leest C, van der Spek P, Foekens
JA, Hoogsteden HC, et al: Gene expression-based classification of
non-small cell lung carcinomas and survival prediction. PLoS One.
5:e103122010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database Issue). D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Viktorsson K and Lewensohn R: Apoptotic
signaling pathways in lung cancer. J Thorac Oncol. 2:175–179. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao M: Some features of lung cancer in
China. Lung Cancer. 10:107–116. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie Y, Zhang Y, Du L, Jiang X, Yan S, Duan
W, Li J, Zhan Y, Wang L, Zhang S, et al: Circulating long noncoding
RNA act as potential novel biomarkers for diagnosis and prognosis
of non-small cell lung cancer. Mol Oncol. 12:648–658. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serresi M, Gargiulo G, Proost N, Siteur B,
Cesaroni M, Koppens M, Xie H, Sutherland KD, Hulsman D, Citterio E,
et al: Polycomb repressive complex 2 is a barrier to KRAS-Driven
inflammation and epithelial-mesenchymal transition in
non-small-cell lung cancer. Cancer Cell. 29:17–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ludlow AT, Wong MS, Robin JD, Batten K,
Yuan L, Lai TP, Dahlson N, Zhang L, Mender I, Tedone E, et al:
NOVA1 regulates hTERT splicing and cell growth in non-small cell
lung cancer. Nat Commun. 9:31122018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaczanowska M and Rydén-Aulin M: The YrdC
protein-a putative ribosome maturation factor. Biochim Biophys
Acta. 1727:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lemiere S, Azar R, Belloc F, Gürsel D,
Pyronnet S, Bikfalvi A and Auguste P: Overexpression of high
molecular weight FGF-2 forms inhibits glioma growth by acting on
cell-cycle progression and protein translation. Exp Cell Res.
314:3701–3711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brewer JW, Hendershot LM, Sherr CJ and
Diehl JA: Mammalian unfolded protein response inhibits cyclin D1
translation and cell-cycle progression. Proc Natl Acad Sci USA.
96:8505–8510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakamura H and Nishimura T: History,
molecular features, and clinical importance of conventional serum
biomarkers in lung cancer. Surg Today. 47:1037–1059. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin Q, Chen XY, Liu WF, Zhu PW, Shi WQ, Li
B, Yuan Q, Min YL, Liu JM and Shao Y: Diagnostic value of CA-153
and CYFRA 21-1 in predicting intraocular metastasis in patients
with metastatic lung cancer. Cancer Med. Jun 20–2019.(Epub ahead of
print).
|
|
37
|
Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B
and Zhang X: Diagnosis value of serum B7-H3 expression in non-small
cell lung cancer. Lung Cancer. 66:245–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu Y, Yang A, Hu S, Zhang J and Yan H:
Significance of human papillomavirus 16/18 infection in association
with p53 mutation in lung carcinomas. Clin Respir J. 7:27–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niklinska W, Burzykowski T, Chyczewski L
and Niklinski J: Expression of vascular endothelial growth factor
(VEGF) in non-small cell lung cancer (NSCLC): Association with p53
gene mutation and prognosis. Lung Cancer. 34 (Suppl 2):S59–S64.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Zhao H, Li J, Zhang A and Wang H:
Downregulation of long non-coding RNA LET predicts poor prognosis
and increases Notch signaling in non-small cell lung cancer.
Oncotarget. 9:1156–1168. 2017.PubMed/NCBI
|
|
41
|
Han L, Zhang EB, Yin DD, Kong R, Xu TP,
Chen WM, Xia R, Shu YQ and De W: Low expression of long noncoding
RNA PANDAR predicts a poor prognosis of non-small cell lung cancer
and affects cell apoptosis by regulating Bcl-2. Cell Death Dis.
6:e16652015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He X, Liu Z, Su J, Yang J, Yin D, Han L,
De W and Guo R: Low expression of long noncoding RNA CASC2
indicates a poor prognosis and regulates cell proliferation in
non-small cell lung cancer. Tumour Biol. 37:9503–9510. 2016.
View Article : Google Scholar : PubMed/NCBI
|