Introduction
Liver cancer is a common malignancy worldwide. Liver
cancer is not sensitive to chemotherapy and radiotherapy. Liver
resection, local cytodestructive therapies and liver
transplantation are considered curative for patients with early
hepatocellular carcinoma (1). The
majority of patients with advanced disease at diagnosis do not
receive curative treatment (2). The
carcinogenesis of liver cancer appears to be multifactorial,
involving multiple genetic changes that may provide clues for
identifying novel therapeutic targets. Vitamin D3 may be involved
in certain types of cancer; previous studies have demonstrated that
a sufficient serum concentration of 25-hydroxyvitamin D, a
metabolite of vitamin D3, may decrease the risk of bladder cancer
(3–6). Vitamin D3 is the biologically active
form of vitamin D, and its ability to regulate cell proliferation,
apoptosis and angiogenesis may influence cancer risk, development,
and progression (7–9). Nuclear vitamin D receptor is a
ligand-dependent nuclear transcription factor that binds vitamin D3
to form a hormone-receptor complex that either initiates or
inhibits the transcription of a target gene by binding to the
promoter (10).
The anticancer effects of vitamin D may be mediated
by changes in micro (mi)RNA expression (11). miRNAs may function as either tumor
suppressors or oncogenes (12).
Multiple deregulated miRNAs have been identified in liver cancer,
including miRNA (miR)-363-3p, miR-187-3p, miR-99a and miR-143
(13–16). miR-15a, located on human chromosome
13q14, is abnormally expressed in numerous types of tumors
(17). and has been demonstrated to
suppress hepatocellular carcinoma (HCC) cell proliferation and
invasion through the regulation of target genes, including BDNF and
cMyb (18,19). The present study investigated the
antiproliferative effects of vitamin D3 and its molecular mechanism
in liver cancer cells.
Materials and methods
Cell culture
HepG2 and Hep3B human hepatoma cells, and 293 human
embryonic kidney cells were obtained from the Key Laboratory of
Environment and Genes Related to Diseases at Xi'an Jiaotong
University. The cells were cultured in Dulbecco's Modified Eagle's
Medium (Thermo Fisher Scientific, Inc.) supplemented with 10% fetal
bovine serum (Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 in a humidified atmosphere. HepG2 cells were
authenticated by short tandem repeat analysis. miR-15a-5p mimic,
mimic control, si-E2F3 and si-control were synthesized by Shanghai
GenePharma Co., Ltd. miR-15a-5p-inhibitor and inhibitor-ctrl were
synthesized by Sangon Biotech Co., Ltd. Cells were transfected with
miR-15a-5p mimic (50 nM), mimic-ctrl (50 nM), miR-15a-5p inhibitor
(1 µg/ml), inhibitor-control (1 µg/ml), si-E2F3 or si-control (50
nM) by using JetPrime transfection reagent (Polyplus-transfection
SA) according to the manufacturer's instructions. The sequences are
presented in Table SI.
MTT assay
Hep3B and HepG2 cells were incubated in 96-well
plates at the density of 3,000 cells/well for 24 h prior to
treatment with 0, 300, 600 or 1,200 nM vitamin D3 at 37°C for 24,
48 and 72 h or prior to transfection with a miR-15a-5p mimic, mimic
control (mimic-ctrl), miR-15a-5p inhibitor, inhibitor control,
small interfering (si)-RNA targeting E2F transcription factor 3
(si-E2F3) or si-control for an additional 24, 48 and 72 h. MTT
(Sigma-Aldrich; Merck KGaA) was added to each well and incubated
for 4 h at 37°C. After discarding the supernatant, 150 µl/well of
DMSO was added, and the absorbance was read at 492 nm using a
microplate reader.
Colony formation assay
Hep3B and HepG2 cells were incubated at the density
of 2,000 cells/well for 24 h and transfected with a miR-15a-5p
mimic, mimic-ctrl, miR-15a-5p inhibitor, inhibitor-control, si-E2F3
or si-control, and cultured for 2 weeks. The colonies were stained
with 0.1% crystal violet for 30 min, rinsed with phosphate buffered
saline (PBS) and images were acquired using Quantity One software
(version 4.3.1; Bio-Rad Laboratories, Inc.). The supernatant was
discarded, crystal violet staining was solubilized using DMSO, and
the absorbance was read at 492 nm on a microplate reader.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from Hep3B and HepG2 cells
following treatment with vitamin D3 or transfection by using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Prime-Script RT reagent kit (Takara Bio, Inc.) was used to
reverse transcribe RNA according to the manufacturers'
instructions. The RT-qPCR assays were conducted with
SYBR® Green Ex Taq II (Takara Bio, Inc.) using a
FTC-3000TM System (Funglyn Biotech Inc.). The reactions were as
follows: Initial denaturation at 95°C for 30 sec, followed by 40
cycles at 95°C for 5 sec and at 60°C for 30 sec. miRNA expression
was normalized against a U6 endogenous control. E2F3 expression was
normalized to β-actin. The 2−ΔΔCq (20) method was used to determine the
relative expression of miR-15a-5p. The primer sequences are
presented in the Table SI.
Plasmid construction
Overexpression of miR-15a-5p in Hep3B and HepG2
cells was induced by the miRNA mimics synthesized by GenePharma
(Shanghai GenePharma Co.). The mimic and control sequences are
presented in Table SI. The
wild-type (WT) and mutant (MUT) 3′-untranslated regions (UTRs) of
the human E2F3 mRNA were synthesized from oligonucleotides
and cloned into the SacI and XhoI sites of a pmirGLO
Vector (Promega Corporation).
Apoptosis assay
Hep3B and HepG2 cells cultured in 12-well plates at
the density of 2×105 cells/well for 24 h, then treated
with 1,200 nM of vitamin D3 at 37°C for 48 h, harvested and washed
with PBS. Following staining with an Annexin V-FITC Apoptosis
Detection Kit (7Sea Biotech) according to the manufacturer's
instructions, apoptosis was evaluated by flow cytometry (ACEA
Bioscience, Inc.) by calculating early and late apoptosis and
analyzed by NovoExpress (ACEA Bioscience, Inc.).
Dual-luciferase reporter assay
miR-15a-5p target genes were identified using
TargetScan (http://www.targetscan.org). The
dual-luciferase reporter assay was conducted in 293 cells seeded in
96-well plates at the density of 5×104 cells/well. After
a 24-h incubation, miR-15a-5p were co-transfected with WT E2F3
3′-UTR or MUT E2F3 3′-UTR or pmirGLO Vector (Promega Corporation)
using JetPrime Polyplus transfection reagent (Polyplus-transfection
SA) according to the manufacturer's instructions. After a 48-h
transfection, Luciferase activity was examined using the Dual-Glo
Luciferase Assay system and results were normalized to Renilla
luciferase activity (Promega Corporation).
Western blotting
Total protein was extracted from Hep3B and HepG2
cells using RIPA lysis buffer (Xi'an Wolsen Biotechnology Co.,
Ltd). Protein concentration was determined using NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). Proteins (30 µg) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. After
blocking with 5% non-fat dry milk in Tris-buffered saline
containing 0.1% Tween-20 for 1 h, the membranes were incubated
overnight at 4°C with primary antibodies against Bcl-2 (cat. no.
12789-1-AP; 1:500; ProteinTech Group, Inc.), Bax (cat. no.
50599-2-Ig; 1:500; ProteinTech Group, Inc.) and β-actin (cat. no.
sc-47778; 1:1,000; Santa Cruz Biotechnology, Inc.) overnight at
4°C. Subsequently, the membranes were washed with TBST and
incubated with secondary goat anti-rabbit or goat anti-mouse
antibody (cat. nos. 111-035-144; 115-035-003; 1:1,000; Jackson
ImmunoResearch Laboratories, Inc.) for 2 h at room temperature. The
protein expression in each sample was normalized to that of
β-actin.
Statistical analysis
Assays were performed in triplicate and the results
are reported as the mean ± standard error of the mean (SEM).
Student's t-test or one-way ANOVA followed by a Tukey's post hoc
test was performed to analyze the differences between the groups.
Statistical analysis was performed using SPSS 13.0 (SPSS, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with vitamin D3 inhibits
liver cancer cell proliferation
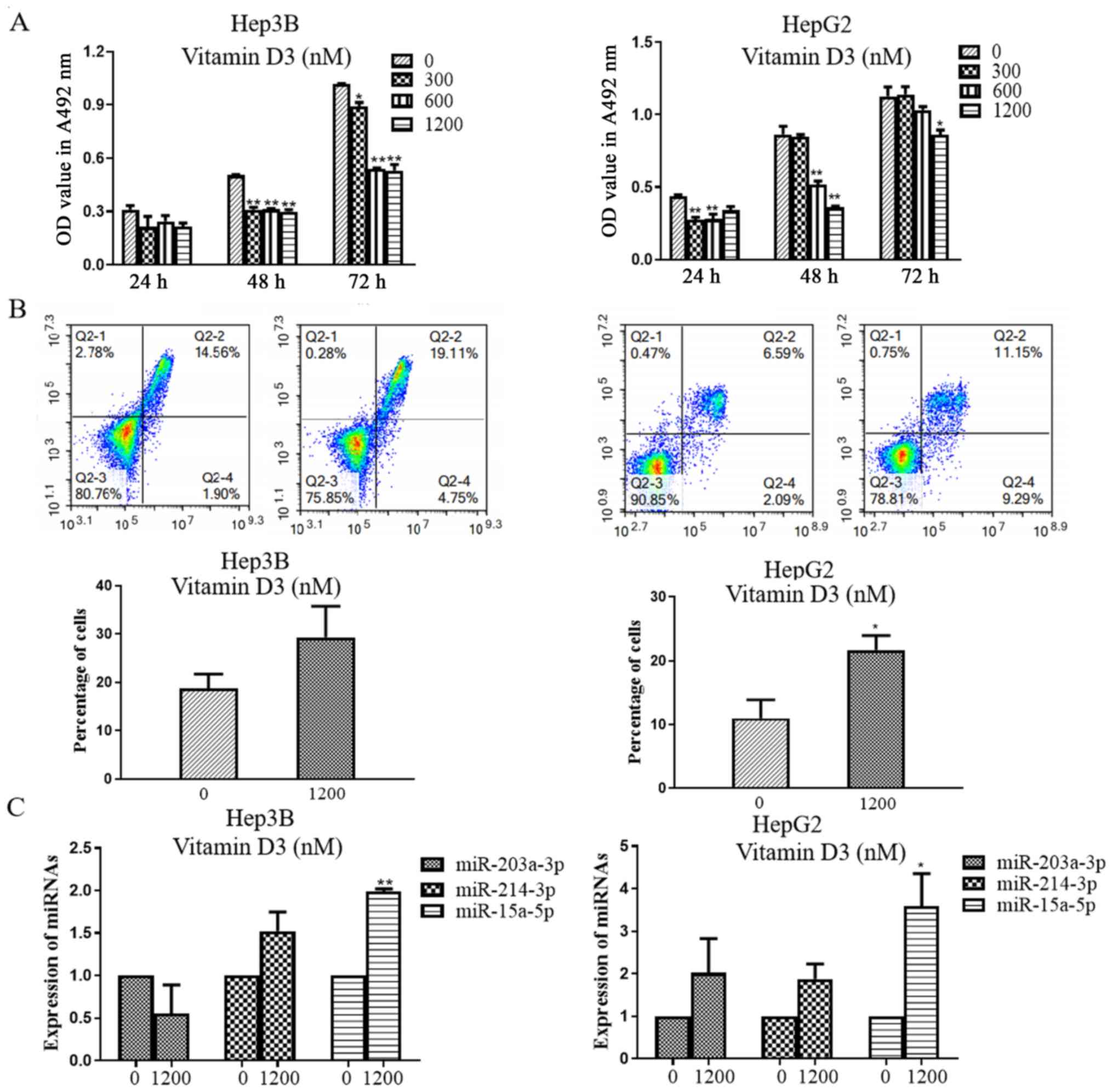
The MTT assay results demonstrated that vitamin D3
significantly inhibited the proliferation of Hep3B and HepG2 cells
compared with the controls (Fig.
1A). The Annexin-V assay results revealed that 1,200 nM vitamin
D3 significantly increased apoptosis in HepG2, but not in Hep3B
cells compared with their respective controls (Fig. 1B). The antiproliferative mechanism of
vitamin D3 was investigated by RT-qPCR, which demonstrated that
miR-15a-5p expression was significantly increased in Hep3B and
HepG2 cells treated with 1,200 nM vitamin D3 and that the
expression levels of miR-203a-3p and miR-214-3p did not change
compared with the controls (Fig.
1C).
miR-15a-5p mimics inhibit liver cancer
cell proliferation
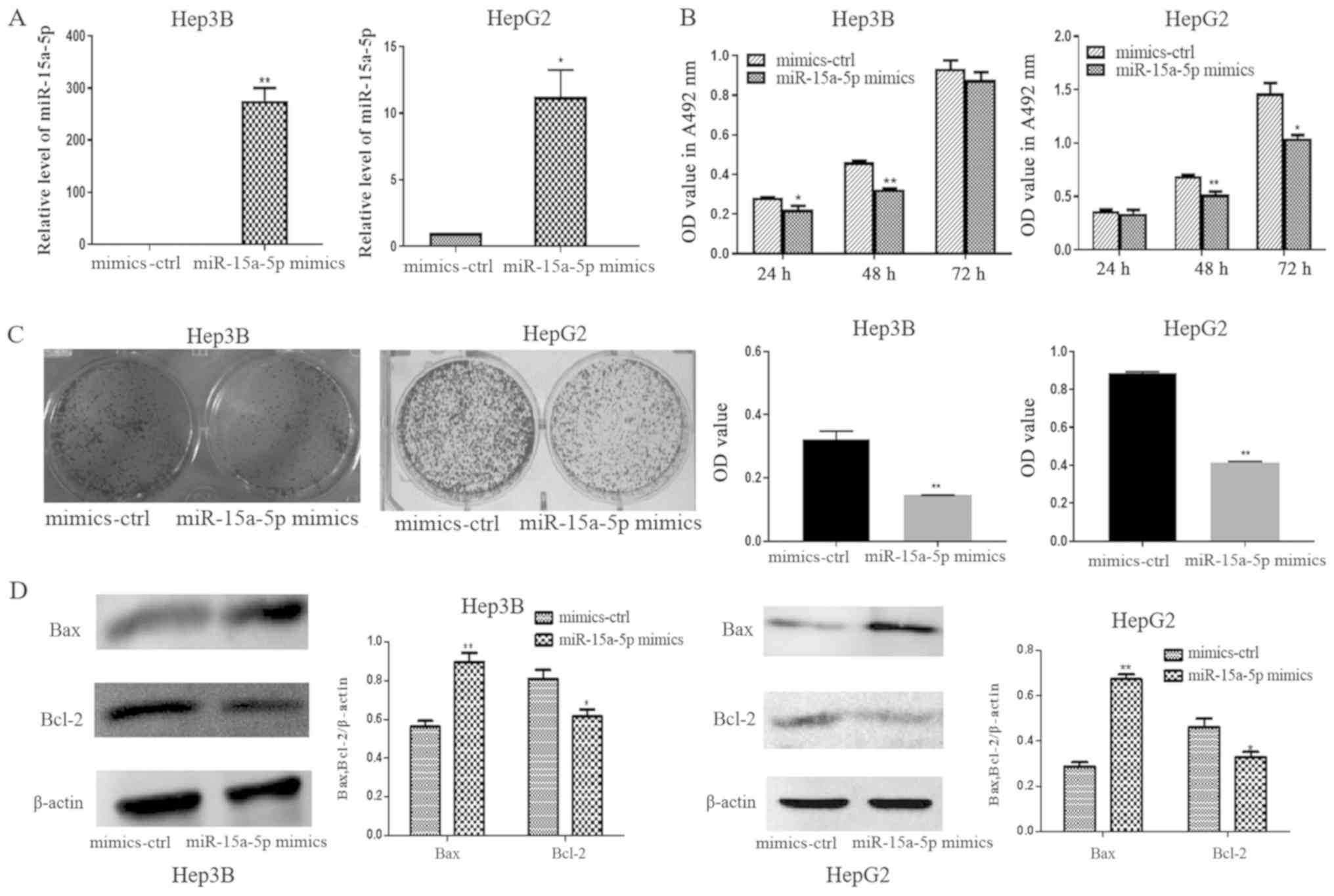
The function of miR-15a-5p was studied by
gain-of-function experiments following transfection of Hep3B and
HepG2 cells with the miR-15a-5p mimic. The expression of miR-15a-5p
in Hep3B and HepG2 cells transfected with the miR-15a-5p mimic was
significantly higher compared with that in cells transfected with
the mimic-ctrl (Fig. 2A). The MTT
and colony formation assays revealed significant proliferation
inhibition following transfection with the miR-15a-5p mimic
compared with mimic-ctrl. No significant differences were observed
between the miR-15a-5p mimic and mimic-ctrl in Hep3B cells at 72 h
(Fig. 2B and C). Bcl-2 was
downregulated and Bax was upregulated in the miR-15a-5p
mimic-transfected Hep3B and HepG2 cells compared with the
mimic-ctrl-transfected cells (Fig.
2D). These findings demonstrated that miR-15a-5p was
downregulated and may serve a role in suppressing cell
proliferation and inducing apoptosis in liver cancer cells.
Inhibition of miR-15a-5p contributes
to carcinogenesis in liver cancer cells
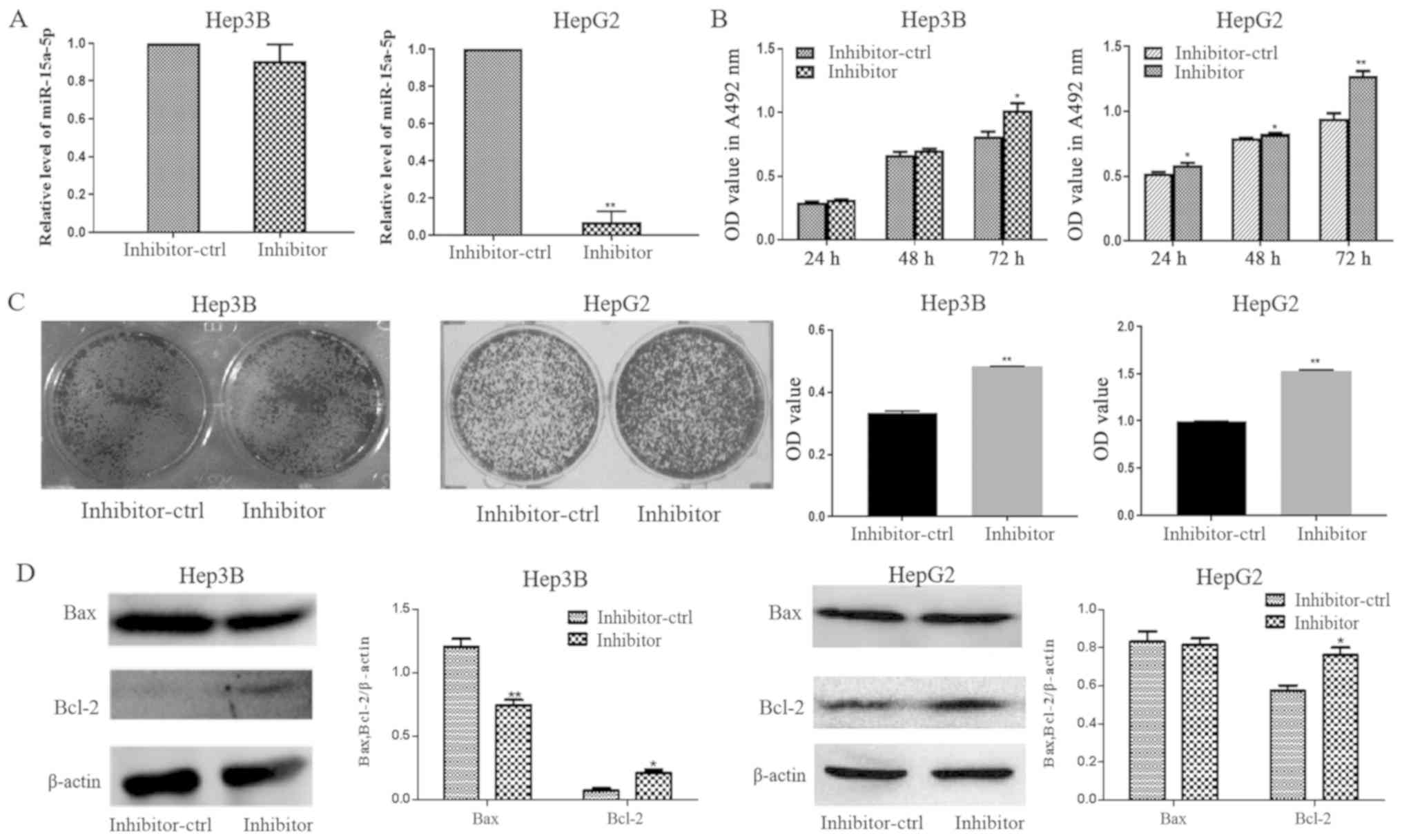
miR-15a-5p expression was decreased in HepG2 cells
transfected with an miR-15a-5p inhibitor compared with that in the
inhibitor control group (Fig. 3A).
The results from MTT assay revealed that vitamin D3 inhibited Hep3B
and HepG2 cell proliferation, and that the inhibitor could partly
rescue the inhibiting effect of vitamin D3, however, the difference
was not statistically significant (Fig.
S1). The proliferation and colony formation of Hep3B and HepG2
cells transfected with the miR-15a-5p inhibitor were increased
compared with the inhibitor control-transfected cells (Fig. 3B and C). Bcl-2 protein expression was
increased in Hep3B and HepG2 cells transfected with the miR-15a-5p
inhibitor, compared with cells transfected with the inhibitor
control (Fig. 3D).
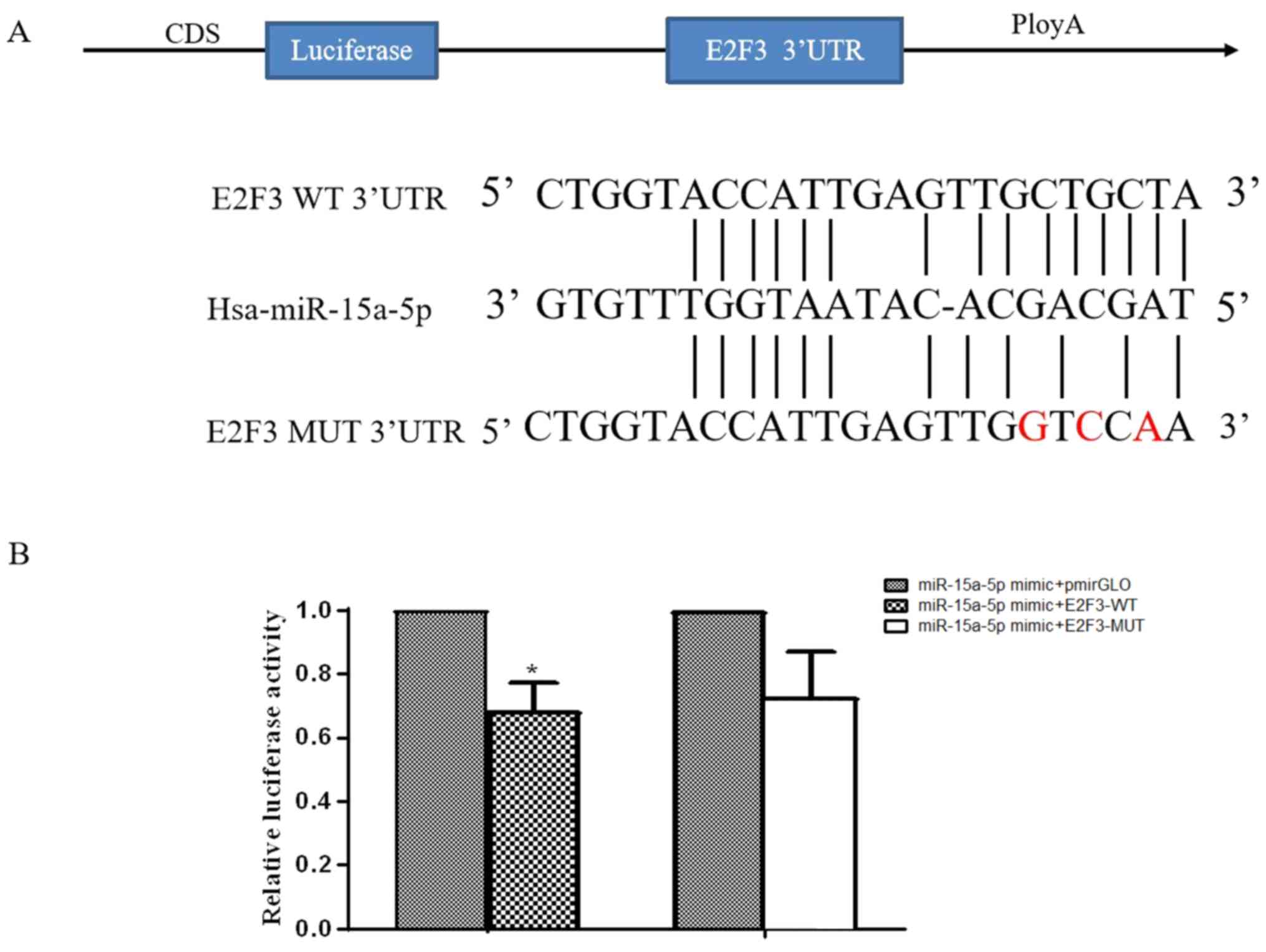
E2F3 is a target of miR-15a-5p
The 3′-UTR of E2F3 contained a potential miR-15a-5p
binding site (Fig. 4A). E2F3
expression has been demonstrated to be upregulated in various types
of cancer (21,22); thus, E2F3 was selected as a candidate
miR-15a-5p target gene. A luciferase reporter assay was performed
in 293 cells co-transfected with miR-15a-5p mimics and E2F3-WT or
-MUT vectors, or the pmirGLO vector. Luciferase activity was
significantly reduced in cells co-transfected with the miR-15a-5p
mimics and E2F3-WT vector compared with co-transfected with the
miR-15a-5p mimics and pmirGLO vector (Fig. 4B), which suggested that miR-15a-5p
directly targeted E2F3.
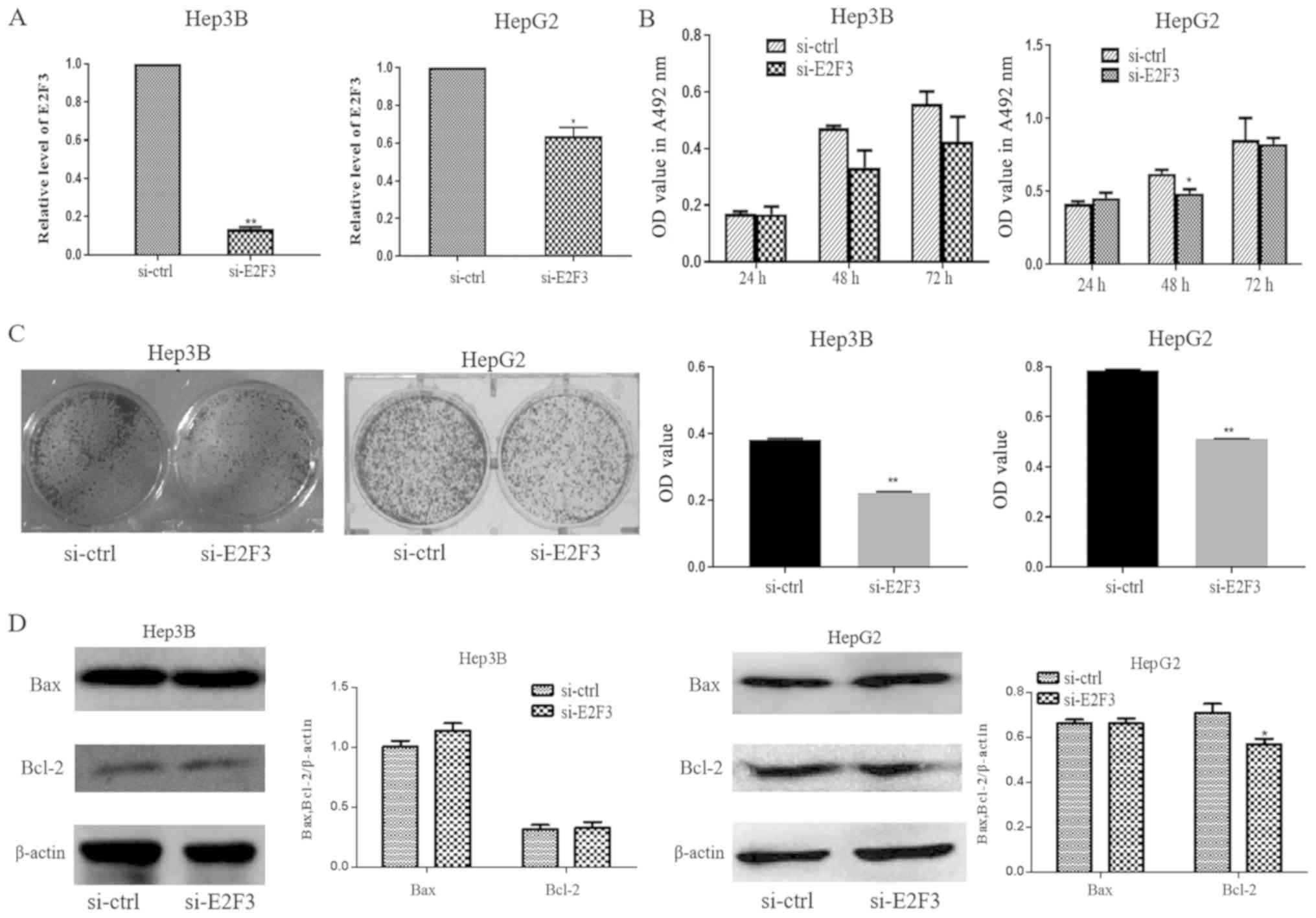
Silencing E2F3 suppresses liver cancer
cell proliferation
E2F3 mRNA expression was significantly decreased in
si-E2F3-transfected cells compared with si-control
(ctrl)-transfected cells (Fig. 5A).
Additionally, MTT assay results demonstrated that cell
proliferation was decreased at 48 h in si-E2F3-transfected HepG2
cells compared with si-ctrl transfected HepG2 cells (Fig. 5B). Hep3B and HepG2 cells transfected
with si-E2F3 formed fewer colonies compared with those of
si-ctrl-transfected cells (Fig. 5C).
Bcl-2 was downregulated in si-E2F3-transfected HepG2 cells
(Fig. 5D). These results indicated
that miR-15a-5p may function as a tumor suppressor by directly
targeting E2F3 transcription and that vitamin D3 may suppress the
proliferation of liver cancer cells by regulating the
miR-15a-5p/E2F3 axis.
Discussion
Vitamin D is a fat-soluble steroid hormone;
physiological events associated with the activation of vitamin D
signaling may influence the prevention and treatment of various
types of cancer (23). Mediation of
the anticancer activity of vitamin D by changes in miRNA expression
has been demonstrated both in vitro and in vivo
(24,25). In the present study, vitamin D3, the
active form of vitamin D, suppressed the proliferation and induced
apoptosis in liver cancer cells, which was consistent with its
activity as a tumor suppressor against the development of liver
cancer. Deregulation of miRNAs, including miR-15a-5p, has been
reported in various types of cancer (18–27). In
the present study, MTT assays in Hep3B and HepG2 cells demonstrated
significant proliferation inhibition following transfection with
the miR-15a-5p mimic compared with that in the controls. The
results suggested that miR-15a-5p functioned as a tumor suppressor
gene and that vitamin D3 may exert its anticancer effects by
increasing miR-15a-5p expression in liver cancer cells.
miRNAs interact with 3′-UTRs to inhibit the
expression of the target mRNA (28).
In the present study, Bioinformatics analysis revealed that the
3′-UTR of E2F3 contained a potential miRNA-15a-5p binding site. The
E2F family of transcription factors regulates the cell cycle,
proliferation and apoptosis (29,30).
E2F3 has been reported to be involved in various types of human
cancer, including nasopharyngeal carcinoma (31), hepatocellular carcinoma (32) and bladder cancer (33). It was demonstrated that E2F3 is
upregulated in HCC compared with normal controls, and that
overexpression of E2F3 could be associated with a poor prognosis in
HCC (34). The 3′-UTR of E2F3 mRNA
includes a number of miRNA seed sequences; in the present study,
the dual luciferase reporter assay revealed that E2F3 was a target
gene of miR-15a-5p. siRNA silenced E2F3 expression, and E2F3
silencing suppressed the proliferation, induced apoptosis and
decreased colony formation in liver cancer cells.
In conclusion, the results of the present study
provided novel evidence for the inhibition of Hep3B and HepG2 cell
proliferation by vitamin D3 and the molecular mechanisms involved.
These results provide a new therapeutic rationale and target for
the early diagnosis and treatment of liver cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
JW conceived the study. YL and SC performed the
experiments. QL and RZ analyzed the data. YL wrote the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellissimo F, Pinzone MR, Cacopardo B and
Nunnari G: Diagnostic and therapeutic management of hepatocellular
carcinoma. World J Gastroenterol. 21:12003–12021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu CY, Chen KF and Chen PJ: Treatment of
liver cancer. Cold Spring Harb Perspect Med. 5:a0215352015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Zhu S, Lin G, Song C and He Z:
Vitamin D enhances omega-3 polyunsaturated fatty acids-induced
apoptosis in breast cancer cells. Cell Biol Int. 41:890–897. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dormoy V, Beraud C, Lindner V, Coquard C,
Barthelmebs M, Brasse D, Jacqmin D, Lang H and Massfelder T:
Vitamin D3 triggers antitumor activity through targeting hedgehog
signaling in human renal cell carcinoma. Carcinogenesis.
33:2084–2093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan L, Matloob AF, Du J, Pan H, Dong Z,
Zhao J, Feng Y, Zhong Y, Huang B and Lu J: Vitamin D stimulates
apoptosis in gastric cancer cells in synergy with trichostatin
A/sodium butyrate-induced and 5-aza-2′-deoxycytidine-induced PTEN
upregulation. FEBS J. 277:989–999. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Chen C, Pan W, Gao M, He W, Mao R,
Lin T and Huang J: Comparative efficacy of vitamin D status in
reducing the risk of bladder cancer: A systematic review and
network meta-analysis. Nutrition. 32:515–523. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Louka ML, Fawzy AM, Naiem AM, Elseknedy
MF, Abdelhalim AE and Abdelghany MA: Vitamin D and K signaling
pathways in hepatocellular carcinoma. Gene. 629:108–116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caputo A, Pourgholami MH, Akhter J and
Morris DL: 1,25-Dihydroxyvitamin D(3) induced cell cycle arrest in
the human primary liver cancer cell line HepG2. Hepatol Res.
26:34–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iseki K, Tatsuta M, Uehara H, Iishi H,
Yano H, Sakai N and Ishiguro S: Inhibition of angiogenesis as a
mechanism for inhibition by 1alpha-hydroxyvitamin D3 and
1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by
azoxymethane in Wistar rats. Int J Cancer. 81:730–733. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Guo Q, Zhang Z, Bai N, Liu Z,
Xiong M, Wei Y, Xiang R and Tan X: VDR status arbitrates the
prometastatic effects of tumor-associated macrophages. Mol Cancer
Res. 12:1181–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeljic K, Supic G and Magic Z: New
insights into vitamin D anticancer properties: Focus on miRNA
modulation. Mol Genet Genomics. 292:511–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng Y and Croce CM: The role of MicroRNAs
in human cancer. Signal Transduct Target Ther. 1:150042016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying J, Yu X, Ma C, Zhang Y and Dong J:
MicroRNA-363-3p is downregulated in hepatocellular carcinoma and
inhibits tumorigenesis by directly targeting specificity protein 1.
Mol Med Rep. 16:1603–1611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dou C, Liu Z, Xu M, Jia Y, Wang Y, Li Q,
Yang W, Zheng X, Tu K and Liu Q: miR-187-3p inhibits the metastasis
and epithelial-mesenchymal transition of hepatocellular carcinoma
by targeting S100A4. Cancer Lett. 381:380–390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li D, Liu X, Lin L, Hou J, Li N, Wang C,
Wang P, Zhang Q, Zhang P, Zhou W, et al: MicroRNA-99a inhibits
hepatocellular carcinoma growth and correlates with prognosis of
patients with hepatocellular carcinoma. J Biol Chem.
286:36677–36685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue F, Yin J, Xu L and Wang B:
MicroRNA-143 inhibits tumorigenesis in hepatocellular carcinoma by
downregulating GATA6. Exp Ther Med. 13:2667–2674. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bandyopadhyay S, Mitra R, Maulik U and
Zhang MQ: Development of the human cancer MicroRNA network.
Silence. 1:62010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Long J, Jiang C, Liu B, Fang S and Kuang
M: MicroRNA-15a-5p suppresses cancer proliferation and division in
human hepatocellular carcinoma by targeting BDNF. Tumour Biol.
37:5821–5828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu B, Sun T, Wu G, Shang-Guan H, Jiang
ZJ, Zhang JR and Zheng YF: MiR-15a suppresses hepatocarcinoma cell
migration and invasion by directly targeting cMyb. Am J Transl Res.
9:520–532. 2017.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng Z, Peng C, Li D, Zhang D, Li X, Cui
F, Chen Y and He Q: E2F3 promotes cancer growth and is
overexpressed through copy number variation in human melanoma. Onco
Targets Ther. 11:5303–5313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun FB, Lin Y, Li SJ, Gao J, Han B and
Zhang CS: MiR-210 knockdown promotes the development of pancreatic
cancer via upregulating E2F3 expression. Eur Rev Med Pharmacol Sci.
22:8640–8648. 2018.PubMed/NCBI
|
|
23
|
Jeon SM and Shin EA: Exploring Vitamin D
metabolism and function in cancer. Exp Mol Med. 50:202018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ting HJ, Messing J, Yasmin-Karim S and Lee
YF: Identification of microRNA-98 as a therapeutic target
inhibiting prostate cancer growth and a biomarker induced by
Vitamin D. J Biol Chem. 288:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kasiappan R, Shen Z, Tse AK, Jinwal U,
Tang J, Lungchukiet P, Sun Y, Kruk P, Nicosia SV, Zhang X and Bai
W: 1,25-Dihydroxyvitamin D3 suppresses telomerase expression and
human cancer growth through MicroRNA-498. J Biol Chem.
287:41297–41309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang ZM, Wan XH, Sang GY, Zhao JD, Zhu QY
and Wang DM: miR-15a-5p suppresses endometrial cancer cell growth
via Wnt/β-catenin signaling pathway by inhibiting WNT3A. Eur Rev
Med Pharmacol Sci. 21:4810–4818. 2017.PubMed/NCBI
|
|
27
|
Kontos CK, Tsiakanikas P, Avgeris M,
Papadopoulos IN and Scorilas A: miR-15a-5p, A novel prognostic
biomarker, predicting recurrent colorectal adenocarcinoma. Mol
Diagn Ther. 21:453–464. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. Plos Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhan L, Zhang Y, Wang W, Song E, Fan Y and
Wei B: E2F1: A promising regulator in ovarian carcinoma. Tumour
Biol. 37:2823–2831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong W, Li J, Wang Y, Meng J and Zheng G:
miR-221 promotes lens epithelial cells apoptosis through
interacting with SIRT1 and E2F3. Chem Biol Interact. 306:39–46.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong Q, Huang J, Wei J and Wu R: Circular
RNA CDR1as sponges miR-7-5p to enhance E2F3 stability and promote
the growth of nasopharyngeal carcinoma. Cancer Cell Int.
19:2522019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang H, Zheng W, Shuai X, Chang RM, Yu L,
Fang F and Yang LY: MicroRNA-424 inhibits Akt3/E2F3 axis and tumor
growth in hepatocellular carcinoma. Oncotarget. 6:27736–27750.
2015.PubMed/NCBI
|
|
33
|
Feber A, Clark J, Goodwin G, Dodson AR,
Smith PH, Fletcher A, Edwards S, Flohr P, Falconer A, Roe T, et al:
Amplification and overexpression of E2F3 in human bladder cancer.
Oncogene. 23:1627–1630. 2003. View Article : Google Scholar
|
|
34
|
Zeng X, Yin F, Liu X, Xu J, Xu Y, Huang J,
Nan Y and Qiu X: Upregulation of E2F transcription factor 3 is
associated with poor prognosis in hepatocellular carcinoma. Oncol
Rep. 31:1139–1146. 2014. View Article : Google Scholar : PubMed/NCBI
|